Abstract
Background
Aminocandin is an investigational echinocandin with excellent activity against Candida species, including Candida albicans and Candida tropicalis. However, few data are available for this agent versus Candida glabrata. We compared the in vitro potency and in vivo efficacy of aminocandin and caspofungin against clinical isolates of C. glabrata including those with reduced caspofungin susceptibility (MIC > 2 mg/L).
Methods
In vitro activity was assessed using microdilution broth susceptibility testing. Three isolates, one with a low and two with elevated caspofungin MICs, were chosen and mice were infected with C. glabrata followed by a single dose of aminocandin or caspofungin (0.5–100 mg/kg), or daily doses of caspofungin (0.07–14.3 mg/kg) begun 1 day after inoculation. Reduction in fungal burden, assessed in kidney tissue on day 8 post-inoculation, was the marker of antifungal response.
Results
Aminocandin was more potent than caspofungin against each isolate with reduced caspofungin susceptibility. Mice infected with the caspofungin-susceptible isolate had significant decreases in tissue burden with low doses of either drug. Higher single doses of aminocandin (≥10 mg/kg) were required to reduce fungal burden against the two isolates with elevated caspofungin MICs. Single dose administration of caspofungin was ineffective against one of these isolates, and higher daily doses were required to reduce fungal burden.
Conclusions
These studies suggest that aminocandin has the potential for extended interval dosing in the treatment of C. glabrata infections caused by susceptible isolates. However, higher doses may be required against isolates with reduced caspofungin susceptibility.
Keywords: echinocandin, candidiasis, murine model
Introduction
Candida species are the most common causes of invasive fungal infections, ranging from oesophageal candidiasis to fulminating life-threatening candidiasis. Invasive candidiasis caused by Candida glabrata is of increasing concern due to the increased incidence and high mortality rates in patients with multiple comorbidities.1–3 Furthermore, concern has also been raised about the utility of the azoles in the treatment of invasive infections caused by this species, as an increasing proportion of C. glabrata clinical isolates within the USA is resistant to fluconazole.4–6 For serious C. glabrata infections, the echinocandins are alternative first-line antifungal agents. In vitro and in vivo studies have demonstrated excellent potency and efficacy of these agents against the Candida species, including C. glabrata,5 findings that appear to be supported by results from clinical trials.7–10 However, concern over the development of resistance to these agents exists as 2% of C. glabrata isolates submitted to the Fungus Testing Laboratory of the University of Texas Health Science Center at San Antonio have caspofungin MIC values of >2 mg/L (A. Fothergill, Department of Pathology, University of Texas Health Science Center at San Antonio, personal communication).
Aminocandin (IP-960, formerly HMR3270, Indevus, Lexington, MA, USA, now NXL201, Novexel, Romainville, France) is an investigational echinocandin with good in vitro potency and in vivo efficacy against many Candida species, including fluconazole-resistant Candida albicans and Candida tropicalis.11,12 Like the other echinocandins, aminocandin is thought to act by non-competitive inhibition of the 1,3-β-d-glucan synthase enzyme complex, resulting in decreased synthesis of the major cell wall component 1,3-β-d-glucan. By inhibiting this fungus-specific target, echinocandins avoid many of the toxicities associated with other classes of antifungal agents.13 Because of the long half-lives of these agents and the excellent tolerability observed in clinical trials and dosage escalation studies,7–10,14–17 extended interval dosing has been proposed as a potential means of avoiding the requirement of daily intravenous therapy. Data from our group and other investigators have demonstrated this to be an effective strategy in murine models of invasive candidiasis.18,19 However, this strategy has not been evaluated for aminocandin against C. glabrata, and data are lacking regarding its utility against strains with reduced susceptibility to this agent and caspofungin. Therefore, we sought to compare the in vitro potency and in vivo efficacy of aminocandin and caspofungin against clinical isolates of C. glabrata, including isolates with reduced susceptibility to these agents.
Materials and methods
In vitro susceptibility testing
Twelve clinical isolates of C. glabrata were obtained from the Fungus Testing Laboratory at the University of Texas Health Science Center at San Antonio, USA. Eleven of the isolates had previously been identified as having markedly decreased susceptibility to caspofungin (MIC ≥ 4 mg/L), while the susceptible isolate, A3 (CAS-S), had a low MIC (≤0.5 mg/L) to both aminocandin and caspofungin. Aminocandin (IP-960, Indevus) and caspofungin (Merck & Co., Inc., Whitehouse Station, NJ, USA) stock solutions were prepared in sterile water and serial 2-fold dilutions were prepared for each antifungal agent, according to the CLSI M27-A3 methodology in RPMI 1640.20 Drug concentrations in the microdilution trays of both antifungal agents ranged from 0.015 to 16 mg/L. Yeast inocula were measured spectrophotometrically and diluted in sterile water to obtain final inocula concentrations between 0.5×103 and 2.5×103 cells/mL. Candida parapsilosis ATCC 22019 (caspofungin MIC 1 mg/L) and Candida krusei ATCC 6258 (caspofungin MIC 0.5 mg/L) were used as control organisms in this study. Trays were incubated at 35°C, and the MIC endpoints were defined as the first concentration of the antifungal agent at which the turbidity in the well was at least 50% less than in the drug-free growth control well.
Animals
Outbred male ICR mice with an average weight of 28 g were maintained five per cage and given food and water ad libitum. Studies were conducted using an isolate with a low MIC to both agents (A3; CAS-S isolate), an isolate with a low aminocandin MIC and an elevated caspofungin MIC (05-62; CAS-R1 isolate), and an isolate with an increased MIC to both agents (04-1748; CAS-R2 isolate). At least eight mice were included in each dose group against each isolate tested. This study was approved by the Institutional Animal Care and Use Committee at the University of Texas Health Science Center at San Antonio (Approval No. 98094-34-C), and all animals were maintained in accordance with the American Association for Accreditation of Laboratory Animal Care.21
Test organisms for in vivo studies
The caspofungin-susceptible isolate of C. glabrata A3 (caspofungin MIC 0.25 mg/L; aminocandin MIC 0.5 mg/L) was obtained from the Fred Hutchinson Cancer Research Center in Seattle. Isolates with reduced caspofungin susceptibility, 05-62 (CAS-R1) and 04-1748 (CAS-R2), were obtained from the University of Texas Health Science Center at San Antonio Fungus Testing Laboratory. Each isolate was subcultured onto Sabouraud dextrose agar from frozen milk stock and grown for 2–3 days prior to animal inoculation. Colony-forming units (cfu) from each isolate were collected and suspended separately in physiological saline with 0.1% Tween 20. Each inocula was washed three times, final inocula concentrations were determined with a haemocytometer, and viability was confirmed by plating dilutions of each inoculum and enumerating the cfu.
Infection model and dosing regimens
On the day prior to infection, mice were made neutropenic by intravenous administration of 5-fluorouracil at 150 mg/kg of body weight. On day 0, mice were infected intravenously with C. glabrata isolates at 108 cfu/mouse. Caspofungin was obtained commercially as lyophilized powder and was reconstituted with sterile distilled water according to the manufacturer’s instructions. Aminocandin powder was reconstituted with a 5% weight/volume mannitol (pH 6–7) as per the instructions from the manufacturer. Mice inoculated with the CAS-S isolate received 0.2 mL of caspofungin intraperitoneally (ip) or aminocandin intravenously (iv) at single doses of 2.5, 10, 40, 60 or 100 mg/kg. Another group of animals received daily ip doses of caspofungin 0.36, 1.4, 5.7, 8.6 or 14.3 mg/kg on days 1 through 7 (cumulative dose corresponding to single doses of 2.5, 10, 40, 60 and 100 mg/kg, respectively). Animals in the control group received a single 0.2 mL dose of 5% mannitol solution iv on day 1. Animals inoculated with the CAS-R1 and CAS-R2 isolates received single doses of caspofungin ip or aminocandin iv at 0.5, 1.0, 2.5, 10, 25, 40, 60 or 100 mg/kg beginning on day 1. Similar to animals infected with the CAS-S isolate, other groups of mice inoculated with the CAS-R1 isolate were treated with daily doses of caspofungin ip at 0.07, 0.14, 0.36, 1.4, 3.6, 5.7, 8.6 or 14.3 mg/kg on days 1 through 7 (cumulative dose corresponding to single doses of 0.5, 1.0, 2.5, 10, 40, 60 or 100 mg/kg, respectively).
Tissue burden
To assess tissue fungal burden, mice were euthanized on day 8 post-inoculation. Kidneys were removed, weighed and homogenized using a tissue homogenizer (Plytron dispensing and mixing technology PT 2100, Kinematica, Cincinnati, OH, USA) in 2 mL of sterile saline containing piperacillin and amikacin at 60 mg/L to suppress bacterial growth. Serial dilutions of homogenate were prepared and plated on Sabouraud dextrose agar. After 24–48 h of incubation at 37°C, colonies were counted and the number of cfu/g kidney tissue was calculated.
Data analysis
Differences in fungal burden endpoints (cfu/g) were assessed for significance using the Mann–Whitney test. A P value of ≤0.05 was considered statistically significant for all comparisons. Mean and median tissue fungal burden values were plotted, and data were fitted to an inhibitory sigmoid model (modified Hill equation) using computer curve-fitting software (Prism 5; GraphPad Software, Inc., San Diego, CA, USA) to calculate the dose resulting in 50% reduction in colony-forming units (ED50) compared with controls.
Results
Antifungal susceptibility testing
Aminocandin was more potent than caspofungin against each C. glabrata isolate tested. Against the isolates with reduced caspofungin susceptibility (MIC values ranged from 4 to >16 mg/L), aminocandin MICs ranged from 0.5 to 4 mg/L (Table 1).
Table 1.
MICs (mg/L) of aminocandin and caspofungin for C. glabrata isolates by microdilution methodology in RPMI
| Isolate | Aminocandin | Caspofungin |
|---|---|---|
| A3 (CAS-S) | 0.5 | 0.25 |
| 05-62 (CAS-R1) | 0.5 | 4 |
| 04-1748 (CAS-R2) | 2 | >16 |
| 04-3525 | 0.125 | >16 |
| 04-3144 | 2 | >16 |
| 04-3477 | 4 | >16 |
| 04-1026 | 4 | >16 |
| 04-569 | 4 | >16 |
| 04-2971 | 2 | 8 |
| 04-1400 | 4 | 8 |
| 04-2880 | 2 | 4 |
| 04-2596 | 1 | >16 |
Tissue burden with CAS-S
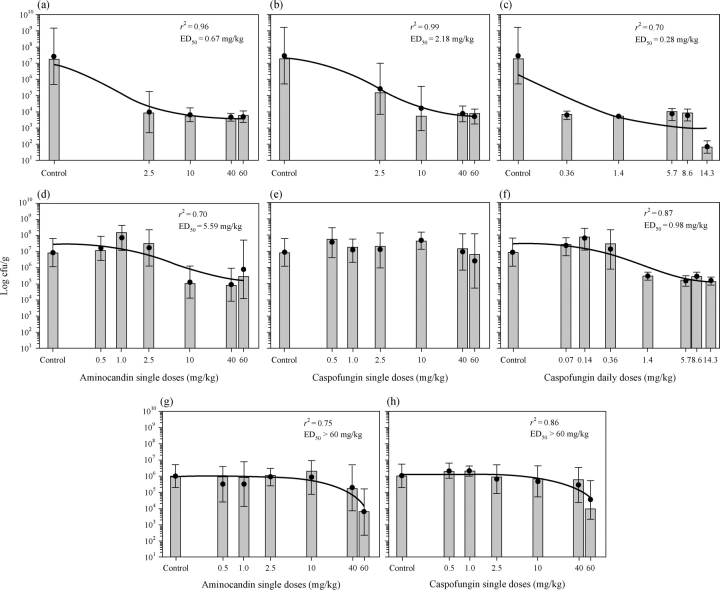
In the treatment of the isolate, A3, with low aminocandin and caspofungin MIC values, aminocandin single doses of 2.5–60 mg/kg were effective in reducing the tissue fungal burden compared with controls (P < 0.05; Table 2). Similarly, all doses of caspofungin, whether administered as a single dose or divided into daily doses, were effective in reducing tissue fungal burden compared with controls. In addition, the ED50 values predicted by the dose–response curves for single dose aminocandin (0.67 mg/kg) and caspofungin (2.18 mg/kg), and caspofungin administered daily (0.28 mg/kg) were less than the lowest doses tested (Figure 1a–c, respectively). These results are consistent with the observed data, which demonstrated that each of the tested doses for these regimens significantly lowered fungal burden compared with control. Acute toxicity with high-dose aminocandin and caspofungin appeared to occur as nine mice treated with a single dose of caspofungin at 100 mg/kg and two animals treated with a single dose of aminocandin 100 mg/kg died immediately after the treatment (data not shown). Although the 100 mg/kg single doses of both aminocandin and caspofungin appeared to result in reductions in tissue fungal burden, these data were not included in the analysis due to the small number of animals that survived. However, daily doses of caspofungin 14.3 mg/kg (cumulative dose corresponding to the caspofungin single dose of 100 mg/kg) were well tolerated, suggesting that the deaths in the high-dose groups were potentially due to toxicity.
Table 2.
Kidney tissue fungal burden in mice inoculated intravenously with isolates of C. glabrata with varying susceptibility to aminocandin and caspofungin
| Single dose—median log10 cfu/g (range) |
Daily dose caspofungin—median log10 cfu/g (range) |
|||
|---|---|---|---|---|
| Isolate and dose (mg/kg) | aminocandin | caspofungin | dose | fungal burden |
| A3 (CAS-S)—Control 7.3 (2.8–10) | ||||
| 2.5 | 3.9 (1.6–5.9)* | 5.2 (3.1–7.7)* | 0.36 | 3.8 (3.3–4.2)* |
| 10 | 3.8 (3.4–4.9)* | 3.7 (3.5–7.8)* | 1.4 | 3.7 (3.5–4.0)* |
| 40 | 3.6 (3.4–4.2)* | 3.9 (3.3–4.9)* | 5.7 | 4.0 (3.1–4.3)* |
| 60 | 3.8 (2.9–4.3)* | 3.9 (2.9–4.2)* | 8.6 | 3.9 (2.8–4.1)* |
| 14.3 | 1.8 (1.3–2.5)* | |||
| 05-62 (CAS-R1)—Control 6.8 (4.8–8.5) | ||||
| 0.5 | 7.1 (5.7–8.5) | 7.7 (5.3–8.3) | 0.07 | 7.4 (6.0–8.0) |
| 1 | 8.2 (6.7–8.7) | 7.2 (5.8–7.8) | 0.14 | 7.8 (6.4–8.5) |
| 2.5 | 7.5 (5.4–8.4) | 7.3 (5.4–8.4) | 0.36 | 7.4 (5.0–8.9) |
| 10 | 5.0 (4.0–7.2)* | 7.6 (6.8–8.8) | 1.4 | 5.4 (5.2–5.9)* |
| 40 | 4.9 (3.6–6.6)* | 7.1 (4.7–8.7) | 5.7 | 5.2 (4.5–5.5)* |
| 60 | 5.4 (4.4–11)* | 6.8 (3.1–8.3) | 8.6 | 5.4 (5.1–6.0)* |
| 14.3 | 5.1 (4.8–5.6)* | |||
| 04-1748 (CAS-R2)—Control 6.0 (4.9–7.3) | ||||
| 0.5 | 5.9 (2.7–6.5) | 6.3 (5.7–7.2) | — | — |
| 1 | 5.9 (3.2–7.3) | 6.3 (5.8–6.9) | — | — |
| 2.5 | 6.1 (4.7–6.6) | 5.9 (3.9–6.8) | — | — |
| 10 | 6.3 (4.1–7.2) | 5.8 (4.3–7.1) | — | — |
| 40 | 5.2 (1.9–7.5) | 5.8 (3.3–6.7) | — | — |
| 60 | 3.8 (1.5–6.1)* | 3.9 (3.3–5.9)* | — | — |
cfu/g, colony-forming units per gram of kidney tissue.
*P < 0.05.
Figure 1.
Sigmoidal dose–response curves for single dose against C. glabrata isolates A3 (CAS-S) (a, b and c), 05-62 (CAS-R1) (d, e and f) and 04-1748 (CAS-R2) (g and h) for single dose administration of aminocandin (a, d and g) and caspofungin (b, e and g), and daily doses of caspofungin (c and f). Symbols (closed circles) represent the means plus or minus the standard deviations of colony-forming units per gram of tissue, and the bars represent the corresponding median values. ED50, dose resulting in 50% reduction in colony-forming units compared with controls.
Tissue burden with CAS-R1
In the treatment of mice inoculated with the C. glabrata isolate with an elevated caspofungin MIC (4 mg/L) but a low aminocandin MIC (0.5 mg/L), isolate 05-62, single doses of aminocandin ≥10 mg/kg resulted in significant decreases in tissue burden compared with control (P < 0.05), while no single dose administration of caspofungin resulted in reductions in fungal burden (Table 2 and Figure 1e). In contrast, caspofungin daily doses of ≥1.4 mg/kg (equal to a cumulative single dose of ≥10 mg/kg) were able to reduce cfu counts within the renal tissue compared with controls (P < 0.05). These results are consistent with the dose–response data that predicted ED50 values of 5.59 mg/kg for single dose aminocandin versus 0.98 mg/kg for daily dose caspofungin (Figure 1d and f). The results also suggest that higher doses of caspofungin may still be useful against an isolate with elevated MICs provided the drug is frequently dosed. Similar to that observed with the susceptible isolate, 7 of 10 mice treated with caspofungin single dose 100 mg/kg and 2 of 9 mice treated with aminocandin single dose 100 mg/kg died on the day of treatment, thus confirming that single doses of 100 mg/kg of each agent to be toxic. Based on these observations, the tissue fungal burden data were not included in the analysis, and single dose administration of 100 mg/kg of either agent was not studied against the remaining isolate. However, as before, daily doses of caspofungin 14.3 mg/kg (corresponding to the caspofungin total dose of 100 mg/kg) were well tolerated.
Tissue burden with CAS-R2 strain
Against clinical isolate 04–1748, which had increased MICs to both aminocandin (2 mg/L) and caspofungin (>16 mg/L), only single doses 60 mg/kg of each agent led to reductions in tissue fungal burden compared with control. These results are consistent with the ED50 values (>60 mg/kg) predicted by the dose–response curves for both agents (Figure 1g and h). Daily dosing of caspofungin was not evaluated for this organism.
Discussion
C. glabrata is a fungal species of increasing clinical importance. This opportunistic pathogen is responsible for 20% to 24% of Candida bloodstream infections in the USA and has been increasingly encountered in hospitals throughout the USA over the past decade as the second most commonly isolated yeast in patients with invasive candidiasis.1,5,6,22 For many, the first choice of antifungal therapy for invasive candidiasis remains fluconazole due to its clinical effectiveness, lower cost and favourable adverse effect profile.23,24 However, C. glabrata resistance to fluconazole and other azoles is becoming increasingly problematic, and breakthrough infections caused by this species in patients receiving fluconazole prophylaxis have been reported.3,5,6,25 Although amphotericin B therapy has been successfully used for the treatment of such infections, this strategy may be associated with significant renal toxicity. Recently, the echinocandins have become increasingly used for the treatment of invasive fungal infections caused by Candida species. Clinical trials have demonstrated these agents to be efficacious and safe for the treatment of invasive candidiasis as well as for empirical therapy and antifungal prophylaxis.7–10,26,27 Aminocandin is a new echinocandin undergoing preclinical development and Phase I trials with potent in vitro activity against many Candida spp. including C. albicans, C. krusei, Candida lusitaniae, C. tropicalis and C. glabrata with MICs ranging from 0.03 to 4 mg/L.28 Phase I single dose escalation studies in healthy volunteers have also demonstrated this agent to be well tolerated.29 Several in vivo studies with aminocandin reported efficacy in the treatment of infections caused by C. albicans and C. tropicalis, including isolates resistant to fluconazole.11,12,30
One of the major limitations of echinocandin therapy is the need for daily intravenous dosing, which may limit prolonged use in severely debilitated or immunocompromised patients due to increased risk of infection. To overcome this limitation, extended interval dosing has been suggested. This strategy is supported by the long half-lives of members of this antifungal class,16,17,31 the excellent safety profiles observed in clinical trials and dosage escalation studies,7–10,14,15 and the concentration-dependent activity reported in the animal models of invasive fungal infections.18,32–35 Furthermore, this strategy has been shown to be effective for micafungin and aminocandin in the animal models of invasive candidiasis. Two studies have shown that aminocandin, administered as a single dose or twice weekly either as prophylaxis or treatment, reduces fungal burden and improves survival against infections caused by susceptible C. albicans.12,36 In a murine model of invasive candidiasis caused by a susceptible C. glabrata isolate, Gumbo et al.18 demonstrated that a single high dose of micafungin 100 mg/kg was effective in reducing fungal burden. The results of the current study are consistent with these data as well as with other studies that have reported concentration-dependent activity of caspofungin and aminocandin with both the Cmax/MIC and AUC/MIC associated with efficacy.30,33 In the current study, single doses of aminocandin and caspofungin ≥2.5 mg/kg were effective in reducing tissue fungal burden after inoculation with a C. glabrata isolate with low MIC values to both agents. Interestingly, single doses of aminocandin and caspofungin 100 mg/kg were associated with rapid deaths in animals in the current study, but such an effect has not been reported with micafungin at this same dose.18
A second question addressed by our study was whether differences in the in vitro potency favouring aminocandin could be reflected by greater in vivo efficacy. Against the 11 isolates of C. glabrata that had elevated caspofungin MIC values, aminocandin appeared to maintain in vitro potency. However, when animals were inoculated with an isolate with an elevated caspofungin MIC, higher single doses of aminocandin (≥10 mg/kg) were required to reduce the fungal burden within the renal tissue, while single doses of caspofungin were ineffective. These data suggest that a C. glabrata isolate with decreased in vitro susceptibility to caspofungin may not respond to single doses of this drug, but single doses of aminocandin could still be effective. Interestingly, daily doses of caspofungin did reduce tissue fungal burden against this isolate, suggesting that more frequent administration may be an alternative to extended interval dosing against isolates with reduced caspofungin susceptibility. The in vivo efficacy of aminocandin was markedly reduced against the isolate with severely reduced in vitro susceptibility to caspofungin, as only the single doses of 60 mg/kg of each agent were able to lead to reductions in fungal burden. This observation, coupled with the toxicity observed with 100 mg/kg single doses of aminocandin and caspofungin, suggests that the strategy of extended interval dosing of aminocandin and caspofungin may be limited against C. glabrata isolates with reduced in vitro susceptibility.
One limitation of this study is that we did not assess the pharmacokinetics of either aminocandin or caspofungin in our model. Other investigators have reported long half-lives in mice (20.2–23.2 h) for both agents when serum concentration data alone are assessed.30,33 When caspofungin serum concentration data were co-modelled with kidney tissue concentrations, the terminal half-life increased significantly (59.2 h).33 This long terminal half-life in mice is similar to that reported in humans for aminocandin (53 h) and caspofungin (40–50 h).16,31 Another potential limitation is that these agents were administered by different routes (iv for aminocandin and ip for caspofungin). However, previous animal studies have demonstrated rapid absorption of caspofungin from the peritoneal space into the bloodstream following ip administration.33,34 Thus, differences in the time to peak concentrations between these two agents that occur between iv and ip administration are negligible. Furthermore, similar pharmacokinetic parameters for caspofungin (e.g. plasma clearance, total exposure as measured by the area under the concentration curve) are expected when equivalent doses up to 5 mg/kg are administered ip or iv in mice.33,37 However, it is unknown if this would be consistent at the higher doses used in this study. Finally, we did not evaluate the efficacy of daily dosing of aminocandin to determine if this strategy would be effective against isolates with reduced susceptibility. As suggested by the daily dose caspofungin data, this strategy may be effective against isolates to which this agent has reduced in vitro potency. However, further studies would need to be conducted to test this hypothesis.
The results of our study as well as those by others demonstrate that single doses of aminocandin, caspofungin and micafungin are effective as treatment against susceptible C. glabrata isolates in murine models of invasive candidiasis. This suggests that extended interval dosing may be a potentially useful strategy against such infections. However, as demonstrated by the data from this study, this strategy may be limited against infections caused by isolates with reduced susceptibilities to these agents as well as potential toxicity associated with high doses (e.g. 100 mg/kg). Thus, although aminocandin has been shown to be well tolerated in single dose escalation studies in healthy volunteers, it is unknown if the maximum tolerated doses will be high enough to allow for extended interval dosing of this echinocandin as empirical therapy. Further investigations are warranted to assess the feasibility of this approach.
Funding
This work was supported by the National Institute of Health contract #N01-AI-25475.
Transparency declarations
N. P. W. has received research support from Pfizer and Schering-Plough. M. G. R. has received contract support from Schering-Plough, Pfizer and Astellas, and has served on the speakers bureau for Astellas and Enzon. T. F. P. has received research support from Merck, Pfizer, Schering-Plough and Nektar Therapeutics, has served on the speakers bureau for Merck and Pfizer, and acted as a consultant for Astellas, Basilea, Merck, Nektar, Pfizer, Schering-Plough and Stiefel Laboratories. J. R. G. has received research support from Pfizer, Schering- Plough, Merck and Astellas, has served on the speakers bureau for Merck and Schering-Plough, and acted as a consultant for Merck, Schering-Plough, Indevus, Vicuron, Nektar and F2G. G. E. B., L. K. N., R. B. and A. W. F. have none to declare.
Acknowledgements
The authors would like to thank the following people from The University of Texas Health Science Center at San Antonio, Department of Medicine, Division of Infectious Diseases, for their assistance in completing the laboratory work: Dora I. McCarthy, Ana C. Vallor, Marcos Olivo and Destiny Molina. Also, thanks to Nova Najarian and Indevus Pharmaceuticals for supplying us with aminocandin, and Chris Lambros at the National Institute of Health for his support on this project and contract.
References
- 1.Pappas PG, Rex JH, Lee J, et al. A prospective observational study of candidemia: epidemiology, therapy, and influences on mortality in hospitalized adult and pediatric patients. Clin Infect Dis. 2003;37:634–43. doi: 10.1086/376906. [DOI] [PubMed] [Google Scholar]
- 2.Malani A, Hmoud J, Chiu L, et al. Candida glabrata fungemia: experience in a tertiary care center. Clin Infect Dis. 2005;41:975–81. doi: 10.1086/432939. [DOI] [PubMed] [Google Scholar]
- 3.Bodey GP, Mardani M, Hanna HA, et al. The epidemiology of Candida glabrata and Candida albicans fungemia in immunocompromised patients with cancer. Am J Med. 2002;112:380–5. doi: 10.1016/s0002-9343(01)01130-5. [DOI] [PubMed] [Google Scholar]
- 4.Pfaller MA, Diekema DJ. Twelve years of fluconazole in clinical practice: global trends in species distribution and fluconazole susceptibility of bloodstream isolates of Candida. Clin Microbiol Infect. 2004;10(Suppl 1):11–23. doi: 10.1111/j.1470-9465.2004.t01-1-00844.x. [DOI] [PubMed] [Google Scholar]
- 5.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20:133–63. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pfaller MA, Diekema DJ, Gibbs DL, et al. Results from the ARTEMIS DISK Global Antifungal Surveillance study, 1997 to 2005: an 8.5-year analysis of susceptibilities of Candida species and other yeast species to fluconazole and voriconazole determined by CLSI standardized disk diffusion testing. J Clin Microbiol. 2007;45:1735–45. doi: 10.1128/JCM.00409-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pappas PG, Rotstein CM, Betts RF, et al. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin Infect Dis. 2007;45:883–93. doi: 10.1086/520980. [DOI] [PubMed] [Google Scholar]
- 8.Reboli AC, Rotstein C, Pappas PG, et al. Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med. 2007;356:2472–82. doi: 10.1056/NEJMoa066906. [DOI] [PubMed] [Google Scholar]
- 9.Kuse ER, Chetchotisakd P, da Cunha CA, et al. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet. 2007;369:1519–27. doi: 10.1016/S0140-6736(07)60605-9. [DOI] [PubMed] [Google Scholar]
- 10.Mora-Duarte J, Betts R, Rotstein C, et al. Comparison of caspofungin and amphotericin B for invasive candidiasis. N Engl J Med. 2002;347:2020–9. doi: 10.1056/NEJMoa021585. [DOI] [PubMed] [Google Scholar]
- 11.Warn PA, Sharp A, Morrissey G, et al. Activity of aminocandin (IP960) compared with amphotericin B and fluconazole in a neutropenic murine model of disseminated infection caused by a fluconazole-resistant strain of Candida tropicalis. J Antimicrob Chemother. 2005;56:590–3. doi: 10.1093/jac/dki268. [DOI] [PubMed] [Google Scholar]
- 12.Ghannoum MA, Kim HG, Long L. Efficacy of aminocandin in the treatment of immunocompetent mice with haematogenously disseminated fluconazole-resistant candidiasis. J Antimicrob Chemother. 2007;59:556–9. doi: 10.1093/jac/dkl525. [DOI] [PubMed] [Google Scholar]
- 13.Wiederhold NP, Lewis RE. The echinocandin antifungals: an overview of the pharmacology, spectrum and clinical efficacy. Expert Opin Investig Drugs. 2003;12:1313–33. doi: 10.1517/13543784.12.8.1313. [DOI] [PubMed] [Google Scholar]
- 14.Sirohi B, Powles RL, Chopra R, et al. A study to determine the safety profile and maximum tolerated dose of micafungin (FK463) in patients undergoing haematopoietic stem cell transplantation. Bone Marrow Transplant. 2006;38:47–51. doi: 10.1038/sj.bmt.1705398. [DOI] [PubMed] [Google Scholar]
- 15.Hiemenz J, Cagnoni P, Simpson D, et al. Pharmacokinetic and maximum tolerated dose study of micafungin in combination with fluconazole versus fluconazole alone for prophylaxis of fungal infections in adult patients undergoing a bone marrow or peripheral stem cell transplant. Antimicrob Agents Chemother. 2005;49:1331–6. doi: 10.1128/AAC.49.4.1331-1336.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cancidas. Cancidas Package Insert. Whitehouse Station, NJ: Merck & Co., Inc.; 2005. [Google Scholar]
- 17.Eraxis. Eraxis Package Insert. New York, NY: Pfizer, Inc.; 2006. [Google Scholar]
- 18.Gumbo T, Drusano GL, Liu W, et al. Once-weekly micafungin therapy is as effective as daily therapy for disseminated candidiasis in mice with persistent neutropenia. Antimicrob Agents Chemother. 2007;51:968–74. doi: 10.1128/AAC.01337-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Najvar LK, Bocanegra R, Wiederhold NP, et al. Therapeutic and prophylactic efficacy of aminocandin (IP960) against disseminated candidiasis in mice. Clin Microbiol Infect. 2008;14:595–600. doi: 10.1111/j.1469-0691.2008.01994.x. [DOI] [PubMed] [Google Scholar]
- 20.Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts—Third Edition: Approved Standard M27-A3. Wayne, PA, USA: CLSI; 2008. [Google Scholar]
- 21.National Academy of Sciences. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- 22.Wisplinghoff H, Bischoff T, Tallent SM, et al. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–17. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 23.Rex JH, Bennett JE, Sugar AM, et al. A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. Candidemia Study Group and the National Institute. N Engl J Med. 1994;331:1325–30. doi: 10.1056/NEJM199411173312001. [DOI] [PubMed] [Google Scholar]
- 24.Rex JH, Pappas PG, Karchmer AW, et al. A randomized and blinded multicenter trial of high-dose fluconazole plus placebo versus fluconazole plus amphotericin B as therapy for candidemia and its consequences in nonneutropenic subjects. Clin Infect Dis. 2003;36:1221–8. doi: 10.1086/374850. [DOI] [PubMed] [Google Scholar]
- 25.Wingard JR, Merz WG, Rinaldi MG, et al. Association of Torulopsis glabrata infections with fluconazole prophylaxis in neutropenic bone marrow transplant patients. Antimicrob Agents Chemother. 1993;37:1847–9. doi: 10.1128/aac.37.9.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walsh TJ, Teppler H, Donowitz GR, et al. Caspofungin versus liposomal amphotericin B for empirical antifungal therapy in patients with persistent fever and neutropenia. N Engl J Med. 2004;351:1391–402. doi: 10.1056/NEJMoa040446. [DOI] [PubMed] [Google Scholar]
- 27.van Burik JA, Ratanatharathorn V, Stepan DE, et al. Micafungin versus fluconazole for prophylaxis against invasive fungal infections during neutropenia in patients undergoing hematopoietic stem cell transplantation. Clin Infect Dis. 2004;39:1407–16. doi: 10.1086/422312. [DOI] [PubMed] [Google Scholar]
- 28.Isham N, Ghannoum MA. Determination of MICs of aminocandin for Candida spp. and filamentous fungi. J Clin Microbiol. 2006;44:4342–4. doi: 10.1128/JCM.01550-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Indevus Pharmaceuticals Inc. 2004 http://www.Indevus.com . (3 March 2008, date last accessed) [Google Scholar]
- 30.Andes D, Marchillo K, Lowther J, et al. In vivo pharmacodynamics of HMR 3270, a glucan synthase inhibitor, in a murine candidiasis model. Antimicrob Agents Chemother. 2003;47:1187–92. doi: 10.1128/AAC.47.4.1187-1192.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sandage B, Cooper G, Najarian N, et al. 15th European Congress of Clinical Microbiology and Infectious Diseases. Copenhagen, Denmark: 2005. Pharmacokinetics and fungicidal activity of aminocandin (HMR3270), a novel echinocandin in healthy volunteers. [Google Scholar]
- 32.Gumbo T, Drusano GL, Liu W, et al. Anidulafungin pharmacokinetics and microbial response in neutropenic mice with disseminated candidiasis. Antimicrob Agents Chemother. 2006;50:3695–700. doi: 10.1128/AAC.00507-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Louie A, Deziel M, Liu W, et al. Pharmacodynamics of caspofungin in a murine model of systemic candidiasis: importance of persistence of caspofungin in tissues to understanding drug activity. Antimicrob Agents Chemother. 2005;49:5058–68. doi: 10.1128/AAC.49.12.5058-5068.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wiederhold NP, Kontoyiannis DP, Chi J, et al. Pharmacodynamics of caspofungin in a murine model of invasive pulmonary aspergillosis: evidence of concentration-dependent activity. J Infect Dis. 2004;190:1464–71. doi: 10.1086/424465. [DOI] [PubMed] [Google Scholar]
- 35.Andes D, Diekema DJ, Pfaller MA, et al. In vivo pharmacodynamic characterization of anidulafungin in a neutropenic murine candidiasis model. Antimicrob Agents Chemother. 2008;52:539–50. doi: 10.1128/AAC.01061-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Najvar LK, Bocanegra R, Wiederhold NP, et al. Therapeutic and prophylactic efficacy of aminocandin (IP960) against disseminated candidiasis in mice. Clin Microbiol Infect. 2008;14:595–600. doi: 10.1111/j.1469-0691.2008.01994.x. [DOI] [PubMed] [Google Scholar]
- 37.Sandhu P, Xu X, Bondiskey PJ, et al. Disposition of caspofungin, a novel antifungal agent, in mice, rats, rabbits, and monkeys. Antimicrob Agents Chemother. 2004;48:1272–80. doi: 10.1128/AAC.48.4.1272-1280.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]