Abstract
1,25-Dihydroxyvitamin D3 [1,25(OH)2D3] and the vitamin D receptor (VDR) are important regulators of autoimmunity. The effect of the VDR on the ability of mice to fight a primary or secondary infection has not been determined. Young and old VDR knockout (KO) mice were able to clear both primary and secondary infections with Listeria monocytogenes. However, the kinetics of clearance was somewhat delayed in the absence of the VDR. Memory T cell development was not different in young VDR KO and wild-type (WT) mice; however, old VDR KO mice had significantly less memory T cells than their WT counterparts but still mounted an adequate immune response as determined by the complete clearance of L. monocytogenes. Although the primary and secondary immune responses were largely intact in the VDR KO mice, the old VDR KO mice had increased cytokines and antibody responses compared with the old WT mice. In particular, old VDR KO mice had elevated antigen non-specific antibodies; however, these magnified immune responses did not correspond to more effective Listeria clearance. The increased antibody and cytokine responses in the old VDR KO mice are consistent with the increased susceptibility of these mice to autoimmunity.
Keywords: aging; antibodies; 1,25-dihydroxyvitamin D3; Listeria monocytogenes
Introduction
The active form of vitamin D, 1,25-dihydroxyvitamin D3 [1,25(OH)2D3], has been shown to have an important role in regulating the immune system. The effects of vitamin D can be observed in many cells of the immune system and the vitamin D receptor (VDR) is found in myeloid and lymphoid cells in resting and activated states (1). It has been shown that 1,25(OH)2D3 suppresses T cell proliferation and decreases the production of the Th1 cytokines IFN-γ, IL-2 and tumor necrosis factor (TNF)-α (2, 3). Th1-driven autoimmune diseases including experimental multiple sclerosis and experimental inflammatory bowel disease are suppressed by in vivo treatment with 1,25(OH)2D3 (4, 5) and 1,25(OH)2D3 inhibits the listeriacidal activity and oxidative burst of IFN-γ-stimulated macrophage in vitro (6), indicating that 1,25(OH)2D3 is an important regulator of Th1-mediated immune responses.
In the absence of the VDR, the immune system develops normal numbers and subsets of T cells and B cells. NKT cell development, however, is blocked in the VDR knockout (KO) mice (7). VDR KO mice have heightened IFN-γ-mediated immune responses and decreased induction of Th2 response. For example, VDR KO mice are more susceptible to experimental inflammatory bowel disease, have heightened IFN-γ responses and decreased IL-4 responses and are more resistant to the Th2 disease experimental allergic asthma (8, 9). VDR KO mice develop larger granulomas when infected with Shistosoma mansoni [Th2 dominated, (8)] and have decreased Leishmania major (Th1 dominated) parasite burdens compared with wild-type (WT) controls (10). However, in vitro killing of Listeria by infected macrophages is not affected by deletion of the VDR (6). The VDR KO mice thus have heightened IFN-γ-mediated immune responses and decreased induction of Th2 responses.
Listeria monocytogenes is a gram-positive intracellular bacterium that causes infections when ingested in contaminated food. This bacterium is commonly used as a model for studying immune responses to intracellular pathogens. IFN-γ, IL-12 and CD8 T cells are critical for clearance of L. monocytogenes infections (11). Both MHC class I and class II are required for the response to Listeria since deletion of either gene leads to more severe infection, reduced IFN-γ production and increased severity of granulomatous lesions (12). While IL-10 is required for the immune response to Listeria, reduction in this cytokine early during infection leads to more rapid clearance of the bacteria (13, 14). CD8+ T cell memory is reduced in the absence of IL-10, accompanied by increased susceptibility to secondary infections, supporting an important role for IL-10 in the response to this disease (15). During secondary L. monocytogenes infection, CD8+/CD44high T cells are responsible for early onset of specific immune responses (16). IFN-γ is required for sterilizing immunity to secondary infection and this is partially dependent on IL-12 production, and while IFN-γ-deficient mice are extremely susceptible to primary infections, they can produce CD8+ T cell-mediated responses during secondary infection (17, 18). In addition, IL-12 deficiency leads to decreased effector responses during primary infection and increased memory following immunization (17). Protection from L. monocytogenes is dependent on classical Th1-driven cytokine responses with induction of memory CD8 T cells for sterilizing immunity.
Aging results in a decrease in antigen-specific immunity to new antigens. The elderly are more susceptible to infectious diseases including L. monocytogenes infection due to a decline in their ability to specifically generate anti-bacterial immunity (19). Increased susceptibility to infections in the elderly has been attributed to reduced T cell proliferation and decreased IL-2 production (20, 21). The number of mature splenic B cells also decreases with age, and reduced titers of antibodies are made following infection (21, 22). Although the aged immune response is reduced in response to new antigenic challenges, there is an increase in self-reactivity (23).
As 1,25(OH)2D3, and in particular the VDR, is an important regulator of Th1-mediated immune responses, we have determined the role of the VDR on the ability of mice to mount an immune response to L. monocytogenes. Our experiments show that young VDR KO mice exhibited a lag in the clearance of L. monocytogenes during primary challenge of the mice. The lag in clearance occurred early post-infection when the VDR KO mice produced significantly higher levels of IL-10 and IFN-γ compared with WT mice. Memory CD8 and CD4 T cell responses, as well as antigen-specific IgG1 and IgG2c developed normally in the young VDR KO and WT mice. The aged VDR KO mouse also effectively cleared L. monocytogenes from the spleen with largely the same kinetics of the aged WT mouse. However, following a primary infection, aged VDR KO mice had slower kinetics of clearance in the liver compared with WT mice. By contrast, antigen-specific as well as total IgG1 and IgG2c were significantly higher following secondary infection in aged VDR KO mice. The aged VDR KO mice produced more IFN-γ despite the fact that the number of memory CD4 and CD8 T cells producing IFN-γ were lower in the mice compared with WT. These results indicate that expression of the VDR is not required for host resistance to L. monocytogenes in either young or old mice. Instead, old VDR KO mice have heightened cytokine and antibody responses that did not correlate with the ability to clear the infection.
Materials and methods
Mice
Female C57BL/6 (WT) and VDR KO mice were bred and maintained at the Pennsylvania State University (University Park, PA, USA). The mice were 2 months (young) or 19 months (old) at the initiation of the experiments. All experimental procedures were approved by the Office of Research Protection's Institutional Animal Care and Use Committee (Pennsylvania State University).
Listeria monocytogenes and immunization
Transgenic recombinant L. monocytogenes expressing ovalbumin (OVA) [gift from Hao Shen, University of Pennsylvania, PA, USA (15)] was incubated overnight in blood heart infusion (BHI) broth. For primary infections, mice received 5 × 106 colony-forming unit (CFU) intra-peritoneal injections of Listeria and were euthanized 2, 4 and 10 days later. For secondary challenge, mice received a low dose of 1 × 104 CFU for primary exposure, and then 30 days later were challenged with 5 × 106 CFU and euthanized 1, 2 and 4 days later.
Assessment of bacterial burden
Spleens and livers of infected animals were harvested and weighed and the tissue was homogenized in 1 ml sterile PBS. CFUs were determined by plating 10-fold dilutions on BHI agar plates and the colonies were counted 48–72 h later.
Flow cytometry
Splenocytes were collected and after lysis of the RBCs were stimulated 10–12 h with phorbol 12-myristate 13-acetate (0.1 μg ml−1) and ionomycin (0.5 μg ml−1); brefeldin A (10 μg ml−1) was added for the final 6 h. Cells were stained with anti-CD44 (PE), anti-CD4 (ECD) and anti-CD8 (PE–Cy5), fixed with 4% PFA, permeabilized with 0.1% saponin and stained with anti-IFN-γ (FITC), all from BD PharMingen (San Diego, CA, USA). Analyses were performed on a FC500 bench top cytometer (Beckman Coulter, Miami, FL, USA). Data were evaluated with WinMDI 2.9 software (Scripps Institute, La Jolla, CA, USA).
Cytokine production
Splenocytes (5 × 106) were incubated 72 h in RPMI-1640 complete media alone or with additional stimulation with OVA (100 μg ml−1) and supernatants were collected 72 h later. ELISAs were performed for cytokines IFN-γ, IL-10, IL-12 and TNF-α (kits purchased from BD Biosciences). The limits of detection were 40 pg ml−1 IFN-γ, 30 pg ml−1 IL-10, 35 pg ml−1 IL-12 and 100 pg ml−1 TNF-α.
Antibody titers
Serum from infected mice was serially diluted and incubated in 96-well plates coated with 1 × 106 Listeria for 2 h. Plates were washed three times, probed with HRP-conjugated anti-IgG2c or anti-IgG1 (BD PharMingen) followed by 3.3′,5.5′-tetramethylbenzidine substrate (BD Biosciences) and analyzed on a HTS 7000 BioAssay Reader (PerkinElmer, Norwalk, CT, USA).
Statistical analysis
Results are expressed as the mean ± standard error of the mean. Experiments in young mice were performed twice with four mice per group and at each time point. Representative data from one experiment are shown. Experiments in old mice were performed once due to the high costs associated with housing mice for 19 months. Analysis was performed using unpaired t-tests and analysis of variances (Prism 4, GraphPad software, San Diego, CA, USA). Statistical significance is considered to be a value of P ≤0.05.
Results
Listeria monocytogenes is cleared from young VDR KO mice following both primary and secondary infections
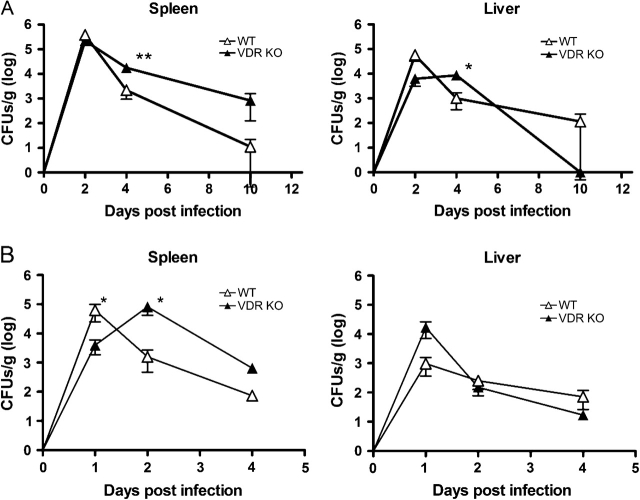
The clearance of L. monocytogenes was tracked in the spleen and liver of young VDR KO and WT mice. Except for day 2, post-infection weights of the spleen and liver of VDR KO and WT mice following primary infection were not significantly different (Supplementary Table S1, available at International Immunology Online). Two days following primary infection, WT and VDR KO mice had equal numbers of bacteria in the spleen (even when the organ weights are taken into account, Supplementary Table S1, available at International Immunology Online, and Fig. 1A). The kinetics of bacterial clearance in the spleen was faster in the WT compared with the VDR KO mice (Fig. 1A). In the liver, VDR KO mice had more Listeria than WT mice at day 4 (Fig. 1A). All VDR KO and WT mice had completely cleared the bacteria in the liver by day 10 post-infection and both groups of mice completely cleared the primary infection by day 14 post-infection (data not shown).
Fig. 1.
Bacterial CFU in the spleen and liver of young WT and VDR KO mice following primary and secondary infections with Listeria monocytogenes. (A) CFU in the spleen and liver following primary infection with 5 × 106 L. monocytogenes. (B) CFU in the spleen and liver following primary infection with 1 × 104 L. monocytogenes and secondary infection 30 days later with 5 × 106 L. monocytogenes. Values are mean ± standard error of the mean of four individual mice at each time point and one of two representative experiments. VDR KO value is significantly different than the corresponding WT value, *P ≤ 0.05, **P ≤ 0.001.
To determine if there were any differences in the recall response, young WT and VDR KO mice were infected with 104 Listeria organisms and then re-challenged 30 days later with 5 × 106 L. monocytogenes. The spleens of the VDR KO mice were significantly heavier at 1 and 2 days post-infection than their WT counterparts (Supplementary Table S1, available at International Immunology Online). The liver was also heavier at day 2 post-infection (Supplementary Table S1, available at International Immunology Online). As expected, clearance of the secondary challenge was faster than clearance of the primary infection in the WT mice (compare Fig. 1A and B). However, as in the primary infection, the kinetics of the response in VDR KO mice was delayed, with more L. monocytogenes recoverable from the spleen (significant) and liver (not significant) of VDR KO mice at 2 days post-infection than WT mice (Fig. 1B). Since the bacterial numbers are calculated per gram of tissue and as noted the spleen and liver of the VDR KO mice were larger than the WT at day 2 post-infection, the differences in bacterial burden at this time point between WT and VDR KO mice are amplified.
Cytokine and antibody responses of young VDR KO and WT mice following primary and secondary infection with L. monocytogenes
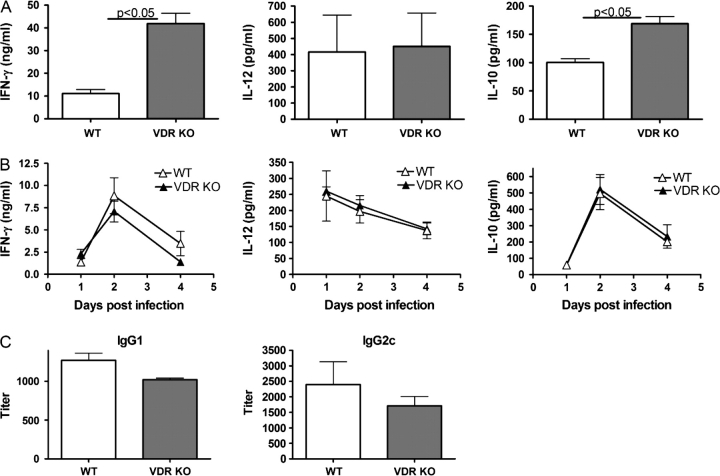
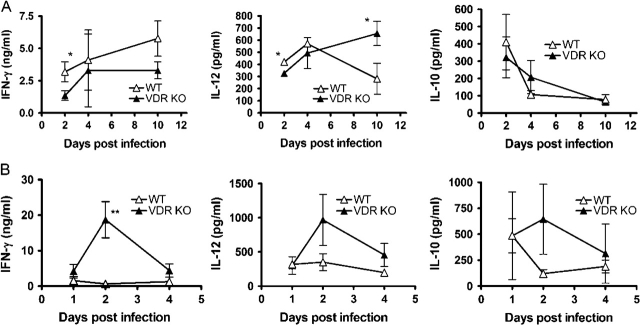
To examine the cytokine response, splenocytes were removed from VDR KO and WT mice cultured alone or re-stimulated in vitro with OVA, following a primary infection with L. monocytogenes, since the bacteria carry a transgene expressing OVA (15). In the absence of re-stimulation, splenocytes produced only low levels of cytokines in all groups (data not shown). OVA stimulation of naive splenocytes did not induce cytokine production (data not shown). Because the spleens of both the WT and VDR KO mice still contained Listeria, the stimulation with OVA was in effect done in the presence of Listeria, therefore inducing production of cytokines from innate immune cells and early T cell responses. Stimulation of the VDR KO and WT splenocytes with OVA induced the same amounts of IFN-γ at all time points except day 4, when VDR KO splenocytes produced significantly higher amounts of IFN-γ than those from WT mice (data not shown for days 2 and 10; Fig. 2A). These early differences may reflect the higher numbers of Listeria present in the VDR KO spleen at these time points. Serum IFN-γ levels were also measured following a primary Listeria infection. At all time points except day 2 post-infection, the levels of IFN-γ in the serum was below the limits of detection. At day 2 post-infection, WT mice had 1240 ± 64 pgml−1 and VDR KO mice had 1665 ± 306 pgml−1 IFN-γ in the serum, which was not significantly different. Production of IL-12 was similar in VDR KO and WT cultures, while IL-10 production was significantly increased in VDR KO cultures at all time points (Fig. 2A; day 2 and day 10 not shown). By contrast, the kinetics and the amounts of IFN-γ, IL-12 and IL-10 generated by OVA-stimulated splenocytes of young WT and VDR KO mice after secondary challenge were comparable at all time points (Fig. 2B). Low levels of IFN-γ were detected in the sera at day 1 post-secondary infection but were not different between WT and VDR KO mice with Listeria (data not shown). We also determined Listeria-specific IgG1 and IgG2c antibody titers in the sera of young VDR KO and WT mice 4 days after the secondary infection and there was no significant difference in the titers of Listeria-specific IgG1 and IgG2c antibodies between the two groups (Fig. 2C).
Fig. 2.
Peripheral immune responses following primary and secondary infection of young WT and VDR KO mice with Listeria monocytogenes. (A) IFN-γ, IL-12 and IL-10 production from whole splenocytes following primary infection and in vitro stimulation with OVA at 4 days post-infection. (B) IFN-γ, IL-12 and IL-10 production following secondary L. monocytogenes infection and in vitro stimulation with OVA. (C) Listeria-specific antibody responses in young VDR KO and WT mice following secondary L. monocytogenes infection. Values are mean ± standard error of the mean of four individual mice and one of two representative experiments. IFN-γ and IL-10 values from VDR KO mice were significantly higher than WT mice, P < 0.05.
Development of memory CD4 and CD8 T cells in young VDR KO mice and WT mice
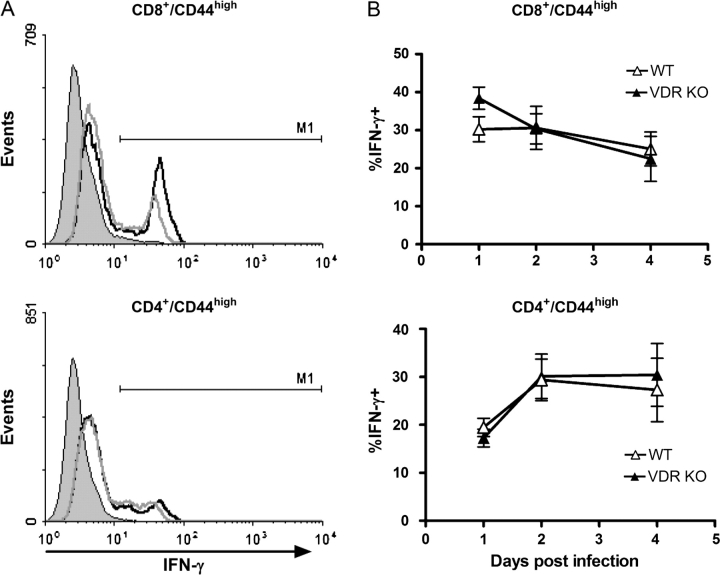
The memory T cell responses of young VDR KO and WT mice were measured following secondary infection with L. monocytogenes. Splenocytes from WT and VDR KO mice were stained for CD4, CD8 and the memory marker CD44. There were similar percentages of memory and naive CD4+ and CD8+ T cells in WT and VDR KO mice (Table 1). The percentage of IFN-γ-producing cells was determined in CD8+/CD44high and CD4+/CD44high T cells (Fig. 3). The percentages of IFN-γ-secreting memory CD8+ and CD4+ T cells were similar at all time points post-infection in the VDR KO and WT mice (Fig. 3B).
Table 1.
Memory T cells in young and old VDR KO and WT mice
| WT (%) | VDR KO (%) | |
| Young | ||
| CD8+/CD44high | 50 ± 5 | 42 ± 6 |
| CD4+/CD44high | 61 ± 4 | 63 ± 5 |
| Old | ||
| CD8+/CD44high | 26 ± 5 | 31 ± 16 |
| CD4+/CD44high | 42 ± 15 | 33 ± 7 |
Values are the mean ± standard error of the mean of n = 4.
Fig. 3.
IFN-γ secretion by memory T cells in young VDR KO and WT mice after secondary exposure to Listeria monocytogenes. Splenocytes were stained for IFN-γ, CD8, CD4 and CD44 following 1–4 days post-secondary infection. (A) CD8+/CD44high (top) or CD4+/CD44high (bottom) T cells were gated and the histograms showing staining for IFN-γ-producing cells presented. The solid peak shows the isotype control staining and IFN-γ expression of VDR KO (black line) and WT (gray line). (B) Average percentage of IFN-γ-producing CD8/CD44high (top) and CD4/CD44high (lower) T cells in young VDR KO and WT mice. Values are mean ± standard error of the mean of four individual mice. Experiments were repeated twice.
Old VDR KO and WT mice clear L. monocytogenes infection
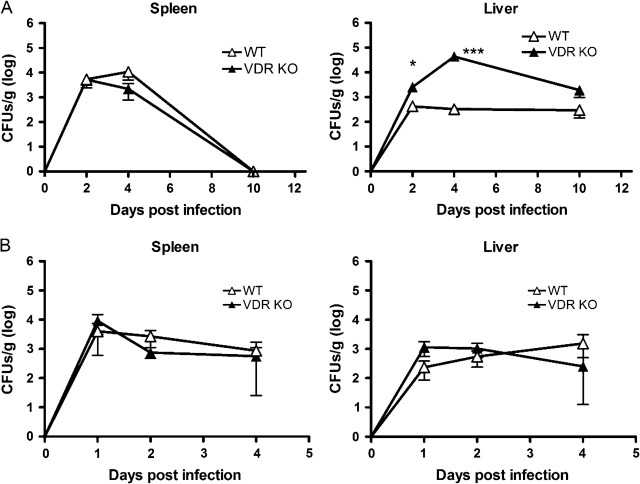
To determine if age affects the ability of VDR null mice to clear Listeria infection, 19-month-old female (old) VDR KO and WT mice were used for the next set of infections. Similar experiments were performed where old WT and VDR KO mice were infected with Listeria as done for the young mice. The spleens and livers of old VDR KO mice infected with Listeria weighed significantly more than their old WT counterparts at the early time points (day 2 and day 4, Supplementary Table S2, available at International Immunology Online). The CFUs per gram of spleen were the same in the old VDR KO and WT mice at day 2 and day 4 post-infection. However, old VDR KO mice had spleens that were twice as big as the old WT spleens and therefore had larger bacterial burdens at day 2 and day 4 post-infection (Fig. 4A and Supplementary Table S2, available at International Immunology Online). By day 10 post-infection, old WT and VDR KO mice cleared L. monocytogenes primary infections from the spleen (Fig. 4A). This was in contrast to the young VDR KO and WT mice, where L. monocytogenes was recoverable at day 10 post-primary infection (Fig. 1A). However, old WT and VDR KO mice showed persistent low grade colonization of the liver following a primary infection (Fig. 4A). Further analysis revealed that the liver of the old VDR KO mice was larger and had significantly more L. monocytogenes at day 2 and day 4 following primary infection, but not at day 10, than the old WT mice (Fig. 4A). Old VDR KO and old WT mice were able to completely clear the primary infection from the liver by 21 days post-infection (data not shown).
Fig. 4.
Bacterial CFU in the spleen and liver of old WT and VDR KO mice following primary and secondary infections with Listeria monocytogenes. (A) CFU in the spleen and liver following primary infection with 5 × 106 L. monocytogenes. (B) CFU in the spleen and liver following primary infection with 1 × 104 L. monocytogenes and secondary infections 30 days later with 5 × 106 L. monocytogenes. Values are mean ± standard error of the mean of three to four individual mice at each time point. VDR KO value is significantly different than the corresponding WT value, *P ≤ 0.05, **P ≤ 0.001, ***P ≤ 0.0001.
As described previously for young mice, additional old VDR KO and WT mice were challenged twice, first with a low dose and then with the challenge dose of L. monocytogenes. We found that in contrast to the results from young mice, secondary challenge of old WT and VDR KO mice did not result in faster clearance of L. monocytogenes compared with the primary infection (compare Fig. 4A and B). There were no differences in the weights of the spleens and livers from VDR KO and WT mice at any time points post-infection (Supplementary Table S2, available at International Immunology Online). The number of L. monocytogenes in the spleen of old mice at day 4 post-secondary infection was not different than at day 4 post-primary infection (Fig. 4). A similar picture was apparent in the livers of old WT mice where the numbers of L. monocytogenes were the same after 1, 2 or 4 days post-secondary and primary infection (Fig. 4). In addition, old VDR KO mice had fewer CFUs in the liver following a secondary Listeria infection compared with the primary infection (Fig. 4). However, there was 100% clearance of L. monocytogenes in the spleens and livers of all old VDR KO and WT mice by 21 days post-infection (data not shown).
Old VDR KO mice have heightened cytokine responses compared with old WT mice
The cytokine response of the old mice was determined following culture alone or re-stimulation in vitro with OVA. Cultures not stimulated with OVA produced undetectable amounts of cytokine. In addition to the OVA, all cultures contained Listeria organisms that had not yet been cleared from the spleens of either the WT or the VDR KO mice prior to the day 10 time point. Splenocytes from old WT and VDR KO mice produced similar levels of IFN-γ and IL-10 at all time points following primary infection. However, the IFN-γ response of the old mice (maximum of 6 ng ml−1) was less than that of young mice (maximum of 40 ng ml−1) following a primary L. monocytogenes infection (compare Figs 2A and 5A). At day 2 post-infection, old WT mice had 206 ± 56 pg ml−1 and old VDR KO mice had 176 ± 90 pg ml−1 IFN-γ in the serum, which was not significantly different. At other times post-infection, IFN-γ was undetectable. At 10 days post-infection, the IL-12 response of VDR KO mice remained high while the WT IL-12 response decreased significantly (Fig. 5A).
Fig. 5.
Cytokine production following primary and secondary infection of old WT and VDR KO mice with Listeria monocytogenes. Old WT and VDR KO mice were evaluated for cytokine production following primary and secondary L. monocytogenes infection. (A) IFN-γ, IL-12 and IL-10 production from whole splenocytes following primary infection and in vitro stimulation with OVA. (B) IFN-γ, IL-12 and IL-10 production following secondary L. monocytogenes infection and in vitro stimulation with OVA. Values are the mean ± standard error of the mean of n = 3–4 individual mice at each time point. VDR KO value is significantly different than the corresponding WT value, *P ≤ 0.05, **P ≤ 0.001.
Secondary infection of old WT mice with L. monocytogenes resulted in a blunted IFN-γ response that was also reflected in the IL-12 response (Fig. 5B). IL-10 responses of stimulated splenocytes from old WT mice were extremely variable but also appeared to show little change following secondary challenge (Fig. 5B). The levels of IL-12 and IL-10 were not significantly different between the two groups although at 2 days post-infection, the VDR KO splenocytes trended toward secreting higher levels of these cytokines. Low levels of IFN-γ were detected in the sera at day 1 post-secondary infection but were not different between WT and VDR KO mice with Listeria (data not shown). Old VDR KO mice secreted significantly more IFN-γ at 2 days post-infection than old WT mice (Fig. 5B). In addition, the old VDR KO mice produced more IFN-γ 2 days post-infection than young VDR KO mice (compare Figs 2B and 5B).
Old VDR KO mice produce high levels of antibody
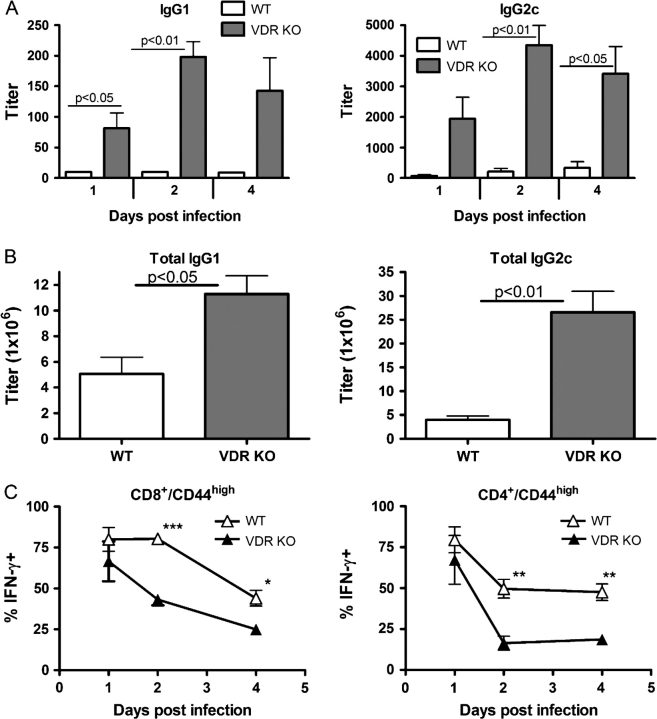
Antibody responses following secondary L. monocytogenes infection were measured in the old mice. Consistent with the literature, old WT mice showed a decreased ability to mount a recall response and produced less antigen-specific IgG1 and IgG2c than young WT mice (compare Figs 2C and 6A; P = 0.0002 and P = 0.052, respectively). Similarly, old VDR KO mice produced significantly less IgG1 than young VDR KO mice (compare Figs 2C and 6A; P = 0.0001). However, at all time points tested, old VDR KO mice produced significantly more Listeria-specific IgG1 and IgG2c compared with WT mice (Fig. 6A). To determine if the VDR KO mice had elevated levels of Listeria non-specific antibodies as well, the total amounts of IgG1 and IgG2c were also measured in old VDR KO and WT mice. We found that old VDR KO mice produced 2-fold more total IgG1 and 5-fold more total IgG2c than old WT mice (Fig. 6B).
Fig. 6.
B cell and T cell memory function in old VDR KO and WT mice following secondary challenge with Listeria monocytogenes. (A) Listeria-specific IgG1 and IgG2c responses following secondary challenge with L. monocytogenes. (B) Total IgG1 and IgG2c in old mice. C) Average percentage of IFN-γ-producing CD8/CD44high and CD4/CD44high T cells in old VDR KO and WT mice. Values are the mean ± standard error of the mean of n = 3–4 individual mice per time point. VDR KO value is significantly different than the corresponding WT value, *P ≤ 0.05, **P ≤ 0.001, ***P ≤ 0.0001.
Memory T cell response in old WT and VDR KO mice
Analysis of the percentage of memory CD4+ and CD8+ T cells (CD8+/CD44high or CD4+/CD44high) in the spleens of old WT and VDR KO mice revealed that there was no significant difference (Table 1). In addition, there were no differences in the number of splenocytes, CD4+ or CD8+ T cells in WT and VDR KO mice (data not shown). However, old WT mice had higher percentages of CD8+ and CD4+ memory T cells that produced IFN-γ compared with old VDR KO mice (Fig. 6C). Old WT mice had higher percentages of IFN-γ-producing CD44high-expressing T cells than their younger counterparts (compare Figs 3B and 6C at all time points except day 4 in CD4+ T cell compartment). Old VDR KO mice had higher percentages than young VDR KO mice early but not later post-infection (compare Figs 3B and 6C).
Discussion
VDR KO mice mount a strong primary and secondary immune response to L. monocytogenes infection that is adequate to completely clear infection in both young and old VDR KO mice. However, young VDR KO mice exhibited delayed L. monocytogenes clearance (compared with young WT mice) that was likely the result of higher levels of both IL-10 and IFN-γ early post-infection. IL-10 and IFN-γ have opposing effects on growth and clearance of L. monocytogenes (13, 18, 24). The early secretion of IL-10 (likely due to macrophage or other innate cells) in the VDR KO host likely reduced the efficacy of the IFN-γ response, leading to delayed kinetics in clearing infections in the spleen and liver. Interestingly, the generation of memory T and B cell responses occurred unimpeded in the VDR KO mice and therefore the observed delayed kinetics of clearance in young VDR KO versus young WT mice was less pronounced in the secondary challenge.
Acquired immunity decreases with age and although old VDR KO and WT mice took longer than young mice to clear a primary and secondary L. monocytogenes infection, they eventually resolved the infections. The age-related delay in clearance of a primary infection was associated with reduced amounts of IFN-γ secreted by the old mice. The increased secretion of IFN-γ by the VDR KO mice following secondary infection may explain the ability of these mice to clear the infection with kinetics that were more similar to WT mice. In addition, although the VDR KO mice had a lower frequency of memory cells secreting IFN-γ, they were able to secrete significantly more IFN-γ following stimulation, suggesting that the VDR KO memory T cells secreted more IFN-γ per cell.
Antibody responses, both antigen specific and non-specific, were higher in old VDR KO than WT mice. However, the high amounts of antibody produced in the old VDR KO mice did not correspond to improvements in host resistance to L. monocytogenes. Overall, the primary and secondary immune response of old VDR KO mice was as effective as that of old WT mice for clearance of L. monocytogenes. The high amounts of cytokines coupled with the large amounts of antibodies produced in the old VDR KO mice suggest that non-specific immune responses and autoimmunity are higher in the absence of the VDR. The heightened immune responses in old VDR KO mice are consistent with the previously described susceptibility of the VDR KO mice to experimental autoimmunity (8, 25).
Based on the effects of 1,25(OH)2D3 on Th1-driven immunity and the heightened IFN-γ and IL-12 responses in the VDR KO host, it is surprising that the VDR KO mice cleared Listeria infections with slower kinetics than their WT counterparts. NKT cells have been shown to be important innate immune cells during L. monocytogenes infection; NKT cell-deficient CD1d KO mice exhibit decreased liver IFN-γ production and increased susceptibility to Listeria infection (26). Approximately 30% of the liver mononuclear cells from WT mice are NKT cells, compared with only 6% NKT cells in VDR KO mice (7). In addition, the remaining NKT cells in the VDR KO mice are of an immature phenotype and produce low levels of cytokines including IFN-γ (7). Therefore, VDR KO mice are essentially NKT cell deficient, suggesting that the delayed kinetics of L. monocytogenes clearance following primary infection is likely a result of low amounts of NKT cell produced IFN-γ, which would predominately affect the response in the liver. 1,25(OH)2D3 differentially regulates IFN-γ production depending on the cell type that produces it. Paradoxically, 1,25(OH)2D3 enhances IFN-γ production from NKT cells and inhibits IFN-γ production from Th1 cells (3, 7), indicating a cell type-specific IFN-γ regulation by 1,25(OH)2D3. It appears that early during an immune response, 1,25(OH)2D3 induces IFN-γ production by NKT cells, followed by inhibition of IFN-γ along with other Th1-mediated immune responses.
The role of vitamin D, 1,25(OH)2D3 and the VDR in B cell development and function has not been extensively studied. B cells express the VDR depending on how they are stimulated and from where they are recovered (27). 1,25(OH)2D3 has been reported to act as a vaccine adjuvant at mucosal sites and to induce antigen-specific IgA (28, 29). More recently, 1,25(OH)2D3 has been shown to inhibit B cell proliferation in vitro depending on when it is added to the culture (27). In addition, memory B cell and plasma cell generation is inhibited by 1,25(OH)2D3 in cultures of human cells (27). Moreover, 1,25(OH)2D3 has been shown to suppress experimental systemic lupus in the MRL/MpJ mouse that is a model of a B cell-mediated autoimmune disorder (30). While we find that B cells from VDR KO mice generated normal amounts of Listeria-specific antibody in young mice, old VDR KO mice produced significantly higher levels of the IFN-γ-driven IgG2c and IL-4/IL-5-driven IgG1. In addition, there were high amounts of antigen-specific and total antibodies in the old VDR KO mice. Increased amounts of antibodies in old VDR KO mice suggest a role for the VDR and 1,25(OH)2D3 as a negative regulator of B cell antibody responses, and vitamin D may play a role in limiting the development of self-reactivity that occurs with aging.
Our data indicate that the expression of the VDR is not required for clearance of L. monocytogenes following either primary or secondary infection, although young VDR KO mice showed delayed kinetics of clearance that corresponded to early production of IL-10. There was a decline in the percentage of memory cells that developed with the age of the mice and VDR KO mice had fewer IFN-γ-producing memory T cells than WT mice. IFN-γ production, however, was higher in cells from old VDR KO mice than old WT mice. Consistent with the increased susceptibility of the VDR KO mice to various autoimmune diseases, old VDR KO mice over-produced IFN-γ, IL-12 and both antigen-specific and non-specific antibodies. Expression of the VDR is therefore critical for the inhibition of cytokine and antibody production but is not required for clearance of the intracellular pathogen L. monocytogenes.
Supplementary data
Supplementary tables S1 and S2 are available at International Immunology Online.
Funding
National Institute of Diabetes and Digestive and Kidney Diseases (DK070781 to M.T.C.).
Glossary
Abbreviations
- BHI
blood heart infusion
- CFU
colony-forming unit
- KO
knockout
- OVA
ovalbumin
- TNF
tumor necrosis factor
- VDR
vitamin D receptor
- WT
wild type
References
- 1.Veldman CM, Cantorna MT, DeLuca HF. Expression of 1,25-dihydroxyvitamin D(3) receptor in the immune system. Arch. Biochem. Biophys. 2000;374:334. doi: 10.1006/abbi.1999.1605. [DOI] [PubMed] [Google Scholar]
- 2.Lemire JM. Immunomodulatory role of 1,25-dihydroxyvitamin D3. J. Cell. Biochem. 1992;49:26. doi: 10.1002/jcb.240490106. [DOI] [PubMed] [Google Scholar]
- 3.Mahon BD, Wittke A, Weaver V, Cantorna MT. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J. Cell. Biochem. 2003;89:922. doi: 10.1002/jcb.10580. [DOI] [PubMed] [Google Scholar]
- 4.Cantorna MT, Hayes CE, DeLuca HF. 1,25-Dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proc. Natl Acad. Sci. USA. 1996;93:7861. doi: 10.1073/pnas.93.15.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cantorna MT, Munsick C, Bemiss C, Mahon BD. 1,25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J. Nutr. 2000;130:2648. doi: 10.1093/jn/130.11.2648. [DOI] [PubMed] [Google Scholar]
- 6.Helming L, Bose J, Ehrchen J, et al. 1alpha,25-Dihydroxyvitamin D3 is a potent suppressor of interferon gamma-mediated macrophage activation. Blood. 2005;106:4351. doi: 10.1182/blood-2005-03-1029. [DOI] [PubMed] [Google Scholar]
- 7.Yu S, Cantorna MT. The vitamin D receptor is required for iNKT cell development. Proc. Natl Acad. Sci. USA. 2008;105:5207. doi: 10.1073/pnas.0711558105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Froicu M, Weaver V, Wynn TA, McDowell MA, Welsh JE, Cantorna MT. A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Mol. Endocrinol. 2003;17:2386. doi: 10.1210/me.2003-0281. [DOI] [PubMed] [Google Scholar]
- 9.Wittke A, Weaver V, Mahon BD, August A, Cantorna MT. Vitamin D receptor-deficient mice fail to develop experimental allergic asthma. J. Immunol. 2004;173:3432. doi: 10.4049/jimmunol.173.5.3432. [DOI] [PubMed] [Google Scholar]
- 10.Ehrchen J, Helming L, Varga G, et al. Vitamin D receptor signaling contributes to susceptibility to infection with Leishmania major. FASEB J. 2007;21:3208. doi: 10.1096/fj.06-7261com. [DOI] [PubMed] [Google Scholar]
- 11.Mombaerts P, Arnoldi J, Russ F, Tonegawa S, Kaufmann SH. Different roles of alpha beta and gamma delta T cells in immunity against an intracellular bacterial pathogen. Nature. 1993;365:53. doi: 10.1038/365053a0. [DOI] [PubMed] [Google Scholar]
- 12.Ladel CH, Flesch IE, Arnoldi J, Kaufmann SH. Studies with MHC-deficient knock-out mice reveal impact of both MHC I- and MHC II-dependent T cell responses on Listeria monocytogenes infection. J. Immunol. 1994;153:3116. [PubMed] [Google Scholar]
- 13.Wagner RD, Maroushek NM, Brown JF, Czuprynski CJ. Treatment with anti-interleukin-10 monoclonal antibody enhances early resistance to but impairs complete clearance of Listeria monocytogenes infection in mice. Infect. Immun. 1994;62:2345. doi: 10.1128/iai.62.6.2345-2353.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foulds KE, Rotte MJ, Seder RA. IL-10 is required for optimal CD8 T cell memory following Listeria monocytogenes infection. J. Immunol. 2006;177:2565. doi: 10.4049/jimmunol.177.4.2565. [DOI] [PubMed] [Google Scholar]
- 15.Dudani R, Chapdelaine Y, Faassen Hv H, et al. Multiple mechanisms compensate to enhance tumor-protective CD8(+) T cell response in the long-term despite poor CD8(+) T cell priming initially: comparison between an acute versus a chronic intracellular bacterium expressing a model antigen. J. Immunol. 2002;168:5737. doi: 10.4049/jimmunol.168.11.5737. [DOI] [PubMed] [Google Scholar]
- 16.Berg RE, Crossley E, Murray S, Forman J. Memory CD8+ T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen. J. Exp. Med. 2003;198:1583. doi: 10.1084/jem.20031051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tripp CS, Kanagawa O, Unanue ER. Secondary response to Listeria infection requires IFN-gamma but is partially independent of IL-12. J. Immunol. 1995;155:3427. [PubMed] [Google Scholar]
- 18.Harty JT, Bevan MJ. Specific immunity to Listeria monocytogenes in the absence of IFN gamma. Immunity. 1995;3:109. doi: 10.1016/1074-7613(95)90163-9. [DOI] [PubMed] [Google Scholar]
- 19.Patel PJ. Aging and cellular defense mechanisms: age-related changes in resistance of mice to Listeria monocytogenes. Infect. Immun. 1981;32:557. doi: 10.1128/iai.32.2.557-562.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Effros RB, Walford RL. The immune response of aged mice to influenza: diminished T-cell proliferation, interleukin 2 production and cytotoxicity. Cell. Immunol. 1983;81:298. doi: 10.1016/0008-8749(83)90237-x. [DOI] [PubMed] [Google Scholar]
- 21.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat. Immunol. 2004;5:133. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 22.Goidl EA, Innes JB, Weksler ME. Immunological studies of aging. II. Loss of IgG and high avidity plaque-forming cells and increased suppressor cell activity in aging mice. J. Exp. Med. 1976;144:1037. doi: 10.1084/jem.144.4.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kyewski B, Wekerle H. Increase of T lymphocyte self-reactivity in aging inbred rats: in vitro studies with a model of experimental autoimmune orchitis. J. Immunol. 1978;120:1249. [PubMed] [Google Scholar]
- 24.Pasche B, Kalaydjiev S, Franz TJ, et al. Sex-dependent susceptibility to Listeria monocytogenes infection is mediated by differential interleukin-10 production. Infect. Immun. 2005;73:5952. doi: 10.1128/IAI.73.9.5952-5960.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Froicu M, Cantorna MT. Vitamin D and the vitamin D receptor are critical for control of the innate immune response to colonic injury. BMC Immunol. 2007;8:5. doi: 10.1186/1471-2172-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arrunategui-Correa V, Kim HS. The role of CD1d in the immune response against Listeria infection. Cell. Immunol. 2004;227:109. doi: 10.1016/j.cellimm.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Chen S, Sims GP, Chen XX, Gu YY, Lipsky PE. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J. Immunol. 2007;179:1634. doi: 10.4049/jimmunol.179.3.1634. [DOI] [PubMed] [Google Scholar]
- 28.Daynes RA, Enioutina EY, Butler S, Mu HH, McGee ZA, Araneo BA. Induction of common mucosal immunity by hormonally immunomodulated peripheral immunization. Infect. Immun. 1996;64:1100. doi: 10.1128/iai.64.4.1100-1109.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Der Stede Y, Verfaillie T, Cox E, Verdonck F, Goddeeris BM. 1alpha,25-dihydroxyvitamin D3 increases IgA serum antibody responses and IgA antibody-secreting cell numbers in the Peyer's patches of pigs after intramuscular immunization. Clin. Exp. Immunol. 2004;135:380. doi: 10.1111/j.1365-2249.2003.02377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deluca HF, Cantorna MT. Vitamin D: its role and uses in immunology. FASEB J. 2001;15:2579. doi: 10.1096/fj.01-0433rev. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.