Abstract
Background
Switching a thymidine analogue to a non-thymidine analogue or changing to a nucleoside-sparing regimen has been shown to partially reverse peripheral lipoatrophy. The current study evaluated both approaches.
Methods
Subjects at 15 AIDS Clinical Trial Group sites receiving thymidine analogue stavudine- or zidovudine-containing regimens with plasma HIV RNA ≤500 copies/mL and lipoatrophy were prospectively randomized to: (i) switch the thymidine analogue to abacavir; (ii) discontinue all antiretrovirals and switch to lopinavir/ritonavir plus nevirapine (LPV/r+NVP); or (iii) delay switching for 24 weeks (ClinicalTrials.gov identifier: NCT00028314). Single-slice computer tomography of mid-thigh and abdominal fat and metabolic and virological/immunological parameters were measured at baseline and weeks 24 and 48.
Results
Among the 101 patients enrolled, there were significant subcutaneous thigh fat and subcutaneous abdominal tissue (SAT) increases over time and decreases in visceral adipose tissue to total adipose tissue (VAT:TAT) ratios for both interventions, and a decrease in VAT for abacavir. CD4 increased in the LPV/r+NVP arm. LPV/r+NVP had a significantly shorter time to grade 3 or higher toxicity (P = 0.007), but discontinuation rates were similar. Glucose levels did not change, but insulin decreased in the LPV/r+NVP arm. Lipids tended to increase in the LPV/r+NVP arm.
Conclusions
Switching stavudine or zidovudine to a non-thymidine analogue or changing to a nucleoside reverse transcriptase inhibitor-sparing regimen is associated with qualitatively similar improvements in thigh fat, SAT and VAT:TAT ratio at 48 weeks. Abacavir also resulted in VAT reductions and LPV/r+NVP resulted in CD4 count increases.
Keywords: nucleoside reverse transcriptase inhibitors, thymidine analogues, stavudine, zidovudine, antiretroviral treatment
Introduction
HIV lipodystrophy is characterized by the accumulation of fat in the abdomen and other regions of the body (lipoaccumulation) and by the loss of subcutaneous adipose tissue (lipoatrophy) of the extremities, buttocks and face.1 The initiation of thymidine-analogue-based antiretroviral treatment is characterized by initial gains in limb and abdominal fat, followed by progressive limb fat loss.2,3 Lipoatrophy is the most characteristic morphological change associated with the treatment of HIV infection and the one with the most profound metabolic and psychological impact for the patient.4,5 Although a clear understanding of all the causes that underlie the development of this problem is still lacking, associations have been seen with a series of host-related factors, including age,6–10 gender,8,9 race,7,10,11 baseline body mass index,7,11 diet, exercise12 and genetic factors. Treatment-related factors are also a significant contributor of lipoatrophy, as this problem is more frequently associated with the use of particular nucleoside combinations (especially stavudine) and protease inhibitors3,6,8,11,13,14 and the duration of treatment.6,9–11,14 Other contributing factors include the duration of HIV infection, the severity of immune depletion6,7,11,15 and the magnitude of immune reconstitution.16
Although the new antiretroviral regimens seem to be less prone to induce the development of these problems, long-term follow-up is still limited.17 Many patients who started antiretroviral treatment in the late 1990s and early 2000s are living with this complication. Cross-sectional studies estimate the prevalence of HIV lipoatrophy to be 30% to 50%,4,6 although these estimates suffer from the lack of a universally accepted definition in spite of numerous attempts to develop one.18 Current therapeutic options include surgery,19–21 switch to a less toxic antiretroviral regimen22–25 and potentially uridine supplementation.26 In general, studies have not compared different strategies.
The primary objective of AIDS Clinical Trial Group (ACTG) A5110 was to investigate whether highly active antiretroviral therapy-associated peripheral fat wasting in subjects with maximal suppression of HIV-1 RNA is reversible by discontinuation of the thymidine analogue and substitution with abacavir, or by a change to a nucleoside analogue-sparing regimen.
Methods
ACTG A5110 was a prospective, restrictively randomized, open-label, controlled pilot study of two interventions to reverse HIV lipoatrophy (ClinicalTrials.gov identifier: NCT00028314). The first intervention involved thymidine analogue substitution with abacavir. The second intervention involved the discontinuation of the current antiretroviral therapy regimen and a change to a nucleoside analogue-sparing regimen. The study was approved by the local Institutional Review Board of all of the institutions and all participants provided informed consent. HLA B5701 status was not determined before enrolment.
Participants
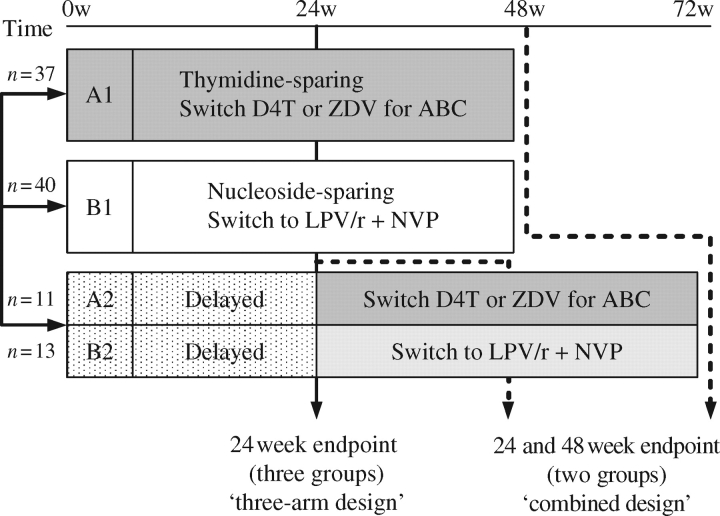
A5110 enrolled subjects with self-reported peripheral fat wasting, including atrophy of the extremities since the initiation of highly active antiretroviral therapy with plasma HIV-1 RNA ≤500 copies/mL receiving zidovudine- or stavudine-containing regimens. The subjects were restrictively randomized into the following four arms in a 2:2:1:1 ratio (Figure 1). Randomization was stratified for receipt of stavudine versus zidovudine at entry.
A1: Immediate discontinuation of stavudine/zidovudine, substituting it with abacavir for 48 weeks, maintaining other antiretrovirals.
B1: Immediate discontinuation of the current antiretroviral treatment regimen and substitution with lopinavir plus low-dose ritonavir+nevirapine (LPV/r+NVP) for 48 weeks. The dose of lopinavir/ritonavir used was four (133.3/33.3 mg) capsules by month, twice daily with food.
A2: Continuation of the current stavudine/zidovudine-containing regimen for 24–28 weeks and then substitution with abacavir for an additional 48 weeks.
B2: Continuation of the current antiretroviral regimen for up to 24–28 weeks and then substitution with LPV/r+NVP for an additional 48 weeks.
Figure 1.
Design of the study. Individuals were randomized to A1, B1, A2 or B2. The assumption was that the population of subjects that delayed switching would be similar at the time of their switch to the population of subjects that switched at randomization, so groups A2 and B2 would be added to A1 and B1 for the ‘combined design’ using the week 24 evaluations as their baseline evaluation for this analysis. w, week; D4T, stavudine; ZDV, zidovudine; ABC, abacavir.
The purpose of this design was to have a control group to help assess the relevance of within-arm changes after switching treatment in all participants. We assumed that the population of subjects that delayed switching would be similar (at the time of their switch) to the population of subjects that switched at week 0. Subjects who had received abacavir before entry were eligible for the study (n = 11), but were assigned directly to arms B1 and B2, the nucleoside-sparing arm. Subjects who were intolerant to or failed therapy with lopinavir/ritonavir or nevirapine or who had to remain on lamivudine for hepatitis B therapy were randomized directly to one of the abacavir arms (n = 9).
After the results of the MITOX study22 were presented demonstrating that the discontinuation of thymidine analogues was associated with improvements of limb fat in subjects with lipoatrophy, it was considered unethical to delay the switch of antiretrovirals in patients with lipoatrophy and the delayed switch arms were discontinued by immediately switching the subjects in the first 24 weeks on those arms to their respective abacavir or LPV/r+NVP arms (version 3.0). As a consequence of this amendment, the targeted sample size was reduced from 150 to 100 subjects.
Measurements
Every 24 weeks, mid-thigh computer tomography (CT) (midpoint of the left femur) and abdominal CT scans (at the interspace between L4 and L5) were acquired using a standardized ACTG protocol and read centrally at Tufts University by a single technician who was unaware of the patient assignment. Fasting blood was obtained and metabolic parameters were measured at the same timepoints.
Fasting assays were performed at Quest Diagnostics Incorporated (Baltimore, MD, USA) on specimens stored at −70°C. Plasma glucose concentrations were measured on specimens stored in sodium fluoride/potassium oxalate using a hexokinase technique. Plasma insulin concentration was measured on heparinized specimens by a two-site chemiluminescent enzyme-labelled immunometric assay using a technique insensitive to proinsulin (DPC Immulite 2000; Quest Diagnostics). Total cholesterol, high density lipoprotein (HDL) cholesterol and triglycerides were measured using enzymatic techniques. Low density lipoprotein (LDL) cholesterol was calculated by the Friedewald equation and not measured directly, so non-HDL cholesterol is presented (calculated as total cholesterol minus HDL cholesterol). Mitochondrial DNA and RNA copies per peripheral blood mononuclear cell (PBMC) were measured in frozen samples by PrimaGen Inc. (Amsterdam, The Netherlands) using their nucleic acid sequence-based amplification (NASBA)-based assay (Retina™ Mitox assay, Primagen Inc.).27 Plasma HIV-1 RNA was measured by the UltraSensitive Roche Amplicor™ HIV-1 Monitoring Assay. CD4 T cell counts were quantified using flow cytometry.
Statistical analysis and considerations
The primary endpoint of the study was the percentage change from baseline in thigh subcutaneous adipose cross-sectional area as measured using CT scanning at 24 weeks. Secondary endpoints included changes in subcutaneous and visceral fat in the abdomen; metabolic parameters, including lipids, glucose and mitochondrial metabolism; and safety (adverse events and virological failure).
Fifty subjects per arms A and B were required to detect a 30% difference from baseline in thigh subcutaneous adipose tissue cross-sectional areas within arms at 24 weeks. This calculation was based on the use of a one-sample t-test and then adjusted using Pitman efficiency (0.864) for use with a Wilcoxon non-parametric test with two-sided α = 0.05 and 80% power. The study had limited power to detect between-arm changes, but the comparisons were planned.
Two types of analyses were performed for this study: (i) a primary analysis based on the ‘three-arm design’; and (ii) an analysis based on a ‘combined design’. In the ‘three-arm design’, data from A2/B2 subjects before they switched treatments were combined into a single control arm to represent the natural history of continued stavudine/zidovudine use. In the ‘combined design’, data from A2/B2 subjects after they switched treatment were combined with data from the A1 and B1 arms, respectively. As the assumption that metabolic and CT parameters would not change during the first 24 weeks in individuals who delayed the switch was confirmed, and the delayed arms were closed after the publication of the MITOX study,22 we preferentially present the results of the ‘combined design’. The week 24 evaluations in the delayed arms (A2/B2) are considered to be the baseline for the combined design (Figure 1). The analysis presented is intent to treat. The subjects who were restrictedly assigned to the treatment arms were included in the analysis. Sensitivity analyses using a last observation carried forward were conducted to evaluate the impact of missing data.
Descriptive statistics are presented to describe the study sample. Fisher's exact test was used to assess associations between categorical variables. Within-arm evaluations are performed using Wilcoxon signed-rank tests and between-arm comparisons are performed using Kruskal–Wallis tests. Graphical methods are used to illustrate trends. Spearman's correlation is used to assess correlation between continuous variables. All significance testing is performed at the α = 0.05 level.
Results
One hundred and one subjects were enrolled between May 2002 and April 2004 (Table 1). Participants were 85% male and 69% white, with a median age of 46 years. Seventy-seven subjects were on stavudine at entry and 24 on zidovudine. Five participants discontinued the study before completion: three in the LPV/r+NVP arm and two in the abacavir arm.
Table 1.
Baseline characteristics
| Three-arm design |
Combined design |
||||
|---|---|---|---|---|---|
| delayed (n = 24) | abacavir (n = 37) | LPV/r+NVP (n = 40) | abacavir (n = 48) | LPV/r+NVP (n = 53) | |
| Male (%) | 92 | 81 | 85 | 81 | 89 |
| White (%) | 71 | 70 | 68 | 69 | 70 |
| Hx IVDU (%) | 21 | 5 | 8 | 13 | 8 |
| Age in years (median) | 46 | 48 | 45 | 48 | 45 |
| CD4 cells/mL (median) | 572 | 598 | 645 | 592 | 603 |
| HIV RNA >200 copies/mL (%) | 4 | 3 | 6 | 2 | 4 |
| Percentage on stavudine | 88 | 78 | 68 | 81 | 72 |
| Weeks of stavudine exposure | 210 (11, 274) | 198 (0, 282) | 95 (0, 274) | 206 (0, 292) | 105 (0, 249) |
| Weeks of zidovudine exposure | 0 (0, 28) | 0 (0, 0) | 0 (0, 33) | 0 (0, 39) | 0 (0, 11) |
| CT measurements | |||||
| thigh subcutaneous adipose tissue (cm2) | 19 (7, 27) | 15 (7, 29) | 20 (11, 29) | 16 (7, 30) | 19 (9, 26) |
| SAT (cm2) | 78 (34, 111) | 60 (39, 170) | 85 (53, 121) | 63 (35, 156) | 74 (51, 119) |
| VAT (cm2) | 94 (50, 146) | 142 (82, 195)a | 115 (71, 160) | 122 (83, 185) | 111 (60, 155) |
| VAT:TAT ratio | 0.53 (0.45, 0.70) | 0.64 (0.46, 0.77) | 0.57 (0.44, 0.68) | 0.63 (0.47, 0.79) | 0.58 (0.44, 0.68) |
| Glucose metabolism | |||||
| glucose (mg/dL) | 89 (84, 96) | 89 (82, 96) | 89 (81, 96) | 90 (82, 98) | 90 (84, 97) |
| insulin (U/mL) | 8.5 (6, 23) | 12 (8, 19) | 14 (9.5, 26) | 12 (6, 19) | 14 (8, 25) |
| Lipid metabolism | |||||
| HDL (mg/dL) | 29 (22, 41) | 34 (30, 41) | 32 (27, 41) | 34 (27, 43) | 31 (26, 41) |
| non-HDL cholesterol (mg/dL) | 173 (139, 190) | 166 (143, 190) | 167 (132, 203) | 168 (143, 197) | 163 (133, 200) |
| LDL cholesterol (mg/dL) | 118 (94, 143) | 120 (105, 143) | 110 (97, 140) | 121 (104, 146) | 111 (96, 139) |
| total cholesterol (mg/dL) | 199 (188, 239) | 201 (174, 217) | 196 (173, 233) | 204 (179, 225) | 194 (170, 230) |
| triglycerides (mg/dL) | 331 (159, 524) | 196 (119, 296) | 288 (148, 428) | 197 (128, 354) | 299 (147, 428) |
| Other | |||||
| venous lactate (mmol/L) | 1.5 (0.9, 1.7) | 1.6 (1, 1.9) | 1.3 (1, 2) | 1.5 (1, 2) | 1.4 (1, 2) |
| mitochondrial RNA (copies/cell) | 1156 (648, 2786) | 1492 (946, 3310) | 1387 (739, 3410) | 1501 (984, 3378) | 1387 (739, 3410) |
| mitochondrial DNA (copies/cell) | 147 (113, 255) | 180 (100, 299) | 170 (106, 223) | 179 (101, 257) | 169 (103, 228) |
Unless stated, the values represent median (IQR). Hx IVDU, history of intravenous drug use.
There were no statistical differences between the treatment arms except for VAT, which was higher in the abacavir arm (aP < 0.05). There were no significant differences between the arms in the ‘combined’ design.
The total weeks of stavudine and zidovudine exposure were calculated as a median (IQR) for the whole group at the time of step 2 randomization.
There was no evidence of clinically significant differences for demographics, CD4 T cell count and HIV-1 RNA, and the metabolic parameters including fasting glucose, insulin, HDL, non-HDL and total cholesterol, triglycerides or mitochondrial RNA/DNA copies (Table 1) among the three arms at baseline. For the combined design, median baseline CD4 T cell count was 592 cells/mm3 for the abacavir arm and 603 cells/mm3 for the LPV/r+NVP arm.
In the three-arm design, visceral adipose tissue (VAT) was higher in the abacavir arm (142 cm2) versus the delayed arm (94 cm2) and the LPV/r+NVP arm (115 cm2). Otherwise, there was no evidence of significant differences in other CT scan measurements among the three original arms. For the combined design, there was no evidence of significant differences in the above baseline measurements between the two treatment arms.
As the delayed arms were closed after the presentation of the MITOX study (see the Methods section) and there were no significant changes in CT measurements or in glucose, lipid and other metabolic parameters between weeks 0 and 24 among individuals that delayed the change in the antiretroviral regimen, the results from the combined design (Figure 1) are presented unless stated otherwise. These results include changes in all individuals for 48 weeks after the switch.
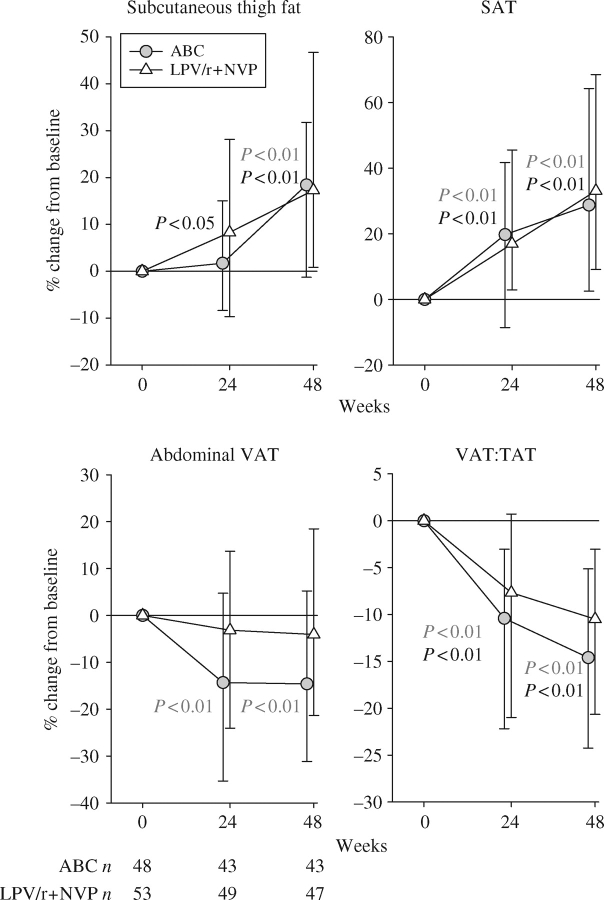
Subcutaneous thigh fat increased significantly in LPV/r+NVP at week 24. Subcutaneous thigh fat increased significantly for both interventions at week 48. At week 24 with the ‘three-arm design’, the median percentage changes were −3% [interquartile range (IQR) −12% to 11%, P = 0.66] in the continuation arm, 0% (IQR −11% to 16%, P = 0.57) in the abacavir arm and +8% (IQR −11% to 31%, P = 0.08) in the LPV/r+NVP arm. For the combined design at 24 weeks, the median percentage changes were 2% (IQR −8% to 15%, P = 0.40) in the abacavir arm and 8% (IQR −10% to 28%, P = 0.02) in the LPV/r+NVP arm. Forty-eight weeks after the switch, subcutaneous thigh fat increased by 18% (P < 0.01) in the abacavir arm and by 17% (P < 0.01) in the LPV/r+NVP arm, but these increments were not significantly different between the arms (Table 2 and Figure 2).
Table 2.
Percentage change from baseline in CT measurements of adipose area in the combined design at weeks 24 and 48
| Abacavir (n = 48) |
LPV/r+NVP (n = 53) |
|||||
|---|---|---|---|---|---|---|
| Measurement | Treatment arm week | n | % change | n | % change | Between arm P value |
| Subcutaneous thigh fat | 24 | 42 | 2 | 48 | 8* | 0.33 |
| 48 | 42 | 18** | 46 | 17** | 0.57 | |
| SAT | 24 | 43 | 20** | 49 | 17** | 0.62 |
| 48 | 43 | 29** | 47 | 33** | 0.6 | |
| VAT | 24 | 43 | −14** | 49 | −3 | 0.1 |
| 48 | 43 | −15** | 47 | −4 | 0.1 | |
| VAT:TAT ratio | 24 | 43 | −10** | 49 | −8** | 0.44 |
| 48 | 43 | −14** | 47 | −11** | 0.38 | |
Within-arm change: *P < 0.05; **P < 0.01.
Figure 2.
Week 24 and 48 results in the combined arms. Changes are expressed as median and IQR % change from baseline. The P values refer to the within-arm changes. The grey P values refer to the abacavir arm and the black P values refer to the LPV/r+NVP arm. ABC, abacavir.
SAT area also increased significantly at weeks 24 and 48 in both arms (Table 2 and Figure 2). VAT area decreased significantly at weeks 24 and 48 in the abacavir arm (week 24: −14%, P < 0.01; week 48: −15%, P = 0.04) (Figure 2); conversely, changes over time in VAT in the LPV/r+NVP arm were not significant. The VAT:total adipose tissue (TAT) ratio decreased significantly in both arms of the study at weeks 24 and 48 after the switch (Figure 2).
The participants had a significant improvement in self-perception of subcutaneous fat as a group using the validated ACTG body image questionnaire, although the improvement in self-perception did not correlate with objective improvements in fat measurements by CT scan. On a scale of 1–4, 61% of the participants reported no changes or improvements in self-perception of subcutaneous leg fat at week 48 of the study versus only 15% at baseline (P < 0.0001, no difference between arms); however, there was no correlation between the perceived changes in the subcutaneous fat in the legs and the objective changes by CT scan (Spearman r = 0.0001, P = 0.98).
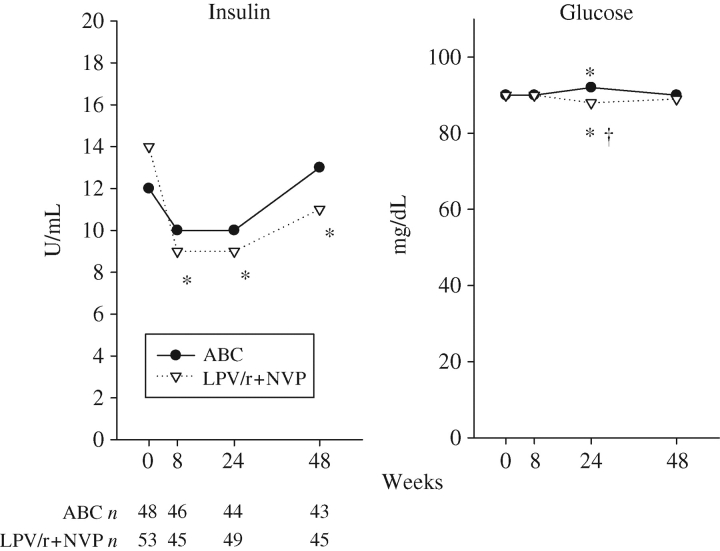
There were minimal statistical differences in glucose levels at week 24, and no difference at weeks 8 and 48 within or between arms. There were significant decreases in insulin levels in the LPV/r+NVP arm at weeks 8, 24 and 48. Insulin levels were not significantly different between arms over time (Figure 3).
Figure 3.
Median glucose and insulin in the combined group. *Significant within-arm change from baseline (P < 0.05). †Significant between-arm change difference (P < 0.05). ABC, abacavir.
Overall, lipids tended to increase in the LPV/r+NVP arm, but changes were clinically irrelevant in the abacavir arm (Figure 4). Non-HDL cholesterol increased significantly in the LPV/r+NVP arm (median at week 48 +20 mg/dL, 11%, within arm P = 0.01) and did not change in the abacavir arm (between arm P = 0.028). Total cholesterol changes paralleled non-HDL cholesterol changes, with stable values in the abacavir arm and modest increases in the LPV/r+NVP arm (+24 mg/dL or 12% increase at week 48, within arm P < 0.001). At week 48, total cholesterol levels were >240 mg/dL in 19/45 (42%) subjects on LPV/r+NVP versus 10/43 (23%) in the abacavir arm. Triglycerides increased more in the LPV/r+NVP arm initially, but after week 24, they tended to decline slightly. At week 48, they had increased by a median value of 56 mg/dL (21%) in the LPV/r+NVP arm and decreased by 6 mg/dL (−1%) in the abacavir arm (between arm P = 0.006). At that time, 27% of the participants in the LPV/r+NVP arm had triglyceride levels >500 mg/dL compared with 14% in the abacavir arm. HDL cholesterol increased modestly in both arms (median 3 mg/dL at week 48, within arm P = 0.01 and P = 0.05, respectively). After 48 weeks, 38% and 42% had HDL cholesterol levels >40 mg/dL in the LPV/r+NVP and abacavir arms, respectively.
Figure 4.
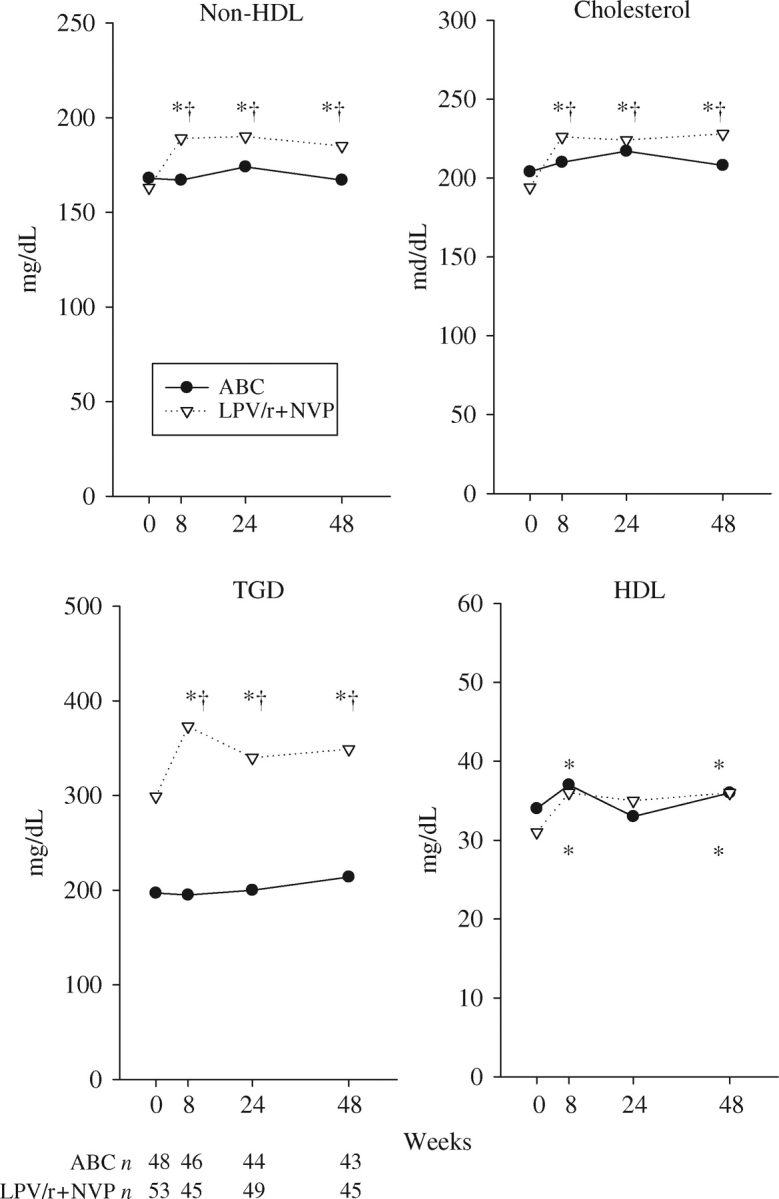
Median lipid/lipoprotein parameters. There were modest increases in total cholesterol and non-HDL cholesterol in the LPV/r+NVP arm. Triglycerides (TGD) increased remarkably in the LPV/r+NVP arm. HDL levels increased slightly in both arms. *Significant within-arm change from baseline (P < 0.05). †Significant between-arm change difference (P < 0.05). ABC, abacavir.
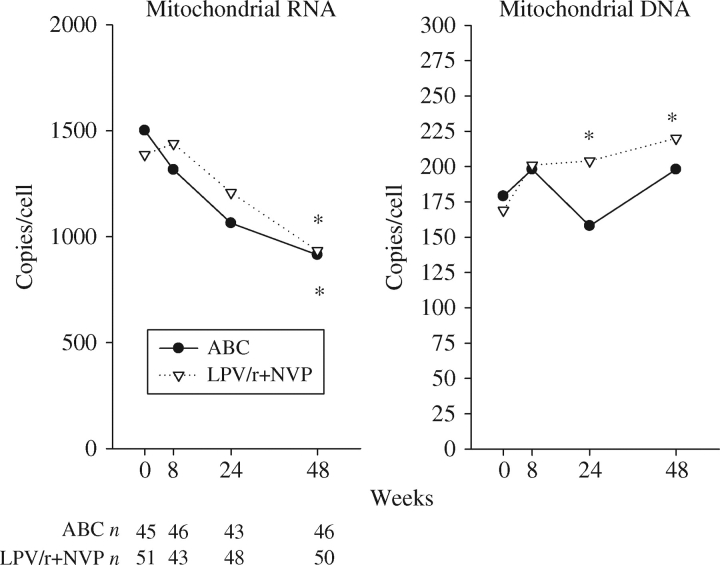
Mitochondrial RNA copy number decreased significantly after 48 weeks in both arms, median=−788 copies/cell in the abacavir arm and −379 copies/cell in the LPV/r+NVP arm (both P < 0.01; Figure 5). Mitochondrial DNA copy number tended to increase more in the lopinavir/ritonavir arm [median=50 copies/cell (P < 0.01)] than in the abacavir arm [median=19 copies/cell (P = 0.19)]. No significant correlations between changes in subcutaneous fat area and changes in PBMC mitochondrial DNA or RNA copy number were noted.
Figure 5.
Median mitochondrial RNA and DNA levels in PBMCs in both arms. *Significant within-arm change from baseline (P < 0.05). ABC, abacavir.
CD4 T cell count increased significantly in the LPV/r+NVP arm (+58 cells/mm3 at week 48, P = 0.03), but changes were not significant [−10 cells/mm3 (P = NS)] in the abacavir arm. From the virological perspective, the switch was safe: there were four virological failures (two in each arm). LPV/r+NVP had a significantly shorter time to grade 3 or higher toxicity (P = 0.007), most of it related to high triglyceride levels, but discontinuation rates were similar for both interventions. There were no abacavir hypersensitivity reactions.
Discussion
In spite of the improvements and better safety profile of the newer antiretroviral regimens, limb and facial lipoatrophy persist among HIV-infected individuals receiving antiretroviral therapy. The options to manage lipoatrophy are limited in the developing world, where drugs such as stavudine are still part of the most frequently used antiretroviral regimens because of their efficacy, relatively low costs as generics and the absence of haematological toxicity. Some small studies have suggested that lowering the dose of stavudine might be the only therapeutic alternative in this particular situation.28 Fortunately, the number of therapeutic options has increased in the developed world. Facial injections of polylactic acid19 and most recently calcium hydroxylapatite29 have resulted in cosmetic improvements; however, the only treatment strategy that has resulted in modest but statistically significant improvements of limb fat has been switching thymidine analogues to abacavir22,30,31 or tenofovir.23 Two studies that evaluated the effects of switching an antiretroviral regimen to a regimen that does not include nucleoside analogues have reported promising results.24,25 Our study evaluated both switching strategies (thymidine-sparing versus non-nucleoside-containing regimen). Our findings indicate that after 48 weeks both strategies significantly and similarly increased subcutaneous fat area in the thigh and abdomen. Both strategies reduced the VAT:TAT ratio, and abacavir switch was associated with a reduction in the absolute area of visceral fat. The changes in visceral fat may have an impact on the long-term cardiovascular risk of these individuals, although they have to be evaluated in conjunction with the changes in lipid parameters.
Fasting glucose and insulin levels were not adversely affected by either switch strategy. The switch to LPV/r+NVP was associated with significant increases in lipids including triglycerides, non-HDL cholesterol and HDL, which might warrant caution, especially in certain populations with an elevated cardiovascular risk.
Both switches were virologically safe. The demonstration of the safety of regimens that do not include a nucleoside analogue is important because it represents a possible alternative for patients with lipoatrophy and antiretroviral options restricted by the previous development of resistance. The results of our study in this regard are consistent with the recently presented treatment-naive trial A5142, which demonstrated that the virological outcomes of a regimen of lopinavir/ritonavir and efavirenz were similar to the outcomes of a regimen of two nucleoside analogues with efavirenz.32 Nonetheless, the particular combination that we used in this trial was associated with increases in lipid fractions, especially triglycerides. For patients with low cardiovascular risk, these switches might represent a reasonable option. As new classes of drugs are developed, such as CCR5 antagonists and integrase inhibitors, some of them with favourable metabolic profiles,33 the selection of combination regimens without nucleosides may become even more attractive.
As mitochondrial toxicity is one of the most frequently proposed mechanisms mediating lipoatrophy,34 there has been considerable interest in using mitochondrial DNA measurements in PBMCs as predictors or monitoring tools of nucleoside-associated toxicity. So far reports have been conflicting. Cote et al.35 found that, in some patients, there was a decline in the mitochondrial to nuclear DNA ratio over time before the development of lactic acidosis, one of the most feared complications of antiretroviral therapy. Cossarizza et al.36 and McComsey et al.,37 in two cross-sectional studies involving HIV-infected children and adults with and without lipoatrophy, found no significant difference in the mitochondrial DNA content of peripheral blood lymphocyte samples and the development of significant fat loss. The main limitation of those studies was their cross-sectional nature. Hoy et al.,38 in the large MITOX trial, found no relationship between mitochondrial DNA measurements and fat improvements after the switch to abacavir. In our study, we observed, as expected, an increase in mitochondrial DNA and a decrease in RNA copy number per cell after the discontinuation of thymidine analogues. The difference between our result and Hoy's may be due to the different methodology used. The measurement of mitochondrial nucleic acid may be useful at the population/study level, as the levels changed in the expected direction in both arms; however, it is important to put those results in perspective as their utility at the individual patient level is more questionable, as the changes in mitochondrial measurement did not correlate with changes in subcutaneous adipose tissue. Confirmation in other studies will clarify the role, if any, of these measurements in clinical practice.
The main limitations of our study are the relatively complex design, the closure of one arm after the results of a successful intervention were presented, the absence of dual energy X-ray absorptiometry measurements that would have allowed us to evaluate whole-body and regional changes in adipose tissue mass and the limited power to evaluate between-arm differences.
In summary, switching stavudine or zidovudine to a non-thymidine analogue or changing to a nucleoside reverse transcriptase inhibitor-sparing regimen is associated with continuous and qualitatively similar improvements in thigh fat area, subcutaneous abdominal tissue (SAT) and VAT:TAT ratio to 48 weeks. Abacavir tended to reduce VAT, while LPV/r+NVP increased CD4 count. Either type of switch will help restore subcutaneous fat in the extremities. The final choice should be based on determining the most appropriate antiretroviral regimen for the given patient, with particular attention being paid to the effect of the regimen on lipid profiles and cardiovascular risk.
Funding
The study was supported by the National Institute of Allergy and Infectious Disease, R21AI063995; Abbott Laboratories Inc. and Boehringer Ingelheim, Inc. provided study medications for the study.
Transparency declarations
None to declare.
Acknowledgements
The A5110 Trial Investigators: Participating ACTG investigators and institutions:
Tania Mikaitis and Allan Tenorio—Northwestern University CTU (A2701, A2702 and A2705) CTU Grant no. AI69471; Teresa Spitz and John Stoneman—Washington University in St Louis (A2101) CTU Grant no. AI069495; Karen T. Tashima and Pamela Poethke—The Miriam Hospital (A2951) CTU Grant no. AI46381; David A. Wininger and Kathy Watson—The Ohio State University (A2301) CTU Grant no. AI069474; Janet Forcht and Charles Gonzalez—New York University/NYC HHC at Bellevue (A0401) CTU Grant no. AI27665 and GCRC Grant no. M01RR00096; William A. O'Brien and Cheryl Mogridge—University of Texas Medical Branch, Galveston (A6301) CTU Grant no. AI32782; Ian Frank and Wayne Wagner—University of Pennsylvania (A6301) CTU Grant no. AI32783; Michael Dube and Deborah O'Connor—Indiana University (A2601) CTU Grant no. AI25859; Lorna Nagamine and Scott Souza—University of Hawaii at Manoa and Queen's Medical Center (A5201) CTU Grant no. AI34853; Carl J. Fichtenbaum and Franette Hyc—University of Cincinnati (A2401) CTU Grant no. AI069513; Ann Conrad—MetroHealth Medical Center (A2503) GCRC Grant no. M01RR000080; David Haas and Janet Nicotera—Vanderbilt University (A3652) CTU Grant no. AI069439; Graham Ray and Steven Johnson—University of Colorado Health Sciences Center (A6101) GCRC Grant no. M01RR00051, CTU Grant no. 1U01AI6945; Neah Kim and Mary Albrecht—Beth Israel Deaconess Medical Center (A0103) CTU Grant no. AI69472-01; Judith S. Currier and Sofia Solis—Harbor General/UCLA (A0601) CTU Grant no. AI27660.
References
- 1.Carr A, Samaras K, Burton S, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998;12:F51–8. doi: 10.1097/00002030-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Mallon PW, Miller J, Cooper DA, et al. Prospective evaluation of the effects of antiretroviral therapy on body composition in HIV-1-infected men starting therapy. AIDS. 2003;17:971–9. doi: 10.1097/00002030-200305020-00005. [DOI] [PubMed] [Google Scholar]
- 3.Dube MP, Parker RA, Tebas P, et al. Glucose metabolism, lipid, and body fat changes in antiretroviral-naive subjects randomized to nelfinavir or efavirenz plus dual nucleosides. AIDS. 2005;19:1807–18. doi: 10.1097/01.aids.0000183629.20041.bb. [DOI] [PubMed] [Google Scholar]
- 4.Bacchetti P, Gripshover B, Grunfeld C, et al. Fat distribution in men with HIV infection. J Acquir Immune Defic Syndr. 2005;40:121–31. doi: 10.1097/01.qai.0000182230.47819.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hansen AB, Lindegaard B, Obel N, et al. Pronounced lipoatrophy in HIV-infected men receiving HAART for more than 6 years compared with the background population. HIV Med. 2006;7:38–45. doi: 10.1111/j.1468-1293.2005.00334.x. [DOI] [PubMed] [Google Scholar]
- 6.Miller J, Carr A, Emery S, et al. HIV lipodystrophy: prevalence, severity and correlates of risk in Australia. HIV Med. 2003;4:293–301. doi: 10.1046/j.1468-1293.2003.00159.x. [DOI] [PubMed] [Google Scholar]
- 7.Lichtenstein KA, Delaney KM, Armon C, et al. Incidence of and risk factors for lipoatrophy (abnormal fat loss) in ambulatory HIV-1-infected patients. J Acquir Immune Defic Syndr. 2003;32:48–56. doi: 10.1097/00126334-200301010-00007. [DOI] [PubMed] [Google Scholar]
- 8.Saves M, Raffi F, Capeau J, et al. Factors related to lipodystrophy and metabolic alterations in patients with human immunodeficiency virus infection receiving highly active antiretroviral therapy. Clin Infect Dis. 2002;34:1396–405. doi: 10.1086/339866. [DOI] [PubMed] [Google Scholar]
- 9.Thiebaut R, Daucourt V, Mercie P, et al. Lipodystrophy, metabolic disorders, and human immunodeficiency virus infection: Aquitaine Cohort, France, 1999. Groupe d'Epidemiologie Clinique du Syndrome d'Immunodeficience Acquise en Aquitaine. Clin Infect Dis. 2000;31:1482–7. doi: 10.1086/317477. [DOI] [PubMed] [Google Scholar]
- 10.Bogner JR, Vielhauer V, Beckmann RA, et al. Stavudine versus zidovudine and the development of lipodystrophy. J Acquir Immune Defic Syndr. 2001;27:237–44. doi: 10.1097/00126334-200107010-00004. [DOI] [PubMed] [Google Scholar]
- 11.Lichtenstein KA, Ward DJ, Moorman AC, et al. Clinical assessment of HIV-associated lipodystrophy in an ambulatory population. AIDS. 2001;15:1389–98. doi: 10.1097/00002030-200107270-00008. [DOI] [PubMed] [Google Scholar]
- 12.Domingo P, Sambeat MA, Perez A, et al. Fat distribution and metabolic abnormalities in HIV-infected patients on first combination antiretroviral therapy including stavudine or zidovudine: role of physical activity as a protective factor. Antivir Ther. 2003;8:223–31. doi: 10.1177/135965350300800306. [DOI] [PubMed] [Google Scholar]
- 13.Joly V, Flandre P, Meiffredy V, et al. Increased risk of lipoatrophy under stavudine in HIV-1-infected patients: results of a substudy from a comparative trial. AIDS. 2002;16:2447–54. doi: 10.1097/00002030-200212060-00010. [DOI] [PubMed] [Google Scholar]
- 14.Martinez E, Mocroft A, Garcia-Viejo MA, et al. Risk of lipodystrophy in HIV-1-infected patients treated with protease inhibitors: a prospective cohort study. Lancet. 2001;357:592–8. doi: 10.1016/S0140-6736(00)04056-3. [DOI] [PubMed] [Google Scholar]
- 15.Heath KV, Hogg RS, Singer J, et al. Antiretroviral treatment patterns and incident HIV-associated morphologic and lipid abnormalities in a population-based cohort. J Acquir Immune Defic Syndr. 2002;30:440–7. doi: 10.1097/00042560-200208010-00010. [DOI] [PubMed] [Google Scholar]
- 16.Ledru E, Christeff N, Patey O, et al. Alteration of tumor necrosis factor-alpha T-cell homeostasis following potent antiretroviral therapy: contribution to the development of human immunodeficiency virus-associated lipodystrophy syndrome. Blood. 2000;95:3191–8. [PubMed] [Google Scholar]
- 17.Gallant JE, Staszewski S, Pozniak AL, et al. Efficacy and safety of tenofovir DF vs. stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004;292:191–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 18.Carr A, Emery S, Law M, et al. An objective case definition of lipodystrophy in HIV-infected adults: a case–control study. Lancet. 2003;361:726–35. doi: 10.1016/s0140-6736(03)12656-6. [DOI] [PubMed] [Google Scholar]
- 19.Valantin MA, Aubron-Olivier C, Ghosn J, et al. Polylactic acid implants (New-Fill) to correct facial lipoatrophy in HIV-infected patients: results of the open-label study VEGA. AIDS. 2003;17:2471–7. doi: 10.1097/00002030-200311210-00009. [DOI] [PubMed] [Google Scholar]
- 20.Moyle GJ, Lysakova L, Brown S, et al. A randomized open-label study of immediate versus delayed polylactic acid injections for the cosmetic management of facial lipoatrophy in persons with HIV infection. HIV Med. 2004;5:82–7. doi: 10.1111/j.1468-1293.2004.00190.x. [DOI] [PubMed] [Google Scholar]
- 21.Humble G, Mest D. Soft tissue augmentation using sculptra. Facial Plast Surg. 2004;20:157–63. doi: 10.1055/s-2004-861758. [DOI] [PubMed] [Google Scholar]
- 22.Martin A, Smith DE, Carr A, et al. Reversibility of lipoatrophy in HIV-infected patients 2 years after switching from a thymidine analogue to abacavir: the MITOX Extension Study. AIDS. 2004;18:1029–36. doi: 10.1097/00002030-200404300-00011. [DOI] [PubMed] [Google Scholar]
- 23.Moyle GJ, Sabin CA, Cartledge J, et al. A randomized comparative trial of tenofovir DF or abacavir as replacement for a thymidine analogue in persons with lipoatrophy. AIDS. 2006;20:2043–50. doi: 10.1097/01.aids.0000247574.33998.03. [DOI] [PubMed] [Google Scholar]
- 24.Boyd MA, Carr A, Ruxrungtham K, et al. Changes in body composition and mitochondrial nucleic acid content in patients switched from failed nucleoside analogue therapy to ritonavir-boosted indinavir and efavirenz. J Infect Dis. 2006;194:642–50. doi: 10.1086/505709. [DOI] [PubMed] [Google Scholar]
- 25.Tebas P, Zhang J, Yarasheski K, et al. Switching to a protease inhibitor-containing, nucleoside-sparing regimen (lopinavir/ritonavir plus efavirenz) increases limb fat but raises serum lipid levels: results of a prospective randomized trial (AIDS clinical trial group 5125s) J Acquir Immune Defic Syndr. 2007;45:193–200. doi: 10.1097/QAI.0b013e318042e204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walker UA, Venhoff N. Uridine in the prevention and treatment of NRTI-related mitochondrial toxicity. Antivir Ther. 2005;10(Suppl 2):M117–23. [PubMed] [Google Scholar]
- 27.Timmermans EC, Tebas P, Ruiter JP, et al. Real-time nucleic acid sequence-based amplification assay to quantify changes in mitochondrial DNA concentrations in cell cultures and blood cells from HIV-infected patients receiving antiviral therapy. Clin Chem. 2006;52:979–87. doi: 10.1373/clinchem.2005.062901. [DOI] [PubMed] [Google Scholar]
- 28.Milinkovic A, Martinez E, Lopez S, et al. The impact of reducing stavudine dose versus switching to tenofovir on plasma lipids, body composition and mitochondrial function in HIV-infected patients. Antivir Ther. 2007;12:407–15. [PubMed] [Google Scholar]
- 29.Silvers SL, Eviatar JA, Echavez MI, et al. Prospective, open-label, 18-month trial of calcium hydroxylapatite (Radiesse) for facial soft-tissue augmentation in patients with human immunodeficiency virus-associated lipoatrophy: one-year durability. Plast Reconstr Surg. 2006;118:34S–45S. doi: 10.1097/01.prs.0000234847.36020.52. [DOI] [PubMed] [Google Scholar]
- 30.Carr A, Workman C, Smith DE, et al. Abacavir substitution for nucleoside analogs in patients with HIV lipoatrophy: a randomized trial. JAMA. 2002;288:207–15. doi: 10.1001/jama.288.2.207. [DOI] [PubMed] [Google Scholar]
- 31.McComsey GA, Ward DJ, Hessenthaler SM, et al. Improvement in lipoatrophy associated with highly active antiretroviral therapy in human immunodeficiency virus-infected patients switched from stavudine to abacavir or zidovudine: the results of the TARHEEL study. Clin Infect Dis. 2004;38:263–70. doi: 10.1086/380790. [DOI] [PubMed] [Google Scholar]
- 32.Riddler SA, Haubrich R, DiRienzo AG, et al. Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med. 2008;358:2095–106. doi: 10.1056/NEJMoa074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teppler H, Azrolan N, Chen J, et al. Differential effect of MK-0518 and efavirenz on serum lipids and lipoproteins in antiretroviral therapy (ART)-naive patients. Abstracts of the Forty-sixth Interscience Conference on Antimicrobial Agents and Chemotherapy; 2006; San Francisco, CA. Washington, DC, USA: American Society for Microbiology; Abstract H-0256a. [Google Scholar]
- 34.Walker U, Brinkman K. NRTI induced mitochondrial toxicity as a mechanism for HAART related lipodystrophy: fact or fiction? HIV Med. 2001;2:163–5. doi: 10.1046/j.1464-2662.2001.00073.x. [DOI] [PubMed] [Google Scholar]
- 35.Cote HC, Brumme ZL, Craib KJ, et al. Changes in mitochondrial DNA as a marker of nucleoside toxicity in HIV-infected patients. N Engl J Med. 2002;346:811–20. doi: 10.1056/NEJMoa012035. [DOI] [PubMed] [Google Scholar]
- 36.Cossarizza A, Pinti M, Moretti L, et al. Mitochondrial functionality and mitochondrial DNA content in lymphocytes of vertically infected human immunodeficiency virus-positive children with highly active antiretroviral therapy-related lipodystrophy. J Infect Dis. 2002;185:299–305. doi: 10.1086/338564. [DOI] [PubMed] [Google Scholar]
- 37.McComsey G, Tan DJ, Lederman M, et al. Analysis of the mitochondrial DNA genome in the peripheral blood leukocytes of HIV-infected patients with or without lipoatrophy. AIDS. 2002;16:513–8. doi: 10.1097/00002030-200203080-00001. [DOI] [PubMed] [Google Scholar]
- 38.Hoy JF, Gahan ME, Carr A, et al. Changes in mitochondrial DNA in peripheral blood mononuclear cells from HIV-infected patients with lipoatrophy randomized to receive abacavir. J Infect Dis. 2004;190:688–92. doi: 10.1086/422602. [DOI] [PubMed] [Google Scholar]