Abstract
Objectives
Based on the assertion that fluorescence spectroscopy detects dimers of the polyene antibiotic amphotericin B (AmB), this technique was recently proposed to analyse the interaction of the drug with cell membranes. However, contradictory results indicate that this ‘dimeric’ fluorescence might actually originate from polyene impurities. We used a highly purified AmB to challenge this last proposal.
Methods
Comparison of the fluorescence of AmB from different origins was made in dimethyl sulphoxide (DMSO); concentration and sodium dodecyl sulphate (SDS) addition dependencies were analysed in water.
Results
Excitation of fluorescence in the absorption band of the AmB monomer (around 410 nm) revealed no difference between the different samples, in contrast with what was observed by excitation in the absorption wavelengths of self-associated AmB (around 325 nm). Furthermore, in this latter case, no concentration dependence was observed, in DMSO or in water. SDS addition increased the fluorescence in water.
Conclusions
The fluorescence of AmB observed by excitation in the absorption wavelengths of self-associated species (around 325 nm) is explainable by the presence of impurities. Fluorescence is probably not appropriate for characterization of the drug interaction with cell membranes.
Keywords: self-association, dimer, membrane, HPLC, toxicity
Introduction
We recently isolated a high purity amphotericin B (AmBHP) using semi-preparative, reverse-phase HPLC of commercially available amphotericin B (AmB).1 A number of peaks that appeared to contain material, polyene in nature, were resolved from AmB. Considerable differences in the relative amounts of these compounds, as well as AmB, were measured between the different commercial formulations. The contribution of such impurities to the fluorescence spectra of AmB is the object of some debate. Specifically, excitation spectra of around 325 nm (emission around 470 nm) are considered to originate either from polyene impurities by some laboratories,2,3 or AmB dimers by others.4,5 The interpretative choice is not without consequences, because AmB fluorescence was used in analyses of the physico-chemical properties of the drug. For instance, heating AmB for 10 min at 45°C induces changes in its state of aggregation, changes that result in decreased in vitro toxicity in mammalian cells and an increased therapeutic index in a murine model.6 Recently, fluorescence was used to evaluate the heat-induced super-aggregated ‘dimeric’ character of AmB-deoxycholate (Fungizone).7 Our purpose was to determine whether using the ‘dimeric’ interpretation was justified. In other words, could a polyene impurity have interfered in the study? We shall see that this was actually the case. To demonstrate it, comparison of the fluorescence of AmB from different origins was made in dimethyl sulphoxide (DMSO); concentration and sodium dodecyl sulphate (SDS) addition dependencies were analysed in water. More generally, we wanted to assess whether the use of AmB fluorescence is an appropriate tool for the study of the interaction of the antibiotic with membranes.
Methods
In the present study, first we compared the fluorescence of two commercial AmB formulations with that of AmBHP. Second, we determined the AmB concentration dependence of fluorescence, together with the UV-visible absorption spectra of the drug dissolved in water and in DMSO. Finally, we measured the influence of detergent addition on the fluorescence of AmB in water.
AmB preparations
Generic AmB (Pharma-Tek® AmB; Pharmatek Laboratories, Inc., San Diego, CA, USA) was obtained as a lyophilized powder containing AmB, sodium deoxycholate and sodium phosphate, respectively, in milligram ratios of 50:41:20.2. Also, United States Pharmacopeia grade AmB (USP AmB: Alpharma ApS, Copenhagen S, Denmark) was obtained as a non-solubilized formulation. AmBHP was prepared from generic AmB by semi-preparative HPLC. Minimal apparent purities, based on absorbance at 405 nm, for Pharma-Tek AmB, USP AmB and AmBHP were, respectively, 88%, 91% and 98%. Nystatin (Sigma Chemical, St Louis, MO, USA) was used as an amphotericin A control. Aliquots of a stock solution of SDS (12 mM) (Sigma L3771) prepared in Milli-Q water were added to designated, concentrated aqueous AmB suspensions, and the mixtures were adjusted to the desired concentrations.
HPLC preparation of AmBHP
HPLC purification of AmB preparations was performed as described previously.1 In brief, a semi-preparative HPLC was performed by applying aliquots of Pharma-Tek AmB reconstituted in HPLC grade water or USP AmB dissolved in DMSO to a 10 mm (I.D.)×250 mm 5 micron AquaC18™ column (Phenomenex, Torrance, CA, USA) to obtain AmBHP.
Comparison of fluorescence spectra of AmB formulations
Absorbance spectra of AmBHP, Pharma-Tek AmB and USP AmB solutions used to adjust final solutions of each drug prior to fluorescence measurements were obtained using a Perkin-Elmer Lambda 11 UV/VIS spectrometer. Each drug was dissolved directly in DMSO immediately before use to obtain a working stock of 2.5 mg/mL (∼2.7 mM) AmB. Aliquots (11–12 µL) of each stock solution were then added to 3 mL DMSO or water to obtain solutions with nearly identical absorbance spectra (data not shown) and final concentrations of ∼10 µM.
Although exhibiting minimal absorbance over the range of interest for AmB, nystatin was added only after the concentration of AmBHP in the samples to which it was added (i.e. in the AmBHP+nystatin sample) had been matched to those of other samples. Nystatin was added to a final concentration of 0.5 µM or ∼5% of the total polyene content. Absorbance spectra of aqueous suspensions of AmB in 0.1 or 1 cm path length quartz cuvettes were recorded on a spectrophotometer, operating with a 2 nm bandwidth, 1 nm interval and 100 nm/min scan rate.
Comparison of the various fluorescence spectra of 10 µM AmB in DMSO was obtained using a Perkin-Elmer LS50B spectrofluorimeter. Each sample was contained in a different matched 3 mL cuvette with a 1 cm light path. Excitation spectra were recorded between 300 and 500 nm, with emission set at either 471 or 560 nm. Excitation slit width was 2.5 nm, whereas emission slit width was set at 5 nm. Emission spectra were recorded from 375 to 700 nm with excitation set to 325, 335, 350 or 408 nm; emission and excitation slit widths, respectively, were 2.5 and 5 nm. Concentration dependence and SDS influence studies were performed using a spectrofluorimeter, Aminco Bowman series 2. Samples were held in a quartz dual path length cuvette (0.25 and 1 cm). The orientation of the cuvette was such as to minimize the inner filter effects. Fluorescence of diluted samples presenting a low absorbance was recorded in a 1 cm quartz cell. The spectrofluorimeter operated with a 4 nm band width, a 1 nm step size and a 60 nm/min scan rate. For a whole series of measurements, photomultiplier high voltage was held constant, between 750 and 1200 m, depending on sample concentration. Base lines with DMSO or SDS were subtracted.
Under some experimental conditions, self-screening (inner filter) effects had to be taken into consideration, and a correction factor had to be applied to fluorescence intensities.8 The corrections introduced are specified in the experimental part.
Results
Fluorescence of AmB formulations in DMSO
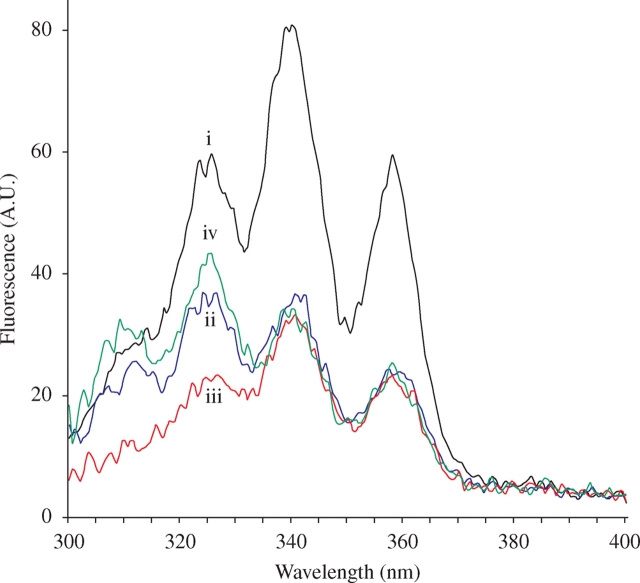
Spectra were recorded at a concentration of ∼10 µM (absorbances adjusted to be the same for all compounds) using excitation wavelengths between 325 and 700 nm. The emission spectra obtained by excitation at 408 nm (in the absorption band of monomeric AmB) did not allow discrimination between the four preparations (Figure 1a). Excitation at 416 nm gave similar results (data not shown). In contrast, emission spectra of the various AmBs obtained by excitation at 325 nm (Figure 1d) were totally different. The AmBHP fluorescence profile was similar to that of USP AmB, but its intensity was four times weaker. Likewise, the AmBHP fluorescence profile was different from that of generic AmB, and its intensity twice as weak. The emissions obtained by excitation at 350 and 335 nm (Figure 1b and c) showed less difference, except for USP AmB fluorescence, which was twice as intense as that of generic AmB and AmBHP. Interestingly, AmBHP combined with nystatin and generic AmB exhibited similar profiles. USP AmB could easily be discriminated from generic AmB and AmBHP. In addition, AmBHP clearly demonstrates a different emission profile compared with all the other test agents. The excitation spectra between 300 and 370 nm obtained by emission at 471 nm (Figure 2) confirmed these results. AmBHP does not emit a pronounced peak around 325 nm, as seen with the other formulations tested.
Figure 1.
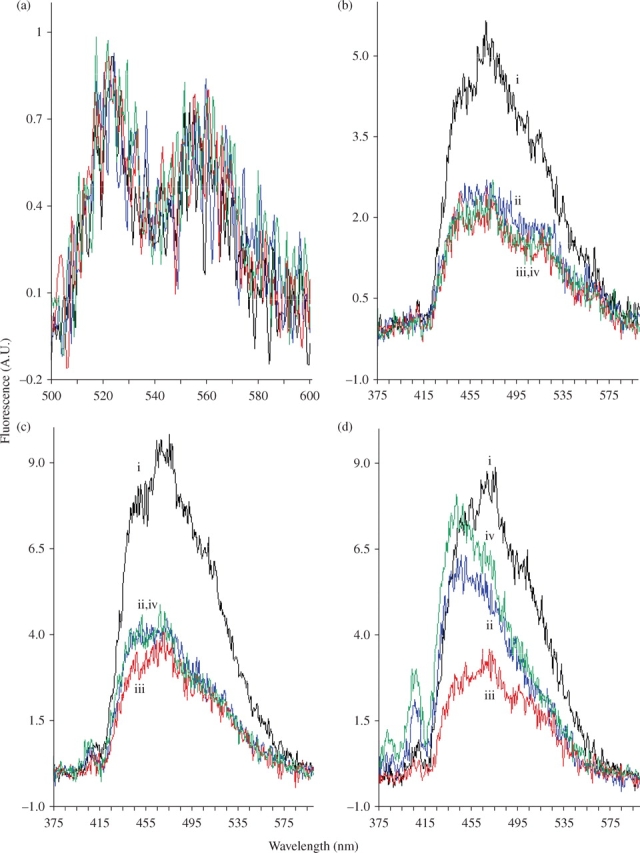
Emission spectra (arbitrary units, A.U.) of USP AmB (i), generic AmB (ii), AmBHP (iii) and AmBHP in the presence of nystatin (iv). Each AmB preparation was dissolved in DMSO to a final concentration of ∼10 µM; nystatin was added to a final concentration of 0.5 µM. (a) 408 nm excitation, (b) 350 nm excitation, (c) 335 nm excitation and (d) 325 nm excitation. Since spectra in (a) are not distinguishable, they are not individually labelled. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Figure 2.
Excitation spectra (arbitrary units, A.U.) of USP AmB (i), generic AmB (ii), AmBHP (iii) and AmBHP in the presence of nystatin (iv). Each AmB preparation was dissolved in DMSO to a final concentration of ∼10 µM; nystatin was added to a final concentration of 0.5 µM. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Generic AmB absorbance and fluorescence concentration dependence in water
Absorbance spectra were recorded between 1 and 200 µM, and fluorescence spectra between 1 and 20 µM with the dual path length cuvette. Fluorescence excitation wavelength was set at 325 nm and emission was recorded at 473 nm. Under these conditions, self-screening (inner filter) effects were negligible on the emission channel. On the excitation channel, for 10 and 20 µM samples, the high absorbance (0.14 and 0.28, respectively) justified the introduction of a correction factor (1.25 and 1.42, respectively).8
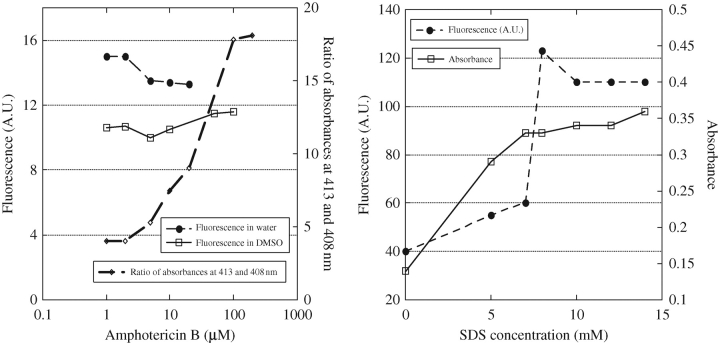
At low (1 µM) concentrations of AmB, a vibronic structure was observed in UV-visible absorption, with the first band located at 409 nm (AmB monomers). With increasing AmB concentrations, new bands appeared at 418 and 340 nm (AmB self-associated species; data not shown). The ratio of UV-visible absorbances at 418 and 408 nm is presented in Figure 3(a). It rapidly increases above 2 µM and reaches a plateau at 100 µM. Concerning fluorescence, its intensity remained approximately constant when the dilution factor was taken into account (Figure 3a).
Figure 3.
Fluorescence (arbitrary units, A.U.) and absorbance dependence on AmB concentration or SDS addition. Fluorescence: excitation wavelength set at 325 nm and emission recorded at 473 nm (data were corrected for the dilution). Absorption: 409 nm. (a) Dependence on AmB concentration in water and DMSO. (b) Influence of increasing SDS concentration (5 µM AmB).
Generic AmB absorbance and fluorescence concentration dependence in DMSO
Absorbance and fluorescence spectra were recorded between 0.5 and 100 µM. Absorbance spectra were identical all along this concentration range (extinction coefficient remained the same). Fluorescence concentration dependence of AmB in DMSO (emission wavelength 325 nm, excitation wavelength 470 nm) is shown in Figure 3(a). Correction factors were applied above 20 µM. Taking into account the dilution factor, fluorescence appeared to remain approximately constant (with higher incertitude for 0.5 µM concentration due to the weakness of the signal).
Generic AmB absorbance and fluorescence in the presence of increasing concentrations of SDS in water
Upon the addition of increasing concentrations of SDS, the intensity of the AmB monomer band at 409 nm increased progressively and shifted to 416 nm (data not shown), whereas absorbance at 340 nm decreased. Concomitantly, the dichroic doublet centred approximately at 340 nm decreased (data not shown). Figure 3(b) shows the influence of the increasing concentration of SDS on 5 µM AmB absorbance and fluorescence intensities. The absorbance (409 nm) of AmB increased progressively with the addition of SDS to concentrations up to 6 M, but plateaued upon the addition of higher concentrations of SDS. Concerning fluorescence, between 7 and 8 M SDS, an abrupt increase in AmB fluorescence occurred, followed by a plateau for higher SDS concentrations.
Discussion
Cytokine release has been a reliable in vitro marker of AmB cellular toxicity. Prostaglandin (PGE2), interleukin (IL)-1-β and tumour necrosis factor (TNF)-α release from murine or human monocytes appears secondary to transcription of the IL-1-β and TNF-α genes, which is mediated, at least in part, by a calcium-calmodulin-dependent activation of the pro-IL-l-p gene.9 AmB formulations appear to differ in their potential for cellular toxicity based on IL-1-β expression. Difference in cytokine expression could not be explained by differences in AmB content. The most common polyene antifungal agents consist of tetraenes (nystatin and amphotericin A), pentaenes (euricidins and filipins), hexaenes and heptaenes (AmB). The fermentation preparation from Streptomyces contains high quantities of amphotericins A and B, which are separated after purification from the fermentation nutrient. We previously hypothesized that amphotericin A was the toxic component in the formulations. Although amphotericin A was ultimately excluded, another polyene product or combination of polyenes could still be responsible.10 Previous in vivo evaluation of AmBHP from raw materials found differences in toxicity. Overall, AmBHP toxicity, at standard doses, is significantly less serious and less frequent, being similar to the lipid-associated AmB formulations when compared with AmB deoxycholate.1
Therefore, impurities could contribute to AmB fluorescence. To add supplementary proof to this hypothesis, we studied the concentration dependence of AmB fluorescence in DMSO and in aqueous media and analysed the effect of SDS addition in aqueous media. At low concentrations, the absorption and fluorescence spectra of the antibiotics nystatin, filipin and AmB present characteristic properties due to the presence of a polyene chain (tetraene, pentaene and heptaene, respectively). The all-trans polyenes exhibit a strong transition in the near ultraviolet or visible wavelengths, with an oscillator strength of typically one or two, increasing in magnitude with chain length, and energy decreasing towards a constant value at long chain length (18 000 cm−1, i.e. 555 nm).11 A well-resolved vibrational structure is observed in these antibiotics, with a strong first peak located at 320 nm for nystatin in ethanol, 361 nm for filipin in N,N-dimethylformamide12 and 416 nm for AmB in DMSO.13 The fluorescence spectra of all-trans polyenes show approximately the same vibrational structures and intensity patterns as do the absorption spectra. The two spectra are, therefore, roughly mirror images.11
In aqueous media, and at concentrations above ∼1 µM, AmB self-association occurs, and new absorption and circular dichroism (CD) spectra have been well described.14 Coupling of the polyene chromophores due to self-association is observed and leads to excitonic coupling and blue-shift of the absorption bands concomitantly with the appearance of a biphasic signal in the CD at ∼325 nm.
AmB fluorescence spectra in DMSO
AmB is soluble in DMSO (in excess of 10 mM) and is only present as a monomer in this solvent. Indeed, below 10 mM, its absorption spectrum is representative of a monomeric form.15 In other words, AmB exhibits a band with a vibronic structure around 400 nm (peaks at 416, 391, 372, 352 nm) and a weak CD similar in shape to the absorption spectrum.14 By excitation in the first band of the vibrational progression (emissions near 416 nm), a weak fluorescence spectrum is observed, with two bands at 525 and 560 nm (Figure 1a). The weakness of this fluorescence is as expected.2 Gruszecki et al.4 also found weak fluorescence (quantum yield 0.0006) for AmB in aqueous medium.
No trace of an excitonic dichroic doublet, which would be representative of AmB self-association, was found around 325 nm (data not shown). Despite the absence of absorption or CD evidence for AmB self-association in DMSO, we observed fluorescence by excitation in the 325 nm region (Figure 1b–d). Surprisingly, whereas fluorescence emission spectra of the different AmB formulations obtained by excitation at 408 or 416 nm were identical in profile and intensity, strong differences were observed by excitation around 325 nm. Between 0.5 and 100 µM, the intensity of this fluorescence (taking into account the dilution factor) remained approximately constant, which confirms the absence of self-association. Since dissolution of AmB in DMSO should lead to no self-association, the fluorescence observed under these conditions should not be assigned to AmB dimers, but rather to an impurity. However, Gruszecki and Herec,16 in a second study, did not evaluate this objective.
There is a well-known precipitous reduction in emission yields with increasing polyene length.17 For instance, the polyene low-temperature fluorescence yields decrease from 0.6 for octatetraene, 0.01 for dodecahexaene and approximately 0.001 for heptaenes. As a consequence, a general problem is encountered in the study of polyene fluorescence.17 The interfering emission from shorter polyenes present as impurities, even in highly purified samples, is undetectable by absorption measurements (the shorter the intensity of the oscillator strength, the shorter the polyene chain) (discussed earlier). This situation is found in the present study: emission spectra are found in the 460 nm region upon excitation between 325 and 350 nm, whereas the spectrum observed by excitation at 416 nm is not visible (Figure 2). Chemical grade AmB contains impurities, although they are not detectable by absorption or CD. This interpretation is in agreement with the results of Petersen and Henshaw.2 These investigators removed a tetraene contaminant from a commercial AmB by reverse-phase HPLC and found that AmB did not exhibit significant intrinsic fluorescence.
Most interestingly, the fluorescence intensity and profile depend on the sample origin. As expected, AmBHP is a significantly less fluorescent sample. From the excitation profile measured at 471 nm, it appears that all samples contain a polyene exhibiting peaks at ~325, 340 and 358 nm in excitation and, approximately, 475 nm in emission. These wavelengths fit well with the pentaene characteristics of filipin, making it a good candidate for AmB formulation impurity.18,19 Also, the fact that the quantum yield of filipin (0.08) is about 100 times higher than that of AmB makes it easily detected by fluorescence.20 Whatever the pentaene impurity (or impurities), USP AmB appears to contain approximately twice as much as generic AmB and AmBHP do. Both generic AmB and AmBHP appear to contain the same amount of pentaene impurity. On the other hand, it appears that generic AmB contains another impurity that is not contained in USP AmB or AmBHP. This additional impurity has a fluorescence excitation that is shifted towards lower wavelengths and maximal emission at ~440 nm. Nystatin, a tetraene, presents fluorescence in these regions.21 Its addition, at a low percentage (5%), to AmBHP leads to a fluorescence spectrum mimicking that of generic AmB (Figure 1c and d). Taken as a whole, these data indicate the presence of tetraene and pentaene impurities in generic AmB formulations.
AmB fluorescence spectra in aqueous media
As mentioned earlier, the analysis of AmB fluorescence in aqueous media is complicated, when compared with that in DMSO, by the fact that AmB is poorly soluble and is, therefore, partly present under self-associated or aggregated forms. Moreover, the concentration of the associated form increases directly with total AmB concentration. As a consequence, absorption and CD spectra not only present bands representative of the monomers (first band around 409 nm) as in DMSO, but also ‘excitonic’ bands around 325 nm representative of the self-associated species.14 Surprisingly, however, the fluorescence spectra of AmB in DMSO (where self-association is not expected to occur) present characteristics similar to those observed in aqueous media.4,5,18
As the ‘excitonic’ bands are in the same region as those of the impurities considered earlier, the possible participation of self-associated AmB dimers to fluorescence is to be considered. Indeed, Gruszecki et al.4 assigned the fluorescence obtained by excitation with short wavelength radiation (below 350 nm) in 2-propanol/water mixtures to dimeric AmB. Their assignment was mainly based on a van't Hoff plot of the ratio of the fluorescence intensity at 352 and 408 nm and the change of this ratio with the solvent composition. Others have argued that these data could alternatively be explained by consideration of the classical equilibrium of AmB self-association in water.3 In the present study, we varied the AmB concentration between 1 and 50 µM, and found that fluorescence remained approximately constant (Figure 3a). However, by scanning this concentration domain, we found a strong increase in the ratio of absorbances at 413 and 408 nm (Figure 3a), indicative of self-association.14 This contradiction indicates that the observed fluorescence is not related to self-association but rather to the presence of tetraene and pentaene impurities, as suggested earlier.
In the present study, we also determined the influence of SDS on the fluorescence of AmB at a concentration of 5 µM. At a concentration (∼8 mM) of SDS at which its micellization occurs, there was a sudden increase in AmB fluorescence emission at 473 nm (excitation at 325 nm) that was accompanied by an increase in the absorbance of AmB around 410 nm (Figure 3b).5 A red-shift to 416 nm in AmB absorbance was also observed, as already described.22 These optical changes are characteristic of monomeric AmB. Therefore, we observed the reverse of what would have been expected if the fluorescence originated from dimeric AmB: addition of the detergent would have destroyed the AmB aggregates and abolished fluorescence. In contrast, if we assign the fluorescence observed at 410 nm to tetraene or pentaene impurities, the fluorescence increase can be favourably compared with that observed with other polyene antibiotics (etruscomycin, nystatin and filipin) in the presence of increasing concentrations of lipids.18–21,23 Selvam and Mishra5 used fluorescence to study the disaggregation of AmB upon addition of sodium deoxycholate. In agreement with our results on SDS, they observed an increase in fluorescence emission at 472 nm (excitation 335 nm) that we can obviously explain, as in our experiment, by the increase in fluorescence of monomeric impurities inserting into the detergent aggregates. However, surprisingly, these authors concluded that the addition of deoxycholate induces the formation of AmB dimeric forms. Stoodley et al.7 applied the assignment of Gruszecki et al.4 and low excitation wavelength fluorescence to compare the aggregation states of AmB and heat-treated AmB in the presence of SDS. They proposed a new basis for examining the super-aggregated character of AmB preparations. However, their results were biased by the use of high AmB concentrations (50 µM) without the introduction of a correction factor for a self-screening effect. As a consequence, their results are difficult to interpret.
Of course, besides the impurity hypothesis, there is still the possibility of some self-associated species of AmB with a very high association constant that accounts for only a minor part of all AmB (self-associated or aggregated) species. The absorbance and CD spectra of such a species in an aqueous medium could easily be hidden behind those of more abundant species. Likewise, spectral band weakness would prevent its detection in DMSO or in the presence of SDS. Very weak absorption bands are observed in DMSO between 300 and 350 nm (approximately at 323 and 336 nm) at an AmB concentration of 0.5 µM, but these bands likely represent the tail of the vibronic progression of the monomers. However, let us admit for the sake of argument that these bands do indeed originate from a self-associated AmB species. Its maximal absorption is approximately 100 times weaker than that of AmB monomers at 415 nm, suggesting that the concentration of the fluorescing species would be around 5 nM. It is quite unlikely that self-association occurs to the same extent at this concentration as at 100 µM.
Finally, two supplementary arguments can be introduced against the ‘dimeric’ interpretation. One, the fluorescence excitation profile of the ‘dimer’ is the same for AmB, Fungizone and heat-treated Fungizone.4,7 In contrast, absorption maxima for the three agents occur, respectively, at 340, 327 and 321 nm. Two, the vibronic structure of the fluorescence spectra does not fit with a bisignated structure resulting from a (putative) excitonic interaction.
Conclusions
On the basis of the arguments presented here, it appears that pentaene or tetraene impurities are the primary determinants of AmB fluorescence around 470 nm when excitation is around 325 nm. As a consequence, fluorescence should not be used under these conditions to study the interaction of AmB with membranes. As for the fluorescence specific to AmB monomers obtained by excitation around 408 nm, it is too weak to make it an appropriate tool for such studies.
Funding
This work was supported in part by the National Institute of Health NIH/NIAID (R41 AI63 935-01A1) entitled ‘High purity amphotericin B: a safer antimycotic in AIDS’ and Cumberland Pharmaceuticals. It was also supported by Centre National de la Recherche Scientifique and Université Pierre et Marie Curie (UMR 7033).
Transparency declarations
J. D. C. and R. E. K. have not received funds for the research presented in this article; however, J. D. C. and R. E. K. are patent holders of amphotericin B high purity and have a license agreement with Cumberland Emerging Technologies, Inc., Nashville, TN, USA, for its development. J.B.: none to declare.
References
- 1.Cleary JD, Chapman SW, Swiatlo E, et al. High purity amphotericin B. J Antimicrob Chemother. 2007;60:1331–40. doi: 10.1093/jac/dkm322. [DOI] [PubMed] [Google Scholar]
- 2.Petersen NO, Henshaw PF. Separation of fluorescent impurities from amphotericin B. Can J Chem. 1981;59:3377–8. [Google Scholar]
- 3.Bolard J, Chéron M. Does fluorescence spectroscopy detect dimers of the polyene antibiotic amphotericin B? J Photochem Photobiol B. 2003;72:101–2. doi: 10.1016/j.jphotobiol.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Gruszecki WI, Gagos M, Herec M. Dimers of polyene antibiotic amphotericin B detected by means of fluorescence spectroscopy: molecular organization in solution and in lipid membranes. J Photochem Photobiol B. 2003;69:49–57. doi: 10.1016/s1011-1344(02)00405-0. [DOI] [PubMed] [Google Scholar]
- 5.Selvam S, Mishra AK. Disaggregation of amphotericin B by sodium deoxycholate micellar aggregates. J Photochem Photobiol B. 2008;93:66–70. doi: 10.1016/j.jphotobiol.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 6.Bau P, Bolard J, Dupouy-Camet J. Heated amphotericin to treat leishmaniasis. Lancet Infect Dis. 2003;4:188. doi: 10.1016/s1473-3099(03)00573-5. [DOI] [PubMed] [Google Scholar]
- 7.Stoodley R, Wasan KM, Bizzotto D. Fluorescence of amphotericin B-deoxycholate (Fungizone) monomers and aggregates and the effect of heat-treatment. Langmuir. 2007;23:8718–25. doi: 10.1021/la7008573. [DOI] [PubMed] [Google Scholar]
- 8.Lakowicz JR. Principles of Fluorescence Spectroscopy, Second Edition. New York: Kluwer Academic/Plenum Publishers; 1999. p. 54. [Google Scholar]
- 9.Rogers PD, Kramer RE, Chapman SW, et al. Amphotericin B-induced IL-1β expression in human monocytic cells is calcium and calmodulin dependent. J Infect Dis. 1999;180:1259–66. doi: 10.1086/315004. [DOI] [PubMed] [Google Scholar]
- 10.Cleary JD, Rogers PD, Chapman SW. Variability in polyene content and cellular toxicity among deoxycholate amphotericin B formulations. Pharmacotherapy. 2003;23:572–8. doi: 10.1592/phco.23.5.572.32209. [DOI] [PubMed] [Google Scholar]
- 11.Hudson B, Kohler B. Linear polyene electronic structure and spectroscopy. Ann Rev Phys Chem. 1974;25:437–60. [Google Scholar]
- 12.Bittman R, Fischkoff SA. Fluorescence studies of the binding of the polyene antibiotics filipin III, amphotericin B, nystatin, and lagosin to cholesterol. Proc Natl Acad Sci USA. 1972;69:3795–9. doi: 10.1073/pnas.69.12.3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petersen NO, Gratton R, Pisters EM. Fluorescence properties of polyene antibiotics in phospholipid bilayer membranes. Can J Chem. 1987;65:238–44. [Google Scholar]
- 14.Bolard J. How do the polyene macrolide antibiotics affect the cellular membrane properties? Biochim Biophys Acta. 1986;864:257–304. doi: 10.1016/0304-4157(86)90002-x. [DOI] [PubMed] [Google Scholar]
- 15.Hammond SM. Biological activity of polyene antibiotics. Prog Med Chem. 1977;14:106–79. doi: 10.1016/s0079-6468(08)70148-6. [DOI] [PubMed] [Google Scholar]
- 16.Gruszecki WI, Herec M. Dimers of polyene antibiotic amphotericin B. J Photochem Photobiol B. 2003;72:103–5. doi: 10.1016/s1011-1344(02)00405-0. [DOI] [PubMed] [Google Scholar]
- 17.Snyder R, Arvidson E, Foote C, et al. Electronic energy levels in long polyenes: S2 .fwdarw. S0 emission in all-trans-1,3,5,7,9,11,13-tetradecaheptaene. J Am Chem Soc. 1985;107:4117–22. [Google Scholar]
- 18.Bittman R, Chen WC, Anderson OR. Stopped-flow kinetic and equilibrium studies of filipin 3 binding to sterols. Biochemistry. 1974;13:1374–79. doi: 10.1021/bi00704a010. [DOI] [PubMed] [Google Scholar]
- 19.Castanho MA, Coutinho A, Prieto MJ. Absorption and fluorescence spectra of polyene antibiotics in the presence of cholesterol. J Biol Chem. 1992;267:204–9. [PubMed] [Google Scholar]
- 20.De Kruijff B, Demel RA. Polyene antibiotic–sterol interactions in membranes of Acholeplasma laidlawii cells and lecithin liposomes. 3. Molecular structure of the polyene antibiotic–cholesterol complexes. Biochim Biophys Acta. 1974;339:57–70. doi: 10.1016/0005-2736(74)90332-0. [DOI] [PubMed] [Google Scholar]
- 21.Coutinho A, Prieto M. Self-association of the polyene antibiotic nystatin in dipalmitoyl-phosphatidylcholine vesicles: a time-resolved fluorescence study. Biophys J. 1995;69:2541–57. doi: 10.1016/S0006-3495(95)80125-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsumori N, Houdai T, Murata M. Conformation and position of membrane-bound amphotericin B deduced from NMR in SDS micelles. J Org Chem. 2007;72:700–6. doi: 10.1021/jo061309p. [DOI] [PubMed] [Google Scholar]
- 23.Strom R, Crifo C, Bozzi A. The interaction of the polyene antibiotic lucensomycin with cholesterol in erythrocyte membranes and in model systems. I. A fluorimetric and spectrophotometric study. Biophys J. 1973;13:568–80. doi: 10.1016/S0006-3495(73)86007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]