Abstract
The trichothecene mycotoxin deoxynivalenol (DON) induces systemic expression of the interleukin-6 (IL-6) and other proinflammatory cytokines in the mouse. The purpose of this study was to test the hypothesis that DON triggers an endoplasmic reticulum (ER) stress response in murine macrophages capable of driving IL-6 gene expression. DON at concentrations up 5000 ng/ml. was not cytotoxic to peritoneal cells. However, DON markedly decreased protein levels but not the mRNA levels of glucose-regulated protein (GRP) 78 (BiP), a chaperone known to mediate ER stress. Inhibitor studies suggested that DON-induced GRP78 degradation was cathepsin and calpain dependent but was proteosome-independent. RNAi-mediated knockdown of GRP78 resulted in increased IL-6 gene expression indicating a potential downregulatory role for this chaperone. GRP78 is critical to the regulation of the two transcription factors, X-box binding protein 1 (XBP1) and activating transcription factor 6 (ATF6), which bind to cAMP-response element (CRE) and drive expression of CRE-dependent genes such as IL-6. DON exposure was found to increase IRE1α protein, its modified products spliced XBP1 mRNA and XBP1 protein as well as ATF6. Knockdown of ATF6 but not XBP1 partially inhibited DON-induced IL-6 expression in the macrophages. Three other trichothecenes (satratoxin G, roridin, T-2 toxin) and the ribosome inhibitory protein ricin were also found to induce GRP78 degradation suggesting that other translation inhibitors might evoke ER stress. Taken together, these data suggest that in the macrophage DON induces GRP78 degradation and evokes an ER stress response that could contribute, in part, to DON-induced IL-6 gene expression.
Keywords: deoxynivalenol (DON); interleukin-6; ER stress, translation inhibition
Membrane and secretory proteins synthesized in the endoplasmic reticulum (ER) need to be folded properly with the assistance of ER chaperones and folding enzymes located in the ER (Anelli and Sitia, 2008). Cytotoxicity, nutrient limitation, and accumulation of unfolded or misfolded proteins can initiate activation of a series of self-defense mechanisms known as the ER stress response or unfolded protein response (UPR) (Schroder, 2008; Zhang and Kaufman, 2006). The ER chaperone GRP 78/BiP serves as a master controller of this stress response (Quinones et al., 2008).
GRP78 interacts with two ER stress sensors known as activating transcription factor 6 (ATF6) and inositol requiring enzyme 1 (IRE1), the latter of which mediates expression of the transcription factor XBP1 (Zhang and Kaufman, 2006). Upon accumulation of unfolded proteins in the ER lumen, GRP78 is titrated away thus releasing the transcription factor ATF6 and the enzyme IRE1 α which mediates expression of the transcription factor XBP1 (Kaufman, 1999; Zhang and Kaufman, 2006; Zhang et al., 2006).
ER stress is involved in inflammation, neurodegeneration, diabetes mellitus, heart diseases, and kidney diseases (Han et al., 2008; Lin et al., 2008; Marciniak and Ron, 2006). Both lipopolysaccharide (Endo et al., 2005, 2006) and proinflammatory cytokines (Nowis et al., 2007; Oliver et al., 2005) induce ER stress leading to expression of acute response proteins. ER stress has also been related to autoimmune diseases including rheumatoid arthritis (Gao et al., 2008; Purcell et al., 2003) and autoimmune myositis (Nagaraju et al., 2005). Accordingly, aberrant ER stress responses can contribute to disease.
Deoxynivalenol (DON) is a trichothecene mycotoxin produced by Fusarium sp. that is prevalent worldwide in cereal-based foods (Pestka and Smolinski, 2005). Toxicological effects associated with trichothecene mycotoxin poisoning in humans and animals include anorexia, gastroenteritis, emesis and hematological disorders. The innate immune system is particularly sensitive to DON with exposure to low trichothecene doses induces rapid, transient upregulation of proinflammatory cytokines causing immune stimulation and exposure to high doses causing apoptosis in lymphoid tissues resulting in immunosuppression (Pestka, 2008).
Chronic dietary exposure of mice to DON results in dramatic elevation in serum IgA, serum IgA immune complexes and IgA deposition in the mouse kidney, all of which mimic the early stages of human IgA nephropathy (Pestka, 2003; Pestka et al., 1989). IgA dysregulation is mediated by DON-induced IL-6 expression which drives differentiation and proliferation of IgA-secreting B cells (Pestka and Zhou, 2000; Yan et al., 1997, 1998). We have observed in peritoneal macrophages that the transcription factor cAMP response element binding protein (CREB) promotes IL-6 mRNA transcription by binding to cAMP response element (CRE) (Jia et al., 2006; Shi and Pestka, 2006, 2009).
Using differential display, it has been demonstrated that DON exposure decreases GRP78 expression in EL-4 T cells (Yang et al., 2000). Downregulation of GRP78 could potentially induce ER stress and activation of ATF6 and XBP1 (via IRE1). Because ATF6 and XBP1 belong to the CREB/ATF family, they can upregulate IL-6 expression by binding to CRE (Hai and Hartman, 2001; Kanemoto et al., 2005; Schroder and Kaufman, 2005). Therefore, activation of one or both of these transcription factors might contribute to DON-induced upregulation of IL-6.
The purpose of this research was to test the hypothesis that DON triggers an ER stress response capable of driving IL-6 gene expression in the murine macrophage. We demonstrate in mouse peritoneal macrophages that DON induces GRP78 degradation but causes upregulation of IRE 1, XBP1, and ATF6 expression. Our results further indicate that these responses contribute in part to induction of IL-6 expression by DON.
MATERIALS AND METHODS
Materials.
All inhibitors were purchased from Calbiochem, Inc. (San Diego, CA).
All chemicals including DON (purity ≥ 98%) and cell culture components were purchased from Sigma-Aldrich, Inc. (St Louis, MO) unless otherwise noted. DON, roridin A, satratoxin G, ricin, and T-2 toxin were dissolved in pyrogen-free water and tunicamycin (10 mg/ml) was dissolved in dimethyl sulfoxide (DMSO) as stock solutions. Inhibitors ALLN, EPO, CATI-I, and CALI-III were dissolved in DMSO at 50, 10, 50, and 25mM concentrations respectively. DON-contaminated labware and cell culture media were detoxified by sodium hypochlorite.
Animals.
Female B6C3F1 mice (7 weeks old) were obtained from Charles River Laboratories, Inc. (Wilmington, MA). Housing, handling, and sample collection procedures conformed to the policies of the Michigan State University All-University Committee on Animal Use and Care in accordance with National Institutes of Health guidelines. Mice were provided free access to food and water.
Peritoneal macrophage cultures.
Mice were injected ip with 1.5 ml of sterile 3% (wt/vol) thioglycollate broth. After 3 days, the animals were euthanized and macrophages collected by peritoneal lavage with ice-cold Hank's balanced salt solution (BSS) (Invitrogen Corporation, Carlsbad, CA). Cells were pelleted by centrifugation at 1100 × g for 5 min, washed with BSS once and resuspended in RPMI-1640 containing 10% (vol/vol) heat-inactivated fetal bovine serum (Atlanta Biologicals, Norcross, GA), 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were cultured at 37°C under 6% CO2 in a humidified incubator for 24 h prior to toxin treatment.
Cytotoxicity.
The potential cytotoxic effects of DON in peritoneal macrophages were assessed by both the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2-tetrazolium bromide (MTT) and Alamar Blue assays. For the former (Mosmann, 1983), macrophages were cultures in 96-well plates and treated with DON for 2–12 h. Filter-sterilized MTT (5 mg/ml in PBS, pH 7.4) was added at 10% (vol/vol) to each well and plates incubated for 3 h at 37°C. After removing medium, 100 μl of DMSO (Sigma) was added to each well and the plate was read at 570 nm using 690 nm as the reference wavelength on a Synergy HT Multi-Mode Microplate Reader (BioTek Instruments, Inc., Winooski, VT). In addition, the Invitrogen Alamar Blue Assay kit (Carlsbad, CA) was used according to the manufacturer's instructions. Briefly, peritoneal macrophages were cultured in 96-well with DON for 0 and 6 h, 10% (vol/vol) AlamarBlue reagent added to each well, and the cultures incubated for 6 h at 37°C. Absorbance was measured at 570 nm using 690 nm as the reference wavelength on the microplate reader.
Experimental design.
In a typical experiment, macrophages were incubated with or without DON in the presence or absence of inhibitors for various time periods and analyzed for expression proteins by Western analysis or specific mRNAs by real-time PCR. For protein collection, cells (1 × 106 /ml) were incubated in 100-mm-diameter cell culture dishes (Corning Life Sciences, Lowell, MA) containing 10 ml of culture medium. Two milliliters of cell suspension (1 × 106 /ml) was incubated in each well of 6-well cell culture plates (Corning Life Sciences) for experiments requiring RNA isolation.
Western blot analysis.
Macrophages were lysed in Tris buffer (10mM, pH 7.4) containing 2% (wt/vol) sodium dodecyl sulfate, protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN), boiled and sonicated. After centrifugation at 18,000 × g for 15 min, supernatants were subjected to Western analysis (Shi and Pestka, 2009) using specific antibodies to GRP78 or IRE1α (Cell Signaling Technology, Inc., Danvers, MA), XBP1, ATF6 (Santa Cruz Biotechnology, Inc.), and β-actin (Sigma-Aldrich). Alexa Fluor 680 goat-anti rabbit and IRDye 800 goat-anti mouse secondary antibodies were purchased from Invitrogen Corporation and Rockland Immunochemicals, Inc. (Gilbertsville, PA), respectively. Infrared fluorescence was measured using an Odyssey Infrared Imaging System (Li-Cor Biosciences, Lincoln, NE).
Real-time PCR.
Total RNA of peritoneal macrophages was extracted using RNeasy Mini kits (Promega, Madison, WI) and mRNAs quantified by real-time PCR. TaqMan primers and probes for IL-6 mRNA were purchased from Applied Biosystems (Foster City, CA). The primer sequences for unspliced (u) and spliced XBP1 (s) were designed as follows: (forward) 5′-tgg ccg ggt ctg ctg agt ccg-3′ (u), 5′-ctg agt ccg cag cag gtg cag-3′ (s); (reverse) 5′-gtc cat ggg aag atg ttc tgg-3′ (u and s). SYBER Green PCR Master Mix (Applied Biosystems) was used for real-time PCR to detect unspliced and spliced XBP1 mRNA. β-2 Microglobulin RNA expression was not affected by DON treatment and thus was used as endogenous control to normalize target gene expression. Target gene expression levels were calculated relative to the control group.
siRNA knockdown.
siRNA cocktails targeting mouse GRP78, XBP1, ATF6, or a comparable scrambled siRNAs were purchased from Dharmacon (Lafayette, CO). siRNA transfection was performed in peritioneal macrophages by electroporation using an Amaxa Nucleofector (Amaxa, Inc., Gaithersburg, MD) (Loniewski et al., 2008; Shi and Pestka, 2008). Briefly, 2 × 106 cells were suspended in 100 μl of electroporation buffer (Amaxa, Inc.) and mixed with 10μM siRNA. Electroporation was performed using program D032 for macrophages according to the manufacturer's protocol. Transfection efficacy was verified by assessing reduction of GRP78, XBP1, or ATF6 proteins by Western blot after 48 h of transfection.
Statistics.
All data were analyzed with SigmaStat v 3.1 (Jandel Scientific, San Rafael, CA) with the criterion for significance set at p < 0.05. Student's t-test was used for comparison of two groups of data; and one-way ANOVA was performed for comparison of multiple groups. Holm-Sidak (if normality test passed) or Dunn (ANOVA on ranks if normality test failed) tests were used for post hoc analysis.
RESULTS
The cytotoxic effects of DON were evaluated in cultured peritoneal macrophages. Both the MTT (Fig. 1A) and Alamar Blue (Fig. 1B) assays revealed that macrophages were remarkably recalcitrant to DON at concentrations up to 5000 ng/ml for 12 h.
FIG. 1.
DON is not cytotoxic to peritoneal macrophages. DON cytotoxicity was assessed in cultured peritoneal macrophages by the (A) MTT and (B) Alamar Blue assays.
Following incubation of peritoneal macrophages with DON (500 ng/ml), GRP78 was reduced markedly after 60 min and decreased further after 180 and 360 min as compared with vehicle-treated cells (Fig. 2A). The effects of different DON concentrations on GRP78 disappearance were assessed (Fig. 2B). DON at 500 ng/ml induced prominent decreases in GRP78 protein within 3 h, whereas as little as 100 ng/ml DON diminished GRP78 protein after 6 h. Based on these findings, a DON concentration of 500 ng/ml was chosen for subsequent studies on GRP78 reduction from the perspective of potential upstream mechanisms and downstream effects.
FIG. 2.
Kinetics and concentration-dependence of DON-induced GRP78 degradation in peritoneal macrophages. Following exposure to DON, peritoneal macrophages were cultured with DON (500 ng/ml) or water vehicle (VH) for various intervals. Total protein was extracted and GRP78 was analyzed by Western blot. (A) Kinetics of GRP78 degradation during exposure to 500 ng/ml DON. (B) Concentration-dependent DON-induced GRP78 degradation. RI indicates relative intensity which is the percentage of the maximal fluorescence in the same lane.
Although DON at 500 ng/ml induced robust time-dependent decreases in GRP78 protein (Fig. 3A), GRP78 mRNA was unaffected in peritoneal macrophages after DON treatment (Fig. 3B). Thus decreased GRP78 protein was not likely to be a direct result of decreased GRP78 gene expression.
FIG. 3.
DON treatment does not affect GRP78 gene expression in peritoneal macrophages. Peritoneal macrophages were treated with DON (500 ng/ml) for 0, 1, 3, 6, or 12 h. (A) GRP78 protein was detected by Western blot. RI indicates relative intensity which is the percentage of the maximal fluorescence in the same lane. (B) Total RNA was collected and GRP78 mRNA was analyzed by real-time PCR. Data are means ± SE.
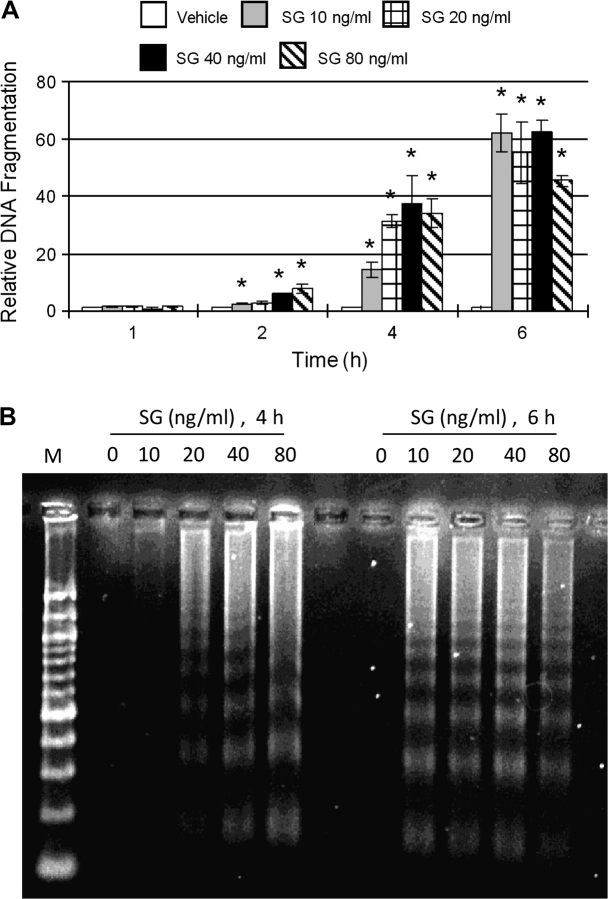
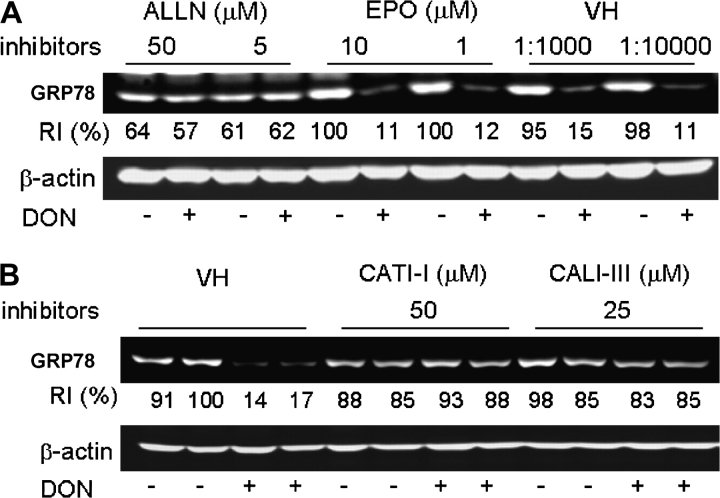
Specific inhibitors were employed to determine if the observed reduction of GRP78 was related to proteolysis. DON-induced GRP78 degradation in peritoneal macrophages was markedly inhibited by preincubation with ALLN which inhibits both cathepsins and calpains, whereas there was no inhibition by the proteasome inhibitor epoxomicin (Fig. 4A). Preincubation with either cathepsin inhibitor I (CATI-I) and calpain inhibitor III (CALI-III) blocked DON-induced GRP78 degradation (Fig. 4B). These data suggest that GRP78 loss was possibly related to an autophagic pathway.
FIG. 4.
DON-induced GRP78 degradation in peritoneal macrophages is cathepsin/calpain dependent. (A) Peritoneal macrophages were incubated with ALLN, epoxomicin (EPO), or equivalent concentrations of DMSO (VH) for 1 h, and then incubated with DON (500 ng/ml) for 3 h. (B) Peritoneal macrophages were incubated with inhibitors to cathepsins (CATI-I) or calpains (CALI-III) or equivalent concentrations of DMSO (VH) for 1 h, and then incubated with DON (500 ng/ml) for 3 h. Total protein was extracted and GRP78 was analyzed by Western blot. RI indicates relative intensity which is the percentage of the maximal fluorescence in the same lane.
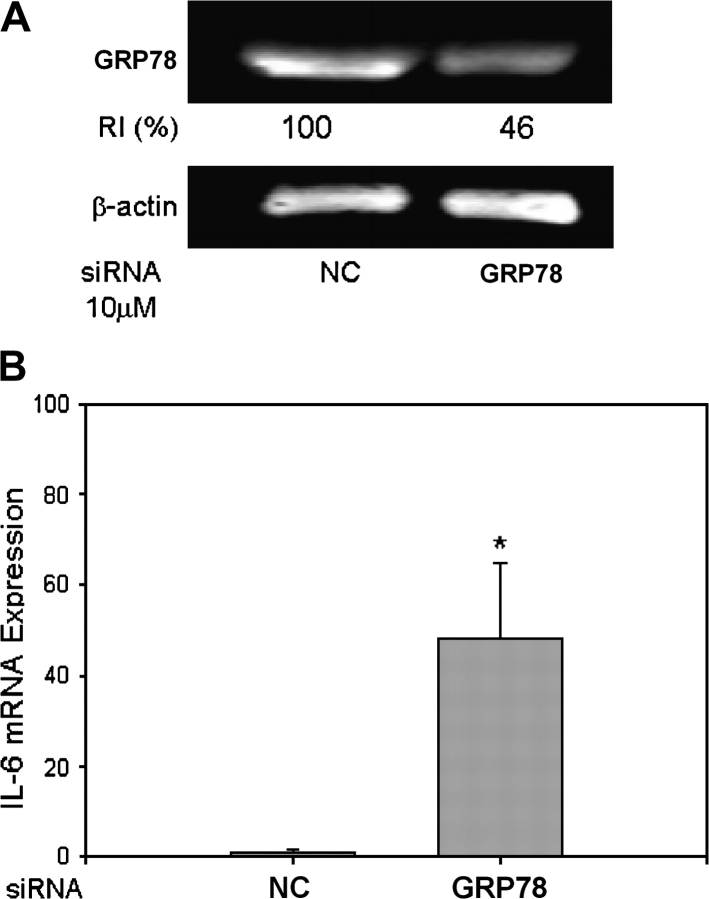
To confirm a relationship between DON-induced GRP78 degradation and IL-6 gene expression, GRP78 was knocked down by siRNA transfection. GRP78 protein reduction was detectable within 48 h after transfection (Fig. 5A) and found to correlate with upregulated IL-6 mRNA expression (Fig. 5B).
FIG. 5.
GRP78 knockdown induces IL-6 gene expression in peritoneal macrophages. siRNA specific to mouse GRP78 or scrambled siRNA (negative control, NC) was transfected by electroporation into peritoneal macrophages. (A) To evaluate knockdown efficiency, total protein was collected after 48 h. GRP78 was measured by Western blot. RI indicates relative intensity which is the percentage of the maximal fluorescence in the same lane. (B) To detect the correlation of GRP78 knockdown and IL-6 gene expression, total RNA was extracted and IL-6 mRNA was analyzed by real-time PCR. Data are means ± SE (n = 3). Asterisk indicates statistically different from control group (p < 0.05).
Concentrations of IRE-1α, an ER stress sensor activated upon dissociation from GRP78, were increased in peritoneal macrophages within 1 and 3 h after DON treatment (Fig. 6A). Because XBP1 RNA is known to be spliced by activated IRE1α, levels of unspliced and spliced XBP1 mRNA were compared following DON treatment for various time intervals. Concentrations of spliced XBP-1 mRNA were upregulated by DON whereas unspliced XBP-1 mRNAs were unaffected (Fig. 6B). DON was further observed to induce modest increases in the transcription factors XBP1 and ATF6 in peritoneal macrophages (Fig. 7).
FIG. 6.
DON upregulates IRE1α, and induces XBP1 mRNA splicing in peritoneal macrophages. Peritoneal macrophages were cultured with DON (500 ng/ml) for various time intervals. (A) Total protein was extracted and assessed for IRE1α . RI indicates relative intensity which is the percentage of the maximal fluorescence in the same lane. (B) Total RNA was extracted and relative concentration of unspliced and spliced XBP1 mRNA determined by real-time PCR. Data are means ± SE (n = 3). Bars with different letters differ (p < 0.05).
FIG. 7.
DON induces XBP1 and ATF6 in peritoneal macrophages. Peritoneal macrophages were cultured with DON (500 ng/ml) for various time intervals. XBP1 and ATF6 were analyzed by Western blot. RI indicates relative intensity which is the percentage of the maximal fluorescence in the same lane.
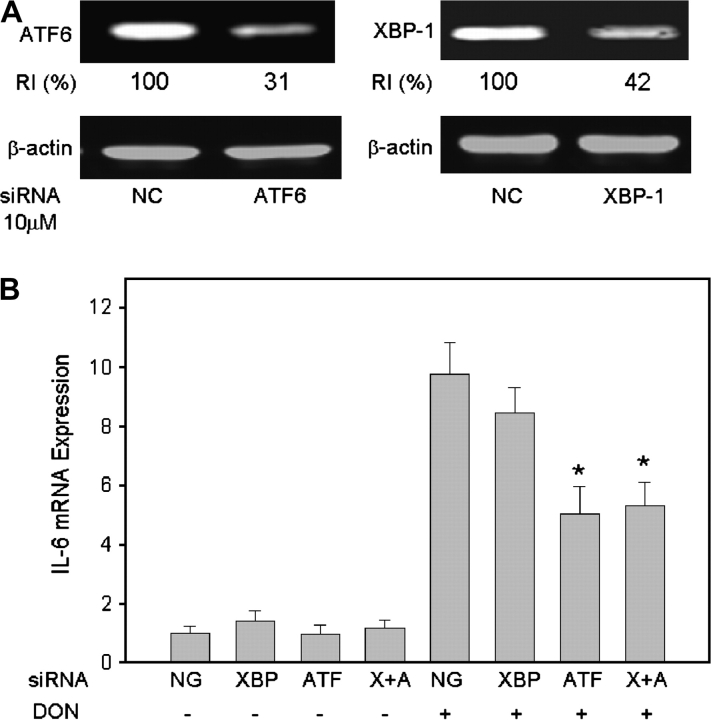
To ascertain whether DON-induced IL-6 gene expression is mediated by components of the ER stress response, ATF6 and/or XBP1 were knocked down by transfection with specific siRNAs. ATF6 and XBP1 protein concentrations decreased markedly 48 h after siRNA transfection compared with negative control (Fig. 8A). Knockdown of transcription factor ATF6 or both ATF-6 and XBP1 decreased DON-induced IL-6 expression, whereas no effect was evident following knockdown of only transcription factor XBP1 (Fig. 8B). This indicates that ATF6, but not XBP1, is involved in IL-6 expression induced by DON.
FIG. 8.
DON-induced IL-6 mRNA expression correlates to ATF6 activation in peritoneal macrophages. siRNA specific to ATF6, XBP1, or scrambled siRNA (negative control, NC) was transfected into peritoneal macrophages by electroporation (A) ATF6 and XBP1 knockdown efficiency. Total protein was collected after 48 h. ATF6 and XBP1 were measured by Western blot. RI indicates relative intensity which is the percentage of the maximal fluorescence in the same lane. (B) Effect of ATF6 and XBP1 knockdown on IL-6 mRNA expression, cells were treated with DON after transfection with siRNA specific to XBP-1 (XBP), ATF6 (ATF), or both (X + A). Total RNA was collected 3 h later and IL-6 mRNA was analyzed by real-time PCR. Data are means ± SE (n = 3). Bars with different letters differ (p < 0.05).
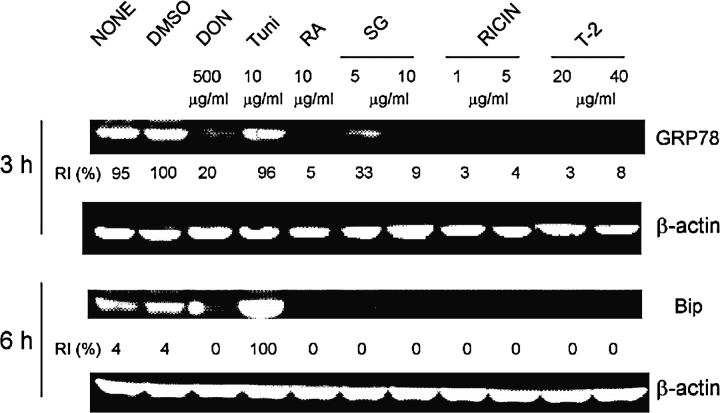
To discern if GRP78 degradation was a general effect induced by other translational inhibitors, GRP78 protein levels were measured in peritoneal macrophages that were treated with three trichothecenes roridin A, satratoxin G, and T-2 toxin, as well as the ribosomal inhibitory protein ricin. Tunicamycin, a classic ER stress inducer, was also used as a positive control. All of the toxin treatments induced GRP78 degradation at 3 and 6 h, whereas tunicamycin upregulated BiP dramatically at 6 h (Fig. 9).
FIG. 9.
GRP78 degradation is induced by different toxins in peritoneal macrophages. Peritoneal macrophages were treated with DON, tunicamycin (Tuni), roridin A (RA), satratoxin G (SG), ricin, and T-2 toxin for 3 or 6 h. GRP78 protein was measured by Western blot. RI indicates relative intensity which is the percentage of the maximal fluorescence in the same lane.
DISCUSSION
The ER stress response is evoked by accumulation and aggregation of nascent, unfolded or misfolded polypeptides in the ER (Ma and Hendershot, 2001). To maintain ER homeostasis, cells initiate processes that augment the ER capacity of protein folding by evoking transient attenuation of protein translation, ER-associated degradation of misfolded proteins and the induction of molecular chaperones. If the stress is not relieved by these responses in badly damaged cells, apoptotic pathways will be activated.
GRP78, which belongs to heat shock protein 70 family, is one of the most well-characterized ER chaperones and plays a critical role in activating ER stress sensors (Zhang and Kaufman, 2006). By recognizing and binding to hydrophobic domain of unfolded proteins, GRP78 stabilizes unfolded proteins thereby enabling modification. Of particular relevance to the model described herein, GRP78 interacts directly with the ER stress sensors ATF6 and IRE1α to maintain them in inactive forms. When unfolded proteins accumulate in the ER lumen, GRP78 is titrated away thereby releasing ATF6 and IRE1 α and enabling them to transduce signals to the cytosol and the nucleus (Kaufman, 1999; Zhang and Kaufman, 2006; Zhang et al., 2006).
Activated IRE1 α will cleave XBP1 mRNA by its inherent RNase activity thereby yielding spliced XBP1 mRNA that codes for a second activated transcription factor capable of modulating gene expression (Ron and Hubbard, 2008). Activated ATF6 translocates from ER to Golgi where it is cleaved (from 90 to 50 kDa) by two proteases, S1P and S2P to become an active transcription factor (Shen et al., 2002). As shown here, DON-induced degradation GRP78 in the peritoneal macrophages closely corresponded to activation and increases in IRE1, XBP1, and ATF6 proteins. It should be noted that although ER stress response is conventionally thought to include eventual upregulation of GRP78 as is observed for tunicamycin, DON did not have this effect. This difference might relate to selective inhibition of GRP78 translation by DON or its selective degradation.
The ER stress response has been previously associated with dysregulation of immune processes. For example, enhanced ER stress response activates transcription factor nuclear factor kappa B (NF-κB) and cyclic AMP response element binding protein H (CREBH) and induce proinflammatory gene expression (Deng et al., 2004; Hu et al., 2006; Hung et al., 2004; Zhang et al., 2006; Li et al., 2005). Activation of XBP1 and ATF6 is also required for differentiation of B lymphocytes into antibody-producing plasma cells (Gass et al., 2002; Iwakoshi et al., 2003). Our results also showed that GRP78 knockdown directly induced IL-6 upregulation, and further indicating that the ATF6 could be upstream of IL-6 expression. Thus the ER stress response is likely to contribute to DON-induced IL-6 gene expression.
The capacity of DON and other ribotoxins to promote GRP degradation might have relevance to cancer chemotherapy. GRP78 is considered anti-apoptotic in the ER stress response and, interestingly, this chaperone is induced in a many types of cancers (Li and Lee, 2006). Both overexpression and siRNA studies have demonstrated that GRP78 contributes to tumor growth and confers drug resistance to cancer cells. There are relatively few reported examples of agents which promote degradation or downregulation of GRP78. Subtilase cytotoxin (SubAB), an AB toxin produced by Shiga-toxigenic Escherichia coli, causes GRP78 cleavage in Vero cells (Morinaga et al., 2008). It is believed that subunit B binds to a surface receptor and that upon internalization, subunit B traffics through the Golgi where it cleaves GRP78. Sorafenib, a multikinase inhibitor that induces apoptosis in human leukemia and other malignant cells, causes reduction of GRP78 protein in U937 human monocytes. The mechanisms of these effects however, are not known (Rahmani et al., 2007). Other natural compounds shown to downregulate GRP78 via as yet unidentified mechanisms include (1) deoxyverrucosidin from Penicillium (Choo et al., 2005), (2) efrapeptin J from the marine fungus Tolypocladium (Hayakawa et al., 2008), and (3) prunustatin A from Streptomyces sp. (Umeda et al., 2005).
A critical question arising from this study relates to how GRP78 is degraded in the macrophage after DON treatment. This could be explained by either a decreased rate of synthesis or increased rate of degradation. Because GRP78 mRNA was unchanged following DON treatment, decreased GRP78 is not likely to be due to reduced expression. Eukaryotic cells have two major avenues for protein degradation, the ubiquitin-proteasome and autophagy-lysosomal pathways (Cecarini et al., 2007; Yorimitsu and Klionsky, 2005).
Protein ubiquitination and proteasome-mediated protein degradation is a key pathway for removal of misfolded and short-lived intracellular proteins (Guerrero et al., 2006; Rubinsztein, 2006). The 26S proteasome is a 2.5-MDa complex composed of two multisubunit subcomplexes: one is a 20S core particle and the other a 19S regulatory particle (Demartino and Gillette, 2007). The core particle is characterized by chymotrypsin-like, trypsin-like, and peptidylglutamyl peptide-hydrolyzing activities (Cuervo and Dice, 1998). Epoxomicin is a potent, highly specific and irreversible inhibitor of chymotrypsin-like, trypsin-like, and peptidylglutamyl peptide-hydrolyzing activities of the proteasome. The inability of epoxomicin to block DON-induced GRP78 degradation, suggests that proteaseome-mediated pathways are not involved.
Autophagy is another mechanism for the degradation of cytoplasmic components during ER stress and putative targets can range from single macromolecules (proteins, lipids and nucleic acids) to entire organelles (Yorimitsu and Klionsky, 2005). Once inside the lysosomal system, the substrates are degraded by cathepsins which is a mixture of more than 80 types of proteases, peptidases and other hydrolases (Cecarini et al., 2007; Cuervo and Dice, 1998; Grinyer et al., 2007). ALLN is an inhibitor of cathepsins, calpains and, at higher concentrations, proteasome. ALLN nearly abolished degradation of GRP78. Subsequent experiments with more specific inhibitors showed that DON-induced GRP78 degradation is cathepsin/calpain dependent. Thus, DON appeared to initiate an autophagy pathway in which GRP78 was degraded.
We have previously demonstrated the dsRNA-activated protein kinase (PKR) plays a critical role in DON-induced proinflammatory gene expression and apoptosis in mononuclear phagocytes (Pestka, 2008). The results presented herein suggest the ER stress response might be a second complementary pathway by which DON and other trichothecenes affect innate immune function. In addition to ATF6 and IRE1α, the PKR-like ER kinase (PERK) is another ER stressor sensor under regulation of GRP78 (Kincaid and Cooper, 2007). PERK, like PKR, can induce phosphorylation of eukaryotic initiation factor 2α (EIF2α) which can result in translational inhibition. Future studies should determine whether DON and other trichothecenes affect PERK activity and whether cross-talk occurs between PERK and PKR exists.
It should be noted that DON's effects in cell cultures are likely to be very complex and will vary depending on cell type, species source and presence or absence of a costimulatory signal. For example, ATF-3, another member of CREB/ATF family that could impact IL-6, is upregulated in DON-treated epithelial cells (Yang et al., 2009). In porcine pulmonary alveolar macrophages, DON induces IL-1β mRNA in unstimulated as well as LPS-stimulated macrophages, whereas tumor necrosis factor-α is overinduced in stimulated porcine macrophages and IL-6 seems to be of less importance (Döll et al., 2009).
Although DON induces apoptosis in several leukocyte cell lines such as RAW 264.7, U937, and Jurkat cells (Pestka et al., 2005; Yang et al., 2000; Zhou et al., 2005a), peritoneal macrophages were remarkably resistant to this DON at concentrations up to 5000 ng/ml for the time periods up to 12 h as indicated by two different cytotoxicity assays. It might be speculated that, in the primary cultures, anti-apoptotic signals such as ERK and AKT phosphorylation might exceed apoptotic signals, however, this requires further exploration .
The concentrations employed for the GRP78 studies (100–2000 ng/ml) here are consistent with those previously used to induce proinflammatory gene expression in mononuclear phagocytes (Gray and Pestka, 2007; Islam et al., 2006; Wong et al., 1998; Zhou et al., 2005b). These concentrations are comparable to those attained in tissues of mice gavaged orally with DON at 5 mg/kg and sufficient to induce proinflammatory cytokine expression in spleen, liver, and lungs (Pestka and Amuzie, 2008).
To summarize, the results presented here suggest that DON induces GRP78 degradation and an ensuing ER stress response. The mechanisms for GRP78 autophagy are unclear. One possibility, however, is that it is translation suppression per se and/or that ribosome disruption evokes misfolding of proteins which are sequestered by GRP78 released from the ER. These GRP-containing complexes could enter into the autophagy pathway and be degraded. The ensuing ER stress-like response potentially contributes to DON-induced IL-6 expression in the macrophage. It is widely recognized that ER stress initially triggers evolutionarily conserved signal-transduction events designed to ameliorate the unfolded proteins accumulation in the ER, but if these events are severe or protracted they can induce apoptosis. Future research will focus on identifying critical events immediately upstream and downstream to GRP degradation as well as the relative contributions of this pathway to the upregulation of inflammatory genes and apoptosis.
FUNDING
Public Health Service Grants (DK058833 and ES03358).
Acknowledgments
We would like to acknowledge the assistance of Mary Rosner in preparation of this manuscript.
References
- Anelli T, Sitia R. Protein quality control in the early secretory pathway. EMBO J. 2008;27:315–327. doi: 10.1038/sj.emboj.7601974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecarini V, Gee J, Fioretti E, Amici M, Angeletti M, Eleuteri AM, Keller JN. Protein oxidation and cellular homeostasis: Emphasis on metabolism. Biochim. Biophys. Acta. 2007;1773:93–104. doi: 10.1016/j.bbamcr.2006.08.039. [DOI] [PubMed] [Google Scholar]
- Choo SJ, Park HR, Ryoo IJ, Kim JP, Yun BS, Kim CJ, Shin-ya K, Yoo ID. Deoxyverrucosidin, a novel GRP78/BiP down-regulator, produced by Penicillium sp. J. Antibiot. (Tokyo) 2005;58:210–213. doi: 10.1038/ja.2005.26. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Dice JF. How do intracellular proteolytic systems change with age? Front Biosci. 1998;3:d25–d43. doi: 10.2741/a264. [DOI] [PubMed] [Google Scholar]
- Demartino GN, Gillette TG. Proteasomes: Machines for all reasons. Cell. 2007;129:659–662. doi: 10.1016/j.cell.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Deng J, Lu PD, Zhang Y, Scheuner D, Kaufman RJ, Sonenberg N, Harding HP, Ron D. Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2. Mol. Cell Biol. 2004;24:10161–10168. doi: 10.1128/MCB.24.23.10161-10168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döll S, Schrickx JA, Dänicke S, Fink-Gremmels J. Deoxynivalenol-induced cytotoxicity, cytokines and related genes in unstimulated or lipopolysaccharide stimulated primary porcine macrophages. J. Toxicol. Lett. 2009;30:97–106. doi: 10.1016/j.toxlet.2008.10.024. [DOI] [PubMed] [Google Scholar]
- Endo M, Mori M, Akira S, Gotoh T. C/EBP homologous protein (CHOP) is crucial for the induction of caspase-11 and the pathogenesis of lipopolysaccharide-induced inflammation. J. Immunol. 2006;176:6245–6253. doi: 10.4049/jimmunol.176.10.6245. [DOI] [PubMed] [Google Scholar]
- Endo M, Oyadomari S, Suga M, Mori M, Gotoh T. The ER stress pathway involving CHOP is activated in the lungs of LPS-treated mice. J. Biochem. 2005;138:501–507. doi: 10.1093/jb/mvi143. [DOI] [PubMed] [Google Scholar]
- Gao B, Lee SM, Chen A, Zhang J, Zhang DD, Kannan K, Ortmann RA, Fang D. Synoviolin promotes IRE1 ubiquitination and degradation in synovial fibroblasts from mice with collagen-induced arthritis. EMBO Rep. 2008;9:480–485. doi: 10.1038/embor.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JN, Gifford NM, Brewer JW. Activation of an unfolded protein response during differentiation of antibody-secreting B cells. J. Biol. Chem. 2002;277:49047–49054. doi: 10.1074/jbc.M205011200. [DOI] [PubMed] [Google Scholar]
- Gray JS, Pestka JJ. Transcriptional regulation of deoxynivalenol-induced IL-8 expression in human monocytes. Toxicol. Sci. 2007;99:502–511. doi: 10.1093/toxsci/kfm182. [DOI] [PubMed] [Google Scholar]
- Grinyer J, Kautto L, Traini M, Willows RD, Te'o J, Bergquist P, Nevalainen H. Proteome mapping of the Trichoderma reesei 20S proteasome. Curr. Genet. 2007;51:79–88. doi: 10.1007/s00294-006-0108-8. [DOI] [PubMed] [Google Scholar]
- Guerrero C, Tagwerker C, Kaiser P, Huang L. An integrated mass spectrometry-based proteomic approach: Quantitative analysis of tandem affinity-purified in vivo cross-linked protein complexes (QTAX) to decipher the 26 S proteasome-interacting network. Mol. Cell Proteomics. 2006;5:366–378. doi: 10.1074/mcp.M500303-MCP200. [DOI] [PubMed] [Google Scholar]
- Hai T, Hartman MG. The molecular biology and nomenclature of the activating transcription factor/cAMP responsive element binding family of transcription factors: Activating transcription factor proteins and homeostasis. Gene. 2001;273:1–11. doi: 10.1016/s0378-1119(01)00551-0. [DOI] [PubMed] [Google Scholar]
- Han K, Choi J, Lee J, Song J, Joe M, Jung M, Hwang J. Therapeutic potential of peroxisome proliferators-activated receptor-alpha/gamma dual agonist with alleviation of endoplasmic reticulum stress for the treatment of diabetes. Diabetes. 2008;57:737–745. doi: 10.2337/db07-0972. [DOI] [PubMed] [Google Scholar]
- Hayakawa Y, Hattori Y, Kawasaki T, Kanoh K, Adachi K, Shizuri Y, Shin-ya K. Efrapeptin J, a new down-regulator of the molecular chaperone GRP78 from a marine Tolypocladium sp. J. Antibiot. (Tokyo) 2008;61:365–371. doi: 10.1038/ja.2008.51. [DOI] [PubMed] [Google Scholar]
- Hu P, Han Z, Couvillon AD, Kaufman RJ, Exton JH. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol. Cell Biol. 2006;26:3071–3084. doi: 10.1128/MCB.26.8.3071-3084.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung JH, Su IJ, Lei HY, Wang HC, Lin WC, Chang WT, Huang W, Chang WC, Chang YS, Chen CC, et al. Endoplasmic reticulum stress stimulates the expression of cyclooxygenase-2 through activation of NF-kappaB and pp38 mitogen-activated protein kinase. J. Biol. Chem. 2004;279:46384–46392. doi: 10.1074/jbc.M403568200. [DOI] [PubMed] [Google Scholar]
- Islam Z, Gray JS, Pestka JJ. p38 Mitogen-activated protein kinase mediates IL-8 induction by the ribotoxin deoxynivalenol in human monocytes. Toxicol. Appl. Pharmacol. 2006;213:235–244. doi: 10.1016/j.taap.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Iwakoshi NN, Lee AH, Vallabhajosyula P, Otipoby KL, Rajewsky K, Glimcher LH. Plasma cell differentiation and the unfolded protein response intersect at the transcription factor XBP-1. Nat. Immunol. 2003;4:321–329. doi: 10.1038/ni907. [DOI] [PubMed] [Google Scholar]
- Jia Q, Zhou HR, Shi Y, Pestka JJ. Docosahexaenoic acid consumption inhibits deoxynivalenol-induced CREB/ATF1 activation and IL-6 gene transcription in mouse macrophages. J. Nutr. 2006;136:366–372. doi: 10.1093/jn/136.2.366. [DOI] [PubMed] [Google Scholar]
- Kanemoto S, Kondo S, Ogata M, Murakami T, Urano F, Imaizumi K. XBP1 activates the transcription of its target genes via an ACGT core sequence under ER stress. Biochem. Biophys. Res. Commun. 2005;331:1146–1153. doi: 10.1016/j.bbrc.2005.04.039. [DOI] [PubMed] [Google Scholar]
- Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- Kincaid MM, Cooper AA. ERADicate ER stress or die trying. Antioxid. Redox. Signal. 2007;9:2373–2387. doi: 10.1089/ars.2007.1817. [DOI] [PubMed] [Google Scholar]
- Li J, Lee AS. Stress induction of GRP78/BiP and its role in cancer. Curr. Mol. Med. 2006;6:45–54. doi: 10.2174/156652406775574523. [DOI] [PubMed] [Google Scholar]
- Li Y, Schwabe RF, DeVries-Seimon T, Yao PM, Gerbod-Giannone MC, Tall AR, Davis RJ, Flavell R, Brenner DA, Tabas I. Free cholesterol-loaded macrophages are an abundant source of tumor necrosis factor-alpha and interleukin-6: Model of NF-kappaB- and map kinase-dependent inflammation in advanced atherosclerosis. J. Biol. Chem. 2005;280:21763–22172. doi: 10.1074/jbc.M501759200. [DOI] [PubMed] [Google Scholar]
- Lin JH, Walter P, Yen TS. Endoplasmic reticulum stress in disease pathogenesis. Annu. Rev. Pathol. 2008;3:399–425. doi: 10.1146/annurev.pathmechdis.3.121806.151434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loniewski K, Shi Y, Pestka J, Parameswaran N. Toll-like receptors differentially regulate GPCR kinases and arrestins in primary macrophages. Mol. Immunol. 2008;45:2312–2322. doi: 10.1016/j.molimm.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Ma Y, Hendershot LM. The unfolding tale of the unfolded protein response. Cell. 2001;107:827–830. doi: 10.1016/s0092-8674(01)00623-7. [DOI] [PubMed] [Google Scholar]
- Marciniak SJ, Ron D. Endoplasmic reticulum stress signaling in disease. Physiol. Rev. 2006;86:1133–1149. doi: 10.1152/physrev.00015.2006. [DOI] [PubMed] [Google Scholar]
- Morinaga N, Yahiro K, Matsuura G, Moss J, Noda M. Subtilase cytotoxin, produced by Shiga-toxigenic Escherichia coli, transiently inhibits protein synthesis of Vero cells via degradation of BiP and induces cell cycle arrest at G1 by downregulation of cyclin D1. Cell Microbiol. 2008;10:921–929. doi: 10.1111/j.1462-5822.2007.01094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival—Application to proliferation and cyto-toxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nagaraju K, Casciola-Rosen L, Lundberg I, Rawat R, Cutting S, Thapliyal R, Chang J, Dwivedi S, Mitsak M, Chen YW, et al. Activation of the endoplasmic reticulum stress response in autoimmune myositis: Potential role in muscle fiber damage and dysfunction. Arthritis Rheum. 2005;52:1824–1835. doi: 10.1002/art.21103. [DOI] [PubMed] [Google Scholar]
- Nowis D, McConnell EJ, Dierlam L, Palamarchuk A, Lass A, Wojcik C. TNF potentiates anticancer activity of bortezomib (Velcade) through reduced expression of proteasome subunits and dysregulation of unfolded protein response. Int. J. Cancer. 2007;121:431–441. doi: 10.1002/ijc.22695. [DOI] [PubMed] [Google Scholar]
- Oliver BL, Cronin CG, Zhang-Benoit Y, Goldring MB, Tanzer ML. Divergent stress responses to IL-1beta, nitric oxide, and tunicamycin by chondrocytes. J. Cell. Physiol. 2005;204:45–50. doi: 10.1002/jcp.20261. [DOI] [PubMed] [Google Scholar]
- Pestka JJ. Deoxynivalenol-induced IgA production and IgA nephropathy-aberrant mucosal immune response with systemic repercussions. Toxicol. Lett. 2003;140–141:287–295. doi: 10.1016/s0378-4274(03)00024-9. [DOI] [PubMed] [Google Scholar]
- Pestka JJ. Mechanisms of deoxynivalenol-induced gene expression and apoptosis. Food Addit. Contam. 2008:1–13. doi: 10.1080/02652030802056626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka JJ, Moorman MA, Warner RL. Dysregulation of IgA production and IgA nephropathy induced by the trichothecene vomitoxin. Food Chem. Toxicol. 1989;27:361–368. doi: 10.1016/0278-6915(89)90141-5. [DOI] [PubMed] [Google Scholar]
- Pestka JJ, Smolinski AT. Deoxynivalenol: Toxicology and potential effects on humans. J. Toxicol. Environ. Health B Crit. Rev. 2005;8:39–69. doi: 10.1080/10937400590889458. [DOI] [PubMed] [Google Scholar]
- Pestka JJ, Uzarski RL, Islam Z. Induction of apoptosis and cytokine production in the Jurkat human T cells by deoxynivalenol: Role of mitogen-activated protein kinases and comparison to other 8-ketotrichothecenes. Toxicology. 2005;206:207–219. doi: 10.1016/j.tox.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Pestka JJ, Zhou HR. Interleukin-6-deficient mice refractory to IgA dysregulation but not anorexia induction by vomitoxin (deoxynivalenol) ingestion. Food Chem. Toxicol. 2000;38:565–575. doi: 10.1016/s0278-6915(00)00041-7. [DOI] [PubMed] [Google Scholar]
- Pestka JJ, Amuzie CJ. Tissue distribution and proinflammatory cytokine gene expression following acute oral exposure to deoxynivalenol: Comparison of weanling and adult mice. Food Chem. Toxicol. 2008;46:2826–2831. doi: 10.1016/j.fct.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell AW, Todd A, Kinoshita G, Lynch TA, Keech CL, Gething MJ, Gordon TP. Association of stress proteins with autoantigens: A possible mechanism for triggering autoimmunity? Clin. Exp. Immunol. 2003;132:193–200. doi: 10.1046/j.1365-2249.2003.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinones QJ, de Ridder GG, Pizzo SV. GRP78: A chaperone with diverse roles beyond the endoplasmic reticulum. Histol. Histopathol. 2008;23:1409–1416. doi: 10.14670/HH-23.1409. [DOI] [PubMed] [Google Scholar]
- Rahmani M, Davis EM, Crabtree TR, Habibi JR, Nguyen TK, Dent P, Grant S. The kinase inhibitor sorafenib induces cell death through a process involving induction of endoplasmic reticulum stress. Mol. Cell Biol. 2007;27:5499–5513. doi: 10.1128/MCB.01080-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, Hubbard SR. How IRE1 reacts to ER stress. Cell. 2008;132:24–26. doi: 10.1016/j.cell.2007.12.017. [DOI] [PubMed] [Google Scholar]
- Rubinsztein DC. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature. 2006;443:780–786. doi: 10.1038/nature05291. [DOI] [PubMed] [Google Scholar]
- Schroder M. Endoplasmic reticulum stress responses. Cell Mol. Life Sci. 2008;65:862–894. doi: 10.1007/s00018-007-7383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu. Rev. Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- Shen J, Chen X, Hendershot L, Prywes R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev. Cell. 2002;3:99–111. doi: 10.1016/s1534-5807(02)00203-4. [DOI] [PubMed] [Google Scholar]
- Shi Y, Pestka JJ. Attenuation of mycotoxin-induced IgA nephropathy by eicosapentaenoic acid in the mouse: dose response and relation to IL-6 expression. J. Nutr. Biochem. 2006;17:697–706. doi: 10.1016/j.jnutbio.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Shi Y, Pestka JJ. Mechanisms for suppression of interleukin-6 expression in peritoneal macrophages from docosahexaenoic acid-fed mice. J. Nutr. Biochem. 2009;20:358–368. doi: 10.1016/j.jnutbio.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeda Y, Chijiwa S, Furihata K, Furihata K, Sakuda S, Nagasawa H, Watanabe H, Shin-ya K. Prunustatin A, a novel GRP78 molecular chaperone down-regulator isolated from Streptomyces violaceoniger. J. Antibiot. (Tokyo) 2005;58:206–209. doi: 10.1038/ja.2005.25. [DOI] [PubMed] [Google Scholar]
- Wong SS, Zhou HR, Marin-Martinez ML, Brooks K, Pestka JJ. Modulation of IL-1beta, IL-6 and TNF-alpha secretion and mRNA expression by the trichothecene vomitoxin in the RAW 264.7 murine macrophage cell line. Food Chem. Toxicol. 1998;36:409–419. doi: 10.1016/s0278-6915(97)00167-1. [DOI] [PubMed] [Google Scholar]
- Yan D, Zhou HR, Brooks KH, Pestka JJ. Potential role for IL-5 and IL-6 in enhanced IgA secretion by Peyer's patch cells isolated from mice acutely exposed to vomitoxin. Toxicology. 1997;122:145–158. doi: 10.1016/s0300-483x(97)00087-5. [DOI] [PubMed] [Google Scholar]
- Yan D, Zhou HR, Brooks KH, Pestka JJ. Role of macrophages in elevated IgA and IL-6 production by Peyer's patch cultures following acute oral vomitoxin exposure. Toxicol. Appl. Pharmacol. 1998;148:261–273. doi: 10.1006/taap.1997.8326. [DOI] [PubMed] [Google Scholar]
- Yang GH, Li S, Pestka JJ. Down-regulation of the endoplasmic reticulum chaperone GRP78/BiP by vomitoxin (Deoxynivalenol) Toxicol. Appl. Pharmacol. 2000;162:207–217. doi: 10.1006/taap.1999.8842. [DOI] [PubMed] [Google Scholar]
- Yang GH, Jarvis BB, Chung YJ, Pestka JJ. Apoptosis induction by the satratoxins and other trichothecene mycotoxins: relationship to ERK, p38 MAPK, and SAPK/JNK activation. Toxicol. Appl. Pharmacol. 2000;164:149–160. doi: 10.1006/taap.1999.8888. [DOI] [PubMed] [Google Scholar]
- Yang H, Park SH, Choi HJ, Moon Y. Epithelial cell survival by activating transcription factor 3 (ATF3) in response to chemical ribosome-inactivating stress. Biochem. Pharmacol. 2009 doi: 10.1016/j.bcp.2008.11.028. doi:10.1016/j.bcp. 2008.11.028. [DOI] [PubMed] [Google Scholar]
- Yorimitsu T, Klionsky DJ. Autophagy: molecular machinery for self-eating. Cell Death. Differ. 2005;12(Suppl. 2):1542–1552. doi: 10.1038/sj.cdd.4401765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Kaufman RJ. The unfolded protein response: A stress signaling pathway critical for health and disease. Neurology. 2006;66(2 Suppl. 1):S102–S109. doi: 10.1212/01.wnl.0000192306.98198.ec. [DOI] [PubMed] [Google Scholar]
- Zhang K, Shen X, Wu J, Sakaki K, Saunders T, Rutkowski DT, Back SH, Kaufman RJ. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell. 2006;124:587–599. doi: 10.1016/j.cell.2005.11.040. [DOI] [PubMed] [Google Scholar]
- Zhou HR, Islam Z, Pestka JJ. Induction of competing apoptotic and survival signaling pathways in the macrophage by the ribotoxic trichothecene deoxynivalenol. Toxicol. Sci. 2005a;87:113–122. doi: 10.1093/toxsci/kfi234. [DOI] [PubMed] [Google Scholar]
- Zhou HR, Jia Q, Pestka JJ. Ribotoxic stress response to the trichothecene deoxynivalenol in the macrophage involves the SRC family kinase hck. Toxicol. Sci. 2005b;85:916–926. doi: 10.1093/toxsci/kfi146. [DOI] [PubMed] [Google Scholar]