Abstract
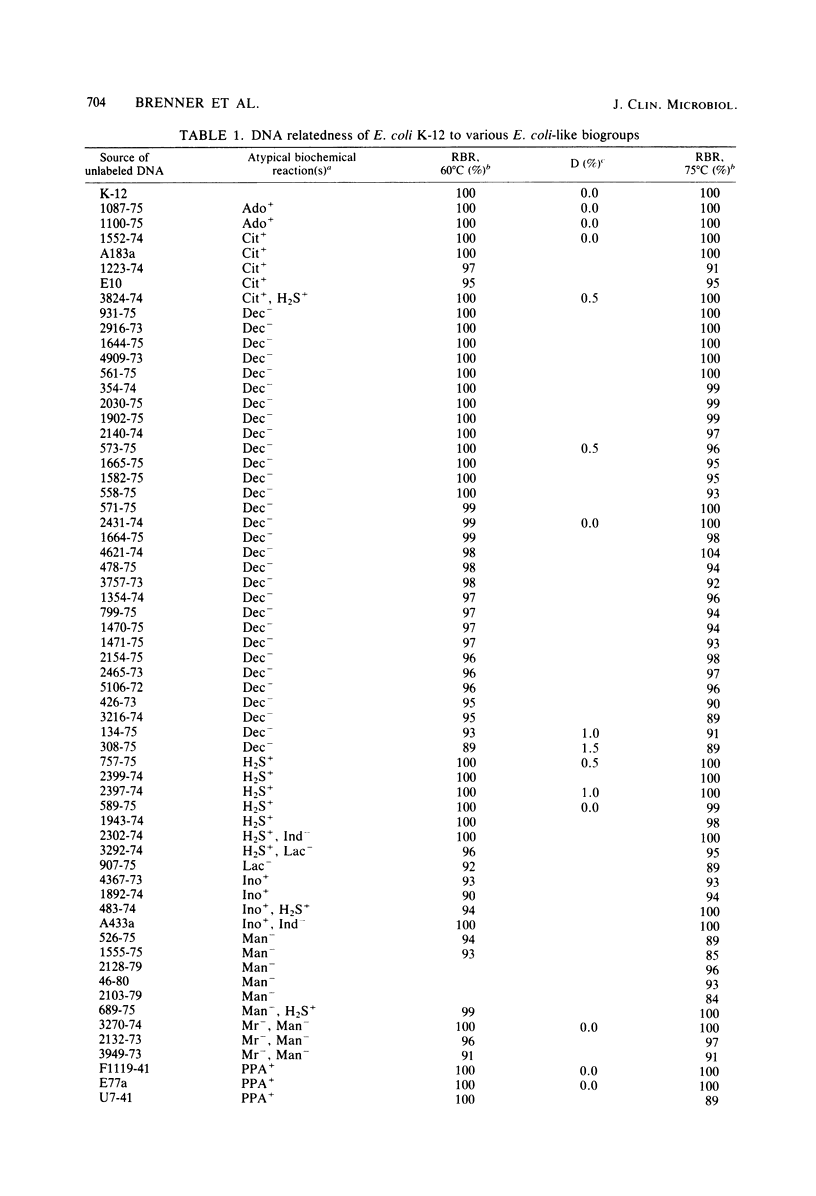
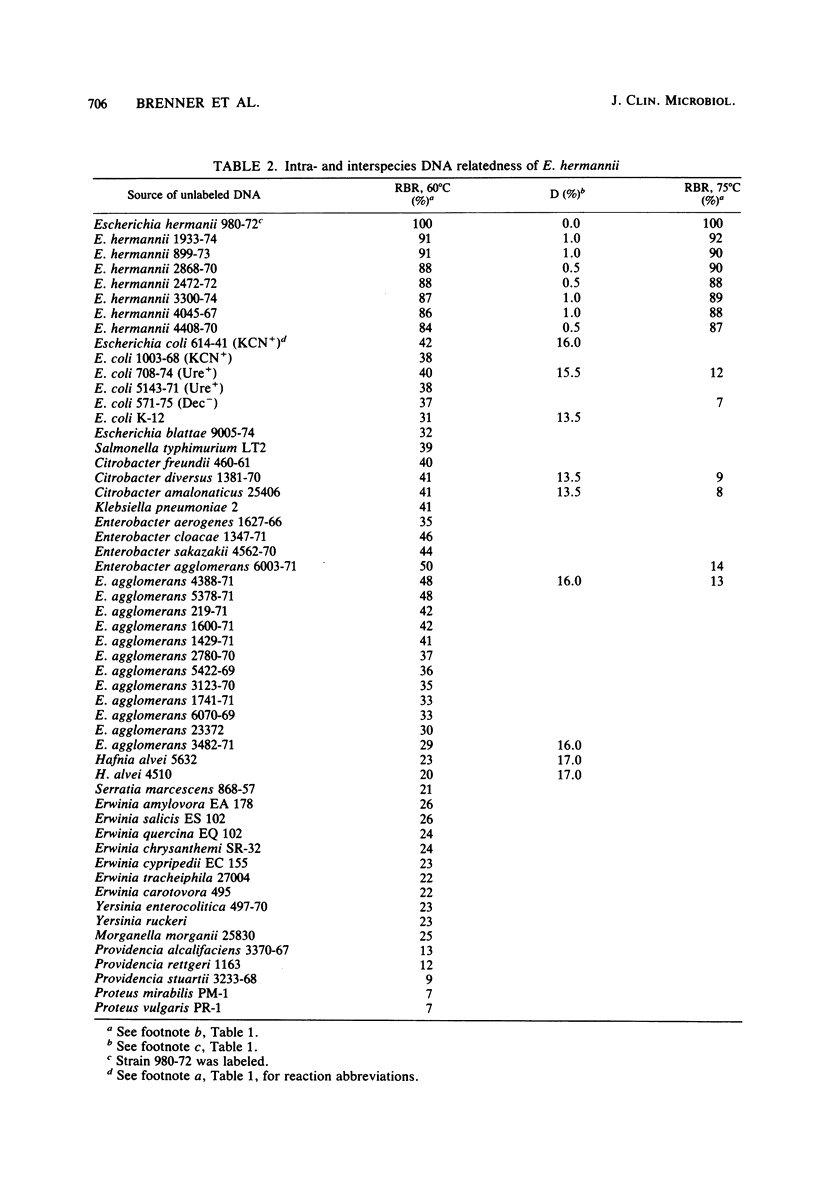
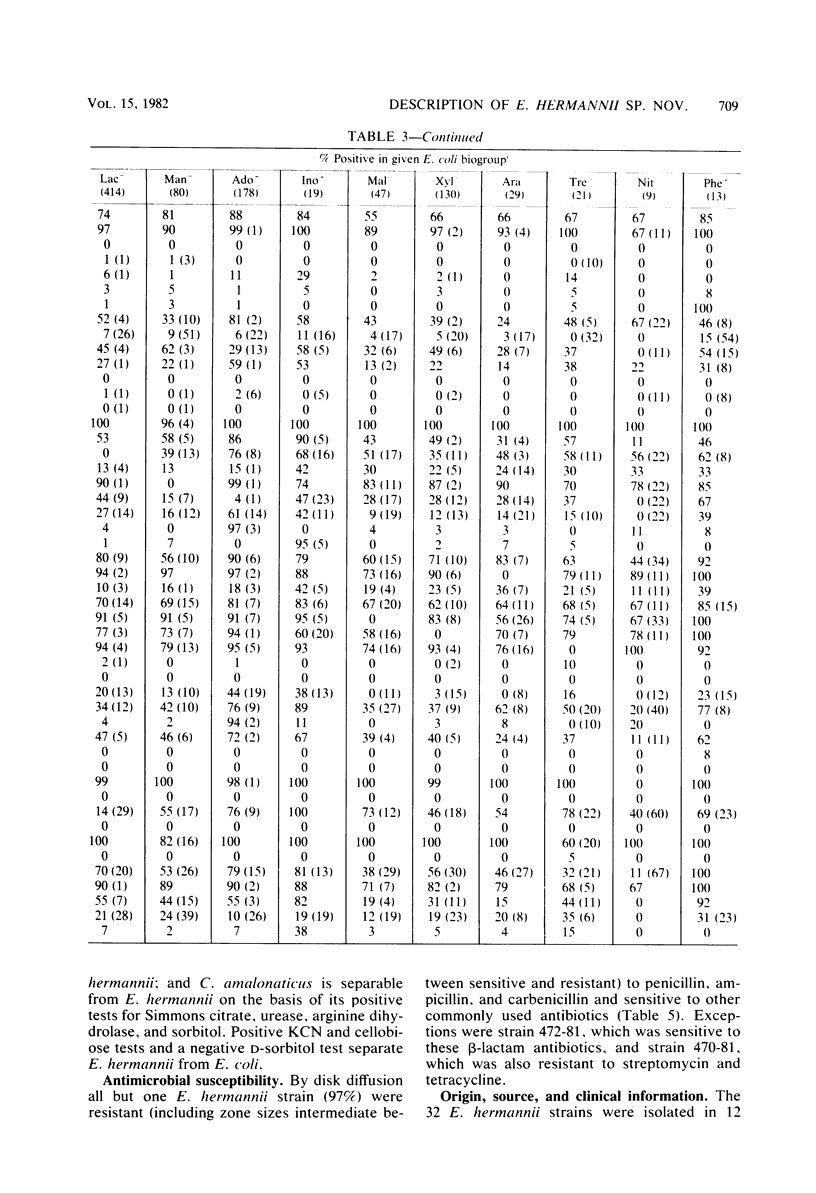
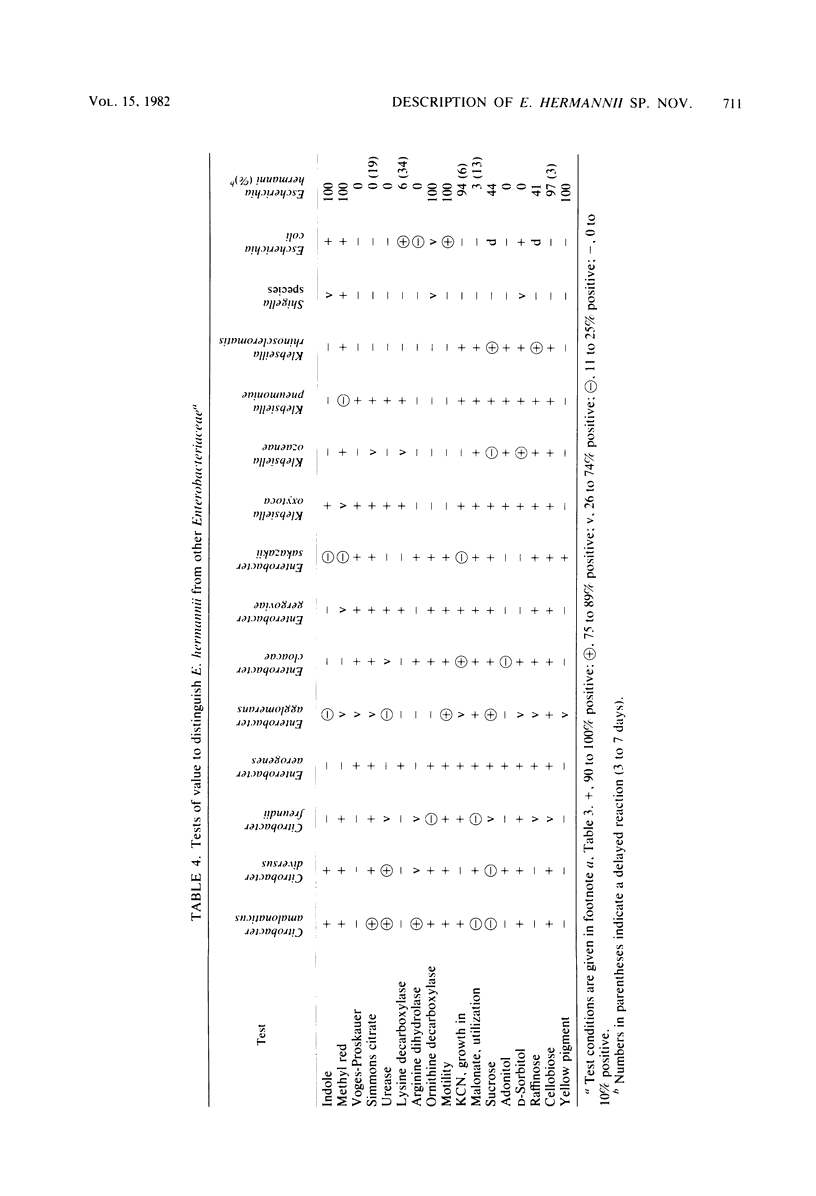
DNA relatedness was used to define the biochemical boundaries of Escherichia coli. A large number of biochemically atypical strains were shown to belong to biogroups of E. coli. These included strains negative in reactions for indole, all three decarboxylases, D-mannitol, lactose, or methyl red and strains positive in reactions for H2S, urea, citrate, KCN, adonitol, myo-inositol, or phenylalanine deaminase. Frequency and source data are presented for these atypical E. coli biogroups. One group of KCN-positive, cellobiose-positive, yellow-pigmented strains was 84 to 91% interrelated but only 35 to 45% related to E. coli. The name Escherichia hermannii sp. nov. is proposed for this group of organisms that was formerly called Enteric Group 11 by the Enteric Section, Centers for Disease Control, Atlanta, GA. Twenty-nine strains of E. hermannii have been isolated in the United States from a variety of clinical sources, principally wounds, sputum, and stools. Three additional strains were isolated from food. E. hermannii strains are gram-negative, oxidase-negative, fermentative, motile rods. In addition to yellow pigment and positive KCN and cellobiose tests, the biochemical reactions characteristic of 32 strains of E. hermannii were as follows: gas from D-glucose, acid from D-glucose, maltose, D-xylose, L-arabinose, L-rhamnose, and D-mannitol; no acid from adonitol or inositol; variable acid production from lactose and sucrose; positive tests for indole, methyl red, and mucate; negative tests for Voges-Proskauer. Simmons citrate, H2S, urea, phenylalanine deaminase, and gelatin hydrolysis; negative or delayed test for L-lysine decarboxylase and negative test for L-arginine dihydrolase; and positive test for ornithine decarboxylase. E. hermannii strains were resistant to penicillin, ampicillin, and carbenicillin and sensitive to other commonly used antibiotics. Wounds account for almost 50% of human isolates of E. hermannii, followed by sputum or lung isolates (ca. 25%) and stool isolates (20%).
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Brenner D. J., Falkow S. Genetics of the Enterobacteriaceae. C. Molecular relationships among members of the Enterobacteriaceae. Adv Genet. 1971;16:81–118. doi: 10.1016/s0065-2660(08)60355-7. [DOI] [PubMed] [Google Scholar]
- Brenner D. J., Fanning G. R., Johnson K. E., Citarella R. V., Falkow S. Polynucleotide sequence relationships among members of Enterobacteriaceae. J Bacteriol. 1969 May;98(2):637–650. doi: 10.1128/jb.98.2.637-650.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner D. J., Fanning G. R., Skerman F. J., Falkow S. Polynucleotide sequence divergence among strains of Escherichia coli and closely related organisms. J Bacteriol. 1972 Mar;109(3):953–965. doi: 10.1128/jb.109.3.953-965.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner D. J., Fanning G. R., Steigerwalt A. G., Orskov I., Orskov F. Polynucleotide sequence relatedness among three groups of pathogenic Escherichia coli strains. Infect Immun. 1972 Sep;6(3):308–315. doi: 10.1128/iai.6.3.308-315.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer J. J., 3rd, Fanning G. R., Huntley-Carter G. P., Holmes B., Hickman F. W., Richard C., Brenner D. J. Kluyvera, a new (redefined) genus in the family Enterobacteriaceae: identification of Kluyvera ascorbata sp. nov. and Kluyvera cryocrescens sp. nov. in clinical specimens. J Clin Microbiol. 1981 May;13(5):919–933. doi: 10.1128/jcm.13.5.919-933.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman F. W., Farmer J. J., 3rd Salmonella typhi: identification, antibiograms, serology, and bacteriophage typing. Am J Med Technol. 1978 Dec;44(12):1149–1159. [PubMed] [Google Scholar]
- Lautrop H., Orskov I., Gaarslev K. Hydrogensulphide producing variants of Escherichia coli. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(5):641–650. doi: 10.1111/j.1699-0463.1971.tb00092.x. [DOI] [PubMed] [Google Scholar]
- Layne P., Hu A. S., Balows A., Davis B. R. Extrachromosomal nature of hydrogen sulfide production in Escherichia coli. J Bacteriol. 1971 Jun;106(3):1029–1030. doi: 10.1128/jb.106.3.1029-1030.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesher R. J., Jones W. H. Urease production from clinical isolates of beta-hemolytic Escherichia coli. J Clin Microbiol. 1978 Sep;8(3):344–345. doi: 10.1128/jcm.8.3.344-345.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- ORSKOV I., ORSKOV F., SOJKA W. J., LEACH J. M. Simultaneous occurrence of E. coli B and Lantigens in strains from diseased swine. Influence of cultivation temperature. Two new E. coli Kantigens: K 87 and K 88. Acta Pathol Microbiol Scand. 1961;53:404–422. [PubMed] [Google Scholar]
- Sato G., Asagi M., Oka C., Ishiguro N., Terakado N. Transmissible citrate-utilizing ability in Escherichia coli isolated from pigeons, pigs and cattle. Microbiol Immunol. 1978;22(6):357–360. doi: 10.1111/j.1348-0421.1978.tb00380.x. [DOI] [PubMed] [Google Scholar]
- Wachsmuth I. K., Davis B. R., Allen S. D. Ureolytic Escherichia coli of human origin: serological, epidemiological, and genetic analysis. J Clin Microbiol. 1979 Dec;10(6):897–902. doi: 10.1128/jcm.10.6.897-902.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington JA I. I., Timm J. A. Unclassified, citrate-positive member of the family Enterobacteriaceae resembling Escherichia coli. J Clin Microbiol. 1976 Aug;4(2):165–167. doi: 10.1128/jcm.4.2.165-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington J. A., 2nd, Maker M. D. Unclassified, lactose-fermenting, urease-producing member of the family Enterobacteriaceae resembling Escherichia coli. J Clin Microbiol. 1975 Jul;2(1):70–71. doi: 10.1128/jcm.2.1.70-71.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



