Abstract
Objectives
The aim of this study was to assess the dose–response of isavuconazole, voriconazole and fluconazole in disseminated Candida tropicalis and Candida krusei infections.
Methods
Mice were immunosuppressed using either one dose [temporarily neutropenic (TN)] or two doses [persistently neutropenic (PN)] of cyclophosphamide. Treatment was started 5 h after infection with oral isavuconazole (6, 15, 30, 60, 90, 120 or 150 mg/kg equivalent active compound), intravenous voriconazole (5, 20 or 40 mg/kg plus grapefruit gavage twice daily) or oral fluconazole (15, 50 or 150 mg/kg) all administered twice daily. Kidney burden was assessed for C. tropicalis, and kidney and brain burden for C. krusei.
Results
Vehicle controls developed a non-lethal infection with high burdens in both models. In the TN models, isavuconazole, voriconazole and fluconazole (>50 mg/kg) reduced kidney burden compared with controls; >60 mg/kg isavuconazole and 50 mg/kg fluconazole were superior to alternative treatments (other than voriconazole 40 mg/kg). Isavuconazole (all doses) reduced brain burden (P < 0.05) in the C. krusei model; fluconazole (all doses) and voriconazole (5 and 20 mg/kg) did not. In the C. krusei kidney burden model, isavuconazole 120 and 150 mg/kg and voriconazole 40 mg/kg were superior to controls and fluconazole. In the C. tropicalis model, PN isavuconazole (all doses), voriconazole (>5 mg/kg) and fluconazole (all doses) reduced kidney burden (P < 0.05). Only isavuconazole (all doses) and 40 mg/kg voriconazole were effective against C. krusei in the brain, isavuconazole and voriconazole reduced tissue burden (P < 0.05). Fluconazole had no significant effect on brain burden even at 150 mg/kg.
Conclusions
Isavuconazole significantly reduced kidney burden in mice infected with C. tropicalis and both kidney and brain burdens in mice infected with C. krusei. Isavuconazole was as effective as voriconazole and much more effective than fluconazole at reducing brain burden.
Keywords: BAL4815, BAL8557, antifungal, mouse
Introduction
There is general agreement that during the 1980s and 1990s, there was an increase in the incidence of invasive fungal infections culminating in Candida being identified as the fourth most common cause of bloodstream infections in the USA. Although these data are useful, they do not include the high incidence of non-invasive disease caused by Candida, such as oesophageal and oropharyngeal candidiasis, with rates of 10% to 25% in renal transplant patients.1,2 Additionally, the data do not reflect the relative increase in the incidence of non-albicans Candida. Candida tropicalis is the second or third most commonly isolated species of Candida3,4 with higher incidence in South America and Asia. Candida krusei, although less common and accounting for less than 5% of bloodstream infections, is similar to C. tropicalis, in that it occurs most frequently in haematological stem cell transplant patients with neutropenia.5 C. krusei is also intrinsically resistant to fluconazole and demonstrates reduced susceptibility to amphotericin B and is more often isolated in patients with refractory disease.6,7
Mortality rates associated with systemic Candida infections remain unacceptably high with attributable mortality up to 40%, which increases to over 50% in patients undergoing myeloablative chemotherapy.8,9
Isavuconazole BAL8557 is the water-soluble triazole precursor of BAL4815 suitable for oral and intravenous (iv) administration.10 Isavuconazole is in Phase III development for the treatment of severe invasive fungal infections having received fast-track status from the FDA. In vitro, the active moiety demonstrates broad-spectrum activity against opportunistic fungi (Candida, Cryptococcus and Aspergillus)11 and the dimorphic fungi.12,13 In vivo, isavuconazole is highly effective against systemic candidiasis and Aspergillus flavus infections.14
In this study, we compared the dose–response of isavuconazole, voriconazole and fluconazole on the tissue burden (kidney and brain) of neutropenic mice with disseminated C. krusei and C. tropicalis infections.
Methods
Candida isolates
Well-characterized clinical isolates of C. krusei (ATCC 6258) and C. tropicalis (FA 8946) were retrieved prior to experiments from long-term storage at −70°C, placed on Sabouraud dextrose agar (SDA) (Oxoid, Basingstoke, UK) and incubated at 37°C for 48 h. Ten morphologically identical colonies were subcultured into Sabouraud dextrose broth (Oxoid) and placed on an orbital mixer at 37°C for 16 h. Blastoconidia were harvested by centrifugation and washed twice with saline. The final inoculum was determined by counting using a haemocytometer and progressive dilution in PBS. The input count was verified by quantitative culture post-infection.
The in vitro susceptibilities using CLSI M27-A2 of these strains to isavuconazole, voriconazole and fluconazole were 0.015, 0.06 and 0.25 mg/L for C. tropicalis FA 8946 and 0.25, 0.5 and 32 mg/L for C. krusei ATCC 6258.
Mice
All experiments were performed under UK Home Office licence 40/2356 entitled Invasive Fungal Diseases and had received local Ethics Review clearance. Male CD1 mice (virus-free) weighing between 23 and 25 g (Charles River UK Ltd, Margate, Kent, UK) were allowed free access to food and water throughout the experiments. Mice were randomized into groups of three or six, as detailed below.
Immunosuppression
Mice were immunosuppressed either temporarily with a single dose or persistently with 200 mg/kg iv cyclophosphamide (Sigma, Poole, UK) every 5 days starting on day −3. This yields a temporary neutropenia (reduces the WBC counts to <2 × 106 WBC/mL until 3 days post-infection) starting on day 0 in the temporarily neutropenic (TN) models or persistent neutropenia throughout the experiment starting on day 0.
Infection
Prior to the experiment, inoculum-finding studies for the isolates were performed using iv injections via the lateral tail vein of 0.2 mL of a range of inocula. These studies determined that an inoculum of 1 × 105 cfu/mouse C. tropicalis or 1 × 107 cfu/mouse C. krusei was required to cause a heavy non-lethal tissue burden in the kidneys. The inoculum was administered on day 0 (3 days post-immunosuppression). Post-infection viability counts were performed to ensure that the correct inoculum had been given.
Antifungal treatment
Isavuconazole was obtained as pure powder from Basilea Pharmaceuticals (Basel, Switzerland) and stored at −20°C until used. Isavuconazole was reconstituted in distilled water for injection immediately prior to use. This stock was further diluted in distilled water to provide treatments of 6, 15, 30, 60, 90, 120 or 150 mg/kg isavuconazole (concentration was corrected to account for potency) delivered in 0.25 mL orally twice daily.
Voriconazole (Vfend, Pfizer Ltd, Sandwich, UK) was reconstituted according to the manufacturer’s instructions in sterile distilled water. It was further diluted in 5% glucose to provide treatments of 5, 20 or 40 mg/kg; all treatments were administered in 0.25 mL iv twice daily. To prevent rapid clearance of drug, all mice in these treatment groups plus their vehicle controls were administered 0.25 mL of grapefruit juice twice daily (administered 30 min before voriconazole), started 3 days before infection and continued throughout the experiment.15
Fluconazole (Diflucan, Pfizer Ltd) was dissolved in sterile saline plus 0.03% Noble agar (Oxoid) and stored for up to 4 days at 4°C before use. The stock solution was further diluted daily immediately before use and administered by gavage twice daily at 15, 50 and 150 mg/kg.
Treatment was initiated 5 h post-infection and continued for 4 days (6 doses) for C. tropicalis and C. krusei or 7 days (12 doses) for C. tropicalis only. Control mice were infected and received water for injection or grapefruit juice orally. All animals were euthanized 101 or 173 h (C. tropicalis only) post-infection. Group sizes used in this study were: C. tropicalis 4 day model, three mice per group; C. tropicalis 7 day model and C. krusei models, six mice per group.
Additional groups of mice were similarly infected and treated with voriconazole (15 mg/kg twice daily) or fluconazole (50 mg/kg twice daily) to ensure adequate drug exposure. Mice were bled by cardiac puncture 3 days post-infection. Drug levels were assessed in bioassays using RPMI MOPS agar (Sigma) and the Candida kefyr San Antonio strain.
Organ culture
The kidneys (brains were also examined in mice infected with C. krusei) were removed and transferred into 2 mL of sterile PBS. The organs were homogenized in a tissue grinder (Polytron, Kinematica AG, Luzern, Switzerland) for 2–3 s. Colony counts were determined using serial 10-fold dilutions plated on the surface of SDA with 0.5% (w/v) chloramphenicol. Plates were incubated at 37°C in a moist atmosphere and examined after 24 h. Single colonies were scored as a negative result, because of the possibility of carry-over contamination. This method detected Candida at >30 cfu/organ.
Statistical analysis
All data analysis was performed using the Kruskal–Wallis test using the computer package StatsDirect (Ashwell, Herts, UK).
Results
C. tropicalis TN model
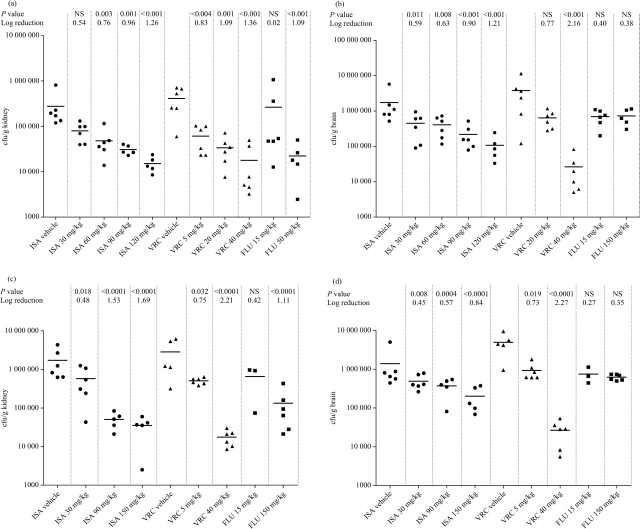
C. tropicalis caused a non-lethal disease with kidney burdens of 1.25 × 107 and 1.52 × 106 cfu/g in the isavuconazole vehicle controls in the 4 and 7 day TN models, respectively (Figure 1a and c). Treatment with isavuconazole (>6 mg/kg), voriconazole (>5 mg/kg) and fluconazole (all doses) reduced kidney burden of C. tropicalis compared with controls (P < 0.05) after both 4 and 7 days. The isavuconazole Emax was achieved at 60 mg/kg after 4 days (2.3 log10 reduction in kidney burden) and 120 mg/kg after 7 days and were similar to the fluconazole Emax (achieved at 50 mg/kg), but superior to the Emax of voriconazole (achieved at 40 mg/kg). Isavuconazole treatment of >60 mg/kg was superior at reducing kidney burden compared with all doses of voriconazole in mice infected with C. tropicalis in both models.
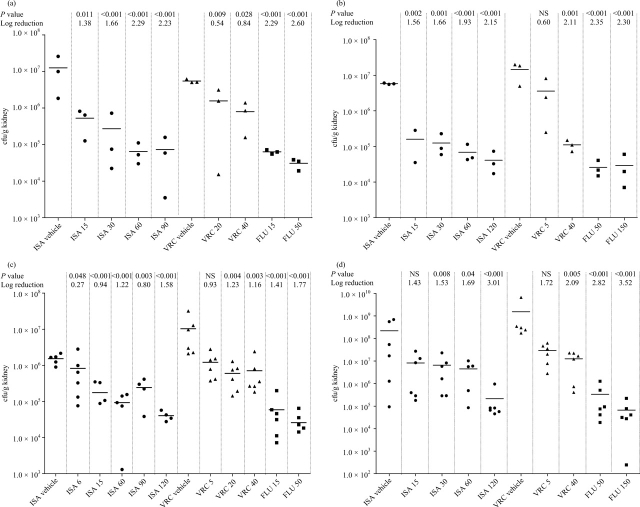
Figure 1.
Tissue burdens following C. tropicalis infection. (a) Kidney burden in TN mice 4 days post-infection. (b) Kidney burden in PN mice 4 days post-infection. (c) Kidney burden in TN mice 7 days post-infection. (d) Kidney burden in PN mice 7 days post-infection. Panels include P values (NS, not significant; P > 0.05) and reduction in burden compared with vehicle control. NB: some treatment groups have not been presented to improve the clarity of the figure (the full set of results is available on request). ISA, isavuconazole; VRC, voriconazole; FLU, fluconazole.
C. tropicalis persistently neutropenic (PN) model
C. tropicalis kidney burdens of 5.7 × 106 and 2.1 × 108 cfu/g in the 4 and 7 day PN models, respectively, were recovered from the isavuconazole vehicle controls (Figure 1b and d). Treatment with isavuconazole (all doses) and fluconazole (all doses) dose dependently reduced the 4 day kidney burden compared with controls (P < 0.05); in contrast, only 40 mg/kg voriconazole was able to significantly reduce the burden. In the 7 day model, isavuconazole (>15 mg/kg), voriconazole (all doses >5 mg/kg) and fluconazole (all doses) dose dependently reduced kidney burden compared with controls. The isavuconazole Emax was achieved at >60 mg/kg. In this part of the study, no treatment sterilized organs.
The effect of persistent neutropenia in these C. tropicalis models is clear with kidney burdens of the vehicle-treated animals >2.0 log10 cfu/g higher than the TN animals after 7 days. Additionally, the burdens increased between days 4 and 7 only in the PN mice.
C. krusei TN model
In this model, isavuconazole vehicle control mice developed a non-lethal infection with high burdens of 4.1 × 105 and 1.7 × 106 log10 cfu/g in kidneys and brain, respectively. Treatment with isavuconazole resulted in an Emax of 1.5 × 104 and 6.07 × 105 in kidneys and brain, respectively (Figure 2a and b). Treatment with isavuconazole, voriconazole and fluconazole dose dependently reduced kidney burden compared with controls (P < 0.05 isavuconazole >30 mg/kg). Voriconazole 40 mg/kg and isavuconazole 120 and 150 mg/kg were superior to other treatments at reducing kidney burden. Treatment with isavuconazole (all doses) significantly reduced brain burden (P < 0.05); in contrast, only the highest dose of voriconazole (40 mg/kg) reduced the brain burden. Fluconazole (all doses) and voriconazole (5 and 20 mg/kg) had no significant effect on brain burden (Figure 2b).
Figure 2.
Tissue burdens following C. krusei infection. (a) Kidney burden in TN mice. (b) Brain burden in TN mice. (c) Kidney burden in PN mice. (d) Brain burden in PN mice. Panels include P values (NS, not significant; P > 0.05) and reduction in burden compared with vehicle control. NB: some treatment groups have not been presented to improve the clarity of the figure (the full set of results is available on request). ISA, isavuconazole; VRC, voriconazole; FLU, fluconazole.
C. krusei PN model
Voriconazole vehicle controls developed a non-lethal infection with high burdens of C. krusei, 3.8 × 106 and 4.9 × 106 log10 cfu/g tissue in kidneys and brain, respectively. Isavuconazole treatment resulted in an Emax of 3.5 × 104 and 2.01 × 105 in kidneys and brain, respectively (Figure 2c and d). Isavuconazole (all doses), voriconazole (all doses) and fluconazole (150 mg/kg only) reduced kidney burdens compared with solvent-treated mice (P < 0.05). Isavuconazole >90 mg/kg and voriconazole 40 mg/kg were significantly superior to all other treatments at reducing the kidney burden. The burden in the brain was significantly reduced by isavuconazole (all doses) and voriconazole (all doses) (P < 0.05). In contrast, fluconazole had no significant effect on brain burden even at the highest dose tested (150 mg/kg).
Persistent neutropenia with C. krusei infection resulted in higher burdens compared with temporary neutropenia in the kidneys (∼1 log10 cfu/g higher burden). An increased burden was also cultured from the brain with ∼0.5 log10 cfu/g higher burdens recovered.
Voriconazole and fluconazole pharmacokinetics
The drug exposures following treatment with 15 mg/kg voriconazole and 50 mg/kg fluconazole were as expected with peak levels of 3.6 and 14.1 mg/L and levels of 0.7 and 14.1 mg/L measured 2 h post-dose, respectively. These levels demonstrate that voriconazole was metabolized as expected and that fluconazole was well absorbed.
Discussion
In these models, isavuconazole demonstrated impressive antifungal activity against C. tropicalis and C. krusei in both TN and PN murine models of disseminated candidiasis. Against both organisms, isavuconazole was as effective as voriconazole, and against C. krusei, isavuconazole was superior to voriconazole (doses <40 mg/kg) and fluconazole even when administered at 150 mg/kg. Of particular interest is the efficacy of isavuconazole at reducing the burden of C. krusei in the brain; all doses tested significantly reduced the burden in both TN and PN mice. Voriconazole at 40 mg/kg is required to have the maximal effect.
These data have reconfirmed that in vivo C. krusei is less responsive to fluconazole compared with other triazoles (though there was some activity with very high doses of fluconazole) and correlate well with in vitro susceptibility data showing intrinsic resistance.
In contrast, the in vitro fluconazole MIC for the C. tropicalis isolate was 0.25 mg/L, and the isolate responded to doses as low as 15 mg/kg in the TN murine model and 50 mg/kg in the PN model.
In this model, we again demonstrated that therapeutic levels of voriconazole remain in the plasma of mice following oral therapy if grapefruit juice is co-administered. The drug exposure following 15 mg/kg voriconazole is similar to the exposure following 50 mg/kg isavuconazole (though much higher maximum concentrations and exposure occur following 150 mg/kg isavuconazole).14 The exposure of isavuconazole (AUC) demonstrated in these models is far exceeded in humans receiving 100 mg daily.
Selection of therapy for C. krusei infections is particularly difficult due to intrinsic resistance to fluconazole and reduced susceptibility to amphotericin B leaving few therapeutic options,6,7 particularly if step-down oral therapy is required. Of particular interest in this model was the efficacy of isavuconazole and voriconazole at reducing the burden in the brain following C. krusei infection (in contrast to fluconazole that had no effect on the burden). In addition, new triazoles such as isavuconazole have good tissue penetration, e.g. for intraocular and cerebral infections, and therefore may be preferable to an echinocandin for invasive candidiasis in sanctuary sites.
These data underline the potential utility of isavuconazole to treat a wide range of infections caused by Candida spp., which needs to be confirmed in clinical trials.
Funding
This work was partially funded by the Fungal Research Trust and Basilea Pharma. P. A. W. is partially funded by The Fungal Research Trust and by federal funds from the National Institute of Allergy and Infectious Diseases under contract no. N01-AI-30041. A. S. is funded by the Fungal Research Trust.
Transparency declarations
P. A. W. and J. M. have received travel grants to attend ICAAC and ECCMID from Basilea Pharma. In the past 5 years, D. W. D. has received grant support from Astellas, Merck, Pfizer, F2G, OrthoBiotech, Indevus, Basilea, The Fungal Research Trust, The Wellcome Trust, The Moulton Trust, The Medical Research Council, The Chronic Granulomatous Disease Research Trust, The National Institute of Allergy and Infectious Diseases and The European Union. He has been an advisor/consultant to Basilea, Vicuron (now Pfizer), Pfizer, Schering Plough, Indevus, F2G, Nektar, Daiichi, Sigma Tau, Astellas, Gilead and York Pharma. He has been paid for talks on behalf of Schering, Astellas, Merck, GSK, Chiron, AstraZeneca and Pfizer. He holds founder shares in F2G Ltd, a University of Manchester spin-out company. Other authors: none to declare.
Acknowledgements
Part of this data was presented at the Forty-seventh Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL, USA, 2007.
References
- 1.Gupta KL, Ghosh AK, Kochhar R, et al. Esophageal candidiasis after renal transplantation: comparative study in patients on different immunosuppressive protocols. Am J Gastroenterol. 1994;89:1062–5. [PubMed] [Google Scholar]
- 2.Abbott KC, Hypolite I, Poropatich RK, et al. Hospitalizations for fungal infections after renal transplantation in the United States. Transpl Infect Dis. 2001;3:203–11. doi: 10.1034/j.1399-3062.2001.30404.x. [DOI] [PubMed] [Google Scholar]
- 3.Hajjeh RA, Sofair AN, Harrison LH, et al. Incidence of bloodstream infections due to Candida species and in vitro susceptibilities of isolates collected from 1998 to 2000 in a population-based active surveillance program. J Clin Microbiol. 2004;42:1519–27. doi: 10.1128/JCM.42.4.1519-1527.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colombo AL, Nucci M, Park BJ, et al. Epidemiology of candidemia in Brazil: a nationwide sentinel surveillance of candidemia in eleven medical centers. J Clin Microbiol. 2006;44:2816–23. doi: 10.1128/JCM.00773-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warnock DW. Trends in the epidemiology of invasive fungal infections. Nippon Ishinkin Gakkai Zasshi. 2007;48:1–12. doi: 10.3314/jjmm.48.1. [DOI] [PubMed] [Google Scholar]
- 6.Pfaller MA, Diekema DJ. Twelve years of fluconazole in clinical practice: global trends in species distribution and fluconazole susceptibility of bloodstream isolates of Candida. Clin Microbiol Infect. 2004;10(Suppl 1):11–23. doi: 10.1111/j.1470-9465.2004.t01-1-00844.x. [DOI] [PubMed] [Google Scholar]
- 7.Pfaller MA, Diekema DJ, Gibbs DL, et al. Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997–2005: an 8.5-year analysis of susceptibilities of Candida and other yeast species to fluconazole and voriconazole by CLSI standardized disk diffusion testing. J Clin Microbiol. 2007;45:1735–45. doi: 10.1128/JCM.00409-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kullberg BJ, Sobel JD, Ruhnke M, et al. Voriconazole versus a regimen of amphotericin B followed by fluconazole for candidaemia in non-neutropenic patients: a randomised non-inferiority trial. Lancet. 2005;366:1435–42. doi: 10.1016/S0140-6736(05)67490-9. [DOI] [PubMed] [Google Scholar]
- 9.Mora-Duarte J, Betts R, Rotstein C, et al. Comparison of caspofungin and amphotericin B for invasive candidiasis. N Engl J Med. 2002;347:2020–9. doi: 10.1056/NEJMoa021585. [DOI] [PubMed] [Google Scholar]
- 10.Schmitt-Hoffmann A, Roos B, Heep M, et al. Single-ascending-dose pharmacokinetics and safety of the novel broad-spectrum antifungal triazole BAL4815 after intravenous infusions (50, 100, and 200 milligrams) and oral administrations (100, 200, and 400 milligrams) of its prodrug, BAL8557, in healthy volunteers. Antimicrob Agents Chemother. 2006;50:279–85. doi: 10.1128/AAC.50.1.279-285.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warn PA, Sharp A, Denning DW. In vitro activity of a new triazole BAL4815, the active component of BAL8557 (the water-soluble prodrug), against Aspergillus spp. J Antimicrob Chemother. 2006;57:135–8. doi: 10.1093/jac/dki399. [DOI] [PubMed] [Google Scholar]
- 12.Guinea J, Pelaez T, Recio S, et al. In vitro antifungal activities of isavuconazole (BAL4815), voriconazole, and fluconazole against 1007 isolates of Zygomycete, Candida, Aspergillus, Fusarium, and Scedosporium species. Antimicrob Agents Chemother. 2008;52:1396–400. doi: 10.1128/AAC.01512-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Illnait-Zaragozi MT, Martinez GF, Curfs-Breuker I, et al. In vitro activity of the new azole isavuconazole (BAL 4815) compared with six other antifungal agents against 162 Cryptococcus neoformans isolates from Cuba. Antimicrob Agents Chemother. 2008;52:1580–2. doi: 10.1128/AAC.01384-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warn PA, Sharp A, Mosquera J, et al. Comparative in vivo activity of BAL4815, the active component of the prodrug BAL8557, in a neutropenic murine model of disseminated Aspergillus flavus. J Antimicrob Chemother. 2006;58:1198–207. doi: 10.1093/jac/dkl396. [DOI] [PubMed] [Google Scholar]
- 15.Sugar AM, Liu XP. Effect of grapefruit juice on serum voriconazole concentrations in the mouse. Med Mycol. 2000;38:209–12. doi: 10.1080/mmy.38.3.209.212. [DOI] [PubMed] [Google Scholar]