Abstract
Treatment with N-methyl-D-aspartate (NMDA) receptor antagonists, such as ketamine (KET) or phencyclidine (PCP), can trigger apoptotic neurodegeneration in neonatal rodents; however, little is known about the behavioral alterations resulting from such treatment. Here, rats were sc treated with saline; 10 mg/kg PCP on postnatal days (PNDs) 7, 9, and 11; 20 mg/kg KET (six injections every 2 h on PND 7); or a regimen of ketamine and 250 mg/kg L-carnitine (KLC) both administered on PND 7 with additional 250 mg/kg doses of L-carnitine given on PNDs 8–11. Postinjection, the home cage behavior of each pup was categorized on PNDs 7–11. Slant board and forelimb hang behaviors were examined on PNDs 8–11 and 12–16, respectively. The initial KET or KLC injections on PND 7 elevated abnormal home cage activity (i.e., paresis and paddling); however, KLC pup behavior returned to normal by the fourth injection, indicating the protective effects of L-carnitine against NMDA antagonist toxicity. PCP treatment caused substantial abnormal home cage activity on each injection day (PNDs 7, 9, and 11). Latencies to turn on the slant board were significantly longer on PND 8 for KET- and PCP-treated pups and PND 10 for PCP-treated pups. On PND 12, the forelimb hang time of PCP-treated pups was significantly shorter. Body weight was decreased on PNDs 8–18 in PCP-treated pups and PNDs 8–10 in KET-treated pups. These data indicate that developmental NMDA antagonist treatment causes short-term behavioral alterations which appear related to motor coordination and may be cerebellar in nature. Furthermore, single PCP injections appear more potent at altering behavior than multiple injections of KET.
Keywords: ketamine, phencyclidine, neurodegeneration, forelimb hang, negative geotaxis, pup behavior
The excitatory neurotransmitter glutamate activates ionotropic (α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid, kainate, and N-methyl-D-aspartate [NMDA]) and metabotropic (G protein linked) receptors and is essential for neuronal differentiation, migration, and survival (reviewed by Meldrum, 2000). Treatment of postnatal day (PND) 7 rats with noncompetitive NMDA receptor antagonists such as MK-801 (dizocilpine), phencyclidine (PCP), or ketamine (KET) results in increased neuronal degeneration (Hayashi et al., 2002; Ikonomidou et al., 1999; Scallet et al., 2004; Wang and Johnson, 2005; Wang et al., 2001). Specifically, multiple injections of 20 mg/kg KET caused increased numbers of Fluoro-Jade, terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling, and silver-stained cells in the hippocampus, thalamus, subiculum, caudate nucleus, and frontal, cingulate, parietal, and retrosplenial cortices (Ikonomidou et al., 1999; Scallet et al., 2004). Single injections of the same or higher doses (25–75 mg/kg) do not appear to cause similar neuronal cell death (Hayashi et al., 2002; Scallet et al., 2004).
Such NMDA antagonist–induced neurodegeneration has been shown to result in behavioral deficits as well. For example, neonatal PCP or MK-801 treatment causes later sensorimotor gating deficits as measured by prepulse inhibition (Harris et al., 2003; Wang et al., 2001) and impairs Morris water maze performance in juvenile and adult rats (Sircar, 2003; Sircar and Rudy, 1998). Neonatal PCP treatment has been described to cause increased sensitivity to later PCP treatment as well as transient deficits in spatial alternation performance (Wang et al., 2001). Repeated neonatal MK-801 treatment results in long-term deficits in radial-arm maze performance (Kawabe et al., 2007). Those behavioral deficits imply that the observed neurodegeneration following developmental NMDA antagonist treatment has long-term effects.
The description of long-term behavioral effects after developmental NMDA antagonist treatment led us to hypothesize that there may be acute effects observable during treatment. Here, the behavioral effects of PCP or KET were evaluated using those treatment regimens previously shown to produce significant neurodegeneration. Rat pups were treated on PND 7 with KET at 2-h intervals for six injections or on PNDs 7, 9, and 11 with PCP. As a preliminary exploration, the potential protective effects of L-carnitine were measured in KET-treated rats since L-carnitine appears to prevent glutamate neurotoxicity (Felipo et al., 1994) and neurodegeneration in the frontal cortex of PND 7 rats (Zou et al., 2008). Home cage behavior of the pups was rated using a comprehensive scoring system on PNDs 7–11 after each treatment. Slant board (negative geotaxis; PNDs 8–11) and forelimb hang (PNDs 12–16) behaviors were examined to assess potential early neurotoxicant-induced dysfunctions.
MATERIALS AND METHODS
Animals
Sprague-Dawley dams (n = 48) had normal vaginal births and on the day of birth (PND 0), each litter was separated by sex, and four males and four females were randomly selected so that each litter was culled to eight. The dams with their natural litters (culled to four/sex/litter) were obtained on PND 0 from the breeding colony at the National Center for Toxicological Research (NCTR/FDA). Each dam was individually housed in a standard polycarbonate cage lined with wood chip bedding and provided with ad libitum food (NIH-31, Purina Mills, St Louis, MO) and water. The colony room was maintained at 22°C ± 1°C (mean ± SE) and 45–55% humidity on a 12-h light/dark cycle (7:00 A.M.–7:00 P.M.). Each pup was paw tattooed on PND 1 and also identified with a nontoxic marker on the dorsal side and tail tip on PND 4. All animal procedures followed the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996) and were approved in advance by the NCTR Institutional Animal Care and Use Committee.
Treatment
Ketamine hydrochloride (100 mg/ml solutions as Ketaset, Fort Dodge Animal Health, Fort Dodge, IA) was diluted with saline to produce 2 mg/ml solutions. PCP (NIDA, Bethesda, MD) and L-carnitine (Sigma-Aldrich Corp., St Louis, MO) were dissolved in 0.9% saline. Ketamine hydrochloride (400 μl) and L-carnitine (500 mg) were diluted with 10 ml of saline to produce 40 mg/ml KET and 500 mg/ml L-carnitine solutions, respectively. These solutions were combined in a 50 ml conical tube to obtain the KLC dose (250 mg/kg L-carnitine and 20 mg/kg ketamine) injected on PND 7. Solutions were made weekly and kept refrigerated. The sc injections were done using a 25-gauge needle.
The within-litter treatment (one pup/sex/treatment/litter) was a particularly important aspect of the experimental design since it is well recognized that differences in maternal care can affect offspring behavior (Barron and Riley, 1985; Fleming et al., 1999) and, at least in rats, pup behavior determines some aspects of maternal care (Marino et al., 2002). Thus, similar to that described by Zissen et al. (2007), overall maternal care was controlled at the litter level in that each dam cared for a litter which contained pups of all treatment groups. However, as noted by Zissen et al. (2007), this cannot prevent or control for differential treatment of individual pups by the dam.
Treatment assignment was based on PND 4 body weight such that all groups had similar average body weights prior to treatment. The four groups were (1) 10 mg/kg PCP at 12:00 P.M. on PNDs 7, 9, and 11; (2) six injections of 20 mg/kg KET on PND 7 (8:00 A.M.–6:00 P.M.), separated by 2-h intervals; (3) six injections of 20 mg/kg KET and 250 mg/kg L-carnitine on PND 7 (8:00 A.M.–6:00 P.M.), separated by 2-h intervals followed by 250 mg/kg L-carnitine at 12:00 P.M. on PNDs 8–11; and (4) six injections of saline at on PND 7 (8:00 A.M.–6:00 P.M.), separated by 2-h intervals followed by saline at 12:00 P.M. on PNDs 8–11. The doses and treatment regimens were based on previous reports indicating that similar treatments caused neurodegeneration in rats (Ikonomidou et al., 1999; Scallet et al., 2004; Wang et al., 2001). The L-carnitine dose was based on studies of its protective effects against 1-methyl-phenylpyridinium ion–induced apoptosis (Wang et al., 2007). Thus, for each of the 48 litters, 1 male and 1 female were assigned to each treatment resulting in 48 pups/sex/treatment.
Body Weight
Body weights of the offspring were recorded on PNDs 4, 7, 8, 9, 10, 11, and 18. On PNDs 8–11, body weights were recorded after behavioral testing and prior to treatment.
Home Cage Pup Behavior
To determine the immediate effects of treatment, home cage behavior was assessed on PNDs 7–11. At each treatment time, the dam was placed in a holding cage. Each pup was then identified and when indicated, injected. Those pups not injected (e.g., PCP-treated pups at 8:00 A.M., 10:00 A.M., 2:00 P.M., and 4:00 P.M. on PND 7 and on PNDs 8 and 10 as well as the KET-treated pups on PNDs 8–11) were handled in a manner similar to the injected pups. Time of the last injection/handling for each litter was recorded, and the dam was returned to the home cage. Time from dam removal to replacement into the home cage was less than 120 s. At 5, 14, 23, and 32 min posttreatment, the behavior of each pup was assessed by one of two experimenters blind to treatment. Thus, there were four observations at five of the six treatment times on PND 7 (i.e., pups were observed after injections/handling at 8:00 A.M., 10:00 A.M., 12:00 P.M., 2:00 P.M., and 4:00 P.M., but not after the 6:00 P.M. injection/handling time). On PNDs 8–11, there were four observations following the 12:00 P.M. treatment time. Each pup was categorized as exhibiting one of 12 different behaviors (see Table 1) which were based on a previous scoring system (Goodwin and Barr, 2005). Only one behavior/pup/observation time was recorded.
TABLE 1.
Behavior
| Behaviora | Definition | No. of instancesb |
| Quiet | No movement except for twitches and respiration | 5929 |
| Immobile | No movement except for respiration with the head up | 234 |
| Activity | Walking, sniffing, and rearing | 3028 |
| Fast activity | Running | 3 |
| Paddling | On dorsal side while moving forelimbs and hind limbs | 418 |
| Partial paddling | On dorsal side with three limbs moving | 340 |
| Maternal interference | Dam interacted with pup (e.g., licking, moving, or stepping on pup) | 241 |
| Paresis | On ventral side with hind limbs moving and stiff forelimbs | 129 |
| Partial paresis | On ventral side with hind limbs moving and one stiff forelimb | 271 |
| Grooming | Grooming any part of the body | 1 |
| Wall climbing | Standing on hind limbs against cage side while forelimbs are alternately moving back and forth | 15 |
| Unidentifiable | Pup could not be identified (typically, as a result of nursing and inability to identify via paw tattoo or markings) | 3175 |
These categories and the scoring system were modified from Goodwin and Barr (2005).
The total number of observations for PNDs 7–11 home cage behavior was 13,244 (four posttreatment observations [5, 14, 23, and 32 min posttreatment] at each of five observation times on PND 7 (8:00 A.M., 10:00 A.M., 12:00 P.M., 2:00 P.M., and 4:00 P.M.), four posttreatment observations at each single treatment time on PNDs 9–11, maximum eight pups each of 48 litters).
Slant Board Behavior (Negative Geotaxis)
Vestibular system integrity and Motor coordination were examined using a slant board test as previously described (Adams et al., 1985). Briefly, between 7:30 and 9:00 A.M. on PNDs 8–11, the dam was removed and each pup was placed on its ventral side with its nose pointing toward the lower end of a sandpaper-covered 45° incline board. Each pup was allowed 60 s to complete a 180° turn. One trial/day was conducted, and the latency to turn or fall from the apparatus was recorded by a tester blind to treatment conditions.
Forelimb Hang Behavior
Muscle strength/coordination was examined using a forelimb hang test as previously described (Cada et al., 2000). Briefly, between 7:30 and 10:00 A.M. on PNDs 12–16, the dam was removed and each pup was placed on a taut string stretched between two blocks of wood spaced 46 cm apart and 41 cm above a padded surface. One trial/day was conducted, and the latency to fall was recorded (maximum 60 s) by a tester blind to treatment conditions.
Statistical Analyses
Body weight.
Offspring body weights were compared using ANOVAs with factors of treatment (control, KET, PCP, and KLC), sex, and the repeated measure of PND (JMP, Version 7.0; SAS Institute Inc., Cary, NC). Tukey post hoc tests were used to further analyze significant main effects or interactions.
Home cage pup behavior.
Data from the five observation times on PND 7 (8:00 A.M., 10:00 A.M., 12:00 P.M., 2:00 P.M., and 4:00 P.M.) were analyzed separately from the single observation time on PNDs 8–11 (12:00 P.M.). Six behaviors were categorized as abnormal activity: fast activity, paddling, partial paddling, paresis, partial paresis, wall climbing. To analyze abnormal activity, each pup at each observation at each time was assigned a “1” if it exhibited any of the six abnormal behaviors or a “0” for any other behavior. Generalized linear models with a log link and Poisson distribution were used to analyze the counts for each of the two data sets (PND 7 only and PNDs 8–11) with factors of treatment, observation time (e.g., 8:00 A.M., 10:00 A.M.) (PND 7 analysis only), minutes posttreatment (e.g., 5, 14, 23, or 32 min), and sex.
Slant board behavior.
Each pup could exhibit one of three outcomes: a successful turn within 60 s, a fall from the apparatus within 60 s, or an incomplete turn. A failure was categorized as a fall or an incomplete turn. The odds of failure were analyzed using a generalized linear model with repeated measures and a binomial distribution and logit link function. To analyze the latency to turn time, a Cox Proportional Hazards model was run in SAS (SAS Version 9.1; SAS Institute Inc.) using treatment, sex, and PND as factors. Pups that fell or did not complete the turn were accounted for in this analysis by adjusting the empirical distribution function.
Forelimb hang behavior.
To analyze the latency to fall, a Cox Proportional Hazards model was run using SAS (SAS Version 9.1, SAS Institute Inc., Cary, NC) with treatment, sex, and PND as factors.
RESULTS
Mortality and Body Weight
There were three deaths. One male KET pup died after the 2:00 P.M. PND 7 home cage behavior observation and before the 4:00 P.M. PND 7 treatment. One male KLC pup died on PND 8 after the 12:00 P.M. home cage behavior observation time and another male KLC pup died on PND 9 after the 12:00 P.M. home cage observation time.
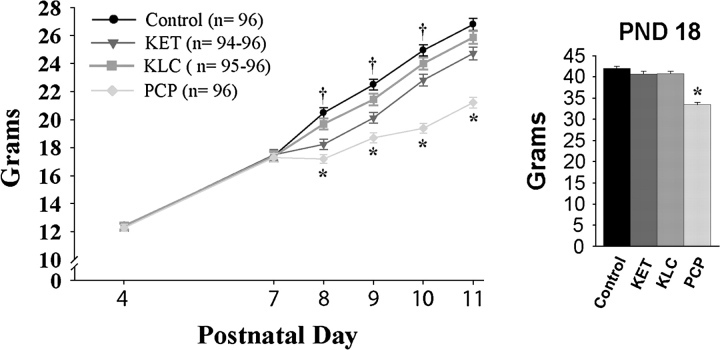
Body weight analysis revealed a significant interaction of treatment with PND (F18,2244 = 75.66, p < 0.0001) (Fig. 1). Post hoc tests indicated that there were no significant body weight differences on PND 4 or 7 (before treatment). However, on PNDs 8, 9, and 10, the KET-treated group weighed less than the control group (p < 0.05). On PNDs 8–18, the PCP-treated group weighed less than the control group (p < 0.05). Post hoc tests of the significant sex by PND interaction (F6,2244 = 3.62, p < 0.002) demonstrated that males were significantly heavier than females on PND 18 only (p < 0.05) (mean ± SE for PND 18 males and females: 40.0 ± 0.5 and 38.4 ± 0.5 g, respectively). There was neither a main effect of sex nor a significant interaction of treatment with sex.
FIG. 1.
Body weight on PNDs 4–18, averaged over sex. The larger graph shows body weight on PNDs 4–11 while the inset graph shows PND 18 body weight. *Indicates the PCP-treated group weighed significantly less than controls. †Indicates the KET-treated group weighed significantly less than controls.
Home Cage Pup Behavior
PND 7 abnormal behavior.
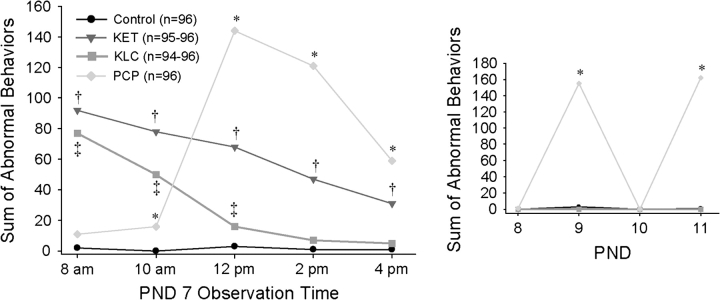
There was a significant interaction of treatment with observation time (p < 0.0001) (Fig. 2, large graph). KET-treated pups had elevated levels of abnormal activity at each of the five observation times (p < 0.001) compared with controls. KLC-treated pups exhibited higher abnormal activity levels at 8:00 A.M., 10:00 A.M., and 12:00 P.M. relative to controls (p < 0.02); by 2:00 P.M., however, their abnormal behavior was within the range of controls. Although the PCP-treated pups had somewhat higher counts of abnormal activity at 10:00 A.M. (p < 0.004), the PCP treatment at 12:00 P.M. caused a significant increase in abnormal activity levels (p < 0.0001) which remained elevated (p < 0.0001) throughout the last two observation times (i.e., 2:00 and 4:00 P.M.).
FIG. 2.
Level of abnormal home cage pup behavior on PNDs 7–11. The larger graph shows abnormal behavior on PND 7 while the inset graph shows abnormal behavior on PNDs 8–11. *Indicates higher levels of abnormal activity in the PCP-treated group relative to the control group. †Indicates higher levels of abnormal activity in the KET-treated group relative to the control group. ‡Indicates higher levels of abnormal activity in the KLC-treated group relative to the control group.
PNDs 8–11 abnormal behavior.
A significant interaction of treatment with PND (p < 0.0001) indicated that PCP-treated pups exhibited elevated levels of abnormal activity on PNDs 9 and 11 (i.e., treatment days) relative to all other treatment groups (p < 0.0001) (Fig. 2, inset graph). Levels of abnormal behavior in KET-, KLC-treated, and control pups did not differ from one another on PNDs 8–11, nor did PCP-treated pups exhibit significant levels of abnormal behavior on PNDs 8 and 10 (i.e., days on which handling, but no PCP treatment, occurred).
Slant Board Behavior (Negative Geotaxis)
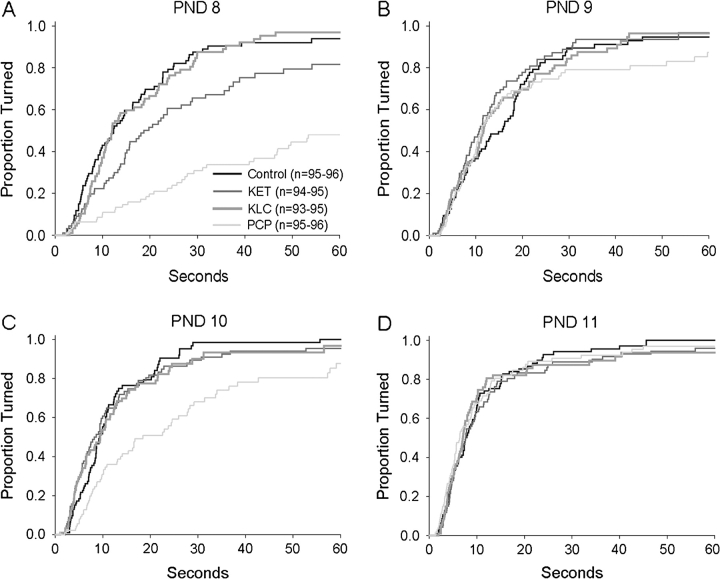
Analysis of the odds of failure (falling or incomplete turn) indicated a significant interaction of treatment by PND (χ2 = 32.05, df = 9, p < 0.0002) (data not shown). Post hoc tests on each PND indicated that on PND 8, the KET- and PCP-treated groups had increased odds of failure (p < 0.05) (mean ± SE for control, KET-, KLC-, and PCP-treated groups: 0.19 ± 0.04, 0.39 ± 0.05, 0.21 ± 0.04, and 0.63 ± 0.05 proportion failing to turn, respectively). On PND 10, the PCP-treated group again had increased odds of failure (p < 0.05) (mean ± SE for control, KET-, KLC-, and PCP-treated groups: 0.17 ± 0.04, 0.21 ± 0.04, 0.22 ± 0.04, and 0.39 ± 0.05 proportion failing to turn, respectively). On PND 11, the proportion failing to turn was less than 0.25; however, relative to the control group, the KLC-treated group was more likely to fail (p < 0.05) (mean ± SE for control, KET-, KLC-, and PCP-treated groups: 0.13 ± 0.03, 0.16 ± 0.04, 0.24 ± 0.04 and 0.14 ± 0.04, respectively).
For illustrative purposes, Figures 3A–3D shows the proportion of each treatment group making a successful turn during the time allotted on PNDs 8–11. Analysis of latency to turn indicated a significant interaction of treatment by PND (χ2 = 68.69, df = 9, p < 0.0001). Post hoc tests indicated that on PND 8, KET- and PCP-treated pups had longer latencies to turn than controls (p < 0.05) (mean ± SE for control, KET, KLC, and PCP groups: 16.8 ± 1.5, 25.4 ± 2.1, 17.2 ± 1.4, and 40.4 ± 2.0 s, respectively) (see also Fig. 3A). On PND 10, the PCP-treated group had a longer latency to turn (p < 0.05) (mean ± SE for control, KET, KLC, and PCP groups: 12.0 ± 1.0, 13.0 ± 1.5, 13.8 ± 1.7, and 25.3 ± 2.3 s, respectively) (see also Fig. 3C). Neither the main effect of sex nor any interactions with sex were significant.
FIG. 3.
Proportion of each treatment group making a successful slant board turn during the allotted 60 s. Each of the four panels (A–D) represents a single test day and is similar to a typical “survival curve.” Here, the “survival” estimates were inverted such that higher curves correspond to a higher proportion of animals making successful turns (e.g., on PND 8, approximately 43% of the control group had turned successfully at the end of 10 s). Note that a smaller proportion of the PCP-treated group successfully turned within the allotted 60-s trial on PNDs 8 and 10. See text for description of statistically significant effects.
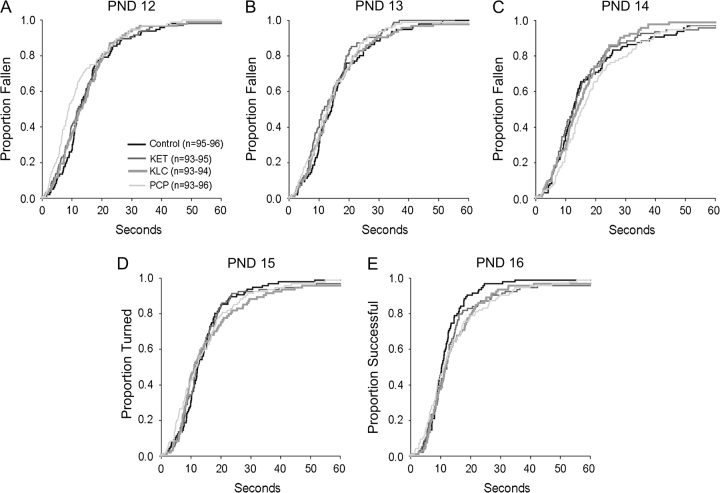
Forelimb Hang Behavior
For illustrative purposes, Figures 4A–4E show the proportion of each treatment group that fell from the forelimb hang apparatus during the time allotted on PNDs 12–16. Analysis of latency to fall indicated a significant interaction of treatment with PND (χ2 = 27.78, df = 12, p < 0.006). Post hoc tests revealed that on PND 12, relative to controls, the PCP-treated group had a shorter latency to fall (p < .05) (mean ± SE for control, KET-, KLC-, and PCP-treated groups: 15.5 ± 1.2, 14.5 ± 1.1, 15.1 ± 1.2, and 13.0 ± 1.1, respectively) (see also Fig. 4A). On PND 16, the KLC- and PCP-treated groups had longer latencies to fall (p < 0.05) (mean ± SE for control, KET-, KLC-, and PCP-treated groups: 12.0 ± 0.9, 14.6 ± 1.3, 15.0 ± 1.2, 15.6 ± 1.3, respectively) (see also Fig. 4D). There was neither a significant main effect of sex nor any significant interactions with sex.
FIG. 4.
Proportion of each treatment group falling from the forelimb hang apparatus during the allotted 60 s. Each of the five panels (A–E) represents a single test day and is similar to a typical “survival curve.” Here, the “survival” estimates were inverted such that higher curves correspond to a higher proportion of animals falling (e.g., on PND 8, approximately 27% of the control group had fallen at the end of 10 s). Note the shorter fall latencies in the PCP-treated group on PND 12 and the longer latencies to fall in the PCP- and KLC-treated groups on PND 16. See text for statistically significant effects.
DISCUSSION
NMDA receptor antagonists can affect psychological and physiological functions in humans, and it is becoming clear that the musculoskeletal system can be altered as well (Brunson et al., 2001; Kakizawa et al., 2000; Wolff and Winstock, 2006). In the current study, neonatal rats were treated with KET, PCP, or ketamine + L-carnitine (KLC) and assessed for home cage pup behavior, slant board behavior, and forelimb hang time on PNDs 7–11, 8–11, and 12–16, respectively. The single 10 mg/kg PCP injection on PNDs 7, 9, and 11 decreased body weight gain and produced high levels of abnormal activity as well as altered slant board and forelimb hang behavior. The PCP effects appeared more severe than those caused by the prolonged 20 mg/kg KET treatment on PND 7. These data suggest that three injections of 10 mg/kg PCP over PNDs 7–11 were more potent at modifying preweaning behaviors than were repeated injections of 20 mg/kg KET on PND 7. The addition of L-carnitine to the KET treatment regimen appeared to ameliorate KET-induced adverse effects on body weight, home cage pup behavior, and slant board behavior. These results more completely describe the acute effects of NMDA antagonist treatment in rat pups and may be associated with the neuronal degeneration known to occur with similar treatment.
Increased levels of abnormal home cage behavior occurred within 5–32 min after the initial PCP and KET injections on PND 7. These abnormal behaviors were primarily paddling and paresis; other behaviors such as wall climbing rarely occurred. This is the first study to quantitatively describe the acute behavioral effects of developmental NMDA antagonist treatment; however, paddling has been described in PND 7 mice treated with 40 mg/kg KET (Young et al., 2005) and in adult rats treated with 2–15 mg/kg PCP (Chen et al., 1959; Sturgeon et al., 1979). Further, KET can cause paresis, ataxia, and temporary paralysis in humans (Wolff and Winstock, 2006). Thus, the specific types of abnormal activity induced in neonatal rats by the NMDA antagonists here resemble those described for similarly treated adults.
The highest level of KET-induced abnormal behavior occurred after the initial injection (i.e., at 8:00 A.M.) on PND 7. Subsequently, levels declined at each 2-h observation time even though each was accompanied by an additional 20 mg/kg injection. Still, the rate of decline was shallow enough such that abnormal behavior levels at the fifth treatment (i.e., 4:00 P.M.) remained elevated. By 12:00 P.M. on the following day, there were no measurable levels of abnormal home cage behavior in the KET-treated group. This pattern of initially high levels of PND 7 abnormal behavior with a decline throughout the day was very similar to that exhibited by the KLC group, albeit to a lesser extent. While the initial injection of ketamine with L-carnitine elevated abnormal behavior levels, the rate of decline throughout the remainder of the day was fairly steep and by the fourth injection (i.e., 2:00 P.M.), abnormal behavior levels were comparable to and remained at control levels. Thus, while the initial KLC treatment was associated with an increase in abnormal home cage behavior, the addition of L-carnitine somewhat ameliorated the effects of KET. Neither KET nor KLC had lasting effects on home cage pup behavior.
The effects of PCP treatment on home cage pup behavior differed quantitatively, but not qualitatively, from those of KET and KLC. Specifically, the single PND 7 injection elevated abnormal behavior levels well above those of the initial KET injection, and 4 h postinjection, abnormal behavior levels were still high. Further, while there appeared to be a type of tolerance to repeated KET treatment on PND 7, PCP treatment on PNDs 9 and 11 elevated abnormal behaviors to the same extent as the initial injection on PND 7. Still, similar to KET and KLC treatments, the effects of PCP on home cage pup behavior had no lasting effects since level of abnormal home cage behavior was indistinguishable from controls on PNDs 8 and 10. These differences in levels of home cage abnormal activity may be related to differences in the half-lives of KET and PCP. In adult rats, iv treatment with PCP results in a half-life of 3–5 h (Shelnutt et al., 1999) whereas a half-life of less than 1 h is obtained with iv or im treatment with KET (Edwards et al., 2002; Williams et al., 2004). Thus, it is not surprising that PCP-induced abnormal home cage behavior was still evident 4 h posttreatment on PND 7.
The slant board and forelimb hang behaviors measured here are typical of those utilized in developmental neurotoxicity studies and were included since developmental PCP treatment has been shown to cause later motor coordination deficits (Sircar, 2003; Sircar and Rudy, 1998; Wang et al., 2001; Wiley et al., 2003). Although home cage pup behavior on PND 8 (conducted approximately 4 h after slant board behavior tests) indicated no abnormal behavior in the PCP- and KET-treated pups, both groups were slower to turn on the slant board on that day. Further, PCP-treated pups were slower to turn on PND 10, but not on PNDs 9 and 11, days on which the test was conducted prior to PCP treatment. This pattern indicates that the behavioral effects of the PCP treatment were still detectable via slant board behavior approximately 20, but not 44, h later. PCP, but not KET, treatment appeared to affect muscle strength as latency to fall in the forelimb hang test was decreased on PND 12. Finally, the repeated saline injections appeared not to affect behavior since slant board and forelimb hang results of the control group here were similar to those of previous studies (Ferguson et al., 2003).
There is some evidence of sexual dimorphism in the effects of PCP and anesthetics such as isoflurane and phenobarbital. The half-life of PCP is longer in adult female Sprague-Dawley rats than in males after iv treatment, and this appears to be due to sex differences in metabolism (Shelnutt et al., 1999). On the other hand, neonatal treatment with isoflurane or phenobarbital resulted in more severe neurotoxicity in male Sprague-Dawley rats when examined at adulthood (Rothstein et al., 2008). There was no evidence of sexual dimorphism in response to the KET, KLC, or PCP treatments in the preweaning assessments conducted in the present study; however, assessments continued through adulthood, and it is possible that sex differences in sensitivity to these compounds may be apparent after puberty.
These NMDA antagonist-induced behavioral alterations may be related to increased cerebellar apoptosis. While apoptotic elimination of cerebellar granule cells is a natural process in rodent pups (Wood et al., 1993), inadequate stimulation of these cells or insufficient mossy fiber connections may increase the rate of apoptotic granule cell elimination. For example, treatment with the competitive NMDA receptor antagonist CGP 39551 on PNDs 7–11 increased DNA fragmentation levels in the inner granule layer and caspase-3 (a proapoptotic protease) levels in the rodent cerebellum (Monti and Contestabile, 2000). Further, developmental treatment with noncompetitive NMDA receptor antagonists such as MK-801 or KET resulted in increased numbers of multiple climbing fiber innervations in the cerebellum, increased apoptotic cerebellar cell death, and mild motor coordination impairments in mice (Kakizawa et al., 2000; Rudin et al., 2005). Thus, the KET and PCP treatments here are likely to have altered cerebellar development which may have resulted in impaired motor coordination expressed as paddling and paresis, longer slant board turn latencies, and shorter forelimb fall latencies.
The mechanism by which L-carnitine ameliorated the adverse effects of KET treatment on body weight and behavior is not clear nor is it clear what effects L-carnitine may have had irrespective of the effects of KET. A group treated solely with L-carnitine (i.e., without KET) was not included as the focus of the study was on the effects of KET and PCP. The protective effects of L-carnitine may be mitochondrially mediated since L-carnitine protects against age-dependent mitochondrial decay (Hagen et al., 2002). NMDA antagonist treatment causes neuronal cell death through the Bax-cytochrome c-caspase pathway (Yoon et al., 2003) in which Bax is translocated into the outer mitochondrial membrane. Thus, L-carnitine may have prevented the KET-induced behavioral modifications by prohibiting the release of cytochrome c which would prevent caspase activation and apoptosis. As evidence, L-carnitine inhibits apoptosis in murine MC3T3-E1 osteoblastic cells by decreasing the release of cytochrome c and the activation of caspase-9 and caspase-3 (Xie et al., 2007).
These results provide a more complete description of the acute behavioral effects of developmental NMDA antagonist treatment. The exact mechanism by which PCP and KET induce such effects is unknown but is likely related to the increased neuronal cell death caused by these compounds and may be regionally specific to the cerebellum. Future studies will examine adolescent and adult behaviors in these subjects as well as cerebellar morphology.
FUNDING
Interagency Agreement #224-93-0001 between the National Center for Toxicological Research (NCTR) / Food and Drug Administration (FDA) and the National Institute for Environmental Health Sciences (NIEHS) / National Toxicology Program (NTP).
Supplementary Material
Acknowledgments
The authors would like to thank Brett Thorn and Jacob Wulff of the Division of Personalized Medicine and Nutrition/NCTR for their excellent statistical analyses. The authors would also like to thank Natalya Sadovova of Toxicologic Pathology Associates, Inc., Lee McVay of the Bionetics Corporation, as well as Joseph (Ruda) Pollard and Melody Smith for their superb technical help. This document has been reviewed in accordance with United States Food and Drug Administration (FDA) policy and approved for publication. Approval does not signify that the contents necessarily reflect the position or opinions of the FDA nor does mention of trade names or commercial products constitute endorsement or recommendation for use. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the FDA.
References
- Adams J, Buelke-Sam J, Kimmel CA, Nelson CJ, Reiter LW, Sobotka TJ, Tilson HA, Nelson BK. Collaborative Behavioral Teratology Study: Protocol design and testing procedures. Neurobehav. Toxicol. Teratol. 1985;7:579–586. [PubMed] [Google Scholar]
- Barron S, Riley EP. Pup-induced maternal behavior in adult and juvenile rats exposed to alcohol prenatally. Alcohol Clin. Exp. Res. 1985;9:360–365. doi: 10.1111/j.1530-0277.1985.tb05560.x. [DOI] [PubMed] [Google Scholar]
- Brunson KL, Khanna A, Cromwell HC, Cohen RW. Effect of the noncompetitive NMDA antagonists MK-801 and ketamine on the spastic Han-Wistar mutant: A rat model of excitotoxicity. Dev. Neurosci. 2001;23:31–40. doi: 10.1159/000048694. [DOI] [PubMed] [Google Scholar]
- Cada AM, Gray EP, Ferguson SA. Minimal behavioral effects from developmental cerebellar stunting in young rats induced by postnatal treatment with alpha-difluoromethylornithine. Neurotoxicol. Teratol. 2000;22:415–420. doi: 10.1016/s0892-0362(99)00085-9. [DOI] [PubMed] [Google Scholar]
- Chen G, Ensor CR, Russell D, Bohner B. The pharmacology of 1-(1-phenylcyclohexyl) piperidine-HCl. J. Pharmacol. Exp. Ther. 1959;127:241–250. [PubMed] [Google Scholar]
- Edwards SR, Minto CF, Mather LE. Concurrent ketamine and alfentanil administration: Pharmacokinetic considerations. Br. J. Anaesth. 2002;88:94–100. doi: 10.1093/bja/88.1.94. [DOI] [PubMed] [Google Scholar]
- Felipo V, Minana MD, Cabedo H, Grisolia S. L-carnitine increases the affinity of glutamate for quisqualate receptors and prevents glutamate neurotoxicity. Neurochem. Res. 1994;19:373–377. doi: 10.1007/BF00971588. [DOI] [PubMed] [Google Scholar]
- Ferguson SA, Gray EP, Cada AM. Early behavioral development in the spontaneously hypertensive rat: A comparison with the Wistar-Kyoto and Sprague-Dawley strains. Behav. Neurosci. 2003;117:263–270. doi: 10.1037/0735-7044.117.2.262. [DOI] [PubMed] [Google Scholar]
- Fleming AS, O'Day DH, Kraemer GW. Neurobiology of mother-infant interactions: Experience and central nervous system plasticity across development and generations. Neurosci. Biobehav. Rev. 1999;23:673–685. doi: 10.1016/s0149-7634(99)00011-1. [DOI] [PubMed] [Google Scholar]
- Goodwin GA, Barr GA. Developmental changes in the behavioral and autonomic effects of kappa opioid receptor stimulation of the midbrain periaqueductal gray. Dev. Psychobiol. 2005;46:47–56. doi: 10.1002/dev.20039. [DOI] [PubMed] [Google Scholar]
- Hagen TM, Moreau R, Suh JH, Visioli F. Mitochondrial decay in the aging rat heart: Evidence for improvement by dietary supplementation with acetyl-L-carnitine and/or lipoic acid. Ann. N. Y. Acad. Sci. 2002;959:491–507. doi: 10.1111/j.1749-6632.2002.tb02119.x. [DOI] [PubMed] [Google Scholar]
- Harris LW, Sharp T, Gartlon J, Jones DN, Harrison PJ. Long-term behavioural, molecular and morphological effects of neonatal NMDA receptor antagonism. Eur. J. Neurosci. 2003;18:1706–1710. doi: 10.1046/j.1460-9568.2003.02902.x. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Dikkes P, Soriano SG. Repeated administration of ketamine may lead to neuronal degeneration in the developing rat brain. Paediatr. Anaesth. 2002;12:770–774. doi: 10.1046/j.1460-9592.2002.00883.x. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- Kakizawa S, Yamasaki M, Watanabe M, Kano M. Critical period for activity-dependent synapse elimination in developing cerebellum. J. Neurosci. 2000;20:4954–4961. doi: 10.1523/JNEUROSCI.20-13-04954.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabe K, Iwasaki T, Ichitani Y. Repeated treatment with N-methyl-d-aspartate antagonists in neonatal, but not adult, rats causes long-term deficits of radial-arm maze learning. Brain Res. 2007;1169:77–86. doi: 10.1016/j.brainres.2007.06.062. [DOI] [PubMed] [Google Scholar]
- Marino MD, Cronise K, Lugo JN, Jr, Kelly SJ. Ultrasonic vocalizations and maternal-infant interactions in a rat model of fetal alcohol syndrome. Dev. Psychobiol. 2002;41:341–351. doi: 10.1002/dev.10077. [DOI] [PubMed] [Google Scholar]
- Meldrum BS. Glutamate as a neurotransmitter in the brain: Review of physiology and pathology. J. Nutr. 2000;130:1007S–1015S. doi: 10.1093/jn/130.4.1007S. [DOI] [PubMed] [Google Scholar]
- Monti B, Contestabile A. Blockade of the NMDA receptor increases developmental apoptotic elimination of granule neurons and activates caspases in the rat cerebellum. Eur. J. Neurosci. 2000;12:3117–3123. doi: 10.1046/j.1460-9568.2000.00189.x. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- Rothstein S, Simkins T, Nunez JL. Response to neonatal anesthesia: Effect of sex on anatomical and behavioral outcome. Neuroscience. 2008;152:959–969. doi: 10.1016/j.neuroscience.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudin M, Ben-Abraham R, Gazit V, Tendler Y, Tashlykov V, Katz Y. Single-dose ketamine administration induces apoptosis in neonatal mouse brain. J. Basic Clin. Physiol. Pharmacol. 2005;16:231–243. doi: 10.1515/jbcpp.2005.16.4.231. [DOI] [PubMed] [Google Scholar]
- Scallet AC, Schmued LC, Slikker W, Jr, Grunberg N, Faustino PJ, Davis H, Lester D, Pine PS, Sistare F, Hanig JP. Developmental neurotoxicity of ketamine: Morphometric confirmation, exposure parameters, and multiple fluorescent labeling of apoptotic neurons. Toxicol. Sci. 2004;81:364–370. doi: 10.1093/toxsci/kfh224. [DOI] [PubMed] [Google Scholar]
- Shelnutt SR, Gunnell M, Owens SM. Sexual dimorphism in phencyclidine in vitro metabolism and pharmacokinetics in rats. J. Pharmacol. Exp. Ther. 1999;290:1292–1298. [PubMed] [Google Scholar]
- Sircar R. Postnatal phencyclidine-induced deficit in adult water maze performance is associated with N-methyl-D-aspartate receptor upregulation. Int. J. Dev. Neurosci. 2003;21:159–167. doi: 10.1016/s0736-5748(03)00026-1. [DOI] [PubMed] [Google Scholar]
- Sircar R, Rudy JW. Repeated neonatal phencyclidine treatment impairs performance of a spatial task in juvenile rats. Ann. N. Y. Acad. Sci. 1998;844:303–309. [PubMed] [Google Scholar]
- Sturgeon RD, Fessler RG, Meltzer HY. Behavioral rating scales for assessing phencyclidine-induced locomotor activity, stereotyped behavior and ataxia in rats. Eur. J. Pharmacol. 1979;59:169–179. doi: 10.1016/0014-2999(79)90279-6. [DOI] [PubMed] [Google Scholar]
- Wang C, McInnis J, Ross-Sanchez M, Shinnick-Gallagher P, Wiley JL, Johnson KM. Long-term behavioral and neurodegenerative effects of perinatal phencyclidine administration: Implications for schizophrenia. Neuroscience. 2001;107:535–550. doi: 10.1016/s0306-4522(01)00384-0. [DOI] [PubMed] [Google Scholar]
- Wang C, Sadovova N, Ali HK, Duhart HM, Fu X, Zou X, Patterson TA, Binienda ZK, Virmani A, et al. L-carnitine protects neurons from 1-methyl-4-phenylpyridinium-induced neuronal apoptosis in rat forebrain culture. Neuroscience. 2007;144:46–55. doi: 10.1016/j.neuroscience.2006.08.083. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Johnson KM. Differential effects of acute and subchronic administration on phencyclidine-induced neurodegeneration in the perinatal rat. J. Neurosci. Res. 2005;81:284–292. doi: 10.1002/jnr.20559. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Buhler KG, Lavecchia KL, Johnson KM. Pharmacological challenge reveals long-term effects of perinatal phencyclidine on delayed spatial alternation in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2003;27:867–873. doi: 10.1016/S0278-5846(03)00146-5. [DOI] [PubMed] [Google Scholar]
- Williams ML, Mager DE, Parenteau H, Gudi G, Tracy TS, Mulheran M, Wainer IW. Effects of protein calorie malnutrition on the pharmacokinetics of ketamine in rats. Drug Metab. Dispos. 2004;32:786–793. doi: 10.1124/dmd.32.8.786. [DOI] [PubMed] [Google Scholar]
- Wolff K, Winstock AR. Ketamine: From medicine to misuse. CNS Drugs. 2006;20:199–218. doi: 10.2165/00023210-200620030-00003. [DOI] [PubMed] [Google Scholar]
- Wood KA, Dipasquale B, Youle RJ. In situ labeling of granule cells for apoptosis-associated DNA fragmentation reveals different mechanisms of cell loss in developing cerebellum. Neuron. 1993;11:621–632. doi: 10.1016/0896-6273(93)90074-2. [DOI] [PubMed] [Google Scholar]
- Xie H, Tang SY, Li H, Luo XH, Yuan LQ, Wang D, Liao EY. L: -Carnitine protects against apoptosis of murine MC3T3-E1 osteoblastic cells. Amino Acids. 2007 doi: 10.1007/s00726-007-0598-9. doi:10.1007/s00726-007-0598-9. [DOI] [PubMed] [Google Scholar]
- Yoon WJ, Won SJ, Ryu BR, Gwag BJ. Blockade of ionotropic glutamate receptors produces neuronal apoptosis through the Bax-cytochrome C-caspase pathway: The causative role of Ca2+ deficiency. J. Neurochem. 2003;85:525–533. doi: 10.1046/j.1471-4159.2003.01724.x. [DOI] [PubMed] [Google Scholar]
- Young C, Jevtovic-Todorovic V, Qin YQ, Tenkova T, Wang H, Labruyere J, Olney JW. Potential of ketamine and midazolam, individually or in combination, to induce apoptotic neurodegeneration in the infant mouse brain. Br. J. Pharmacol. 2005;146:189–197. doi: 10.1038/sj.bjp.0706301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zissen MH, Zhang G, McKelvy A, Propst JT, Kendig JJ, Sweitzer SM. Tolerance, opioid-induced allodynia and withdrawal associated allodynia in infant and young rats. Neuroscience. 2007;144:247–262. doi: 10.1016/j.neuroscience.2006.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X, Sadovova N, Patterson TA, Divine RL, Hotchkiss CE, Ali SF, Hanig JP, Paule MG, Slikker W, Jr, Wang C. The effects of L-carnitine on the combination of, inhalation anesthetic-induced developmental, neuronal apoptosis in the rat frontal cortex. Neuroscience. 2008;151:1053–1065. doi: 10.1016/j.neuroscience.2007.12.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.