Abstract
In 1991, a group of expert scientists at a Wingspread work session on endocrine-disrupting chemicals (EDCs) concluded that “Many compounds introduced into the environment by human activity are capable of disrupting the endocrine system of animals, including fish, wildlife, and humans. Endocrine disruption can be profound because of the crucial role hormones play in controlling development.” Since that time, there have been numerous documented examples of adverse effects of EDCs in invertebrates, fish, wildlife, domestic animals, and humans. Hormonal systems can be disrupted by numerous different anthropogenic chemicals including antiandrogens, androgens, estrogens, AhR agonists, inhibitors of steroid hormone synthesis, antithyroid substances, and retinoid agonists. In addition, pathways and targets for endocrine disruption extend beyond the traditional estrogen/androgen/thyroid receptor–mediated reproductive and developmental systems. For example, scientists have expressed concern about the potential role of EDCs in increasing trends in early puberty in girls, obesity and type II diabetes in the United States and other populations. New concerns include complex endocrine alterations induced by mixtures of chemicals, an issue broadened due to the growing awareness that EDCs present in the environment include a variety of potent human and veterinary pharmaceutical products, personal care products, nutraceuticals and phytosterols. In this review we (1) address what have we learned about the effects of EDCs on fish, wildlife, and human health, (2) discuss representative animal studies on (anti)androgens, estrogens and 2,3,7,8-tetrachlorodibenzo-p-dioxin–like chemicals, and (3) evaluate regulatory proposals being considered for screening and testing these chemicals.
Keywords: endocrine disruptors, androgens, estrogens, dioxins, PCBs, Pharmaceuticals, Mixtures, Screening and Multigenerational Testing
It has been approximately 15 years since the first World Wildlife Federation (WWF) Wingspread Conference focused on endocrine-disrupting chemicals (EDCs), and 10 years since the U.S. Environmental Protection Agency (USEPA) was given a mandate under the Food Quality Protection and Safe Drinking Water Acts to develop test protocols to screen for endocrine effects of chemicals. This review article will briefly highlight some of the major scientific events that have taken place since the inception of the EDCs issue. In this review, we will (1) discuss EDCs effects on invertebrates, fish, wildlife, and human health, (2) highlight some representative studies on (anti)androgens, estrogens and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)–like chemicals, and (3) evaluate regulatory proposals being considered for screening and testing these chemicals.
An initial impetus for studies of EDCs arose from the Wingspread work session in 1991 on “Chemically Induced Alterations in Sexual Development: The Wildlife /Human Connection” (Colborn and Clement, 1992). A consensus from that meeting, reached by a panel of expert scientists was that “Many compounds introduced into the environment by human activity are capable of disrupting the endocrine system of animals, including fish, wildlife, and humans. Endocrine disruption can be profound because of the crucial role hormones play in controlling development” (Colborn and Clement, 1992). These scientists also “estimated with confidence” that developmental impairments in humans have resulted from exposure to EDCs that are present in the environment as a result of human activities. Within a 5-year span several international organizations held meetings to discuss the EDC issue and research objectives. For example, starting in 1996 the USEPA held a series of workshops highlighting the new EDC issue and research needs in collaboration with the WWF and Chemical Manufacturers Association (Ankley and Giesy, 1998; Ankley et al., 1998; DeVito et al., 1999; Gray et al., 1997a) The European Union (Weybridge Report, European Commission,1996), Australia, and several Asian countries including Korea, Japan held similar meetings.
Subsequent studies have strengthened the documentation of effects of EDCs on animals. Although most of the effects in humans are due to occupational exposures or pharmaceutical usage, hundreds if not thousands of publications showing associations between background EDC exposures and adverse effects in humans are now available in the peer-reviewed literature. Many of the associations between EDCs and human health effects are controversial, but others, like the effects of polychlorinated biphenyls (PCBs) on neurological and immune function are quite widely accepted. Furthermore, animal laboratory studies corroborate many of these adverse effects observed in the field and, in some cases, provide mechanisms to explain the effects.
About 10 years ago the discussion of “endocrine disrupters” broadened from a focus on environmental estrogens to include additional mechanisms of endocrine toxicity; mechanisms that in many cases may be of equal or greater concern than estrogens. Numerous “new” areas of interest and concern have arisen since the original 1991 Wingspread conference and regulatory mandates. These areas include processes potentially disrupted by new classes of anthropogenic chemicals that act as antiandrogens, androgens, inhibitors of steroid hormone synthesis, antithyroid substances, and retinoid agonists. Within the last few years, scientists also have expressed concern about the potential role of EDCs in increasing trends in obesity and type II diabetes in the United States and other populations. In addition, although research has documented the complexity of the multiple mechanisms by which a single chemical can alter the endocrine milieu, recent evidence demonstrates the need to investigate complex endocrine alterations induced by mixtures of chemicals. We are now not only concerned about pesticides and other toxic substances in the environment, but the issue has broadened considerably due to the growing awareness that the list of EDCs present in the environment from human activities includes potent human and veterinary pharmaceutical products, personal care products, nutraceuticals and phytosterols. Among the drugs found as pharmaceuticals in the environment (PiEs) are potent, long lasting estrogens, antibiotics, β-blockers, antiepileptics, androgenic steroids, and lipid regulating agents. Some PiEs have been linked to dramatic effects in wildlife, including death in bald eagles (http://www.fws.gov/southeast/news/2002/12-03SecPoisoningFactSheet.pdf), threatened extinction of species of vultures in Asia (Shultz et al., 2004) and sex reversal and infertility in several species of fish (WHO, 2002.).
The 1996 legislation mandated that the USEPA both establish validated screening and testing procedures for estrogens and other substances as deemed appropriate and consider combinations of chemicals rather than evaluate the potential risk on a chemical by chemical basis. Both of these mandates encompass new areas of investigation in the EDC field. Furthermore, reports of nonmonotonic (e.g., U-shaped) dose–response relationships, ultra-low dose effects and nonthreshold effects for EDCs continue to challenge some of the basic assumptions of toxicology and risk assessment. Although the focus of this debate has centered on the low dose effects of Bisphenol A, U-shaped dose–response curves are well known from many in vitro and a few in vivo studies. It appears that the basic tenet of toxicology from Ames and Gold (2000) that “dose alone determines the poison” is too limited for some EDCs because the timing of exposure can dictate not only the effect, but also whether the effects are adverse versus beneficial, or permanent versus transient. In vivo, however, the U-shaped effects of EDCs are generally limited to one or two effects whereas all other effects display “normal” nonmonotonic dose responses. Furthermore, EDCs that induce cellular and molecular alterations of endocrine function at low dosage levels produce a cascade of effects increasing in severity resulting in adverse alterations at higher doses. The effects in the high dose groups may be different from those seen at low doses and the low dose effects are often causally linked to the high dose adverse effects of the chemical. This is true for androgens, natural estrogens, xenoestrogens, and antiandrogens.
The present review will focus on some of the new scientific issues in regard to EDCs that have surfaced since the initial Wingspread conference. The review will focus on effects of endocrine-active chemicals on the androgen, estrogen, and AhR-mediated signaling pathways. Reviews of other hormonal pathways potentially affected by EDCs, such as the thyroid system, can be found elsewhere (Guillette, 2006; Howdeshell, 2002; Tabb and Blumberg, 2006; Zoeller and Crofton, 2005).
EDC EFFECTS ON FISH AND WILDLIFE, DOMESTIC ANIMALS, AND HUMAN HEALTH
Invertebrates, Fish, and Wildlife
Endocrine-disrupting compounds have had dramatic effects on some invertebrate, fish, and wildlife populations. The diversity in life histories and habitats of animals in the environment introduce the possibility of species or life-stage–specific responses to EDCs that may not be evident in controlled laboratory experiments. Chemicals including organotins, organochlorine pesticides, PCBs, and steroidal androgens and estrogens have all been associated with endocrine-mediated dysfunction in fish and wildlife populations. Wildlife studies can identify emerging contaminant problems, as well as inform about mechanisms of toxicity from laboratory work.
Great strides have been made in establishing cause and effect linkages between EDC exposures and observed effects in wildlife despite the inherent challenges. The complexity of environmental exposures often precludes identification of a single chemical responsible for observed population level effects. Additionally, it can be difficult to identify the underlying mechanism of the effect. These obstacles can be overcome by using a weight-of-evidence approach to assess the entire body of work surrounding observed wildlife effects.
Criteria have been developed for assessing the strength of the causal linkage between contaminant exposure and population level effect (Ankley et al., 1998; WHO, 2002). These criteria assess the cause–effect connection based on concepts of temporality, strength of association, consistency of observation, biological plausibility, and evidence of recovery (WHO, 2002). There are several cases where strong evidence supports a causal linkage between exposure and effect (Table 1). Two examples discussed in detail below are organotin-mediated imposex in marine snails and feminization of sewage effluent-exposed male fish.
TABLE 1.
Field and Lab Criteria for Determining a Causal Linkage between Endocrine Disruptors and Wildlife Effects (Ankley and Giesy, 1998; Gray et al., 1998; WHO, 2002)
| Field criteria |
Lab criteria |
||||||||||
| Animal | Notable effect | Chemical(s) | Exposure–effect correlation | Effect involved in multiple populations | Specific effect(s) observed in exposed population | Exposure correlates with effect onset | Correlation of effect with exposure overtime | Correlation of decrease in effect with exposure remediation | Replication of field effects in lab species with relevant concentrations | Identification of mechanism of action | Replication of effect with like-acting chemicals |
| Marine snails | Imposex | TBT | + | + | + | + | + | + | + | +/− | + |
| Predatory birds | Egg shell thinning | p,p′-1,1-Dichloro-2,2-bis(p-chlorophenyl) ethylene (DDE) | + | + | + | + | + | + | + | +/− | + |
| Fish | Feminization | Sewage effluent | + | + | + | + | + | + | + | + | |
| Fish | Masculinization | Pulp/paper mill effluent | + | + | + | + | + | + | + | + | |
| Lake Trout | Low hatchability of fertilized eggs | TCDD-like compounds | + | + | + | + | + | + | + | +/− | + |
| Mink/otter | Reproductive failure | PCBs | + | + | + | + | + | + | + | +/− | + |
| Seals | Reproductive failure | PCBs | + | + | + | + | + | + | +/− | ||
| Seals | Immune dysfunction | PCBs | + | + | + | + | + | + | +/− | ||
| Alligator | Demasculinization | p,p′-DDE and other organochlorine pesticides | + | + | + | +/− | |||||
| Frogs | Hermaphroditism/ Demasculinization | Atrazine | +/− | + | + | + | + | ||||
| Polar bears | Demasculinization | PCBs | + | + | + | ||||||
| Florida panthers | Cryptorchidism | Agricultural chemicals | + | + | + | ||||||
Note. + indicates that data support the listed criteria; +/− indicate that some data support the listed criteria, but more work is needed to confirm.
Exposure to the biocide tributyltin (TBT) causes imposex, or pseudohermaphroditism in female prosobranch gastropods (for reviews see, Ankley and Giesy, 1998; WHO, 2002). Imposex is the imposition of male sex organs, including penis and vas deferens, onto female snails and can lead to reproductive failure in some species (Horiguchi, 2006). It has been documented worldwide in around 150 species (Horiguchi, 2006). Due to these effects, use of TBT was restricted during the 1990′s leading to subsequent recovery in multiple marine snail populations (Jorundsdottir et al., 2005). High levels of TBT, however, are still found in some aquatic ecosystems because all usages have not been banned. The observation of imposex in these organisms has been associated in some studies with increased titers of testosterone in snail tissue (Spooner et al., 1991). Although there are several competing hypotheses for this effect, including aromatase inhibition, altered metabolism of testosterone, and disruption of neuropeptide signaling; a definitive mechanism of action has yet to be fully confirmed (Horiguchi, 2006; Sternberg et al., 2008). New studies indicate that TBT causes imposex in invertebrates by acting as a retinoid X receptor (RXR) agonist, and displays an affinity for both invertebrate and vertebrate retinoid receptors (Nishikawa et al., 2004).
Exposure to sewage effluent has been associated with induction of vitellogenin in male fish and the occurrence of intersex in wild fish (Jobling et al., 2002a, b). Vitellogenin is an estrogen-responsive egg yolk protein precursor not normally expressed in male fish. A gradient of effect exists with fish closest to the sewage outflow demonstrating the most severe responses (Harries et al., 1999). Initial attention on feminized fish focused primarily on studies from the United Kingdom, however, the phenomenon seems to be relatively global in nature (Ankley et al., 2007; WHO, 2002). For example, in the United States, the Potomac River has been identified as a hot spot for feminized smallmouth bass (Chambers and Leiker, 2006).
Caged fish and laboratory-based exposures confirm that sewage effluent is responsible for the observed increases in vitellogenin (WHO, 2002) and reproductive failure (Martinovic et al., 2007). Although no single chemical has been identified as the culprit, chemical fractionation studies of sewage effluent have shown that synthetic and natural estrogens are often present in biologically relevant quantities (Desbrow et al., 1998; WHO, 2002). These estrogenic compounds include synthetic pharmaceuticals and natural hormones in wastewater, such as ethinyl estradiol and 17β-estradiol. Various laboratory studies have shown that exposure to relatively low levels of these steroids will induce vitellogenin and can cause the development of intersex gonads (ovotestis) in fish and amphibians (Hutchinson et al., 2006). Furthermore, a recent study by Kidd et al. (2007) showed that levels of ethinyl estradiol sufficient to cause vitellogenin induction in fish also caused a substantial decrease in the sustainability of the wild fish populations.
In some instances, there is moderate evidence for a causal linkage between EDC exposure and effects in wildlife; for example, PCB-induced reproductive and immune dysfunction in Baltic seals (Table 1). Great Lakes trout populations were adversely affected by PCB and dioxin exposures in the 1960–1970′s, PCB-exposed cormorants displayed crossed-bills, great blue herons were infertile, and mink fed PCB contaminated fish from the Great Lakes either died after high exposures or were infertile at lower exposure levels. Proposed associations, which remain weak and also require elucidation, include reproductive and developmental anomalies in frogs exposed to atrazine and cryptorchidism in Florida panthers (Table 1).
Domestic Animals
Naturally occurring compounds with estrogenic and other endocrine activities are widespread in nature. Over 400 species of plants contain known estrogens such as isoflavonoids or coumestans, or are suspected of being estrogenic based on biological grounds. Plants also may contain other compounds, in addition to estrogens, that can affect reproductive performance, such as antiandrogens in oil from saw palmetto (Gray et al., 2001a).
Some plant estrogens occur at sufficient concentrations to cause reproductive alterations in domestic animals. “Clover disease,” which is characterized by dystocia, prolapse of the uterus and infertility, is observed in sheep grazed on clover containing potent plant estrogens. Permanent infertility can be produced in ewes by much lower amounts of estrogen over a longer time period than are needed to produce clover disease (Adams, 1995).
In domestic animals, feeds contaminated with the zearalenone-producing fungus (Fusarium spp.) induce adverse reproductive effects in a wide variety of domestic animals, including impaired fertility in cows and hyperestrogenism in swine (Meyer et al., 2000) and turkeys (Gray et al., 2001a).
Humans
Several chemicals are known to directly affect the endocrine system by altering development or reproduction in humans. Although other chemicals affect the reproductive system and indirectly alter endocrine function resulting in endocrine disruption and disruption of other components of the reproductive system. The examples of anthropogenic chemicals known to interact with the human endocrine system discussed herein includes accidental exposures to pesticides, toxic substances and pharmaceuticals with adverse side effects. In addition, we also discuss a few cases where pesticides or their metabolites and plant derived chemicals have been found to possess sufficient endocrine activity to be considered for use as pharmaceuticals, although no adverse effects have been associated with use of these chemicals. Some of the chemicals discussed are hormone agonists or antagonists or directly alter hormone synthesis. Others affect multiple cell types in the reproductive system including those like the Sertoli cell, cells that have both endocrine (secretes several hormones including antimullerian hormone and inhibin) and nonendocrine functions (support germ cell development).
Reproductive Effects of Chemicals in Humans Resulting from Occupational or Accidental Exposures or Pharmacological Side Effects
Accidental, occupational and therapeutic exposures to EDCs have produced adverse effects in humans (e.g., effects seen with PCBs (AhR and non-AhR pathways), TCDD, 2-bromopropane (likely affects the endocrine system indirectly), 4,4′-diaminostilbene-2,2′ disulfonic acid (DAS) (possible estrogenic mechanism), diethylstilbestrol (DES, estrogenic mechanism), the androgenic drugs danazol and methyltestosterone, and aminoglutethimide (drug that was taken that inhibits aromatase directly) (reviewed in Gray et al., 2001a, b).
Developmental Reproductive Toxicants Acting via an Endocrine Mechanism
In Utero
In a very well-documented example of EDC effects in humans, in utero exposure to the estrogenic chemical DES alters reproductive development. Specifically, DES taken during pregnancy to prevent premature birth, which it failed to do, caused developmental alterations in the daughters including clear cell adenocarcinoma of the vagina, and gross structural abnormalities of the cervix, uterus, and fallopian tube. Women who were exposed to DES in utero are more likely to have spontaneous abortions, ectopic pregnancies and premature births (Steinberger and Lloyd, 1985). Developmental DES exposure has been reported to cause women to have less well-established sex-partner relationships; reduced sexual desire, excitability, and coital functioning, and an increased masculine pattern of cerebral lateralization on a verbal task (reviewed by Gray et al., 2001a). Although far less prevalent and certain (Joffe, 2003), there are reports that males are also affected by developmental exposure to DES, displaying underdevelopment or absence of the vas deferens, epididymis, and seminal vesicles and persistence of the Mullerian ducts (Steinberger and Lloyd, 1985). They also reported that DES causes epididymal cysts, hypotrophic testes and infertility, and, in some males reduced ejaculate volume and numbers of motile sperm (Steinberger and Lloyd, 1985).
In utero exposure to the androgenic drug, danazol, also is contraindicated during pregnancy. Brunskill (1992) reported that of 94 completed pregnancies, 37 resulted in the birth of normal males, 34 in nonvirilized females, and 23 in virilized females. Virilization occurred in a proportion of female fetuses with a pattern of clitoromegaly, fused labia and urogenital sinus formation. The abnormality has not been reported where danazol therapy had been discontinued before the eighth week of pregnancy. Although more common in the higher dosages, virilization was reported in one case with a 200 mg daily dosage. Similar effects have been reported for other androgenic drugs (Grumbach and Ducharme, 1960; Saunders, 1968). The anticonvulsant drug aminoglutethimide, which inhibits aromatase and the production of estrogens from androgens, has also been associated with pseudohermaphroiditism in daughters (LeMaire et al., 1972).
Pubertal
In another more recent example of unintended effects of EDCs in humans, estrogen-containing shampoos (Tiwary, 1998) and skin oils (Henley et al., 2007) were shown to be causative factors for pseudoprecocious puberty in girls and gynecomastia in boys (Felner and White, 2000). Some of the shampoos, for example, contained up to 4 mg of estradiol per 100 g. Others contained up to 2 g of the much less potent estriol per 100 g (Tiwary, 1998).
Virilization in young children as young as 17 months of age is uncommon and is produced by androgens (Kunz et al., 2004), which may be from endogenous or exogenous sources. Recently, the increased availability and use of commercial androgen products for cutaneous application has increased the risk of virilization in children through skin contact and passive absorption.
Reproductive Toxicity in Adults Resulting from an Endocrine Mechanism
Clinical studies by The National Institute of Occupation Safety and Health documented sexual impotence in chemical factory workers exposed to DAS, a DES-like stilbene derivative. These studies were carried out in response to complaints of impotence and decreased libido among male workers involved in the manufacture of DAS, a key ingredient in the synthesis of dyes and fluorescent whitening agents. The data from the studies showed that workers manufacturing DAS had lower serum testosterone levels and reduced libido (Grajewski et al., 1996; Whelan et al., 1996) as compared with control workers. They found that “Current and former DAS workers had lower mean total testosterone (TT) levels compared with additives workers (458 and 442, respectively, vs. 556 ng/dl; p = 0.05 and 0.04). Current and former DAS workers were 3.6 (95% CI, 0.5–24.4) and 2.2 (95% CI, 0.3–18.0) times more likely than additives workers to have lowest quartile TT levels (< 386 ng/dl) after adjustment for age and body mass index. Duration of employment in DAS production was negatively related to the workers’ testosterone levels.” Studies in rodents suggest that DAS has estrogenic activity, possibly providing a possible mechanistic explanation for the complaints of impotency in factory workers exposed to these substances (Smith and Quinn, 1992).
The o,p′-dichlorodiphenyltrichloroethane (DDT) metabolite, o,p′-Ortho,para,dichlorodiphenyl dichloroethane (DDD) has historically been considered as an environmental contaminant. However, o,p′-DDD also has clinical uses due to its ability to alter endocrine function and it has been reported to induce both beneficial and adverse side effects. o,p′-DDD (Mitotane), has been used to treat adrenal steroid hypersecretion associated with adrenal tumors in humans and pets (Reine, 2007). It has been reported to act by inhibiting P450 steroidogenic enzymes and also has some estrogenic activity in humans (Nader et al., 2006). In addition, mitotane treatment restored menstruation in women with high androgen levels of spanomenorrhea associated with hypertrichosis with pregnancies occurring in about a third of the treated women (Hayes and Laws, 1991). However, Mitotane treatment has been associated with atrophy of the spermatogenic tubules of the testis in men (Sparagana, 1987). Sparangana studied a patient who developed impotency due to primary testicular failure at the time that he was treated with Mitotane. A testicular biopsy, performed after the drug was discontinued, showed normal appearing Leydig cells and atrophy of the seminiferous tubules with the picture of a maturation arrest. In the four and one half years since he last received mitotane, the patient's libido has slowly improved and his plasma testosterone, gonadotropins and LH response to gonadotropin-releasing hormone have become essentially normal. He proposed that mitotane was cytotoxic to the testis as it is to the adrenal cortex.
Reproductive Toxicity in Adults that Indirectly Produces Endocrine Alterations
Yet another example of a chemical altering reproductive and endocrine function in humans is 2-bromopropane (CERHR, NTP, 2003) (Kim et al., 1996). Korean electric workers exposed to solvents containing this chemical displayed reproductive and endocrine abnormalities. Specifically, women showed secondary amenorrhea, primary ovarian failure with high follicle–stimulating hormone levels, hot flashes and undetectable estradiol levels (below 13.6 pg/ml the LOD). Exposed men displayed azoospermia, some degree of oligospermia, or reduced sperm motility (Kim et al., 1996) but had normal libido and based upon hormonal levels it appears that the germ cells and not the Leydig cells were directly affected by 2-bromopropane (2003).
Dibromochloropropane (DBCP) is another human reproductive toxicant that indirectly disrupts endocrine function in humans (Lag et al., 1989; Potashnik and Porath, 1995; Potashnik and Yanai-Inbar, 1987; Slutsky et al., 1999; Whorton and Foliart, 1983; Whorton and Milby, 1980; Whorton et al., 1979). The pesticide DBCP acts directly on the germ cells as a mutagen. Occupational exposures during manufacturing and application in several countries (Teitelbaum, 1999) resulted in permanent oligospermia, azoospermia, and infertility, and indirectly elevated FSH levels and nonsignificant reductions in testosterone. Unlike 2-bromopropane, above, few women were exposed to DBCP. In rodents, in utero DBCP severely disrupts fetal testis development (Warren et al., 1988). Effects on sperm counts and FSH levels persist in some workers for at least 17 years. Despite the ban on the use of DBCP, this pesticide remains persistent in soil and continues to be detected as a groundwater contaminant in areas of past high use, in particular California's Central Valley (Clark and Snedeker, 2005; Teitelbaum, 1999). Although case–control, cohort and ecological epidemiology studies have not found significant, positive associations between DBCP exposure and cancer in exposed populations, concerns persist due to the cited limitations in these studies (Clark and Snedeker, 2005).
EDCs in Humans and Effects at Background Exposure Levels
In contrast to the above examples, background human EDC exposure–effect relationships have only occasionally approached a significant degree of certainty. There are literally thousands of studies on the potential effects of EDCs on human health. Although many of the have shown associations among EDC and reproductive alterations (sperm abnormalities, shortened anogenital distance in boys, altered sex ratio, testis cancer, cryptorchidism, hypospadias, testes cancer, prostate cancer, advanced onset of puberty, breast cancer, endometriosis, obesity, attention deficit disorder, uterine cancer, and allergies) many others have failed to find such associations and, for this reason, there remains a great deal of uncertainty about the effects of background levels of EDCs on human reproduction. Although it is very important that scientists continue to evaluate the effects of background levels of EDCs on human disease, it is more important to prevent as opposed to detect the effects of EDCs on humans, fish, and wildlife. As stated by Lucier (2007; http://domesticpolicy.oversight.house.gov/documents/20070925143750.pdf), “We should keep in mind that a positive finding in an epidemiology or clinical study is, in reality, a failure of preventive medicine policy.”
In contrast, the effects of the PCBs on human neurological and immune development, is one case where many scientists have concluded that the data are robust enough to conclude that they approach the level of cause and effect (reviewed in Brouwer et al., 1999; Selgrade, 2007). Even here, there remains considerable uncertainty. In contrast to background levels of PCBs, children accidentally exposed to high levels of PCBs and dibenzofurans display neurobehavioral abnormalities from exposure in utero and during early postnatal development (Guo et al., 1995). These changes included low birth weight, malformations, and reduced IQ and cognitive abilities. Furthermore, the exposed males exhibited abnormal sperm morphology, reduced sperm motility, and reduced in vitro sperm fertilizing capacity (Guo et al., 1995). Selgrade (2007) recently concluded that “suppression of immune responses in rodents is predictive of suppression of immune responses in humans and that there is a relationship between immune suppression following developmental exposure to the toxicants and enhanced risk of infectious or neoplastic disease in humans” for PCBs as well as cigarette smoke and arsenic.
Adverse Effects of EDCs in Laboratory Animals
In Vivo Studies on Select Classes of EDCs
The following section briefly reviews selected examples of the effects of EDCs on, mostly, rodent reproductive development. Although this review does not include a discussion of the in vitro and short-term studies examining the mode(s) of action of the selected chemicals, such studies are essential to confirm mechanisms of action hypothesized from in vivo studies. Specific EDC classes considered here include antiandrogens, androgens, estrogens and AhR agonists (such as TCDD).
Environmental antiandrogens.
Several environmental chemicals adversely affect male development by interfering with androgen signaling during the critical periods of sexual differentiation and maturation. Depending on the timing of exposure (e.g., in utero or peripubertal), these antiandrogens affect androgen-sensitive organs and processes within the male rat, leading to histological lesions within the reproductive organs, delayed puberty (Monosson et al., 1999), hypospadias/cryptorchidism, reduced fertility, and testicular tumors. Mechanistic work has identified some antiandrogenic chemicals to be androgen receptor (AR) antagonists, e.g., the DDT metabolite p,p′-DDE (Kelce et al., 1995), the fungicides vinclozolin (Gray et al., 1994; Kelce et al., 1997), procymidone (Gray et al., 2006; Hosokawa et al., 1993; Ostby et al., 1999) and prochloraz, the herbicide linuron, and polybrominated diphenyl ether (PBDE) flame retardants (Stoker et al., 2005).
Some chemicals can produce antiandrogenic effects via depression of testicular testosterone production. The fungicide prochloraz induces malformations in male offspring and delays the onset of puberty after peripubertal exposure, with mechanistic work revealing that it is not only an AR antagonist but also acts as a potent steroid synthesis inhibitor (Blystone et al., 2007a, b; Noriega et al., 2005; Wilson et al., 2004c). Similarly, linuron, is both an AR antagonist (Lambright et al., 2000) and it reduces testosterone production (Hotchkiss et al., 2004; Wilson et al., 2004c). The AR antagonism and reduced testosterone production displayed by these chemicals (Hotchkiss et al., 2004; Wilson et al., 2004c) likely has a cumulative effect on the male offspring, but the relative contribution of each mechanism requires further clarification.
Several phthalate esters, used as plasticizers in many commercial products, are antiandrogens, which gives cause for concern because there is widespread exposure to these chemicals (Silva et al., 2004). Malformations of male reproductive organs occur after in utero exposure to some phthalates (e.g., dibutyl phthalate, butyl benzyl phthalate, DEHP, di-iso-nonyl phthalate) and, through a mechanism that has yet to be established, these chemicals reduce fetal rat testosterone production (Foster, 2006; Gray et al., 2000; Mylchreest et al., 1998; Parks et al., 2000; Sharpe, 2006; Wilson et al., 2004c). In addition to reduced testosterone, phthalate exposure also reduces insl-3 gene expression (Wilson et al., 2004c); the insl-3 peptide hormone is necessary for transabdominal testis decent (Klonisch et al., 2004).
The spectrum of malformations that result from disrupting the androgen signaling pathway varies depending on the mechanism of action. For example, hypospadias is prominent at high doses of vinclozolin (whose metabolites are AR antagonists), whereas high levels of phthalates (which reduce fetal testosterone and insl-3 expression) induce epididymal agenesis. However, linuron and prochloraz, two chemicals that one would expect to display similar effects based upon similar dual mechanisms, differ in the relative rates of hypospadias and epididymal agenesis that they induce (Gray et al., 2006). Together this area of EDC research has characterized the effects of several antiandrogens and identified relevant and diverse mechanisms of action pertinent to human health risk assessment because this pathway is highly conserved among vertebrates. Disruptions of this pathway either via genetic mutations or steroid-like chemical exposures results in profound examples of pseudohermaphroditism in experimental animals and humans (Quigley et al., 1995).
The reproductive system is also sensitive to phthalate exposure during the peripubertal and neonatal stages of life. Rats treated with diethyl hexyl phthalate (DEHP) orally or by injection as neonates display permanent alterations lesions of the testis and sperm numbers (Cammack et al., 2003; Dostal et al., 1988). When administered during peripubertal life, di-n-phthalate esters with side chains with three to six carbons (Foster et al., 1981) disrupt testis structure and function, reduces testosterone production, delays pubertal onset and reduces androgen-dependent organ weights in male rats (Gray et al., 1999). These effects are not limited to Murid and Cricetid rodents like the rat and hamster, respectively, and have been seen in several other mammalian species including the guinea pig (Gray et al., 1982), ferret (Lake et al., 1976) and rabbit (Higuchi et al., 2003).
Sjoberg et al. (1986) found an age sensitivity in response with the adults more resistant to testicular damage than pubertal animals. When he administered DEHP in the diets of 25-, 40-, and 60-day-old male rats for 14 days, testis weight was reduced in males in 25-day-old males (at 1 g/kg/day) and 40 (at 1.7 g/kg/day) and all testis tubules were damaged in 25-day-old males (at 1.7 g/kg/day). No testicular effects were observed in 60-day-old animals (Sjoberg et al., 1986).
Environmental androgens.
It was noted in the 1970′s that female fish of several species living in rivers contaminated with pulp mill effluent (PME) displayed masculinized sexual traits and starting in 2001 two different research teams reported that PME displayed androgenic activity in in vitro assays (Jenkins et al., 2003; Parks et al., 2001). Since then, androgenic activity has been detected in PME from other rivers in Florida, the Baltic Sea, the Great Lakes and New Zealand (Ellis et al., 2003; Larsson and Forlin, 2002; Parks et al., 2001). PME effluents from sites on the Fenholloway River in Florida include a chemical mixture that binds AR and induces androgen-dependent gene expression in vitro. This mode of action is consistent with the observation of masculinized female mosquitofish (Gambusia holbrooki) collected from contaminated sites on the river. Male-biased sex ratios of fish embryos have been reported near a pulp mill in broods of eelpout (Zoarces viviparus) in the vicinity of a large kraft pulp mill on the Swedish Baltic coast, suggesting that masculinizing compounds in the effluent were affecting gonadal differentiation and promoting skewed sex ratios. Although androgenic chemicals have been isolated from PME, efforts to date have not conclusively identified the chemicals in PME responsible for masculinization of the female fish (Durhan et al., 2002).
Effluents from cattle concentrated animal feeding operations (CAFO) in Nebraska and Ohio have also been shown to display androgenicity. Orlando et al. (2004) found that water associated with a CAFO in Nebraska exhibited androgenic activity and found that fish (fathead minnow; Pimephales promelas) collected at the site displayed small gonads, fewer embryos (Orlando et al., 2007) and morphological differences compared with fish from a reference site. Durhan et al. (2006) detected the synthetic androgens 17α-trenbolone and 17β-trenbolone in water samples associated with a beef CAFO in Ohio, where trenbolone acetate implants were used to stimulate weight gain; samples collected from a direct discharge from the feedlot displayed significant androgenic activity in vitro. Complementary laboratory studies revealed both trenbolone isomers were androgenic in the fathead minnow (Ankley et al., 2003; Jensen et al., 2006) and the rat (Hotchkiss et al., 2007; Wilson et al., 2002b). For example, in utero administration of 17β-trenbolone to dams resulted in masculinized female rat offspring, displaying increased anogenital distance (AGD), vaginal agenesis and induced male sex accessory tissues in females (Hotchkiss et al., 2007; Wilson et al., 2002b).
Environmental estrogens.
Much of the interest in environmental estrogens grew out of reports of reproductive cancers, reproductive dysfunction, and potential behavioral alterations of women who had been developmentally exposed to the pharmaceutical estrogen, DES. Estrogens are critical for female reproduction both developmentally and in adulthood. Two different estrogen receptors (α, β) have been characterized in mammals with 3 different receptors (α, β1, β2) identified in teleost fish (Hawkins et al., 2005). Differential expression of these receptors in tissues as well as different binding affinities for various ligands suggests diversity in physiological function.
Studies with mammals have shown that estrogenic compounds are quite varied in structure and potency, including pharmaceuticals (ethinylestradiol, DES), insecticides (methoxychlor, DDT, Chlordecone), surfactants (octyphenol, nonylphenol), phytoestrogens (genistein, coumesterol), sun screens, and plastics (bisphenol A) (Gray et al., 2001a).
High dose oral in utero exposure to potent estrogens such as ethinyl estradiol induces reproductive tract malformations in rats with females affected at lower doses than male offspring. In contrast, estrogenic pesticides or toxic substances have not been shown to induce similar structural malformations. Perinatal oral maternal exposure to weaker estrogenic chemicals like methoxychlor defeminizes the central nervous system (CNS) of the female rat such that they are acyclic and infertile and have a shortened reproductive life span due to an early onset of irregular estrous cycles. Male offspring from such studies are fertile but occasionally display some effects on reproductive tissues at higher dosage levels. Although one laboratory has reported that ip injections of pregnant rats with high doses of methoxychlor during gonadal differentiation induces testicular lesions and reduced fertility in offspring, with such effects being transmitted through several generations (Anway et al., 2005), other investigators have not observed similar testicular effects or reduced fertility in F1 males using oral exposures (a more relevant exposure route for risk assessment) during this period of development (Gray et al., 1989).
Exposure of developing female rodents (rats and hamsters) to ethinyl estradiol, methoxychlor, chlordecone, and zearalenone, for example, can result in altered reproductive behaviors, sexually dimorphic nonreproductive behavior, neuroendocrine function (estrous cycles, onset of puberty), fertility, and reproductive tract morphology. Developing males are not immune to the adverse effects of exogenous estrogens. Studies with ethinyl estradiol in males showed altered testicular development among other effects (Howdeshell et al., 2007).
Oral exposure to all potent and may xenoestrogens after weaning of female rats causes pseudoprecocious puberty (early vaginal opening), decreased food intake, and body weight, altered activity, and altered reproductive behavior in both males and females (Gray et al., 1989; Gray and Ostby, 1998). Finally, there are ongoing discussions about the possibility of low dose effects of this class of chemicals, especially Bisphenol A, and this continues to be an active area of research and debate about the reproducibility and relevance of the low dose findings given the lack of adverse effects at any dose level (CERHR, NTP Bisphenol A Expert Panel Final Nov 26, 2007) (Guillette, 2006).
Environmental AhR agonists.
Exposure to Ah receptor agonists such as TCDD, PCBs, and PCDFs has been causally linked to developmental/reproductive toxicity in humans (Brouwer et al., 1999; Guo et al., 1995), nonhuman primates (Rier et al., 2001), rodents (see below), mink (Hornshaw et al., 1983), fish (Ankley and Giesy, 1998), and other wildlife species (Ankley and Giesy, 1998). Agonists of the AhR (which is a cellular steroid hormone-like receptor) form a complex with the receptor that acts as a transcription factor by binding to specific DREs (dioxin response elements) on specific genes. Some, if not all of the toxicity of TCDD, appears to result from activation of the Ah receptor. TCDD is an “endocrine disrupter” that acts on multiple components of the endocrine axis. TCDD exposure alters the levels of many hormones and growth factors, and their receptors (see Part II, Draft EPA Dioxin Risk Assessment document: http://www.epa.gov/ncea/pdfs/dioxin/part2/dritoc.pdf).
In addition to the effects of these chemicals seen in humans from accidental exposures, exposure to very low doses of TCDD produces infertility in rodent progeny (Bjerke and Peterson, 1994; Bjerke et al., 1994a, b; Mably et al., 1992). Exposure to a single low dose of TCDD ranging from 50 ng to 2 μg/kg during sex differentiation of the rat or Syrian hamster results in a number of unusual reproductive alterations in male and female progeny (Gray and Ostby, 1995; Gray et al., 1995, 1997b, c; Wolf et al., 1999). In female rats, gestational exposure to TCDD induced clefting of the phallus with a mild degree of hypospadias in females and a permanent “thread” of tissue across the opening of the vagina of the progeny. Female progeny, treated earlier in gestation displayed reduced fecundity, a high incidence of constant estrus, and cystic endometrial hyperplasia at middle-age. Female hamsters, exposed in utero with TCDD also displayed clitoral clefting, reduced fertility as a result of several functional reproductive problems, but they did not display the vaginal thread. In TCDD-exposed male rat and hamster offspring, puberty was delayed, ejaculated and epididymal sperm numbers were reduced, whereas the reductions in ventral prostate, seminal vesicle, and testis size, displayed during peripubertal life, were attenuated with age. When administered chronically prior to and throughout gestation, TCDD delays puberty in the male rat at doses as low as 2.4 ng/kg/day (Bell et al., 2007).
Mating behavior was normal in male hamsters, whereas male rats had some difficulty achieving intromissions. No malformations were noted in male rat or hamster offspring at these dosage levels. Furthermore, a PCB congener, 169, is an Ah receptor agonist with a toxic equivalency factor of about 0.001, as compared with the potency of TCDD. PCB 169 treatment during pregnancy alters reproductive development of Long Evans hooded male and female rats in a manner almost identical to TCDD (Gray and Ostby, 1998).
TCDD and related compounds also have been linked to endometriosis in nonhuman primates (Rier et al., 2001) and women (Louis et al., 2005). Exposure to TCDD is associated with a dose-dependent increase in the incidence and severity of endometriosis in the rhesus monkey and it was reported that the serum levels of TCDD and specific dioxin-like PHAH congeners were increased in TCDD-treated rhesus monkeys with endometriosis 13 years after the TCDD exposure.
Other classes of vertebrates are adversely affected by low concentrations of TCDD and related compounds. These chemicals are toxic to some embryonic fish (Zabel et al., 1995) and avian (Sanderson et al., 1994) species in the field and in the laboratory.
NEW AREAS OF RESEARCH
Other Pathways Affected by EDCs
It has become apparent that endocrine disruption includes mechanisms and targets beyond the traditional estrogen/androgen/thyroid (EAT) receptor–mediated systems. Examples of “nontraditional” endocrine disruption include various inhibitors of the steroidogenic pathway including fetal Leydig cell androgen production and aromatase inhibitors (Gray et al., 2006). The herbicide, atrazine, binds to a new class of membrane-bound progesterone receptors, inducing neuronal mast cell degranulation in vitro (Mizota and Ueda, 2006) and several EDCs have been reported to interact with membrane-bound estrogen receptors. However, the physiological role of these receptors and the biological significance of these in vitro observations have yet to be defined and for these reasons, binding of these receptors can only be hypothetically linked to adverse effects in vivo.
Recently, nitrates have come into focus as potential endocrine disrupters. Although historically linked to methemoglobinemia, nitrates are capable of disrupting gonadal steroidogenesis and thyroid function (Guillette, 2006). In invertebrates, other receptor systems such as the retinoid receptor systems (RXR) and ecdysteroids (EcR) play critical roles in reproduction and growth (Rodriguez et al., 2006).
Recent evidence also suggests that EDCs may disrupt terminal metabolism of endogenous and exogenous materials by altering the activating hepatic enzymes. For example, the pregnane X receptor receptor in the liver is upregulated by numerous EDCs including nonylphenol, DDT, and methoxychlor in a way that alters the metabolism of various xenobiotics and endogenous hormones (Kretschmer and Baldwin, 2005). This altered metabolism may affect androgen and estrogen levels as well as the balance of bound and free thyroid ligands (Kretschmer and Baldwin, 2005).
In addition to novel mechanisms of action, nonreproductive targets of EDCs have been identified. These targets include the cardiovascular system, the digestive system, and adipose tissue (WHO, 2002). Immune function, long known to be sensitive to steroids, has also been identified as an EDC target (Inadera, 2006). Hormone receptors have been identified on various immune cells and cytokine receptors localized to various endocrine tissues and the brain. In addition, increasing incidence of autoimmune diseases, sex differences in immune function, and documented immunomodulatory effects of sex steroids have highlighted the potential for endocrine-active chemicals to affect this dynamic system. Examples of chemicals interfering with immune function via endocrine interactions have been described for numerous compounds including androgens (Hotchkiss and Nelson, 2007), estrogens, organotins, and dioxins (Inadera, 2006). Selgrade (2007) stated that there is now sufficient data, at least for the effects of toxicants on the developing immune system, to conclude that the concern raised about human risk by the rodent data is justified (Selgrade, 2007).
Another target of EDCs is the CNS. Not only are some sexually dimorphic behaviors affected permanently by exposure to chemicals with androgenic or estrogenic activities (Gray et al., 2006) neural development also can be altered by exposure to thyroid-active EDCs (Schantz and Widholm, 2001). The hypothalamic-pituitary-adrenal axis also is a potential target of EDCs. Chemical activation of this system by PCBs or through interactions with the glucocorticoid receptors (Guillette, 2006; Johansson et al., 1998) can have adverse effects on a number of different systems, thereby expanding the number of potential targets for EDCs.
Mixtures
Mixtures of EDCs in the environment present a major emerging issue for research and risk assessment. Toxicological studies have typically focused on single chemical exposures and their effects; however, exposure to mixtures in the environment is the rule, not the exception (Kolpin et al., 2002). It is essential to develop and validate methods to accurately predict effects of endocrine-disrupting mixtures in order to protect humans and wildlife from the risk associated with potentially cumulative effects of these mixtures.
The evidence from both in vitro and in vivo studies generally indicates that mixtures of EDCs with the same mechanism of action display additive effects, however, chemical mixtures also can act in an antagonistic or synergistic manner. Initially, studies focused on estrogenic chemicals. For example, in vitro studies using the yeast estrogen screen assay (Payne et al., 2000), as well as the breast cancer cell proliferation assay (Payne et al., 2001; Silva et al., 2002) found that mixtures of xenoestrogens elicit additive activation of the estrogen receptor. Furthermore, Silva et al. (2002) showed that chemicals present at their no observed effect concentrations contribute to a cumulative effect of the mixture. In vivo studies by Brian et al. (2005) demonstrated that a mixture of five estrogenic compounds induced vitellogenin in male fish corresponding to predictions based on the concept of concentration addition.
An increasing incidence of male reproductive tract developmental abnormalities highlights the importance of assessing antiandrogenic mixtures for their potential effects (Skakkebaek et al., 2001). Recent studies in our own lab have demonstrated that in utero exposure to mixtures of chemicals that target the androgen signaling pathway at multiple sites (e.g., AR antagonists and inhibitors of testosterone) elicit dose-additive effects on the male rat reproductive tract (Gray et al., 2001, 2006; Hotchkiss et al., 2004; Howdeshell et al., 2007; Rider et al., 2008). The fact that chemicals that act on different fetal tissues via diverse cellular mechanism of action produce additive effects indicates that the current framework for conducting cumulative risk assessments should not only consider including chemicals from different classes with the same “mechanism of toxicity”, but also should include chemicals that disrupt differentiation of the same fetal tissue at different sites in the androgen signaling pathway.
In vivo studies (Birkhoj et al., 2004; Gray et al., 2001b, 2006; Hotchkiss et al., 2004; Nellemann et al., 2003) have repeatedly found that mixtures of AR antagonists elicit additive effects on young male rats exposed to the chemicals in utero. Dose-additive effects also have been demonstrated in vitro using AR activation assays (Birkhoj et al., 2004; Nellemann et al., 2003). It is likely, for example, that androgen-dependent tissues cannot discriminate in terms of cellular responses, between the blockade of the AR and significantly lowering the concentration of the ligand (T and/or dihydrotestosterone). It would be reasonable to expect, therefore, that both of these actions could produce dose-additive adverse responses in the differentiating tissues.
The finding that mixtures of chemicals targeting endocrine signaling at multiple levels adhere to dose additivity was also observed with mixtures of thyroid disrupting chemicals (Crofton et al., 2005). Overall, current mixtures research points to two major conclusions: (1) individual chemicals present below their NOAELs contribute to overall mixture effects and (2) true risk associated with exposure to EDCs can only be determined when the cumulative effects of chemicals that target a common signaling pathway are taken into consideration.
Cross-Species Comparisons
Assessment of the validity of cross-species extrapolation is problematic on several issues. Fish and wildlife are often viewed as “sentinel species.” Hence, estrogenic and androgenic effects in animals in the environment are generally assumed to be of concern to other species, including humans. However, preliminary cross-species studies suggest that surrogate laboratory species may not always be relevant to the species of concern and that data from human health risk assessments may not accurately reflect the risk to fish and wildlife. Current prescreening protocols only include mammalian receptor-based assays, and thus may not provide as accurate an assessment for fish and wildlife as would similar assays utilizing receptors obtained from nonmammalian species.
Assessing the impact of EDCs on fish and wildlife has been facilitated by molecular cloning and gene synthesis (Table 2; Wilson et al., 2004). Generation of cDNA libraries from specific tissues can effectively immortalize and clone the genes of a species, frequently utilizing only one animal or surgical specimen. (Wilson et al., 2004b). These libraries act as repositories for genes associated with endocrine function, as well as providing a source of material for future studies. Additionally, databases of gene sequences derived from analysis of overlapping gene fragments provide information for the synthesis and expression of an entire gene without utilization of additional animals or tissues. Both approaches are useful in generating an unlimited source of receptor proteins in a controlled environment. These proteins can then be subjected to binding assays using similar protocols, to determine if EDCs affect steroid hormone receptors equally across species (Wilson et al., 2007). These binding assays can utilize nonmammalian recombinant receptors, in various stages of purification, or expressed proteins in living cells. Both approaches provide valuable data with regard to ligand binding, but provide little information about how ligand–receptor–EDC interactions might affect gene regulation. Current mammalian transcriptional activation assays, utilizing mammalian receptor and receptor specific reporters in homologous cells lines, are more fully developed than for lower vertebrates. However, generation of homologous cell lines and species-specific reporter constructs would facilitate similar studies of alteration of gene induction in fish and wildlife species.
TABLE 2.
Strategy for Ongoing Studies with Vertebrate and Invertebrate Estrogen and ARs
| Invertebrate |
Reptiles and amphibians |
Fish |
Bird |
Mammal |
||||||
| Mud snail | Water flea | Northern Leopard Frog | Giant Salamander | American Alligator | Rainbow trout | Fathead minnow | Japanese Quail | Chimpanzee | Human | |
| Obtain animal tissues | X | X | X | N/A | N/A | N/A | X | N/A | N/A | N/A |
| Prepare complimentary DNA library | X | X | X | N/A | N/A | N/A | X | N/A | N/A | N/A |
| Isolate ER or AR | Ongoing | N/A | N/A | N/A | ERα, AR | N/A | N/A | N/A | ||
| Sequence ER or AR | ERα1 | ERα1 | ERα3, AR2 | ERα, AR | ERα1 | AR1 | ERα1,AR1 | |||
| Express ER or AR | ERα | ERα | ERα | Erα, AR | ERα | AR | ERα, AR | |||
| ER or AR Function | Ongoing | AR | Erα, AR | AR | ERα, AR | |||||
| Compare function across species | AR | ERα4, AR | ARongoing | ERα4, AR | ||||||
Note. ERα1 = sequence obtained from GenBank. AR2 = expression vector generously provided by Takeo and Yamashita (1999); ERα3 = expression vector generously provided by F. Pakdel (Pakdel et al., 2000), ERα4 = manuscript in preparation, N/A = not applicable—sequence obtained from outside sources or GenBank. Mud snail = Ilyanassa obsolete, Water flea = Daphnia magna, Northern Leopard Frog = Rana pipiens, Japanese Quail = Coturnix japonica, Giant salamander = Andrias davidianus, Rainbox trout = Oncorhynchus mykiss, fathead minnow = Pimephales promelas, American alligator = Alligator mississippiensis, Chimpanzee = Pan troglodytes.
The utility of any species in addressing EDC questions is enhanced if species are from taxa not commonly studied, have the potential for laboratory and field studies, have been affected by EDCs in the wild and can help resolve conflicting reports of receptor binding. Studies of the fungicide vinclozolin and its metabolites have reinforced the validity of this approach to species selection. Despite the high degree of homology in the ligand binding domain of human and the fathead minnow AR, subtle differences in the binding affinities for these EDCs have been documented between mammals and fish, and suggest that across species, the impact of a given EDC may vary (Wilson et al., 2004b, 2007). Similar studies comparing the estrogen receptor (ER) binding affinity of plasticizers across animal classes have also documented differences, and established the need for additional studies assessing the effect of EDCs on other receptors (e.g., the estrogen receptor) of diverse species (Rider et al., in press). This is needed to fully assess the default assumption that the interaction of EDCs with steroid hormone receptors in one species can be extrapolated to another.
REGULATORY SCREENING AND TESTING
Mammalian Screening and Testing
One response of the USEPA to the Safe Drinking Water and Food Quality and Protection Acts of 1996 was to form an advisory group, the Endocrine Disruptor Screening and Testing Advisory Committee (EDSTAC), to help formulate a tiered screening and testing strategy for EDCs (http://www.epa.gov/scipoly/oscpendo/edspoverview/finalrpt.htm). The screening battery recommended by EDSTAC was designed to detect alterations in developmental and reproductive processes controlled by the hypothalamic-pituitary-gonadal (HPG) and hypothalamic-pituitary-thyroid (HPT) axes. Based on a “weight-of-evidence” analysis, chemicals positive in Tier 1 screening (T1S) would be considered as potential EDCs and subjected to testing (Tier 2). Because T1S would be less expensive and time consuming than Tier 2 testing, equivocal effects in T1S could be replicated or evaluated further in additional short-term assays before more extensive Tier 2 testing was initiated. The goal of T1S is to use assays sensitive enough to detect EDCs, whereas issues of “dose–response, relevance of the route of exposure, sensitive life stages and adversity” would be resolved in the Tier 2 testing phase.
In vitro T1S assays that are under development would evaluate chemicals for AR and ER binding with rat AR and ER cytosolic, rat AR recombinant, and human ERα recombinant receptors, ER or AR dependent gene expression (Wilson et al., 2002a, 2004a) or cell proliferation (Soto et al., 2004) assays, aromatase enzyme inhibition, and inhibition of steroidogenesis in the H295R cell line (Heneweer et al., 2004). Due to limitations of in vitro assays, it is necessary to include in vivo assays in the screening battery. In vitro assays alone cannot account for absorption, distribution, metabolism, and excretion (ADME), and they yield false negative and false-positive results. In particular, many false negatives result from the inability of the in vitro assays to activate EDCs metabolically. In addition, in vitro assays at high concentrations lack specificity and produce many false-positive results. Because in vivo assays not only account for ADME, but also can integrate all of the endocrine and nonendocrine toxicities, the combination of both in vivo and in vitro assays is recommended for screening.
To avoid the limitations described above, the EDSTAC proposed three short-term in vivo mammalian assays for the T1S battery: the uterotropic, Hershberger, and pubertal female rat assays (Gray, 1998a). Estrogen agonists and antagonists would be detected using the 3-day uterotropic assay using subcutaneous administration of the test compound. Based on the evaluation of four variations of the uterotropic assay protocol in organization for economic cooperation and development (OECD) interlaboratory studies, all of the protocols have produced acceptable responses without regard to rat strain, diet, or housing conditions (Owens and Koeter, 2003; Owens et al., 2006). The selected uterotropic assays for estrogens and antiestrogens use either the intact juvenile or the castrated ovariectomized adult/juvenile female rat that is dosed either by oral or subcutaneous routes.
The second in vivo assay in T1S, the Hershberger assay, detects antiandrogenic activity simply by weighing androgen-dependent tissues in the castrated male rat (Gray, 1998a, b; Hershberger et al., 1953; Owens et al., 2006, 2007). In this assay, weights of the ventral prostate, Cowper's glands, seminal vesicles (with coagulating glands and fluids), glans penis, and levator ani/bulbocavernosus muscles are measured in castrated, testosterone-treated (or untreated) male rats after 10 days of oral treatment with the test compound. This assay is very sensitive for detection of androgens and antiandrogens. Other useful end points that help reveal the mode of action and specificity of the response include weights of the adrenal, liver, and kidney, and measurements of serum (collected by cardiac puncture) levels of testosterone and luteinizing hormone. The Hershberger assay shows high sensitivity and specificity to chemicals with AR-mediated activity. Weak antiandrogenic pesticides such as p,p′-DDE and linuron are easily detected in the Hershberger assay (Lambright et al., 2000; Parks et al., 2000; Yamasaki et al., 2003). Chemicals such as finasteride, which inhibit 5α reductase activity, also are active in this assay. 5α Reductase inhibitors dramatically reduce male accessory sex gland weights with less effect on the levator ani/bulbocavernosus muscle, which has low levels of this enzyme (Blohm et al., 1986). Chemicals that are positive in the Hershberger assay often produce adverse effects after in utero exposure and during puberty.
Both the rat uterotropic and Hershberger assays have been used for several decades to screen chemicals for estrogenicity and androgenicity (Dorfman and Dorfman, 1962). Because estrogens are required for uterine growth and androgens for sex accessory gland growth in both rats and humans, chemicals that are positive in the rat assays for these endocrine activities can be expected to produce predictable responses in humans. These endocrine pathways are highly conserved; in fact, many drugs with antiestrogenic activity or antiandrogenic activity have been shown to produce the anticipated responses in both species. The OECD-led effort to standardize and validate the Uterotrophic and Hershberger assay has been completed with several publications on the different phases available, and OECD test guidelines for both these assays have been peer-reviewed, public comments addressed and are listed as draft Test Guidelines on the OECD web site: http://www.oecd.org/document/62/0,3343,en_2649_34377_2348606_1_1_1_1,00.html#Hershberger.
The third in vivo mammalian/rat assay included in the proposed EDSTAC T1S battery, the pubertal female rat assay, has been used in our laboratory for nearly two decades (Gray, 1998a; Gray et al., 1988a, b, 1989). This assay detects alterations in thyroid hormone status, HPG function, inhibition of steroidogenesis, and direct effects of estrogens, and antiestrogens. In this assay, weanling female rats are dosed daily by gavage for 21 days while the age at vaginal opening (puberty) is monitored. The females are necropsied at about 42 days of age and measurements include serum thyroid hormones, uterine and ovarian weight, and histology (reviewed by Goldman et al., 2000) (http://www.epa.gov/oscpmont/oscpendo/pubs/assayvalidation/pubertal_female_pr.htm).
Alternative in vivo assays were also discussed by EDSTAC and are being evaluated by the EPA. If they are of sufficient sensitivity, specificity, and relevance, they might replace or augment current T1S assays.
One promising alternative assay also used extensively in our laboratory is the pubertal male rat assay (Gray et al., 1999; Kelce et al., 1995; Monosson et al., 1999; Stoker et al., 2000), which detects alterations in thyroid function, HPG maturation, steroidogenesis, and altered steroid hormone function. Intact weanling males are exposed to the test substance for approximately 30 days during which the age at puberty is determined by measuring the age at preputial separation, and reproductive tissues are evaluated and serum taken for optional hormonal analyses at necropsy. We suggest measurement of serum testosterone be included because it would assist in determining chemical mode of action (e.g., separating AR antagonist vs. inhibitors of hormone synthesis) and enhance the sensitivity of the assay to chemicals that inhibit steroid hormone synthesis: http://www.epa.gov/oscpmont/oscpendo/pubs/assayvalidation/pubertal_male_pr.htm.
The EDSTAC recommended that the EPA develop and evaluate an in utero lactational screening assay due to the unique sensitivity of the fetal reproductive system to disruption by some toxicants. For example, TCDD alters sexual differentiation of male and female rats and hamsters at dosage levels approximately two orders of magnitude below those required to produce adverse effects in pubertal or adult rats (Gray et al., 1997b, c; Wolf et al., 1999). One version of the proposed in utero lactational assay now being evaluated by the EPA takes about 80 days and uses approximately 10 litters per group (120–150 pups). In this protocol, androgens and antiandrogens can be detected in approximately 2 to 3 wk, and EDCs with antithyroid activity can be detected in infant or weanling offspring after 4–5 weeks of maternal treatment. However, an EPA Science Advisory Panel recently concluded the above protocol is more suitable for testing than use as a screening assay due to the size and duration of the study but that efforts to streamline such an assay for use in screening were worthy of consideration, http://www.epa.gov/scipoly/sap/meetings/index.htm#january. A scaled-down protocol that might be useful for screening with in utero and lactational exposure could include five to six dose groups over a broad dose range, three to four litters per dose group and carefully examine all the pups of both sexes (i.e., Rider et al., 2008). If effects were noted, then this would be followed by a definitive multigenerational study to define NOAELs.
Tier 2 Testing as Defined by EDSTAC and the USEPA
The purpose of Tier 2 testing is to provide “definitive” information for hazard characterization of endocrine-disrupting agents. This information adds to the body of knowledge essential for risk assessment for an EDC by
Either confirming or refuting the observations noted in the T1S screens/assays. The Tier 2 in vivo assays data can expand on the information derived from the in vitro and in vivo screens and use a route of exposure that will be reflective of likely exposure of humans to the agent under investigation.
For the first time in the tiered process, studies involving mammals utilize an in utero exposure paradigm that includes exposures in critical developmental windows and assessments not evaluated in standard prenatal developmental toxicity studies. For many EDCs, this is the most sensitive life stage for the induction of adverse effects leading to potentially permanent changes in phenotype and function that would not be noted in adult animal toxicity studies.
Identifying in the experimental animal species (most likely the rat) the activity of suspected EDCs for end points for which concerns have been raised in humans (e.g., hypospadias, cryptorchidism) as well as identify other endocrine-like activity associated with androgens, estrogens, and antithyroid acting agents.
Not only detecting activity, but also providing the appropriate dose–response information for use in any risk assessment on individual chemicals.
When the EPA first proposed a Tier 2 test for mammals it noted that the multigeneration study (Fig. 1) was the only one likely to have an appropriate exposure period covering the major developmental life stages of interest. EPA also had built into the protocol, appropriate times for evaluations of effects, especially those that might show latency (e.g., an exposure in utero only producing a readily identifiable change at adulthood). Even though the improvements in protocol study design in the most recent EPA test guidelines (http://www.epa.gov/opptsfrs/publications/OPPTS_Harmonized/870_Health_Effects_Test_Guidelines/Series/870-3800.pdf) did incorporate more endocrine-mediated end points (e.g., sperm analyses, indices of puberty in males and females), EDSTAC realized that some improvements would have to be made to the end points incorporated in the multigeneration protocol to make the assay more comprehensive in the detection of effects (e.g., improved pathology and endocrine assessment, sexually dimorphic phenotypic assessments). It was also noted that many registrants and producers could bypass T1S screening and go directly to Tier 2 (e.g., for pesticides), but that the current protocols might be insensitive to both detection and providing accurate dose–response information.
FIG. 1.
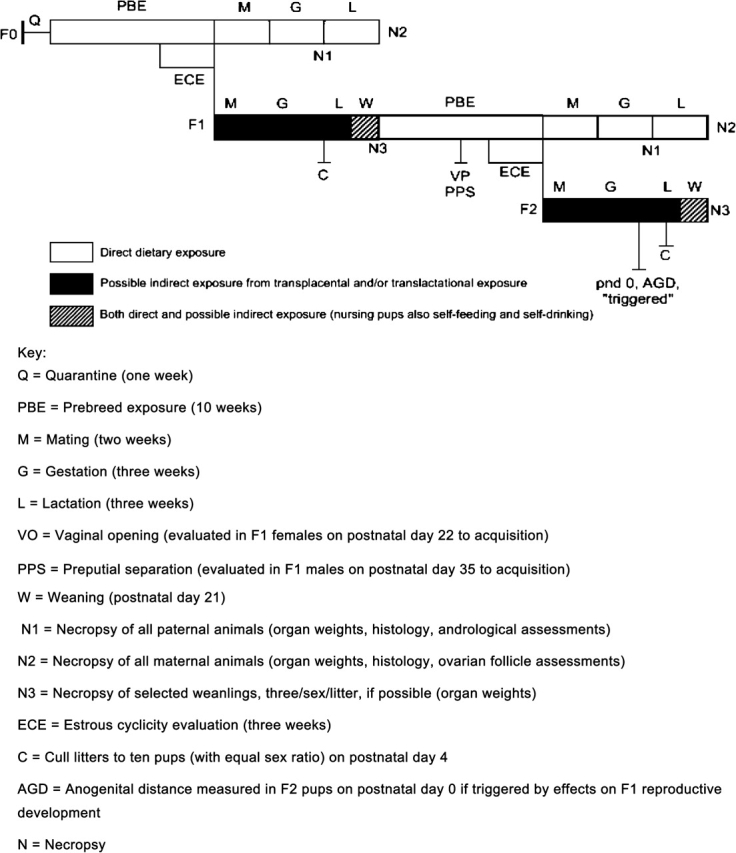
Schematic of a standard multigenerational test.
There is also a large gap (in terms of comprehensiveness and cost) between the T1S screens and Tier 2 tests. Many investigators had successfully used a transgenerational approach (usually employing approximately 10 litters per dose group but analyzing every pup in the litter on reaching adulthood) which employed fewer animals, and was significantly cheaper than a standard multigeneration study in identifying adverse endocrine-mediated end points or found them at lower dose levels than seen in conventional multigeneration studies (e.g., linuron, Gray et al., 1999; Lambright et al., 2000; McIntyre et al., 2000; di-iso-nonyl phthalate, Gray et al., 1999; Waterman et al., 2000).
The use of a lower litter number but increasing the number of pups per litter examined provides greater statistical power over the conventional multigeneration study with 20 litters per dose group, but evaluation of only one male and one female offspring per litter at adulthood. The degree to which the statistical power increases with examination of multiple pups per litter is dependent upon the degree to which pups within a litter differ from one another (the intralitter correlation coefficient [ICC]). We have calculated ICCs and determined how examining multiple male pups in a litter affected the power calculations for a number of reproductive end points from several of our studies. In these studies, dams were exposed to EDCs in utero and the offspring examined later in life. In the data shown here as an example, DEHP was administered to the dam orally from day 8 of gestational to day 18 of lactation, half the male rat offspring being further exposed from 18 to 65 days of age and all the males were examined thoroughly (Gray et al., unpublished data). The results of the DEHP study indicate (Table 3; Fig. 2) that sampling two to three pups from 15 litters, or all of the pups from ten litters/dose provides the same or greater statistical power as sampling one pup from 20 litters. More information on these calculations is provided in the text below.
TABLE 3.
Calculation of Cox's Ratios and ICC From proc Mixed Analyses
| Adult necropsy weight data | Litter variance | Pups-residual variance | Cox's ratio 4 × (pups/litters) | ICC litter/(litter + pups) |
| Epididymis | 5021 | 5167 | 4.116 | 0.493 |
| Liver | 4.95 | 6.22 | 5.031 | 0.443 |
| Body | 2579 | 3481 | 5.399 | 0.426 |
| Kidney | 23,537 | 32,670 | 5.552 | 0.419 |
| Cowper's glands | 1129 | 1694 | 6.002 | 0.400 |
| Testis | 21,506 | 32,376 | 6.022 | 0.399 |
| LABC | 10,417 | 15,736 | 6.042 | 0.398 |
| Glans penis | 31.2 | 56.5 | 7.244 | 0.356 |
| Seminal vesicle | 25,980 | 71,543 | 11.015 | 0.266 |
| Ventral prostate | 5346 | 23,258 | 17.402 | 0.187 |
| Variable pubertal necropsya | Litter variance | Pups-residual variance | Cox's ratio 4 × (pups/litters) | ICC litter/(litter + pups) |
| Body weight, day 18 | 14.225 | 7.131 | 2.005 | 0.666 |
| Body weight, day 44 | 348.2 | 201.02 | 2.309 | 0.634 |
| Body weight final | 753.87 | 492.14 | 2.611 | 0.605 |
| AGD, day 2 | 0.05503 | 0.06889 | 5.007 | 0.444 |
| Glans penis weight | 44.4 | 70.85 | 6.383 | 0.385 |
| Epididymis weight | 839.64 | 1708.64 | 8.140 | 0.329 |
| Testis weight | 23,297 | 61,044 | 10.481 | 0.276 |
| LABC | 2666 | 7417.5 | 11.129 | 0.264 |
| Seminal vesicle weight | 4925 | 14,445 | 11.732 | 0.254 |
| Adrenal weight | 8.214 | 34 | 16.719 | 0.193 |
| Age at preputial separation | 1.391 | 8.0744 | 23.219 | 0.147 |
| Weight at preputial separation | 107.00 | 720.00 | 26.916 | 0.129 |
| Cowper's glands | 14.9954 | 189.57 | 50.568 | 0.073 |
Note. LABC, levator ani bulbocavernosus weight. Power curves flatten out when the number of pups sampled per litter exceeds Cox's ratio (Bergerud, 1995). End points are ordered based upon ICC values, ranging from high (pups more correlated within the litter) to low (pups less correlated within the litter).
The “pubertal” necropsy included all the males dosed by gavage from 18 to 65 days of age with the same dose level administered to the dam during gestation and lactation. For males necropsied as adults, only the dam was treated and exposure was discontinued on day 18 of lactation. The lower the ICC, the less related are the values of the pups within litters.
FIG. 2.
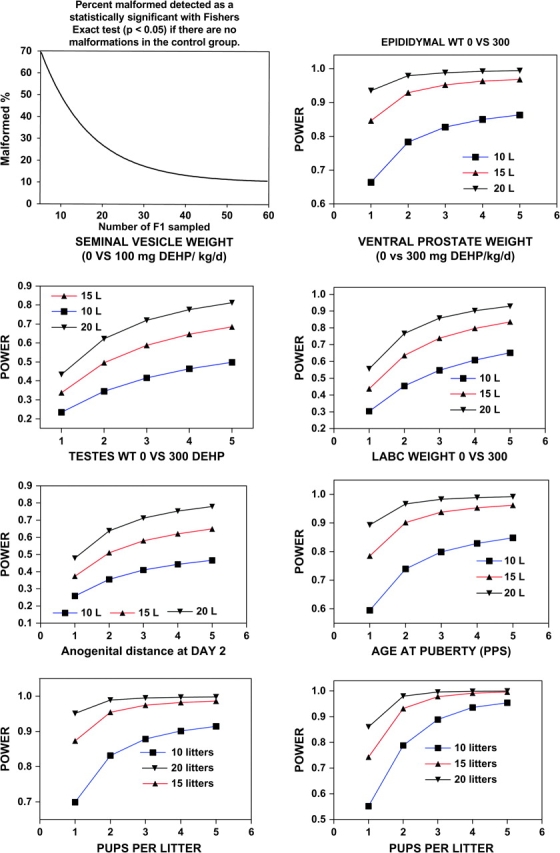
Power curves for reproductive organ weights, malformation rates, anogenital distance and age at preputial separation (puberty). The data from our transgenerational study with DEHP were analyzed using PROC MIXED available on SAS to obtain estimates of components of variation (for litters vs. pups within litters) that make up the overall variability of the means in order to assess the implications for statistical power of using data from multiple pups per litter. Following this analysis, we calculated power to detect the treatment effects as described by Raudenbush and Xiao-Feng (2001). The objective of this was to determine the proportion of the overall error variance due to litter-to-litter variability versus the proportion accounted for by the pups within litters. This retrospective analysis can be useful for designing optimal sampling strategies for future studies. It basically allows one to determine how much the statistical power of a study is enhanced by examining several pups from the same litter rather than using only one pup/per sex/litter. Because the pups in the DEHP treated litters do not respond identically, the more variable the pups are within the litter the more power is enhanced, and hence the standard error of the mean is reduced, by examining multiple pups from the same litter.
Using more pups per litter also addresses the issue of statistical power to detect malformations that occur at low incidences in the lower dosage groups. It is not just the continuous variables but also the malformations that can have improved detection and provide better assessments of dose–response relationships (Fig. 2).
These studies also did not employ triggered end points, but made measurements in all offspring and used other sensitive measures of endocrine activity (e.g., anogenital distance in F1 offspring, assessment of areola and nipple retention in preweaning F1 pups and at adulthood) not currently incorporated into standard multigeneration studies. Such transgenerational studies may offer an intermediate tier between T1S and Tier 2. Although they do not evaluate the reproductive performance of the F1 offspring, such studies could serve as a less expensive intermediate tier to re-enforce the occurrence of endocrine activity that was flagged in T1S, before embarking on any modified multigeneration study, or perhaps could act as a bridging study from an older multigeneration study.
Whatever improvement may be required in the current EPA multigeneration protocols that would serve as the definitive Tier 2 test it would be prudent to think that all the information obtained in T1S should be used in tailoring the study design to move away from a “one size fits all” approach. If no T1S data are obtained, Tier 2 must represent a comprehensive evaluation of activity with sufficient statistical and experimental power to be able to detect events likely to have an endocrine-mediated mode of action (e.g., reproductive tract malformations). The current studies with limited end points and numbers of offspring examined per litter, have much poorer resolving power than, for example, the current prenatal developmental toxicity study to detect birth defects, such that incidences of reproductive tract malformations in excess of 25% with a very low control background incidence cannot be detected.
Newer efforts to move to a more tailored approach to studying reproductive toxicity have been proposed by the Agricultural and Chemical Safety Assessment committee of ILSI (Cooper et al., 2006). This life-stage approach, although never having been tested, contains many end points and approaches that have been used successfully in the past and acknowledges that a flexible approach needs to be taken based on all the available information to adequately test for reproductive toxicity, including those aspects of postnatal development that have been of serious concern in the evaluation of EDCs. However, only three chemicals are being selected with known reproductive, neuro- or immunotoxicity for evaluation in the protocol. This approach is based on many of the transgenerational types of studies that have been previously published. The authors also acknowledge the weakness in having too few pups to examine in every litter and advocate testing more, in different cohorts that would evaluate neuro- and immunotoxicity.
Whilst this represents a new initiative to incorporate the best science into testing, the protocol presented in the publication unfortunately fell short of the ideal, definitive test for EDCs. In particular, the different cohorts of animals are examined at different times so that at sexual maturity (usually deemed to be ∼90 days of age in the rat) there is only 1 cohort available with 1 male and 1 female representative of the original litter, which is no better than we have with the current multigeneration protocol. In common with the transgenerational studies, there is no routine evaluation of the reproductive performance of the F1 offspring. These shortcomings have been discussed and an improved protocol was presented to a USEPA Science Advisory Panel in, 2007 and this effort is being pursued by the OECD.
Cooper et al. (2006) advocated the use of triggers to incorporate additional end points into the design. Unfortunately, our experience with triggered end points in the current EPA multigeneration study design has not been good. In many instances, this is not a fault of the experimenters, but more because of lack of time for evaluation of data. So, for example, male pubertal indices are captured on the study around postnatal day 45. With the normal staggering of individuals on a reproduction study (not all are born on the same day) it would be several days, or even longer if an effect is noted, before all the males had complete evaluations of puberty. These data would then need to be statistically analyzed corrected for body weight and any other confounders and then a decision made on the conduct of a triggered endpoint (which may involve a Contract Research Organization contacting sponsors for approval). This may have to be done within 40 days, which places significant pressure on the scientists conducting these studies and may not be reliably achievable. A much better approach would be the application of “opt out” triggers. That is, all the end points for a study would be collected unless there was sufficient information and justification not to undertake a specific measurement. As the protocol currently exists in its published form (Cooper et al., 2006), this would not suffice as a replacement for the current multigeneration study, but could be adapted to make it meet this need and we are optimistic that this approach will serve as a useful template which can in time be fashioned into a very useful protocol.
Power Calculations, Analysis of Components of Variation, and Intralitter correlation coefficients
The data for our DEHP transgenerational study were analyzed using PROC MIXED available on SAS to obtain estimates of components of variation (for litters vs. pups within litters) that make up the overall variability of the means in order to calculate power to detect the treatment effects (Raudenbush and Xiao-Feng, 2001) (Fig. 2; Table 3). Because the pups in the DEHP treated litters do not respond identically, the more variable the pups are within the litter the more power is enhanced by examining multiple pups from the same litter. We also employed Cox's Ratio as a general rule to determine how many pups per litter could be sampled to enhance the power of the study (Bergerud, 1995) (http://www.for.gov.bc.ca/hre/biopamph/pamp50.pdf). ICC also were calculated to describe the degree of similarity of pups within litters for the multiple end points measured in the current study. According to Cox's Rule Of Thumb (Bergerud, 1995) there is little or no increase in power when the number of subsamples (pups in this case) is greater than Cox's Ratio [Cox's ratio = 4 × (variation due to pups within litters/variation due to litters)]. The power curves flatten out when the number of pups/litter sampled is increased, indicating that the most efficient design would not sample any more pups per litter beyond this Ratio. When the components of variance were estimated for reproductive measures it is clear that, even in the most conservative case, sampling all the male pups in each litter would enhance the power of a transgenerational reproduction study. As examples, power curves were calculated for the control and high dose group (300 mg/kg/day) for one to five pups per litter and 10, 15, and 20 (standard sample size) litters per dose.
Power calculations also were calculated for categorical effects based upon the numbers of malformed males versus males without malformations per dose group using SigmaStat 3.1 software (Fig. 2). If 20 animals per dose group are examined for malformations then lesions occurring at an incidence of 25% or greater can be detected whereas an incidence of 10% can be detected if all the pups are examined from 20 litters. If only ten males per group are examined, as recommended for histopathological analyses in some regulatory agency test guidelines, then effects are only detected statistically if about 50% or more of the tissues/organs are affected; a level of statistical power that many would consider inadequate.
Nonmammalian Screening and Testing
As discussed previously, there is compelling evidence for adverse effects of EDCs on invertebrates, fish, and wildlife. This has provided one impetus for the development of EDC screening and testing assays with nonmammalian species; however, there also are examples where nonmammalian models may prove superior to rodents in detecting some EDC MOA.
The class of animals that has received the most attention in terms of EDC testing is fish. Many different methodological approaches have been applied in fish EDC tests; an experimental design that has been used quite frequently is a 21-day assay initiated with reproductively mature adults. Different freshwater small fish models, including the Japanese medaka (Oryzias latipes) and zebrafish (Danio rerio), have been used in 21-day EDC screening assays (Seki et al., 2006); however in the United States, the small fish species of choice for the 21-day design has been the fathead minnow (Pimephales promelas; status of this and other assays reviewed at (http://www.epa.gov/endo/pubs/assayvalidation/status.htm). The 21-day Tier 1 fathead minnow test features a range of apical and mechanistic end points designed not only to detect the adverse reproductive effects of exposure to the test chemicals, but target specific classes of EDCs (Ankley et al., 2001). For example, an endpoint in the assay that is exquisitely sensitive to (and specific for) estrogenic EDCs is induction of vitellogenin (egg yolk protein) in males, which, under normal physiological conditions, do not express the protein (Ankley et al., 2001; Seki et al., 2006). Decreases in plasma vitellogenin concentrations in females have been shown to be indicative of inhibitors of steroidogenic enzymes, including CYP19 aromatase (Ankley et al., 2005). The 21-day assay also is effective in identifying androgenic EDCs, in that female fish exposed to AR agonists exhibit readily apparent morphological masculinization (Ankley et al., 2001; Seki et al., 2006).
Fish also are being used in longer-term (e.g., Tier 2) tests to detect full life-cycle and multigenerational effects of EDCs. Species that have received the most attention in the United States in this regard have been an estuarine fish, the sheepshead minnow (Cyprinodon variegates), and the Japanese medaka, which is attractive for this work due to its relatively rapid life cycle (Ankley and Johnson, 2004). The medaka also is widely used in Japan for long-term EDC testing, as is the zebrafish in some European countries (Peterson et al., 2001). Responses assessed in the longer-term EDC assays with fish are similar to the apical and diagnostic end points used in the 21-day reproduction tests, but typically also include measurements reflective of chemical effects on the endocrine system during early life stages, such as gonadal development and sexual differentiation in F1 and, perhaps, F2 generations (Ankley and Johnson, 2004).
Amphibian species have received significant attention in the development and implementation of EDC testing programs as recommended by EDSTAC. Amphibian metamorphosis is almost certainly the most visually obvious manifestation of effects on HPT function in a vertebrate; from this, it was initially recommended that a simple tail resorption assay with the model lab species Xenopus laevis could serve as the basis of a screen for chemicals that act as agonists or antagonists of thyroid hormone function. Subsequent research suggested that the tail resorption endpoint was for various reasons problematic, but metamorphic frogs nonetheless were a good model for detecting thyroid-active EDCs (Degitz et al., 2005; Opitz et al., 2006). Currently the endpoint that seems best suited for this purpose is thyroid gland histology, collected in conjunction with some indication of developmental stage; this basic approach (with X. laevis) is being used for EDC screening programs being implemented both by the USEPA and Organization for Economic Cooperation and Development (Degitz et al., 2005; Opitz et al., 2006).
There has been interest in North America in the development of a full life-cycle test with amphibian species that could serve as a tool (e.g., in Tier 2) for assessing the long-term ecological risk of EDCs. Research in this area has focused more on Xenopus tropicalis than X. laevis, due in large part to the more rapid life cycle of the former species (Degitz, unpublished data). In addition to considering responses controlled by thyroid function, such a test would feature end points (e.g., vitellogenin production, gonad histopathology) related to disruption of the HPG axis by (anti-)estrogenic/androgenic chemicals (Degitz, unpublished data).
In addition to fish and amphibians, there have been efforts with other nonmammalian vertebrates to develop standardized assays suitable for assessing the effects of EDCs. A notable effort in this regard involves birds. Full life-cycle tests with birds have been used for some time for pesticide registration; with some adaptation, these basic tests would be applicable to Tier 2 testing of EDCs (Touart, 2004). For example, to detect possible transgenerational effects, it has been proposed that the test design encompass two generations of animals, with (dietary) chemical exposures starting with sexually mature adults, proceeding through a complete F1 generation, and culminating with production of F2 young. Either of two species would be amenable to this type of experimental design; however, due to their relatively more rapid life cycle, the Japanese quail (Coturnix japonica) likely would be a more resource-effective model than the northern bobwhite quail (Colinus virginianus) for routine testing. In addition to apical responses like fecundity and fertility, end points specifically indicative of endocrine-disruptive effects, like gonad development and differentiation and certain changes in behavior, are being incorporated into the two-generation avian test design (Touart, 2004).
There also have been efforts to develop standardized tests for EDCs with different invertebrate species (deFur, 2004). A challenge in this area has been definition of what invertebrate tests are intended to detect because the role, if any, of many of the mammalian steroidal and protein hormones is uncertain in invertebrates. If the assays are to be used to identify chemicals that affect the vertebrate HPG axis, then there is some question as to whether functional aspects of the axis are sufficiently conserved across animal phyla to expect that sensitive, biologically meaningful tests could be developed in invertebrate models. On the other hand, invertebrates possess a variety of endocrine systems/function that could be adversely affected by chemicals, which often would not be “flagged” as EDCs using vertebrate tests (deFur, 2004). Under this scenario there is an easily justifiable need for standardized tests for EDCs using invertebrate models. One test recommended for inclusion in the formal EPA testing program for EDCs is a life-cycle test with Mysid shrimp. Other invertebrates that have received significant attention relative to full life-cycle tests for EDCs include daphnids and copepods (deFur, 2004).
CONCLUSIONS
The field of endocrine disrupters has come a long way since its initial impetus in 1991. Many of the concepts advanced in this area have now been embraced by the general toxicological community resulting in useful enhancements of testing assays, new mechanism-based screening assays and incorporation of toxicant-induced endocrine alterations into risk assessment. Concepts such as the special susceptibility of the developing organism and early induction of latent effects, for example, are now widely held and the mechanism-based approach to toxicology testing and screening has been incorporated into many other branches of toxicology. Parallel to the EDCs there also is and emerging field of “developmental basis of adult health,” or “fetal programming of adult disease” (de Boo and Harding, 2006; Young, 2001). This hypothesis is a variation of the many of the EDC concepts, but applies to potential adverse effects on endpoints such as blood pressure, pancreatic function, insulin sensitivity, and even brain and lung function. The concept is the same: exposure during development may induce profound changes in adult function, and extends that concept past the endocrine system.
Since the Wingspread Conference of 1991, there have been numerous examples of adverse effects of EDCs in fish, wildlife, domestic animals, and humans. Endocrine systems have been disrupted by anthropogenic chemicals including antiandrogens, androgens, estrogens, AhR agonists, inhibitors of steroid hormone synthesis, antithyroidal compounds, and retinoid agonists. In addition, potential pathways and targets for endocrine disruption extend beyond the traditional EAT receptor–mediated reproductive and developmental systems.
Although much has been learned over the past 15 plus years, future research needs to address critical issues that have arisen since the early 1990′s. For example, reports of nonmonotonic (e.g., U-shaped), and low dose effects and nonthreshold effects for EDCs continue to be a challenge for risk assessment. Future well-designed studies are required to address the biological plausibility of low dose effects as well as the ramifications for risk assessment. Furthermore, new mechanisms of endocrine action continue to be discovered. Membrane-bound steroid receptors, novel inhibitors of steroidogenesis, and altered metabolism are just a few of the new areas of investigation.
In addition to the remaining questions of the field, there are multiple emerging areas to be investigated. One of these areas involves mixtures toxicology. It is clear that, rather than focusing on single chemical exposures, data are required to determine and predict potential cumulative effects of EDCs. Pharmaceuticals and veterinary drugs in the environment also present a new challenge. Pharmaceuticals, be they from personal, medical, or agricultural uses have been detected in the environment and these chemicals may be disrupting both target and nontarget systems in exposed populations of wildlife and fish (Ankley et al., 2007). The potential role of EDCs in nontraditional biological pathways also warrants further investigation. Studies implicating EDCs in diabetes, obesity, and behavioral and immune deficiencies have underscored the need to elucidate these areas of endocrine disruptor research. These new areas of research may eventually alter the screening and testing batteries currently proposed by regulatory agencies such as the USEPA and OECD. Until that time, there are fundamental questions remaining about the proposed batteries of screens and tests as described above. Finally, from a regulatory standpoint, current interest in using toxicogenomic data for risk assessment requires further evaluation. Inherent problems with variability, reproducibility, and biological significance continue to challenge application of these promising technologies. Risk assessors have traditionally required that changes in gene expression be validated by some other measure such as endocrine or biochemical “biomarkers” such that a causal link can be established between mechanistic data and an effect that is clearly adverse. Furthermore, one then needs to identify a “point of departure” or the degree of change in the mechanistic endpoint that consistently produces an adverse effect.
In summary, research on EDCs has progressed significantly and now provides concrete examples of chemicals in the environment adversely affecting endocrine systems in diverse fauna, ranging from invertebrates to humans. Many of the concepts introduced in the consensus statement from the Wingspread Conference in 1991 by Dr Theo Colborn (Colborn and Clement, 1992) and the other workshop participants are now generally accepted by the scientific community which in turn has improved our understanding of how “upstream,” cellular and molecular alterations in toxicity pathways produce adverse effects “downstream” at higher dose levels and how exposure to EDCs during critical developmental periods can result in adverse effects later in life. Although much has been learned, the field is relatively new, and many relevant and timely questions remain. Some of the important areas of emphasis in the field are presented in Table 4 “Where do we go from here?”. The continued introduction of novel chemicals (drugs, toxic substances, pesticides, nanomaterials, etc.) into the environment requires ongoing research and monitoring to ensure the safeguarding of the environment for generations to come.
TABLE 4.
Where Do We Go from Here?
| 1. Ecosystem health |
| 1.a. Better characterization of the extent and population impacts on fish of estrogenic municipal effluents, and further research concerning the possible impacts on fish and wildlife of other known EDCs entering the environment (e.g., from pulp and paper mills, CAFOs). |
| 1.b. Clearer delineation of exact chemicals responsible for effluent estrogenicity (i.e., ethinyl estradiol vs. E2 vs. nonylphenol) as a basis for identifying mitigation options. |
| 1.c. Identification of pharmaceuticals that could possibly cause ecological effects through properties similar to EDCs, that is, high-potency chemicals focused on biological pathways (other than the HPG axis) that have a substantial degree of phylogenetic conservation. |
| 2. EDC screening |
| 2.a. Evaluation of the EPA T1S assay suite to determine “holes” and redundancies with respect to identifying HPG-active chemicals (i.e., we aren't going to be able to apply the full suite of tests to very many chemicals-too expensive); an understanding of endpoint/species extrapolation will be critical here. |
| 2.b. Improve predictiveness of screens and tests for EDC activities. |
| 2.c. Enhance and expand the in vitro assays for T1S to replace animal-derived proteins with recombinant receptors, adapt in vitro assays for high throughput. |
| 2.d. Determine the need for in vitro assays using nonmammalian pathways and develop the assays for screening, as warranted. |
| 2.e. Develop an in utero lactational screening assay for possible inclusion in T1S. |
| 2.f. Implement a T1S battery that meets or exceeds the EDSTAC 1998 recommendations. |
| 3. Multigenerational and transgenerational testing including a postnatal examination of offspring |
| 3.a. Enhanced multigenerational assays that include sensitive and reliable end points, that use animals more efficiently (fewer litters/dose, examine more pups/litter, increase histopathological examinations to include all F1 animals) without losing statistical power. |
| 4. Clearer communication and cross-disciplinary training on the following: |
| 4.a Appropriate experimental designs and statistical analyses of postnatal study data for scientists from different disciplines as well as understanding of basic toxicological issues (endocrinology, toxicogenomics, proteomics, epigenetics, etc.) so that research resources are not wasted. It is evident from the literature that special attention needs to be given to assure that studies that examine more than one pup per litter account for this in the analysis of the data. Furthermore, investigators that cross-foster pups should do so properly, retaining both prenatal and postnatal litter information. |
| 4.b Use of reproductive and developmental toxicity data in risk assessment. Promote an understanding of the difference between and no observed effect level and a NOAEL and the need to causally link effects not generally recognized as adverse to clear adverse effects. |
| 5. Maintain an integrated biological approach to study endocrine action (beware of systems biology and the drilling down to molecular levels in vitro as cells and tissues live in complex environments). Integrate EDC latent effects research with other projects on the fetal basis of adult disease. |
| 6. Risk assessment on EDCs |
| 6.a. Develop a scientifically defensible and rational framework for cumulative risk assessments, incorporating the knowledge that dose additivity applies to chemicals that target the same endocrine pathway via very different cellular mechanisms. This redefines what we mean by mode of action and encompasses multiple mechanisms inducing the same phenotypic changes. |
| 6.b. Expanded use of endocrine data in risk assessments not only to support mode of action but also as critical effects. |
FUNDING
USEPA/NCSU Cooperative Training agreement (CT826512010) to A.K.H, C.V.R, C.R.B.; Integrated Graduate Education, Research and Training grant from the National Science Foundation to C.L.G.
Acknowledgments
We would like to thank Dr. Chris Wiesen, Odum Institute for Research in Social Science, University of North Carolina at Chapel Hill, for statistical assistance with the calculation of power from the proc mixed output from SAS.
References
- Adams NR. Organizational and activational effects of phytoestrogens on the reproductive tract of the ewe. Proc. Soc. Exp. Biol. Med. Soc. Exp. Biol. Med. 1995;208:87–91. doi: 10.3181/00379727-208-43837. [DOI] [PubMed] [Google Scholar]
- Ames BN, Gold LS. Paracelsus to parascience: the environmental cancer distraction. Mutation research. 2000;447:3–13. doi: 10.1016/s0027-5107(99)00194-3. [DOI] [PubMed] [Google Scholar]
- Ankley GT, Brooks BW, Huggett DB, Sumpter JP. Repeating History: pharmaceuticals in the environment. Environ. Sci. Technol. 2007;41:8211–8217. doi: 10.1021/es072658j. [DOI] [PubMed] [Google Scholar]
- Ankley G, Mihaich E, Stahl R, Tillitt D, Colborn T, McMaster S, Miller R, Bantle J, Campbell P, Denslow N, et al. Overview of a workshop on screening methods for detecting potential [anti-] estrogenic/androgenic chemicals in wildlife. Environ. Toxicol. Chem. 1998;17:68–87. [Google Scholar]
- Ankley GT, Giesy JP. Pensacola: SETAC Press; 1998. Endocrine Disruptors in Wildlife: A Weight of Evidence Perspective. [Google Scholar]
- Ankley GT, Jensen KM, Durhan EJ, Makynen EA, Butterworth BC, Kahl MD, Villeneuve DL, Linnum A, Gray LE, Cardon M, et al. Effects of two fungicides with multiple modes of action on reproductive endocrine function in the fathead minnow (Pimephales promelas) Toxicol. Sci. 2005;86:300–308. doi: 10.1093/toxsci/kfi202. [DOI] [PubMed] [Google Scholar]
- Ankley GT, Jensen KM, Kahl MD, Korte JJ, Makynen EA. Description and evaluation of a short-term reproduction test with the fathead minnow (Pimephales promelas) Environ. Toxicol. Chem. SETAC. 2001;20:1276–1290. [PubMed] [Google Scholar]
- Ankley GT, Jensen KM, Makynen EA, Kahl MD, Korte JJ, Hornung MW, Henry TR, Denny JS, Leino RL, Wilson VS, et al. Effects of the androgenic growth promoter 17-beta-trenbolone on fecundity and reproductive endocrinology of the fathead minnow. Environ. Toxicol. Chem. SETAC. 2003;22:1350–1360. [PubMed] [Google Scholar]
- Ankley GT, Johnson RD. Small fish models for identifying and assessing the effects of endocrine-disrupting chemicals. ILAR J. 2004;45:469–483. doi: 10.1093/ilar.45.4.469. [DOI] [PubMed] [Google Scholar]
- Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–1469. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell DR, Clode S, Fan MQ, Fernandes A, Foster PM, Jiang T, Loizou G, MacNicoll A, Miller BG, Rose M, et al. Toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in the developing male Wistar(Han) rat. II: Chronic dosing causes developmental delay. Toxicol. Sci. 2007;99:224–233. doi: 10.1093/toxsci/kfm141. [DOI] [PubMed] [Google Scholar]
- Bergerud W. Power analysis and sample sizes for randomized block designs with subsampling. Biom. Inf. B. C. Min. For. Range Res. Pamphlet. 1995;50:1–4. [Google Scholar]
- Birkhoj M, Nellemann C, Jarfelt K, Jacobsen H, Andersen HR, Dalgaard M, Vinggaard AM. The combined antiandrogenic effects of five commonly used pesticides. Toxicol. Appl. Pharmacol. 2004;201:10–20. doi: 10.1016/j.taap.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Bjerke DL, Brown TJ, MacLusky NJ, Hochberg RB, Peterson RE. Partial demasculinization and feminization of sex behavior in male rats by in utero and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin is not associated with alterations in estrogen receptor binding or volumes of sexually differentiated brain nuclei. Toxicol. Appl. Pharmacol. 1994a;127:258–267. doi: 10.1006/taap.1994.1160. [DOI] [PubMed] [Google Scholar]
- Bjerke DL, Peterson RE. Reproductive toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in male rats: Different effects of in utero versus lactational exposure. Toxicol. Appl. Pharmacol. 1994;127:241–249. doi: 10.1006/taap.1994.1158. [DOI] [PubMed] [Google Scholar]
- Bjerke DL, Sommer RJ, Moore RW, Peterson RE. Effects of in utero and lactational 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure on responsiveness of the male rat reproductive system to testosterone stimulation in adulthood. Toxicol. Appl. Pharmacol. 1994b;127:250–257. doi: 10.1006/taap.1994.1159. [DOI] [PubMed] [Google Scholar]
- Blohm TR, Laughlin ME, Benson HD, Johnston JO, Wright CL, Schatzman GL, Weintraub PM. Pharmacological induction of 5 alpha-reductase deficiency in the rat: Separation of testosterone-mediated and 5 alpha-dihydrotestosterone-mediated effects. Endocrinology. 1986;119:959–966. doi: 10.1210/endo-119-3-959. [DOI] [PubMed] [Google Scholar]
- Blystone CR, Furr J, Lambright CS, Howdeshell KL, Ryan BC, Wilson VS, Leblanc GA, Gray LE., Jr Prochloraz inhibits testosterone production at dosage below those that affect androgen-dependent organ weights or the onset of puberty in the male Sprague Dawley rat. Toxicol. Sci. 2007a;97:65–74. doi: 10.1093/toxsci/kfm004. [DOI] [PubMed] [Google Scholar]
- Blystone CR, Lambright CS, Howdeshell KL, Furr J, Sternberg RM, Butterworth BC, Durhan EJ, Makynen EA, Ankley GT, Wilson VS, et al. Sensitivity of fetal rat testicular steroidogenesis to maternal prochloraz exposure and the underlying mechanism of inhibition: Prochloraz reduced fetal testis testosterone. Toxicol. Sci. 2007b;97:512–519. doi: 10.1093/toxsci/kfm055. [DOI] [PubMed] [Google Scholar]
- Brian JV, Harris CA, Scholze M, Backhaus T, Booy P, Lamoree M, Pojana G, Jonkers N, Runnalls T, Bonfa A, et al. Accurate prediction of the response of freshwater fish to a mixture of estrogenic chemicals. Environ. Health Perspect. 2005;113:721–728. doi: 10.1289/ehp.7598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer A, Longnecker MP, Birnbaum LS, Cogliano J, Kostyniak P, Moore J, Schantz S, Winneke G. Characterization of potential endocrine-related health effects at low-dose levels of exposure to PCBs. Environ. Health Perspect. 1999;107(Suppl. 4):639–649. doi: 10.1289/ehp.99107s4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunskill PJ. The effects of fetal exposure to danazol. Br. J. Obstet. Gynaecol. 1992;99:212–215. doi: 10.1111/j.1471-0528.1992.tb14501.x. [DOI] [PubMed] [Google Scholar]
- Cammack JN, White RD, Gordon D, Gass J, Hecker L, Conine D, Bruen US, Friedman M, Echols C, Yeh TY, et al. Evaluation of reproductive development following intravenous and oral exposure to DEHP in male neonatal rats. Int. J. Toxicol. 2003;22:159–174. doi: 10.1080/10915810305098. [DOI] [PubMed] [Google Scholar]
- CERHR, NTP. NTP-CERHR monograph on the potential human reproductive and developmental effects of 2-bromopropane (2-BP) Ntp. Cerhr. Mon. 2003:i–III11. [PubMed] [Google Scholar]
- Chambers DB, Leiker TJ. USGS; 2006. A Reconnaissance for Emerging Contaminants in the South Branch Potomac River, Cacapon River, and Williams River Basins, West Virginia. [Google Scholar]
- Clark HA, Snedeker SM. Critical evaluation of the cancer risk of dibromochloropropane (DBCP) J. Environ. Sci. Health. 2005;23:215–260. doi: 10.1080/10590500500234996. [DOI] [PubMed] [Google Scholar]
- Colborn T, Clement C. Princeton, NJ: Princeton Scientific Publishing Co., Inc.; 1992. Chemically-Induced Alterations in Sexual and Functional Development: The Wildlife/Human Connection. [Google Scholar]
- Cooper RL, Lamb JC, Barlow SM, Bentley K, Brady AM, Doerrer NG, Eisenbrandt DL, Fenner-Crisp PA, Hines RN, Irvine LF, et al. A tiered approach to life stages testing for agricultural chemical safety assessment. Crit. Rev. Toxicol. 2006;36:69–98. doi: 10.1080/10408440500541367. [DOI] [PubMed] [Google Scholar]
- Crofton KM, Craft ES, Hedge JM, Gennings C, Simmons JE, Carchman RA, Carter WH, Jr, DeVito MJ. Thyroid-hormone-disrupting chemicals: Evidence for dose-dependent additivity or synergism. Environ. Health Perspect. 2005;113:1549–1554. doi: 10.1289/ehp.8195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boo HA, Harding JE. The developmental origins of adult disease (Barker) hypothesis. Aust. N. Z. J. Obstet. Gynaecol. 2006;46:4–14. doi: 10.1111/j.1479-828X.2006.00506.x. [DOI] [PubMed] [Google Scholar]
- deFur PL. Use and role of invertebrate models in endocrine disruptor research and testing. ILAR J. 2004;45:484–493. doi: 10.1093/ilar.45.4.484. [DOI] [PubMed] [Google Scholar]
- Degitz SJ, Holcombe GW, Flynn KM, Kosian PA, Korte JJ, Tietge JE. Progress towards development of an amphibian-based thyroid screening assay using Xenopus laevis. Organismal and thyroidal responses to the model compounds 6-propylthiouracil, methimazole, and thyroxine. Toxicol. Sci. 2005;87:353–364. doi: 10.1093/toxsci/kfi246. [DOI] [PubMed] [Google Scholar]
- Desbrow C, Routledge E, Brighty G, Sumpter J, Waldock M. Identification of estrogenic chemicals in STW effluent. 1. Chemical fractionation and in vitro biological screening. Environ. Sci. Technol. 1998;32:1549–1558. [Google Scholar]
- DeVito M, Biegel L, Brouwer A, Brown S, Brucker-Davis F, Cheek AO, Christensen R, Colborn T, Cooke P, Crissman J, et al. Screening methods for thyroid hormone disruptors. Environ. Health Perspect. 1999;107:407–415. doi: 10.1289/ehp.99107407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfman RI, Dorfman AS. Assay of subcutaneously administered androgens on the chick's comb. Acta. Endocrinol. 1962;41:101–106. doi: 10.1530/acta.0.0410101. [DOI] [PubMed] [Google Scholar]
- Dostal LA, Chapin RE, Stefanski SA, Harris MW, Schwetz BA. Testicular toxicity and reduced Sertoli cell numbers in neonatal rats by di(2-ethylhexyl)phthalate and the recovery of fertility as adults. Toxicol. Appl. Pharmacol. 1988;95:104–121. doi: 10.1016/s0041-008x(88)80012-7. [DOI] [PubMed] [Google Scholar]
- Durhan EJ, Lambright C, Wilson V, Butterworth BC, Kuehl OW, Orlando EF, Guillette LJ, Jr, Gray LE, Ankley GT. Evaluation of androstenedione as an androgenic component of river water downstream of a pulp and paper mill effluent. Environ. Toxicol. Chem. SETAC. 2002;21:1973–1976. [PubMed] [Google Scholar]
- Durhan EJ, Lambright CS, Makynen EA, Lazorchak J, Hartig PC, Wilson VS, Gray LE, Ankley GT. Identification of metabolites of trenbolone acetate in androgenic runoff from a beef feedlot. Environ. Health Perspect. 2006;114(Suppl. 1):65–68. doi: 10.1289/ehp.8055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RJ, van den Heuvel MR, Bandelj E, Smith MA, McCarthy LH, Stuthridge TR, Dietrich DR. In vivo and in vitro assessment of the androgenic potential of a pulp and paper mill effluent. Environ. Toxicol. Chem. SETAC. 2003;22:1448–1456. [PubMed] [Google Scholar]
- European Commission. (1996). European Workshop on the Impact of Endocrine Disrepters on Human Health and Wildlife. Proceedings from a workshop, December 2–4, 1996, Weybridge, UK. Report reference EUR 17549. Brussels, Belgium: European Commission. [Google Scholar]
- Felner EI, White PC. Prepubertal gynecomastia: Indirect exposure to estrogen cream. Pediatrics. 2000;105:E55. doi: 10.1542/peds.105.4.e55. [DOI] [PubMed] [Google Scholar]
- Foster PM. Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. Int. J. Androl. 2006;29:140–147. doi: 10.1111/j.1365-2605.2005.00563.x. [DOI] [PubMed] [Google Scholar]
- Foster PM, Lake BG, Cook MW, Thomas LV, Gangolli SD. Structure-activity requirements for the induction of testicular atrophy by butyl phthalates in immature rats: Effect on testicular zinc content. Adv. Exp. Med. Biol. 1981;136(Pt A):445–452. doi: 10.1007/978-1-4757-0674-1_33. [DOI] [PubMed] [Google Scholar]
- Goldman JM, Laws SC, Balchak SK, Cooper RL, Kavlock RJ. Endocrine-disrupting chemicals: Prepubertal exposures and effects on sexual maturation and thyroid activity in the female rat. A focus on the EDSTAC recommendations. Crit. Rev. Toxicol. 2000;30:135–196. doi: 10.1080/10408440091159185. [DOI] [PubMed] [Google Scholar]
- Grajewski B, Whelan EA, Schnorr TM, Mouradian R, Alderfer R, Wild DK. Evaluation of reproductive function among men occupationally exposed to a stilbene derivative: I. Hormonal and physical status. Am. J. Ind. Med. 1996;29:49–57. doi: 10.1002/(SICI)1097-0274(199601)29:1<49::AID-AJIM7>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Gray LE., Jr Tiered screening and testing strategy for xenoestrogens and antiandrogens. Toxicol. Lett. 1998a;102-103:677–680. doi: 10.1016/s0378-4274(98)00287-2. [DOI] [PubMed] [Google Scholar]
- Gray LE., Jr Xenoendocrine disrupters: Laboratory studies on male reproductive effects. Toxicol. Lett. 1998b;102-103:331–335. doi: 10.1016/s0378-4274(98)00327-0. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Kelce WR, Monosson E, Ostby JS, Birnbaum LS. Exposure to TCDD during development permanently alters reproductive function in male Long Evans rats and hamsters: Reduced ejaculated and epididymal sperm numbers and sex accessory gland weights in offspring with normal androgenic status. Toxicol. Appl. Pharmacol. 1995;131:108–118. doi: 10.1006/taap.1995.1052. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Kelce WR, Wiese T, Tyl R, Gaido K, Cook J, Klinefelter G, Desaulniers D, Wilson E, Zacharewski T, et al. Endocrine Screening Methods Workshop report: Detection of estrogenic and androgenic hormonal and antihormonal activity for chemicals that act via receptor or steroidogenic enzyme mechanisms. Reprod. Toxicol. 1997a;11:719–750. doi: 10.1016/s0890-6238(97)00025-7. [DOI] [PubMed] [Google Scholar]
- Gray LE, Lambright C, Parks L, Tyl RW, Orlando EF, Guillette LJ, Wolf CJ, Seeley JC, Chang T-Y, Wilson V, et al. New York: Springer-Verlag; 2001a. Antiandrogenic Effects of Environmental Endocrine Disruptors. [Google Scholar]
- Gray LE, Jr, Ostby JS. In utero 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) alters reproductive morphology and function in female rat offspring. Toxicol. Appl. Pharmacol. 1995;133:285–294. doi: 10.1006/taap.1995.1153. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Ostby J. Effects of pesticides and toxic substances on behavioral and morphological reproductive development: Endocrine versus nonendocrine mechanisms. Toxicol. Ind. Health. 1998;14:159–184. doi: 10.1177/074823379801400111. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Ostby J, Ferrell J, Rehnberg G, Linder R, Cooper R, Goldman J, Slott V, Laskey J. A dose-response analysis of methoxychlor-induced alterations of reproductive development and function in the rat. Fundam. Appl. Toxicol. 1989;12:92–108. doi: 10.1016/0272-0590(89)90065-1. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Ostby JS, Ferrell JM, Sigmon ER, Goldman JM. Methoxychlor induces estrogen-like alterations of behavior and the reproductive tract in the female rat and hamster: Effects on sex behavior, running wheel activity, and uterine morphology. Toxicol. Appl. Pharmacol. 1988b;96:525–540. doi: 10.1016/0041-008x(88)90012-9. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Ostby J, Furr J, Price M, Veeramachaneni DN, Parks L. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol. Sci. 2000;58:350–365. doi: 10.1093/toxsci/58.2.350. [DOI] [PubMed] [Google Scholar]
- Gray LE, Ostby J, Furr J, Price M, Wolf CJ, Lambright C, Parks L, Veeramachaneni DNR, Wilson V, Hotchkiss A, Orlando E, Guillette L. Effects of environmental antiandrogens in experimental animals. Hum. Reprod. Update. 2001a;7:248–264. doi: 10.1093/humupd/7.3.248. [DOI] [PubMed] [Google Scholar]
- Gray LE, Ostby J, Furr J, Price M, Wolf CJ, Lambright C, Parks L, Veeramachaneni DNR, Wilson V, Hotchkiss A, et al. Effects of environmental antiandrogens in experimental animals. Hum. Reprod. Update. 2001b;7:248–264. doi: 10.1093/humupd/7.3.248. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Ostby JS, Kelce WR. Developmental effects of an environmental antiandrogen: The fungicide vinclozolin alters sex differentiation of the male rat. Toxicol. Appl. Pharmacol. 1994;129:46–52. doi: 10.1006/taap.1994.1227. [DOI] [PubMed] [Google Scholar]
- Gray LE, Ostby JS, Kelce WR. A dose-response analysis of the reproductive effects of a single gestational dose of 2,3,7,8-tetrachlorodibenzo-p-dioxin in male Long Evans Hooded rat offspring. Toxicol. Appl. Pharmacol. 1997b;146:11–20. doi: 10.1006/taap.1997.8223. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Ostby J, Monosson E, Kelce WR. Environmental antiandrogens: Low doses of the fungicide vinclozolin alter sexual differentiation of the male rat. Toxicol. Ind. Health. 1999;15:48–64. doi: 10.1177/074823379901500106. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Ostby J, Sigmon R, Ferrell J, Rehnberg G, Linder R, Cooper R, Goldman J, Laskey J. The development of a protocol to assess reproductive effects of toxicants in the rat. Reprod. Toxicol. 1988a;2:281–287. doi: 10.1016/0890-6238(88)90032-9. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Wilson VS, Stoker T, Lambright C, Furr J, Noriega N, Howdeshell K, Ankley GT, Guillette L. Adverse effects of environmental antiandrogens and androgens on reproductive development in mammals. Int. J. Androl. 2006;29:96–104. doi: 10.1111/j.1365-2605.2005.00636.x. discussion 105–108. [DOI] [PubMed] [Google Scholar]
- Gray LE, Wolf C, Mann P, Ostby JS. In utero exposure to low doses of 2,3,7,8-tetrachlorodibenzo-p-dioxin alters reproductive development of female Long Evans hooded rat offspring. Toxicol. Appl. Pharmacol. 1997c;146:237–244. doi: 10.1006/taap.1997.8222. [DOI] [PubMed] [Google Scholar]
- Gray TJ, Rowland IR, Foster PM, Gangolli SD. Species differences in the testicular toxicity of phthalate esters. Toxicol. Lett. 1982;11:141–147. doi: 10.1016/0378-4274(82)90119-9. [DOI] [PubMed] [Google Scholar]
- Grumbach MM, Ducharme JR. The effects of androgens on fetal sexual development: Androgen-induced female pseudohermaphrodism. Fertil. Steril. 1960;11:157–180. doi: 10.1016/s0015-0282(16)33722-0. [DOI] [PubMed] [Google Scholar]
- Guillette LJ., Jr Endocrine disrupting contaminants—beyond the dogma. Environ. Health Perspect. 2006;114(Suppl. 1):9–12. doi: 10.1289/ehp.8045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo YL, Lambert GH, Hsu CC. Growth abnormalities in the population exposed in utero and early postnatally to polychlorinated biphenyls and dibenzofurans. Environ. Health Perspect. 1995;103(Suppl. 6):117–122. doi: 10.1289/ehp.95103s6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harries JE, Janbakhsh A, Jobling S, Mattiessen P, Sumpter JP, Tyler CR. Estrogenic potency of effluent from two sewage treatment works in the United Kingdom. Environ. Toxicol. Chem. 1999;18:932–937. [Google Scholar]
- Hawkins MB, Godwin J, Crews D, Thomas P. The distributions of the duplicate oestrogen receptors ER-beta a and ER-beta b in the forebrain of the Atlantic croaker (Micropogonias undulatus): Evidence for subfunctionalization after gene duplication. Proceedings. 2005;272:633–641. doi: 10.1098/rspb.2004.3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes WJ, Laws ER. Handbook of Pesticide Toxicology. San Diego: Academic Press, Inc; 1991. [Google Scholar]
- Heneweer M, van den Berg M, Sanderson JT. A comparison of human H295R and rat R2C cell lines as in vitro screening tools for effects on aromatase. Toxicol. Lett. 2004;146:183–194. doi: 10.1016/j.toxlet.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Henley DV, Lipson N, Korach KS, Bloch CA. Prepubertal gynecomastia linked to lavender and tea tree oils. N. Engl. J. Med. 2007;356:479–485. doi: 10.1056/NEJMoa064725. [DOI] [PubMed] [Google Scholar]
- Hershberger LG, Shipley EG, Meyer RK. Myotrophic activity of 19-nortestosterone and other steroids determined by modified levator ani muscle method. Proc. Soc. Exp. Biol. Med. Soc. Exp. Biol. Med. 1953;83:175–180. doi: 10.3181/00379727-83-20301. [DOI] [PubMed] [Google Scholar]
- Higuchi TT, Palmer JS, Gray LE, Jr, Veeramachaneni DN. Effects of dibutyl phthalate in male rabbits following in utero, adolescent, or postpubertal exposure. Toxicol. Sci. 2003;72:301–313. doi: 10.1093/toxsci/kfg036. [DOI] [PubMed] [Google Scholar]
- Horiguchi T. Masculinization of female gastropod mollusks induced by organotin compounds, focusing on mechanism of actions of tributyltin and triphenyltin for development of imposex. Environ. Sci. 2006;13:77–87. [PubMed] [Google Scholar]
- Hornshaw TC, Aulerich RJ, Johnson HE. Feeding Great Lakes fish to mink: Effects on mink and accumulation and elimination of PCBS by mink. J. Toxicol. Environ. Health. 1983;11:933–946. doi: 10.1080/15287398309530396. [DOI] [PubMed] [Google Scholar]
- Hosokawa S, Murakami M, Ineyama M, Yamada T, Yoshitake A, Yamada H, Miyamoto J. The affinity of procymidone to androgen receptor in rats and mice. J. Toxicol. Sci. 1993;18:83–93. doi: 10.2131/jts.18.83. [DOI] [PubMed] [Google Scholar]
- Hotchkiss AK, Furr J, Makynen EA, Ankley GT, Gray LE., Jr In utero exposure to the environmental androgen trenbolone masculinizes female Sprague-Dawley rats. Toxicol. Lett. 2007;174:31–41. doi: 10.1016/j.toxlet.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Hotchkiss AK, Nelson RJ. An environmental androgen, trenbolone, affects delayed-type hypersensitivity and reproductive tissues in male mice. J. Toxicol. Environ. Health. 2007;70:138–140. doi: 10.1080/15287390600755091. [DOI] [PubMed] [Google Scholar]
- Hotchkiss AK, Parks-Saldutti LG, Ostby JS, Lambright C, Furr J, Vandenbergh JG, Gray LE., Jr A mixture of the “antiandrogens” linuron and butyl benzyl phthalate alters sexual differentiation of the male rat in a cumulative fashion. Biol. Reprod. 2004;71:1852–1861. doi: 10.1095/biolreprod.104.031674. [DOI] [PubMed] [Google Scholar]
- Howdeshell KL. A model of the development of the brain as a construct of the thyroid system. Environ. Health Perspect. 2002;110(Suppl. 3):337–348. doi: 10.1289/ehp.02110s3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howdeshell KL, Furr J, Lambright CR, Wilson VS, Ryan BC, Gray LE., Jr Gestational and lactational exposure to ethinyl estradiol, but not bisphenol a, decreases androgen-dependent reproductive organ weights and epididymal sperm abundance in the male Long Evans hooded rat. Toxicol. Sci. 2007 doi: 10.1093/toxsci/kfm306. 10.1093/toxsci/kfm306. [DOI] [PubMed] [Google Scholar]
- Hutchinson TH, Ankley GT, Segner H, Tyler CR. Screening and testing for endocrine disruption in fish-biomarkers as “signposts,” not “traffic lights,” in risk assessment. Environ. Health Perspect. 2006;114(Suppl. 1):106–114. doi: 10.1289/ehp.8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inadera H. The immune system as a target for environmental chemicals: Xenoestrogens and other compounds. Toxicol. Lett. 2006;164:191–206. doi: 10.1016/j.toxlet.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Jenkins RL, Wilson EM, Angus RA, Howell WM, Kirk M. Androstenedione and progesterone in the sediment of a river receiving paper mill effluent. Toxicol. Sci. 2003;73:53–59. doi: 10.1093/toxsci/kfg042. [DOI] [PubMed] [Google Scholar]
- Jensen KM, Makynen EA, Kahl MD, Ankley GT. Effects of the feedlot contaminant 17alpha-trenbolone on reproductive endocrinology of the fathead minnow. Environ. Sci. Technol. 2006;40:3112–3117. doi: 10.1021/es052174s. [DOI] [PubMed] [Google Scholar]
- Jobling S, Beresford N, Nolan M, Rodgers-Gray T, Brighty GC, Sumpter JP, Tyler CR. Altered sexual maturation and gamete production in wild roach (Rutilus rutilus) living in rivers that receive treated sewage effluents. Biol. Reprod. 2002a;66:272–281. doi: 10.1095/biolreprod66.2.272. [DOI] [PubMed] [Google Scholar]
- Jobling S, Coey S, Whitmore JG, Kime DE, Van Look KJW, McAllister BG, Beresford N, Henshaw AC, Brighty G, Tyler CR, et al. Wild intersex roach (Rutilus rutilus) have reduced fertility. Biol. Reprod. 2002b;67:515–524. doi: 10.1095/biolreprod67.2.515. [DOI] [PubMed] [Google Scholar]
- Joffe M. Infertility and environmental pollutants. Br. Med. Bull. 2003;68:47–70. doi: 10.1093/bmb/ldg025. [DOI] [PubMed] [Google Scholar]
- Johansson M, Nilsson S, Lund BO. Interactions between methylsulfonyl PCBs and the glucocorticoid receptor. Environ. Health Perspect. 1998;106:769–772. doi: 10.1289/ehp.98106769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorundsdottir K, Svavarsson J, Leung KM. Imposex levels in the dogwhelk Nucella lapillus (L.)—Continuing improvement at high latitudes. Mar. Pollut. Bull. 2005;51:744–749. doi: 10.1016/j.marpolbul.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Kelce WR, Lambright CR, Gray LE, Jr, Roberts KP. Vinclozolin and p,p'-DDE alter androgen-dependent gene expression: In vivo confirmation of an androgen receptor-mediated mechanism. Toxicol. Appl. Pharmacol. 1997;142:192–200. doi: 10.1006/taap.1996.7966. [DOI] [PubMed] [Google Scholar]
- Kelce WR, Stone CR, Laws SC, Gray LE, Kemppainen JA, Wilson EM. Persistent DDT metabolite p,p′-DDE is a potent androgen receptor antagonist. Nature. 1995;375:581–585. doi: 10.1038/375581a0. [DOI] [PubMed] [Google Scholar]
- Kidd KA, Blanchfield PJ, Mills KH, Palace VP, Evans RE, Lazorchak JM, Flick RW. Collapse of a fish population after exposure to a synthetic estrogen. Proc Natl Acad Sci U S A. 2007;104:8897–8901. doi: 10.1073/pnas.0609568104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Jung K, Hwang T, Jung G, Kim H, Park J, Kim J, Park J, Park D, Park S, et al. Hematopoietic and reproductive hazards of Korean electronic workers exposed to solvents containing 2-bromopropane. Scand. J. Work Environ. Health. 1996;22:387–391. doi: 10.5271/sjweh.159. [DOI] [PubMed] [Google Scholar]
- Klonisch T, Fowler PA, Hombach-Klonisch S. Molecular and genetic regulation of testis descent and external genitalia development. Dev. Biol. 2004;270:1–18. doi: 10.1016/j.ydbio.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT. Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999-2000: A national reconnaissance. Environ. Sci. Technol. 2002;36:1202–1211. doi: 10.1021/es011055j. [DOI] [PubMed] [Google Scholar]
- Kretschmer XC, Baldwin WS. CAR and PXR: Xenosensors of endocrine disrupters? Chem. Biol. Interact. 2005;155:111–128. doi: 10.1016/j.cbi.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Kunz GJ, Klein KO, Clemons RD, Gottschalk ME, Jones KL. Virilization of young children after topical androgen use by their parents. Pediatrics. 2004;114:282–284. doi: 10.1542/peds.114.1.282. [DOI] [PubMed] [Google Scholar]
- Lag M, Soderlund EJ, Brunborg G, Dahl JE, Holme JA, Omichinski JG, Nelson SD, Dybing E. Species differences in testicular necrosis and DNA damage, distribution and metabolism of 1,2-dibromo-3-chloropropane (DBCP) Toxicology. 1989;58:133–144. doi: 10.1016/0300-483x(89)90003-6. [DOI] [PubMed] [Google Scholar]
- Lake BG, Brantom PG, Gangolli SD, Butterworth KR, Grasso P. Studies on the effects of orally administered di-(2-ethylhexyl) phthalate in the ferret. Toxicology. 1976;6:341–356. doi: 10.1016/0300-483x(76)90038-x. [DOI] [PubMed] [Google Scholar]
- Lambright C, Ostby J, Bobseine K, Wilson VS, Hotchkiss AK, Mann PC, Gray LEJ. Cellular and molecular mechanisms of action of linuron: An antiandrogenic herbicide that produces reproductive malformations in male rats. Toxicol. Sci. 2000;56:389–399. doi: 10.1093/toxsci/56.2.389. [DOI] [PubMed] [Google Scholar]
- Larsson DG, Forlin L. Male-biased sex ratios of fish embryos near a pulp mill: Temporary recovery after a short-term shutdown. Environ. Health Perspect. 2002;110:739–742. doi: 10.1289/ehp.02110739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMaire WJ, Cleveland WW, Bejar RL, Marsh JM, Fishman L. Aminoglutethimide: A possible cause of pseudohermaphroiditism in females. Am. J. Dis. Child. 1972;124:421–423. doi: 10.1001/archpedi.1972.02110150119020. [DOI] [PubMed] [Google Scholar]
- Louis GM, Weiner JM, Whitcomb BW, Sperrazza R, Schisterman EF, Lobdell DT, Crickard K, Greizerstein H, Kostyniak PJ. Environmental PCB exposure and risk of endometriosis. Hum. Reprod. 2005;20:279–285. doi: 10.1093/humrep/deh575. [DOI] [PubMed] [Google Scholar]
- Mably TA, Bjerke DL, Moore RW, Gendron-Fitzpatrick A, Peterson RE. In utero and lactational exposure of male rats to 2,3,7,8-tetrachlorodibenzo-p-dioxin. 3. Effects on spermatogenesis and reproductive capability. Toxicol. Appl. Pharmacol. 1992;114:118–126. doi: 10.1016/0041-008x(92)90103-y. [DOI] [PubMed] [Google Scholar]
- Martinovic D, Hogarth WT, Jones RE, Sorensen PW. Environmental estrogens suppress hormones, behavior, and reproductive fitness in male fathead minnows. Environ. Toxicol. Chem. SETAC. 2007;26:271–278. doi: 10.1897/06-065r.1. [DOI] [PubMed] [Google Scholar]
- McIntyre BS, Barlow NJ, Wallace DG, Maness SC, Gaido KW, Foster PM. Effects of in utero exposure to linuron on androgen-dependent reproductive development in the male Crl:CD(SD)BR rat. Toxicol. Appl. Pharmacol. 2000;167:87–99. doi: 10.1006/taap.2000.8998. [DOI] [PubMed] [Google Scholar]
- Meyer K, Usleber E, Martlbauer E, Bauer J. [Occurrence of zearalenone, alpha- and beta-zearalenol in bile of breeding sows in relation to reproductive performance] Berl. Munch. Tierarztl. Wochenschr. 2000;113:374–379. [PubMed] [Google Scholar]
- Mizota K, Ueda H. Endocrine disrupting chemical atrazine causes degranulation through Gq/11 protein-coupled neurosteroid receptor in mast cells. Toxicol. Sci. 2006;90:362–368. doi: 10.1093/toxsci/kfj087. [DOI] [PubMed] [Google Scholar]
- Monosson E, Kelce WR, Lambright C, Ostby J, Gray LE., Jr Peripubertal exposure to the antiandrogenic fungicide, vinclozolin, delays puberty, inhibits the development of androgen-dependent tissues, and alters androgen receptor function in the male rat. Toxicol. Ind. Health. 1999;15:65–79. doi: 10.1177/074823379901500107. [DOI] [PubMed] [Google Scholar]
- Mylchreest E, Cattley RC, Foster PM. Male reproductive tract malformations in rats following gestational and lactational exposure to di(n-butyl) phthalate: An antiandrogenic mechanism? Toxicol. Sci. 1998;43:47–60. doi: 10.1006/toxs.1998.2436. [DOI] [PubMed] [Google Scholar]
- Nader N, Raverot G, Emptoz-Bonneton A, Dechaud H, Bonnay M, Baudin E, Pugeat M. Mitotane has an estrogenic effect on sex hormone-binding globulin and corticosteroid-binding globulin in humans. J. Clin. Endocrinol. Metab. 2006;91:2165–2170. doi: 10.1210/jc.2005-2157. [DOI] [PubMed] [Google Scholar]
- Nellemann C, Dalgaard M, Lam HR, Vinggaard AM. The combined effects of vinclozolin and procymidone do not deviate from expected additivity in vitro and in vivo. Toxicol. Sci. 2003;71:251–262. doi: 10.1093/toxsci/71.2.251. [DOI] [PubMed] [Google Scholar]
- Nishikawa J, Mamiya S, Kanayama T, Nishikawa T, Shiraishi F, Horiguchi T. Involvement of the retinoid X receptor in the development of imposex caused by organotins in gastropods. Environ. Sci. Technol. 2004;38:6271–6276. doi: 10.1021/es049593u. [DOI] [PubMed] [Google Scholar]
- Noriega NC, Ostby J, Lambright C, Wilson VS, Gray LE., Jr Late gestational exposure to the fungicide prochloraz delays the onset of parturition and causes reproductive malformations in male but not female rat offspring. Biol. Reprod. 2005;72:1324–1335. doi: 10.1095/biolreprod.104.031385. [DOI] [PubMed] [Google Scholar]
- Opitz R, Hartmann S, Blank T, Braunbeck T, Lutz I, Kloas W. Evaluation of histological and molecular endpoints for enhanced detection of thyroid system disruption in Xenopus laevis tadpoles. Toxicol. Sci. 2006;90:337–348. doi: 10.1093/toxsci/kfj083. [DOI] [PubMed] [Google Scholar]
- Orlando EF, Bass DE, Caltabiano LM, Davis WP, Gray LE, Jr, Guillette LJ., Jr Altered development and reproduction in mosquitofish exposed to pulp and paper mill effluent in the Fenholloway River, Florida USA. Aquat. Toxicol. 2007;84:399–405. doi: 10.1016/j.aquatox.2007.06.018. [DOI] [PubMed] [Google Scholar]
- Orlando EF, Kolok AS, Binzcik GA, Gates JL, Horton MK, Lambright CS, Gray LE, Jr, Soto AM, Guillette LJ., Jr Endocrine-disrupting effects of cattle feedlot effluent on an aquatic sentinel species, the fathead minnow. Environ. Health Perspect. 2004;112:353–358. doi: 10.1289/ehp.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostby J, Kelce WR, Lambright C, Wolf CJ, Mann P, Gray LE., Jr The fungicide procymidone alters sexual differentiation in the male rat by acting as an androgen-receptor antagonist in vivo and in vitro. Toxicol. Ind. Health. 1999;15:80–93. doi: 10.1177/074823379901500108. [DOI] [PubMed] [Google Scholar]
- Owens W, Gray LE, Zeiger E, Walker M, Yamasaki K, Ashby J, Jacob E. The OECD program to validate the rat Hershberger bioassay to screen compounds for in vivo androgen and antiandrogen responses: Phase 2 dose-response studies. Environ. Health Perspect. 2007;115:671–678. doi: 10.1289/ehp.9666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens W, Koeter HB. The OECD program to validate the rat uterotrophic bioassay: An overview. Environ. Health Perspect. 2003;111:1527–1529. doi: 10.1289/ehp.6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens W, Zeiger E, Walker M, Ashby J, Onyon L, Gray LE., Jr The OECD program to validate the rat Hershberger bioassay to screen compounds for in vivo androgen and antiandrogen responses. Phase 1: Use of a potent agonist and a potent antagonist to test the standardized protocol. Environ. Health Perspect. 2006;114:1259–1265. doi: 10.1289/ehp.8751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakdel F, Metivier R, Flouriot G, Valotaire Y. Two estrogen receptor (ER) isoforms with different estrogen dependencies are generated from the trout ER gene. Endocrinology. 2000;141:571–580. doi: 10.1210/endo.141.2.7296. [DOI] [PubMed] [Google Scholar]
- Parks LG, Lambright CS, Orlando EF, Guillette LJ, Jr, Ankley GT, Gray LE., Jr Masculinization of female mosquitofish in Kraft mill effluent-contaminated Fenholloway River water is associated with androgen receptor agonist activity. Toxicol. Sci. 2001;62:257–267. doi: 10.1093/toxsci/62.2.257. [DOI] [PubMed] [Google Scholar]
- Parks LG, Ostby JS, Lambright CR, Abbott BD, Klinefelter GR, Barlow NJ, Gray LE., Jr The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicol. Sci. 2000;58:339–349. doi: 10.1093/toxsci/58.2.339. [DOI] [PubMed] [Google Scholar]
- Payne J, Rajapakse N, Wilkins M, Kortenkamp A. Prediction and assessment of the effects of mixtures of four xenoestrogens. Environ. Health Perspect. 2000;108:983–987. doi: 10.1289/ehp.00108983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne J, Scholze M, Kortenkamp A. Mixtures of four organochlorines enhance human breast cancer cell proliferation. Environ. Health Perspect. 2001;109:391–397. doi: 10.1289/ehp.01109391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson GI, Norrgren L, Holbech H, Lundgren A, Kovisto S. Vol. 2001. Uppsala: Nordic Council of Ministers; 2001. Suitability of Zebrafish as a Test Organism for Detection of Endocrine-Disrupting Chemicals. [Google Scholar]
- Potashnik G, Porath A. Dibromochloropropane (DBCP): A 17-year reassessment of testicular function and reproductive performance. J. Occup. Environ. Med. 1995;37:1287–1292. doi: 10.1097/00043764-199511000-00007. [DOI] [PubMed] [Google Scholar]
- Potashnik G, Yanai-Inbar I. Dibromochloropropane (DBCP): An 8-year reevaluation of testicular function and reproductive performance. Fertil. Steril. 1987;47:317–323. doi: 10.1016/s0015-0282(16)50012-0. [DOI] [PubMed] [Google Scholar]
- Quigley CA, De Bellis A, Marschke KB, el-Awady MK, Wilson EM, French FS. Androgen receptor defects: Historical, clinical, and molecular perspectives. Endocr. Rev. 1995;16:271–321. doi: 10.1210/edrv-16-3-271. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Xiao-Feng L. Effects of study duration, frequency of observation, and sample size on power in studies of group differences in polynomial change. Psychol. Methods. 2001;6:387–401. [PubMed] [Google Scholar]
- Reine NJ. Medical management of pituitary-dependent hyperadrenocorticism: Mitotane versus trilostane. Clin. Tech. Small Anim. Pract. 2007;22:18–25. doi: 10.1053/j.ctsap.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Rider CV, Furr J, Wilson VS, Gray LE., Jr A mixture of seven antiandrogens induces reproductive malformations in rats. Int. J. Androl. 2008 doi: 10.1111/j.1365-2605.2007.00859.x. In press. [DOI] [PubMed] [Google Scholar]
- Rier SE, Turner WE, Martin DC, Morris R, Lucier GW, Clark GC. Serum levels of TCDD and dioxin-like chemicals in Rhesus monkeys chronically exposed to dioxin: Correlation of increased serum PCB levels with endometriosis. Toxicol. Sci. 2001;59:147–159. doi: 10.1093/toxsci/59.1.147. [DOI] [PubMed] [Google Scholar]
- Rodriguez EM, Medesani DA, Fingerman M. Endocrine disruption in crustaceans due to pollutants: A review. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007;146:661–671. doi: 10.1016/j.cbpa.2006.04.030. [DOI] [PubMed] [Google Scholar]
- Sanderson JT, Elliott JE, Norstrom RJ, Whitehead PE, Hart LE, Cheng KM, Bellward GD. Monitoring biological effects of polychlorinated dibenzo-p-dioxins, dibenzofurans, and biphenyls in great blue heron chicks (Ardea herodias) in British Columbia. J. Toxicol. Environ. Health. 1994;41:435–450. doi: 10.1080/15287399409531855. [DOI] [PubMed] [Google Scholar]
- Saunders FJ. Effects of sex steroids and related compounds on pregnancy and on development of the young. Physiol. Rev. 1968;48:601–643. doi: 10.1152/physrev.1968.48.3.601. [DOI] [PubMed] [Google Scholar]
- Schantz SL, Widholm JJ. Cognitive effects of endocrine-disrupting chemicals in animals. Environ. Health Perspect. 2001;109:1197–1206. doi: 10.1289/ehp.011091197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki M, Fujishima S, Nozaka T, Maeda M, Kobayashi K. Comparison of response to 17 beta-estradiol and 17 beta-trenbolone among three small fish species. Environ. Toxicol. Chem. SETAC. 2006;25:2742–2752. doi: 10.1897/05-647r.1. [DOI] [PubMed] [Google Scholar]
- Selgrade MK. Immunotoxicity—The risk is real. Toxicol. Sci. 2007 doi: 10.1093/toxsci/kfm244. [DOI] [PubMed] [Google Scholar]
- Sharpe RM. Pathways of endocrine disruption during male sexual differentiation and masculinization. Best Pract. Res. Clin. Endocrinol. Metab. 2006;20:91–110. doi: 10.1016/j.beem.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Shultz S, Baral HS, Charman S, Cunningham AA, Das D, Ghalsasi GR, Goudar MS, Green RE, Jones A, Nighot P, et al. Diclofenac poisoning is widespread in declining vulture populations across the Indian subcontinent. Proceedings. 2004;271(Suppl. 6):S458–S460. doi: 10.1098/rsbl.2004.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva E, Rajapakse N, Kortenkamp A. Something from “nothing”—Eight weak estrogenic chemicals combined at concentrations below NOECs produce significant mixture effects. Environ. Sci. Technol. 2002;36:1751–1756. doi: 10.1021/es0101227. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Barr DB, Reidy JA, Malek NA, Hodge CC, Caudill SP, Brock JW, Needham LL, Calafat AM. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999-2000. Environ. Health Perspect. 2004;112:331–338. doi: 10.1289/ehp.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoberg P, Lindqvist NG, Ploen L. Age-dependent response of the rat testes to di(2-ethylhexyl) phthalate. Environ. Health Perspect. 1986;65:237–242. doi: 10.1289/ehp.65-1474698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: An increasingly common developmental disorder with environmental aspects. Hum. Reprod. 2001;16:972–978. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- Slutsky M, Levin JL, Levy BS. Azoospermia and oligospermia among a large cohort of DBCP applicators in 12 countries. Int. J. Occup. Environ. Health. 1999;5:116–122. doi: 10.1179/oeh.1999.5.2.116. [DOI] [PubMed] [Google Scholar]
- Smith ER, Quinn MM. Uterotropic action in rats of amsonic acid and three of its synthetic precursors. J. Toxicol. Environ. Health. 1992;36:13–25. doi: 10.1080/15287399209531620. [DOI] [PubMed] [Google Scholar]
- Soto AM, Calabro JM, Prechtl NV, Yau AY, Orlando EF, Daxenberger A, Kolok AS, Guillette LJ, Jr, le Bizec B, Lange IG, et al. Androgenic and estrogenic activity in water bodies receiving cattle feedlot effluent in Eastern Nebraska, USA. Environ. Health Perspect. 2004;112:346–352. doi: 10.1289/ehp.6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparagana M. Primary hypogonadism associated with o,p′ DDD (mitotane) therapy. J. Toxicol. 1987;25:463–472. doi: 10.3109/15563658708992649. [DOI] [PubMed] [Google Scholar]
- Spooner N, Gibbs PE, Bryan GW, Goad LJ. The effect of tributyltin upon steroid titres in female dogwhelk, nucella lapillus, and the development of imposex. Mar. Environ. Res. 1991;32:37–49. [Google Scholar]
- Steinberger E, Lloyd JA. Chemicals Affecting the Development of Reproductive Capacity. New York: Raven Press; 1985. [Google Scholar]
- Sternberg RM, Hotchkiss AK, Leblanc GA. The contribution of steroidal androgens and estrogens to reproductive maturation of the eastern mud snail Ilyanassa obsoleta. Gen. Comp. Endocrinol. 2008 doi: 10.1016/j.ygcen.2007.12.002. 10.1016/j.ygcen.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Stoker TE, Cooper RL, Lambright CS, Wilson VS, Furr J, Gray LE. In vivo and in vitro anti-androgenic effects of DE-71, a commercial polybrominated diphenyl ether (PBDE) mixture. Toxicol. Appl. Pharmacol. 2005;207:78–88. doi: 10.1016/j.taap.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Stoker TE, Parks LG, Gray LE, Cooper RL. Endocrine-disrupting chemicals: Prepubertal exposures and effects on sexual maturation and thyroid function in the male rat. A focus on the EDSTAC recommendations. Crit. Rev. Toxicol. 2000;30:197–202. doi: 10.1080/10408440091159194. [DOI] [PubMed] [Google Scholar]
- Takeo J, Yamashita S. Two distinct isoforms of cDNA encoding rainbow trout androgen receptors. J. Biol. Chem. 1999;274:5674–5680. doi: 10.1074/jbc.274.9.5674. [DOI] [PubMed] [Google Scholar]
- Tabb MM, Blumberg B. New modes of action for endocrine-disrupting chemicals. Mol. Endocrinol. 2006;20:475–482. doi: 10.1210/me.2004-0513. [DOI] [PubMed] [Google Scholar]
- Teitelbaum DT. The toxicology of 1,2-dibromo-3-chloropropane (DBCP): A brief review. Int. J. Occup. Environ. Health. 1999;5:122–126. doi: 10.1179/oeh.1999.5.2.122. [DOI] [PubMed] [Google Scholar]
- Tiwary CM. Premature sexual development in children following the use of estrogen- or placenta-containing hair products. Clin. Pediatrics. 1998;37:733–739. doi: 10.1177/000992289803701204. [DOI] [PubMed] [Google Scholar]
- Touart LW. Factors considered in using birds for evaluating endocrine-disrupting chemicals. ILAR J. 2004;45:462–468. doi: 10.1093/ilar.45.4.462. [DOI] [PubMed] [Google Scholar]
- Warren DW, Ahmad N, Rudeen PK. The effects of fetal exposure to 1,2-dibromo-3-chloropropane on adult male reproductive function. Biol. Reprod. 1988;39:707–716. doi: 10.1095/biolreprod39.3.707. [DOI] [PubMed] [Google Scholar]
- Waterman SJ, Keller LH, Trimmer GW, Freeman JJ, Nikiforov AI, Harris SB, Nicolich MJ, McKee RH. Two-generation reproduction study in rats given di-isononyl phthalate in the diet. Reprod. Toxicol. 2000;14:21–36. doi: 10.1016/s0890-6238(99)00067-2. [DOI] [PubMed] [Google Scholar]
- Whelan EA, Grajewski B, Wild DK, Schnorr TM, Alderfer R. Evaluation of reproductive function among men occupationally exposed to a stilbene derivative: II. Perceived libido and potency. Am. J. Ind. Med. 1996;29:59–65. doi: 10.1002/(SICI)1097-0274(199601)29:1<59::AID-AJIM8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- WHO . ICPS global assessment of the state-of-the-science of endocrine disruptors. In: Damstra T, Barlow S, Bergman A, Kavlock R, Kraak Gv d, editors. International Programme on Chemical Safety, WHO/PCS/EDC/02.2. Geneva, Switzerland: World Health Organization; 2002. [Google Scholar]
- Whorton D, Milby TH, Krauss RM, Stubbs HA. Testicular function in DBCP exposed pesticide workers. J. Occup. Med. 1979;21:161–166. [PubMed] [Google Scholar]
- Whorton MD, Foliart DE. Mutagenicity, carcinogenicity and reproductive effects of dibromochloropropane (DBCP) Mutat. Res. 1983;123:13–30. doi: 10.1016/0165-1110(83)90044-1. [DOI] [PubMed] [Google Scholar]
- Whorton MD, Milby TH. Recovery of testicular function among DBCP workers. J. Occup. Med. 1980;22:177–179. [PubMed] [Google Scholar]
- Wilson VS, Cardon MC, Thornton J, Korte JJ, Ankley GT, Welch J, Gray LE, Jr, Hartig PC. Cloning and in vitro expression and characterization of the androgen receptor and isolation of estrogen receptor alpha from the fathead Minnow (Pimephales promelas) Environ. Sci. Technol. 2004;38:6314–6321. doi: 10.1021/es049771j. [DOI] [PubMed] [Google Scholar]
- Wilson VS, Bobseine K, Gray LEJ. Development and characterization of a cell line that stably expresses an estrogen-responsive luciferase reporter for the detection of estrogen receptor agonist and antagonists. Toxicol. Sci. 2004a;81:69–77. doi: 10.1093/toxsci/kfh180. [DOI] [PubMed] [Google Scholar]
- Wilson VS, Bobseine K, Lambright CR, Gray LE., Jr A novel cell line, MDA-kb2, that stably expresses an androgen- and glucocorticoid-responsive reporter for the detection of hormone receptor agonists and antagonists. Toxicol. Sci. 2002a;66:69–81. doi: 10.1093/toxsci/66.1.69. [DOI] [PubMed] [Google Scholar]
- Wilson VS, Cardon MC, Gray LE, Jr, Hartig PC. Competitive binding comparison of endocrine-disrupting compounds to recombinant androgen receptor from fathead minnow, rainbow trout, and human. Environ. Toxicol. Chem. SETAC. 2007;26:1793–1802. doi: 10.1897/06-593R.1. [DOI] [PubMed] [Google Scholar]
- Wilson VS, Cardon MC, Thornton J, Korte JJ, Ankley GT, Welch J, Gray LE, Jr, Hartig PC. Cloning and in vitro expression and characterization of the androgen receptor and isolation of estrogen receptor alpha from the fathead Minnow (Pimephales promelas) Environ. Sci. Technol. 2004b;38:6314–6321. doi: 10.1021/es049771j. [DOI] [PubMed] [Google Scholar]
- Wilson VS, Lambright C, Furr J, Ostby J, Wood C, Held G, Gray LE., Jr Phthalate ester-induced gubernacular lesions are associated with reduced insl3 gene expression in the fetal rat testis. Toxicol. Lett. 2004c;146:207–215. doi: 10.1016/j.toxlet.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Wilson VS, Lambright C, Ostby J, Gray LE., Jr In vitro and in vivo effects of 17beta-trenbolone: A feedlot effluent contaminant. Toxicol. Sci. 2002b;70:202–211. doi: 10.1093/toxsci/70.2.202. [DOI] [PubMed] [Google Scholar]
- Wolf CJ, Ostby JS, Gray LE., Jr Gestational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) severely alters reproductive function of female hamster offspring. Toxicol. Sci. 1999;51:259–264. doi: 10.1093/toxsci/51.2.259. [DOI] [PubMed] [Google Scholar]
- Yamasaki K, Sawaki M, Ohta R, Okuda H, Katayama S, Yamada T, Ohta T, Kosaka T, Owens W. OECD validation of the Hershberger assay in Japan: Phase 2 dose response of methyltestosterone, vinclozolin, and p,p′-DDE. Environ. Health Perspect. 2003;111:1912–1919. doi: 10.1289/ehp.6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LE. Imprinting of genes and the Barker hypothesis. Twin Res. 2001;4:307–317. doi: 10.1375/1369052012632. [DOI] [PubMed] [Google Scholar]
- Zabel EW, Walker MK, Hornung MW, Clayton MK, Peterson RE. Interactions of polychlorinated dibenzo-p-dioxin, dibenzofuran, and biphenyl congeners for producing rainbow trout early life stage mortality. Toxicol. Appl. Pharmacol. 1995;134:204–213. doi: 10.1006/taap.1995.1185. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Crofton KM. Mode of action: Developmental thyroid hormone insufficiency—Neurological abnormalities resulting from exposure to propylthiouracil. Crit. Rev. Toxicol. 2005;35:771–781. doi: 10.1080/10408440591007313. [DOI] [PubMed] [Google Scholar]


