Abstract
We found in a previous proteomic study dramatic differences of fumarase in the kidney between Dahl salt-sensitive rats and salt-insensitive consomic SS-13BN rats. Fumarase catalyzes the conversion between fumarate and L-malate in the tricarboxylic acid cycle. Little is known about the pathophysiological significance of fumarate metabolism in cardiovascular and renal function including salt-induced hypertension. The fumarase gene is located on the chromosome substituted in the SS-13BN rat. Sequencing of fumarase cDNA indicated the presence of lysine at amino acid position 481 in Dahl salt-sensitive rats and glutamic acid in Brown Norway and SS-13BN rats. Total fumarase activity was significantly lower in the kidneys of Dahl salt-sensitive rats compared to SS-13BN rats, despite an apparent compensatory increase in fumarase abundance in Dahl salt-sensitive rats. Intravenous infusion of a fumarate precursor in SS-13BN rats resulted in a fumarate excess in the renal medulla comparable to that seen in Dahl salt-sensitive rats. The infusion significantly exacerbated salt-induced hypertension in SS-13BN rats (140 ± 3 mmHg vs. 125 ± 2 mmHg in vehicle control at day 5 on a 4% NaCl diet, P<0.05). In addition, the fumarate infusion increased renal medullary tissue levels of H2O2. Treatment of cultured human renal epithelial cells with the fumarate precursor also increased cellular levels of H2O2. These data suggested a novel role for fumarate metabolism in salt-induced hypertension and renal medullary oxidative stress.
Keywords: hypertension, gene, tricarboxylic acid cycle, kidney, oxidative stress, rat
Introduction
The Dahl salt-sensitive (SS) rat is a genetic model of human salt-sensitive forms of hypertension.1,2 The consomic SS-13BN rat has the same genomic makeup as the SS rat except chromosome 13, which is introgressed from the Brown Norway (BN) rat and substantially attenuates salt-sensitive hypertension and renal injury.3
A tree-like network of molecular, biochemical, and physiological mechanisms is likely involved in the development of Dahl salt-sensitive hypertension and renal injury.4 Comparative analysis of SS and SS-13BN rats has revealed several new components of this regulatory network. Examples include increased levels of superoxide and H2O2,5,6 dysregulation of 11β-hydroxysteroid dehydrogenase, and alterations of glucocorticoid metabolism4 in the renal medulla of SS rats compared to SS-13BN rats. Additional mechanisms and particularly sequence variations of specific genes involved in the SS phenotypes remain to be discovered or validated.4,7,8,9
Fumarase was one of the proteins exhibiting dramatic differences between SS and SS-13BN rats according to a recent proteomic study.10 The analysis indicated a consistent and substantial difference in the isoelectric point of fumarase in SS and SS-13BN rats as reflected by a significant shift of the protein spot on two-dimensional gels. Fumarase catalyzes the reversible conversion between fumarate and L-malate in the tricarboxylic acid cycle in mitochondria. Rare loss-of-function mutations of fumarase in humans cause accumulation of fumarate and are associated with the development of hereditary leiomyomatosis, renal cell cancer, or encephalopathy.11,12,13 Little else is known about the pathophysiological significance of fumarase and fumarate metabolism in mammals.
In the present study, we discovered a sequence variation in fumarase between SS and BN alleles and demonstrated that fumarate excess exacerbated salt-induced hypertension and increased cellular levels of H2O2.
Methods and Materials
Animals
Male SS and consomic SS-13BN rats were obtained and maintained as described previously.3,4,9,10 Rats were fed a purified AIN-76A rodent diet (Dyets) containing 0.4% NaCl and had free access to water. The diet was switched to one containing 4% NaCl (Dyets) as required by specific protocols. Rats were 6 to 8 weeks old when entering the present study. The animal protocols were approved by the Institutional Animal Care and Use Committee at the Medical College of Wisconsin.
Molecular cloning and sequencing of fumarase
Molecular cloning and sequencing was performed essentially as we described previously.4,14,15 Briefly, for cDNA sequencing, total RNA was extracted from rat liver, reverse-transcribed using oligo-dT primer, and PCR amplified using the following primers: forward, 5’-ctttccatccacgtacagcagcacc-3’; reverse, 5’-taagtcactttggacccagcatgt-3’. The PCR product of approximately 1.5 kb was recovered and inserted into the T-easy vector (Promega). The plasmid was propagated in competent E. coli, extracted, and sequenced with three reads to obtain the fumarase cDNA sequence containing the complete protein-coding region. For the analysis of genomic DNA, the primers used to amplify a 658 bp segment of the fumarase gene were: forward, 5-ttggctaaaactctcccctcctt-3; reverse, 5-ttcacccactcatcaaactgctc-3. The forward primer binds to an intron of the gene, ensuring amplification from genomic DNA. A second set of primers were used for the sequencing reaction: forward, 5-aaaattttagtgctgccttaga-3; reverse, 5-ttcacccactcatcaaactgc-3.
Fumarase activity assay
Fumarase activity is conventionally measured in the direction of L-malic acid to fumaric acid due to the ease of the assay.16,17 Tissue specimens were homogenized and sonicated extensively in a cold Hepes solution (20 mM, pH 7.5, V/W 3:1). The homogenate was centrifuged at 500×g, 4°C, for 5 min, and then at 18,000×g, 4°C, for 10 min. Protein concentrations of the extracts were measured using the Bio-Rad Dc protein assay and adjusted to be the same across all samples. The reaction recipe was 20 µl tris-acetate (pH 7.5), 178 µl L-malic acid (50 mM prepared in tris-acetate, pH 7.5), and 2 µl of the tissue homogenate. Absorbance at 240 nm was monitored at 30 second intervals using the kinetic mode of a microplate reader.
Fumarate, L-malate, and succinate assay
Fumarate and L-malate levels were measured essentially as described previously.18 Tissue specimens were crushed in liquid nitrogen and homogenized in 3.5 volumes (V/W) of 8% perchloric acid in 40% ethanol. The homogenate was centrifuged at 40,000×g, 4°C, for 10 min. The pellet was extracted with 2.5 volumes of 6% perchloric acid and the supernatants combined. The extract was neutralized with 0.75 volumes of 3M K2CO3 with 0.5M triethanolamine and centrifuged. The supernatant was heated at 96°C for 20 min and centrifuged again. The final supernatant was stored at −80°C as the tissue extract. The reaction recipe consisted of 200 µl of the reaction buffer (400 mM hydrazine hydrate, 5 mM EDTA, 10 mM MgSO4, 100 mM Tris-acetate, pH 8.5), 2 µl of NAD (80 µg/µl), 2 µl of tissue extract, 15 units of NAD-malate dehydrogenase, and 3 units of fumarase. The chemicals and enzymes were from Sigma. Each tissue extract sample was assayed with three sets of duplicate reactions. The first set did not contain any enzymes. The second and the third sets contained malate dehydrogenase. The conversion of NAD to NADH was monitored using a fluorescent microplate reader with excitation and emission wavelengths of 360 nm and 465 nm, respectively, and operating on the kinetic mode. The reactions were incubated at room temperature for 60 min until the fluorescent intensity reached a plateau, indicating that the endogenous L-malate had been completely metabolized. Fumarase was then added to the third set of reactions, and the measurement of fluorescent signals in all the reactions continued. The difference between the reactions with both enzymes (the third set of reactions) and with malate dehydrogenase alone (the second set) represented the tissue level of fumarate. The difference between the reactions with malate dehydrogenase alone and without any enzymes (the first set) represented the tissue level of L-malate. Serial dilutions of fumarate and L-malate were used as standards. Succinate levels were measured using a Succinate Assay Kit (Megazyme) with modifications including the use of a microplate format and fluorescent monitoring.
Western blot
Western blotting was performed as described previously.4,10,19,20 Fumarase antibody was from Santa Cruz Biotechnology.
Chronic intravenous infusion and blood pressure measurement in conscious rats
Chronic instrumentation in conscious rats was performed as described previously.4,10,15,21 Briefly, an arterial catheter and an infusion catheter were implanted into the left femoral artery and vein, respectively. The venous line was continuously infused with normal saline at 8 µl/min. Rats were allowed one week to recover before the initiation of daily blood pressure recording through the arterial catheter. After a baseline period, normal saline was replaced by diethyl-fumarate or vehicle control at the concentrations described in the Results section.
H2O2 assay
Acid extraction of tissue or cell samples was performed as described above for the fumarate and L-malate assay. H2O2 levels in the neutralized acid extract were measured as catalase-inhibitable signals using an Amplex Red assay kit (Invitrogen) similar to that described previously.6
Cell culture
HK2 and HRE cells were obtained from and cultured as suggested by ATCC and Cambrex, respectively. HK2 cells are immortalized human kidney epithelial cells with characteristics of proximal tubular as well as medullary cells.10 HRE cells are primary cultures of human renal epithelial cells.
Statistics
Data were analyzed using student t-test or multiple-group analysis of variance. Data are shown as mean ± SEM.
Results
K481E Variation in Fumarase in SS and SS-13BN Rats
Fh1, the rat gene encoding fumarase is located on rat chromosome 13q25 at approximately 91,520,000 to 91,545,000 base pair positions (http://rgd.mcw.edu). Chromosome 13 is the only chromosome in SS-13BN rats that is of the BN origin.3
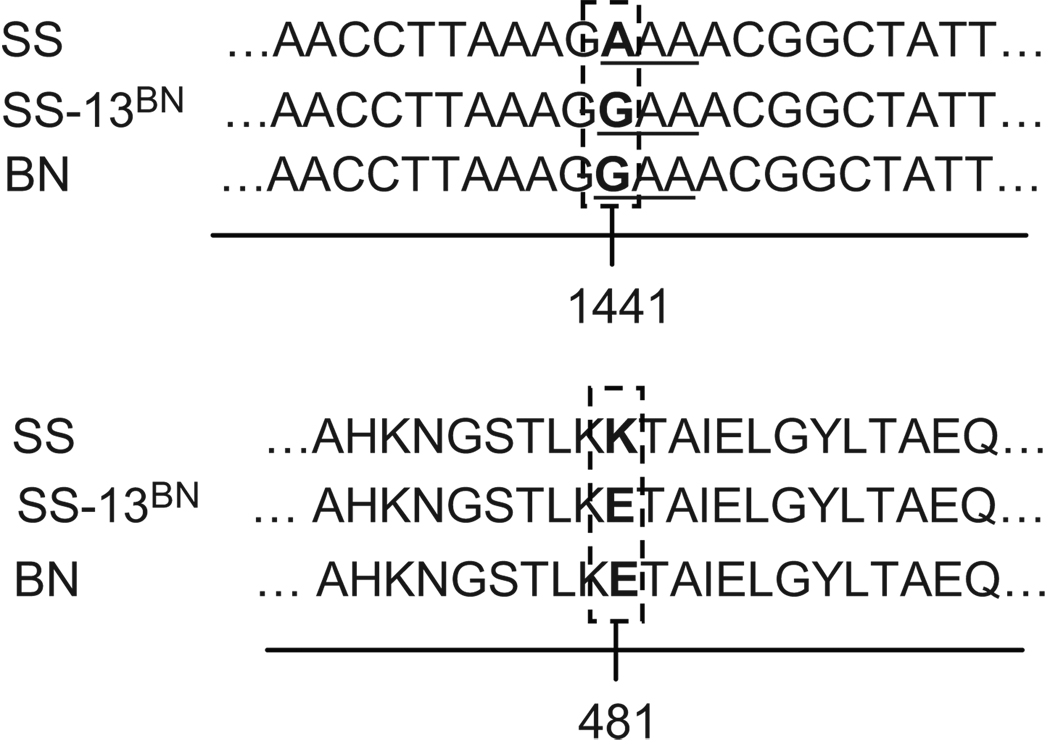
The entire protein-coding region of fumarase cDNA was cloned from SS, SS-13BN, and BN rats, and sequenced. The nucleotide at position 1441, relative to the start codon, was A in SS but G in SS-13BN and BN (Figure 1). The difference was confirmed in 5 SS rats, 4 SS-13BN rats, and 3 BN rats. Rats of each strain were derived from multiple breeding pairs. The rest of the cDNA sequence was identical across the three rat strains. The same nucleotide difference was found in genomic DNA. The nucleotide difference at 1441 would be translated into lysine (K) in SS fumarase but glutamic acid (E) in SS-13BN or BN fumarase at the amino acid position 481 (Figure 1).
Figure 1. An amino acid difference between SS and BN fumarase.
Sequencing of the entire protein-coding region of fumarase cDNA indicated a single nucleotide difference between SS rats and SS-13BN and BN rats. The codon AAA in SS fumarase encodes lysine (K), while the codon GAA in SS-13BN and BN fumarase encodes glutamic acid (E).
Fumarate Excess in SS Rats
Fumarase catalytic activity was measured in the renal medulla of 6 week old SS and SS-13BN rats on the 0.4% NaCl diet. The renal medulla is a kidney region that has been shown to play an important role in long-term blood pressure regulation and the development of Dahl salt-sensitive hypertension.22,23
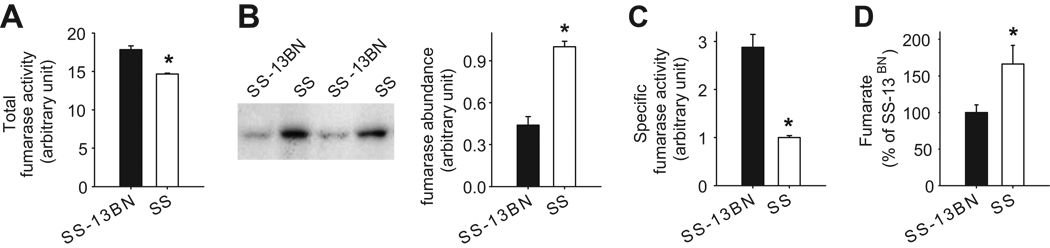
Fumarase activity was consistently and significantly higher in SS-13BN rats than in SS rats by 22% (n=4, P<0.05) (Figure 2A). The protein abundance of fumarase, estimated by Western blotting, was 2.3 folds higher in SS (Figure 2B). The specific activity of fumarase, calculated as total activity normalized by fumarase protein abundance, was 2.9 folds higher in SS-13BN (Figure 2C).
Figure 2. Lower fumarase activity and higher fumarate levels in the renal medulla of SS rats compared to SS-13BN rats.
A. Total fumarase activity was lower in SS rats than in SS-13BN rats. Fumarase activity was measured in renal medullary homogenate in 6 week old, male SS and SS-13BN rats maintained on the 0.4% NaCl diet. n=4, *, P<0.05. B. Fumarase abundance was higher in SS rats than in SS-13BN rats. Fumarase abundance was measured by Western blotting in the same rats used for the activity assay. n=4, *, P<0.05. C. Specific activity of fumarase was much lower in SS rats than in SS-13BN rats. Fumarase activity shown in panel A was normalized by fumarase abundance shown in panel B for each individual rat. n=4, *, P<0.05. D. Fumarate levels were higher in SS rats than in SS-13BN rats. Fumarate levels were measured in acid extracts of renal medulla tissues from rats similar to those described above. n=5, *, P<0.05.
Fumarate and L-malate are the substrate and the product of fumarase in the tricarboxylic acid cycle, respectively. Levels of fumarate and L-malate were measured in acid extracts of the renal medulla. Fumarate levels in the renal medulla, normalized by tissue weight, were 50% higher in SS rats than in SS-13BN rats (166 ± 26 pmol/mg tissue in SS vs. 111 ± 11 pmol/mg tissue in SS-13BN, n=5, P<0.05) (Figure 2D). L-malate levels were slightly lower in SS rats (1500 ± 23 pmol/mg tissue in SS vs. 1611 ± 40 pmol/mg tissue in S S-13BN, n=5, P<0.05). Succinate precedes fumarate in the tricarboxylic acid cycle. Succinate levels in the renal medulla were not significantly different between SS and SS-13BN rats (100 ± 23% in SS vs. 91 ± 25% in SS-13BN, n=5, NS).
The differential patterns of fumarase activity and abundance in the renal cortex were similar to those in the renal medulla. Fumarase activity in the renal cortex was significantly higher in SS-13BN rats than in SS rats by 28 ± 2% (n=4, P<0.05). Fumarase abundance was significantly higher in SS rats than in SS-13BN rats by 1.7 ± 0.2 folds (n=4, P<0.05). The specific fumarase activity in the cortex, normalized by Western blot density, was 2.5 ± 0.4 folds higher in SS-13BN. Fumarate levels in the cortex, however, were not significantly different between SS (100 ± 11%) and SS-13BN (111 ± 15%, n=5, NS).
Infusion of fumarate exacerbated salt-induced hypertension in SS-13BN rats
SS-13BN rats were prepared for chronic intravenous infusion and blood pressure measurement. Three groups of rats were studied. After two days of stable baseline recording, one group of rats received a vehicle infusion (0.5% ethanol in normal saline) (n=6), and another group received an intravenous infusion of diethyl fumarate at the dose of 0.2 mmol/kg body weight/day (n=7), while the third group received diethyl-fumarate at the dose of 1 mmol/kg/day (n=5). Diethyl-fumarate is cell membrane-permeable and can be converted to fumarate in the cell. Seven days later, the dietary salt content was increased from 0.4% to 4%, and the intravenous infusion and blood pressure recording were continued for another 5 days.
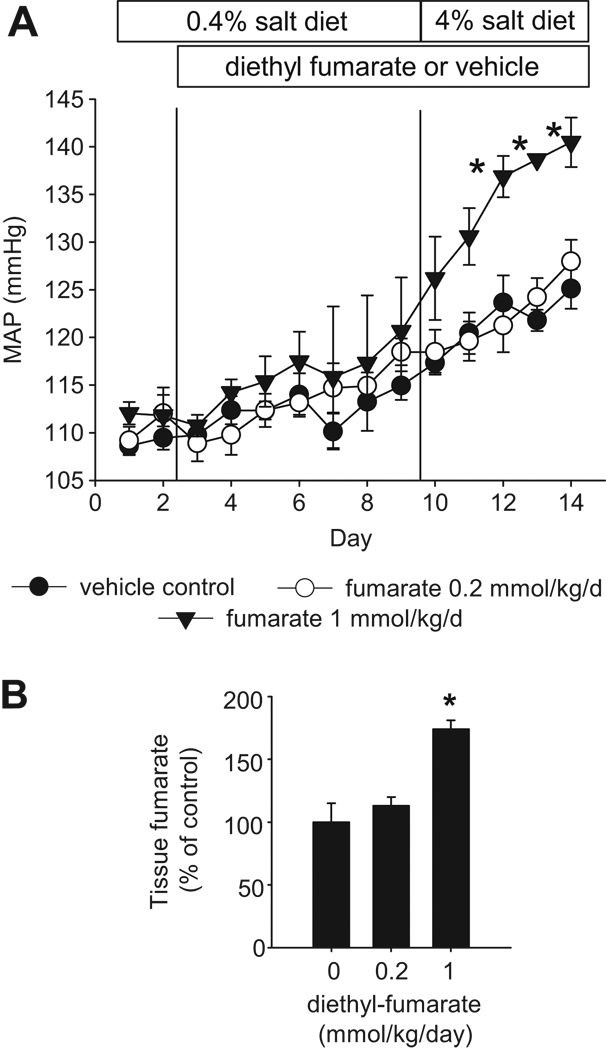
As shown in Figure 3A, intravenous infusion of diethyl fumarate at 1 mmol/kg/day significantly exacerbated high salt-induced hypertension in SS-13BN rats. Mean arterial pressure (MAP) was increased by about 10 mmHg after 5 days on the 4% NaCl diet in control rats and rats receiving diethyl-fumarate at 0.2 mmol/kg/day. The increase of MAP was approximately 20 mmHg in rats receiving 1 mmol/kg/day of diethyl-fumarate.
Figure 3. Intravenous infusion of diethyl-fumarate exacerbated salt-induced hypertension in SS-13BN rats.
Diethyl-fumarate, a cell membrane-permeable precursor of fumarate, was infused intravenously to chronically instrumented SS-13BN rats at 0.2 or 1 mmol/kg body weight/day (n=7 and 5, respectively). Vehicle control (n=6) was 0.5% ethanol in normal saline. A. Diethyl-fumarate at 1 mmol/kg/day significantly exacerbated hypertension induced by a 4% NaCl diet. n=5–7, *, P<0.05 vs. vehicle control. B. Diethyl-fumarate at 1 mmol/kg/day significantly increased renal medullary levels of fumarate. n=5–7, *, P<0.05 vs. vehicle control.
Concomitantly, tissue fumarate levels in the renal medulla were 74% higher in rats receiving diethyl-fumarate at 1 mmol/kg/day (n=5–7, P<0.05) (Figure 3B). The increase in renal medullary levels of fumarate resulting from the infusion of diethyl-fumarate resembled the higher level of fumarate observed in the renal medulla of SS rats compared to SS-13BN rats (see Figure 2D). Diethyl-fumarate at the dose of 0.2 mmol/kg/day did not significantly affect renal medullary levels of fumarate, probably because fumarase in SS-13BN rats was sufficient for metabolizing fumarate infused at the lower dose. Fumarase protein abundance in the renal medulla receiving the infusion of 1 mmol/kg/day of diethyl-fumarate was not significantly different from control (100 ± 9% in control vs. 105 ± 11% in treated, NS).
Fumarate increased H2O2 in vivo and in cultured human cells
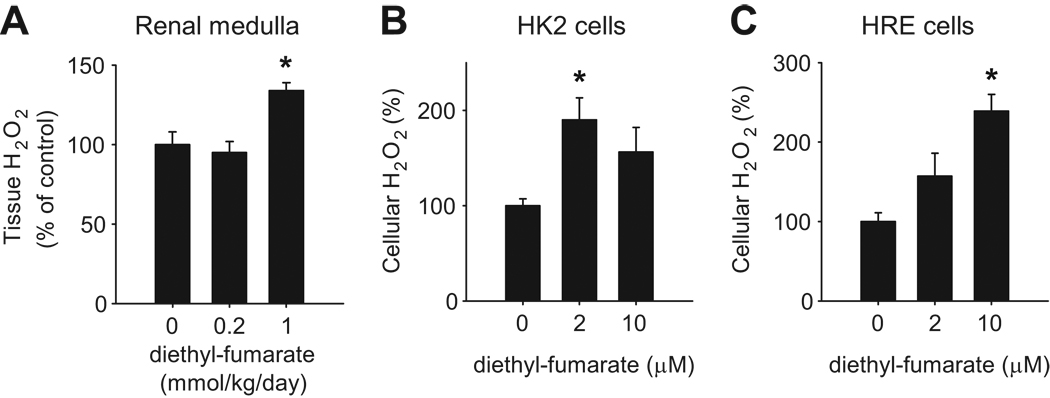
The renal medulla of SS rats has been shown to contain higher levels of H2O2 than SS-13BN rats, which contributes to salt-induced hypertension.6 Interestingly, renal medullary levels of H2O2 were significantly elevated in SS-13BN rats receiving diethyl-fumarate at 1 mmol/kg/day (Figure 4A).
Figure 4. Diethyl-fumarate increased H2O2 levels in vivo and in vitro.
A. SS-13BN rats receiving diethyl-fumarate at 1 mmol/kg/day as described in Figure 3 exhibited higher levels of H2O2 in the renal medulla. n=5–7, *, P<0.05 vs. vehicle control. B. Treatment of HK2 cells, a human kidney cell line, with diethyl-fumarate at 2 µM significantly increased cellular levels of H2O2. n=4, *, P<0.05 vs. vehicle. C. Treatment of HRE cells, a primary culture of human renal epithelial cells, with diethyl-fumarate at 10 µM significantly increased cellular levels of H2O2. n=4, *, P<0.05 vs. vehicle.
The effect of diethyl-fumarate on H2O2 levels was confirmed in cultured human renal epithelial cells. Cellular levels of H2O2 were increased significantly in HK2 cells treated with 2 µM diethyl-fumarate and in HRE cells treated with 10 µM diethyl-fumarate (Figure 4B, 4C).
Discussion
The present study discovered an amino acid sequence difference in fumarase between SS and BN alleles, demonstrated a novel functional role of fumarate metabolism in salt-induced hypertension, and provided insights into possible mechanisms.
The K481E amino acid difference was the first amino acid variation identified between SS and SS-13BN rats. The variation likely contributes to the much higher specific activity of fumarase in SS-13BN rats. The differences in fumarase activity and abundance were observed in both the renal medulla and the renal cortex, which is consistent with a genetically determined mechanism. It is possible, however, that the amino acid difference leads to additional differences in protein modification that collectively cause the change in catalytic activity. The DIGE analysis indicated the presence of at least five fumarase spots, with spots in SS having more basic isoelectric points than SS-13BN.10 Bioinformatic analysis indicated that the single amino acid difference was consistent with, but might not be sufficient to explain, the shift of isoelectric point. In addition, fumarate levels were higher in SS rats only in the renal medulla, and fumarate infusion did not induce a compensatory increase in fumarase abundance in the medulla of SS-13BN rats. These data suggest that fumarate metabolism might be regulated by multiple mechanisms. The precise effect of the K481E variation on fumarase structure and fumarate metabolism warrants further analysis. Moreover, gene transfer study would be required to determine the definitive role of the K481E variation in the SS phenotypes.
Abnormalities in the tricarboxylic acid cycle and related metabolic pathways might represent an important mechanism of blood pressure regulation that is not yet fully appreciated. It has been shown that excess succinate, which is upstream of fumarate in the tricarboxylic acid cycle, can cause hypertension in mice.24 We have shown in the present study that excess of fumarate could dose-dependently exacerbate salt-induced hypertension in rats. The importance of maintaining normal fumarate metabolism was supported by the apparent compensatory increase in fumarase abundance in SS rats. The increase in abundance, however, was not sufficient to bring total fumarase activity up to the level seen in SS-13BN rats. It would be interesting to determine whether fumarate excess affects the levels of other intermediates of the tricarboxylic acid cycle, although succinate levels in the renal medulla were not significantly different between SS and SS-13BN rats. In humans, fumarase mutations cause fibroid syndromes, and hypertension is associated with an increased risk of fibroids.25 Alterations of other metabolic pathways may also be involved in the development of Dahl salt-sensitive hypertension. For example, the renal proteomic differences between SS and SS-13BN rats suggested an up-regulation of fatty acid metabolism in SS rats.10
Metabolic abnormalities might contribute to SS phenotypes through changes in bioenergetics or mechanisms other than bioenergetics. Production of reactive oxygen species might be one of such mechanisms. It has been shown that the renal medulla of SS rats had higher levels of reactive oxygen species including superoxide anions and H2O2.5,6 Pharmacological inhibition of oxidative stress, including reduction of superoxide and H2O2 specifically in the renal medulla, attenuates salt-induced hypertension in SS rats.5,6,26 The present study provided in vivo and in vitro evidence suggesting that excess fumarate might contribute to elevation of reactive oxygen species. Increase of reactive oxygen species has been proposed as a possible link between fumarase mutations and tumorigenesis.27 Fumarase deficiencies have been shown to block succinate dehydrogenase, presumably through fumarate accumulation.11 Succinate dehydrogenase deficiencies could cause an increase in superoxide anion.27 In addition, fumarase C in E. coli is part of the soxRS regulon of antioxidant defense.28,29
Fumarate excess has also been shown to inhibit prolyl hydroxylase and stabilize hypoxia-inducible factor 1α (HIF1α).18,30 It has been suggested that elevation of HIF1α might contribute to the development of tumors in patients with fumarase mutations. It remains to be determined if HIF1α stabilization contributes to the role of fumarate excess in salt-induced hypertension and renal injury. The concentrations of diethyl-fumarate that increased H2O2 levels in cultured cells shown in the present study were lower than or at the low end of the concentrations previously reported to increase HIF1α.18 Patients with fumarase mutations often exhibit greater than 50% loss of fumarase activity.11,12 The difference of fumarase activity between SS and SS-13BN rats was more modest and the effect of these differences on HIF1α is unknown.
Perspectives
Abnormalities in fumarate metabolism appear to be a novel mechanism contributing to oxidative stress and salt-induced hypertension. The findings suggest several new directions of future research that could further delineate the regulatory tree4 underlying the molecular pathophysiology of hypertension.
Acknowledgments
Sources of Funding
The study was supported by NIH R01 HL077263 (M.L.), N01-HV-28182 (A.S.G.), P01 HL082798 (A.W.C.), and P01 HL29587 (A.W.C.).
Footnotes
Disclosures
None
References
- 1.Rapp JP. Dahl salt-susceptible and salt-resistant rats. Hypertension. 1982;4:753–763. doi: 10.1161/01.hyp.4.6.753. [DOI] [PubMed] [Google Scholar]
- 2.Tobian L. Salt and hypertension. Lessons from animal models that relate to human hypertension. Hypertension. 1991;17:152–158. doi: 10.1161/01.hyp.17.1_suppl.i52. [DOI] [PubMed] [Google Scholar]
- 3.Cowley AW, Jr, Roman RJ, Kaldunski ML, Dumas P, Dickhout JG, Greene AS, Jacob HJ. Brown Norway chromosome 13 confers protection from high salt to consomic Dahl S rat. Hypertension. 2001;37:456–461. doi: 10.1161/01.hyp.37.2.456. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y, Singh RJ, Usa K, Netzel BC, Liang M. Renal medullary 11 beta-hydroxysteroid dehydrogenase type 1 in Dahl salt-sensitive hypertension. Physiol Genomics. 2008;36:52–58. doi: 10.1152/physiolgenomics.90283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor NE, Glocka P, Liang M, Cowley AW., Jr NADPH oxidase in the renal medulla causes oxidative stress and contributes to salt-sensitive hypertension in Dahl S rats. Hypertension. 2006;47:692–698. doi: 10.1161/01.HYP.0000203161.02046.8d. [DOI] [PubMed] [Google Scholar]
- 6.Taylor NE, Cowley AW., Jr Effect of renal medullary H2O2 on salt-induced hypertension and renal injury. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1573–R1579. doi: 10.1152/ajpregu.00525.2005. [DOI] [PubMed] [Google Scholar]
- 7.Rapp JP. Genetic analysis of inherited hypertension in the rat. Physiol Rev. 2000;80:135–172. doi: 10.1152/physrev.2000.80.1.135. [DOI] [PubMed] [Google Scholar]
- 8.Cowley AW., Jr The genetic dissection of essential hypertension. Nat Rev Genet. 2006;7:829–840. doi: 10.1038/nrg1967. [DOI] [PubMed] [Google Scholar]
- 9.Liang M, Lee NH, Wang H, Greene AS, Kwitek AE, Kaldunski ML, Luu TV, Frank BC, Bugenhagen S, Jacob HJ, Cowley AW., Jr Molecular networks in Dahl salt-sensitive hypertension based on transcriptome analysis of a panel of consomic rats. Physiol Genomics. 2008;34:54–64. doi: 10.1152/physiolgenomics.00031.2008. [DOI] [PubMed] [Google Scholar]
- 10.Tian Z, Greene AS, Usa K, Matus IR, Bauwens J, Pietrusz JL, Cowley AW, Jr, Liang M. Renal regional proteomes in young Dahl salt-sensitive rats. Hypertension. 2008;51:899–904. doi: 10.1161/HYPERTENSIONAHA.107.109173. [DOI] [PubMed] [Google Scholar]
- 11.Bourgeron T, Chretien D, Poggi-Bach J, Doonan S, Rabier D, Letouzé P, Munnich A, Rötig A, Landrieu P, Rustin P. Mutation of the fumarase gene in two siblings with progressive encephalopathy and fumarase deficiency. J Clin Invest. 1994;93:2514–2518. doi: 10.1172/JCI117261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomlinson IP, Alam NA, Rowan AJ, Barclay E, Jaeger EE, Kelsell D, Leigh I, Gorman P, Lamlum H, Rahman S, Roylance RR, Olpin S, Bevan S, Barker K, Hearle N, Houlston RS, Kiuru M, Lehtonen R, Karhu A, Vilkki S, Laiho P, Eklund C, Vierimaa O, Aittomäki K, Hietala M, Sistonen P, Paetau A, Salovaara R, Herva R, Launonen V, Aaltonen LA. Multiple Leiomyoma Consortium. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet. 2002;30:406–410. doi: 10.1038/ng849. [DOI] [PubMed] [Google Scholar]
- 13.Eng C, Kiuru M, Fernandez MJ, Aaltonen LA. A role for mitochondrial enzymes in inherited neoplasia and beyond. Nat Rev Cancer. 2003;3:193–202. doi: 10.1038/nrc1013. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Park F, Pietrusz JL, Jia G, Singh RJ, Netzel BC, Liang M. Suppression of 11beta-hydroxysteroid dehydrogenase type 1 with RNA interference substantially attenuates 3T3-L1 adipogenesis. Physiol Genomics. 2008;32:343–351. doi: 10.1152/physiolgenomics.00067.2007. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Mladinov D, Pietrusz JL, Usa K, Liang M. Glucocorticoid response elements and 11 beta-hydroxysteroid dehydrogenases in the regulation of endothelial nitric oxide synthase expression. Cardiovasc Res. 2009;81:140–147. doi: 10.1093/cvr/cvn231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beeckmans S, Van Driessche E. Pig heart fumarase contains two distinct substrate-binding sites differing in affinity. J Biol Chem. 1998;273:31661–31669. doi: 10.1074/jbc.273.48.31661. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka KR, Valentine WN. Fumarase activity of human leukocytes and erythrocytes. Blood. 1961;17:328–333. [PubMed] [Google Scholar]
- 18.Koivunen P, Hirsilä M, Remes AM, Hassinen IE, Kivirikko KI, Myllyharju J. Inhibition of hypoxia-inducible factor (HIF) hydroxylases by citric acid cycle intermediates: possible links between cell metabolism and stabilization of HIF. J Biol Chem. 2007;282:4524–4532. doi: 10.1074/jbc.M610415200. [DOI] [PubMed] [Google Scholar]
- 19.Liang M, Pietrusz JL. Thiol-related genes in diabetic complications: a novel protective role for endogenous thioredoxin 2. Arterioscler Thromb Vasc Biol. 2007;27:77–83. doi: 10.1161/01.ATV.0000251006.54632.bb. [DOI] [PubMed] [Google Scholar]
- 20.Tian Z, Greene AS, Pietrusz JL, Matus IR, Liang M. MicroRNA-target pairs in the rat kidney identified by microRNA microarray, proteomic, and bioinformatics analysis. Genome Res. 2008;18:404–411. doi: 10.1101/gr.6587008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Usa K, Singh RJ, Netzel BC, Liu Y, Raff H, Liang M. Renal interstitial corticosterone and 11-dehydrocorticosterone in conscious rats. Am J Physiol Renal Physiol. 2007;293:F186–F192. doi: 10.1152/ajprenal.00484.2006. [DOI] [PubMed] [Google Scholar]
- 22.Cowley AW., Jr Role of the renal medulla in volume and arterial pressure regulation. Am J Physiol. 1997;273:R1–R15. doi: 10.1152/ajpregu.1997.273.1.R1. [DOI] [PubMed] [Google Scholar]
- 23.Cowley AW., Jr Renal medullary oxidative stress, pressure-natriuresis, and hypertension. Hypertension. 2008;52:777–786. doi: 10.1161/HYPERTENSIONAHA.107.092858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He W, Miao FJ, Lin DC, Schwandner RT, Wang Z, Gao J, Chen JL, Tian H, Ling L. Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature. 2004;429:188–193. doi: 10.1038/nature02488. [DOI] [PubMed] [Google Scholar]
- 25.Okolo S. Incidence, aetiology and epidemiology of uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2008;22:571–588. doi: 10.1016/j.bpobgyn.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Meng S, Cason GW, Gannon AW, Racusen LC, Manning RD., Jr Oxidative stress in Dahl salt-sensitive hypertension. Hypertension. 2003;41:1346–1352. doi: 10.1161/01.HYP.0000070028.99408.E8. [DOI] [PubMed] [Google Scholar]
- 27.Rustin P. Mitochondria, from cell death to proliferation. Nat Genet. 2002;30:352–353. doi: 10.1038/ng0402-352. [DOI] [PubMed] [Google Scholar]
- 28.Liochev SI, Fridovich I. Fumarase C, the stable fumarase of Escherichia coli, is controlled by the soxRS regulon. Proc Natl Acad Sci USA. 1992;89:5892–5896. doi: 10.1073/pnas.89.13.5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park SJ, Gunsalus RP. Oxygen, iron, carbon, and superoxide control of the fumarase fumA and fumC genes of Escherichia coli: role of the arcA, fnr, and soxR gene products. J Bacteriol. 1995;177:6255–6262. doi: 10.1128/jb.177.21.6255-6262.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isaacs JS, Jung YJ, Mole DR, Lee S, Torres-Cabala C, Chung YL, Merino M, Trepel J, Zbar B, Toro J, Ratcliffe PJ, Linehan WM, Neckers L. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell. 2005;8:143–153. doi: 10.1016/j.ccr.2005.06.017. [DOI] [PubMed] [Google Scholar]