Abstract
Objectives
Community-associated methicillin-resistant Staphylococcus aureus is responsible for an increasing number of skin infections. Over-the-counter topical wound care products may play a role in the prevention of these infections, but limited data are available regarding their activity. The current study utilized a modified time–kill design to evaluate the activity of three over-the-counter topical wound care products (benzethonium chloride/essential oils, neomycin/polymyxin B and polymyxin B/gramicidin) against four unique isolates (three USA 300 and one USA 400).
Methods
All experiments were performed using commercially available formulations. Bactericidal activity was defined as a sustained 3 log10 reduction in cfu/mL from the initial inoculum. Reductions in bacterial counts between agents were determined using analysis of variance.
Results
At 10 min, the reduction (mean ± SD) in log10 cfu/mL for all strains was 2.87 ± 1.22, 1.86 ± 0.76 and 0.143 ± 0.82 for benzethonium chloride/essential oils, neomycin/polymyxin B and polymyxin B/gramicidin, respectively. By 24 h, bactericidal activity was observed against two strains each for neomycin/polymyxin B and polymyxin B/gramicidin. Benzethonium chloride/essential oils was bactericidal against all strains by 6 h. At 24 h, all three agents were superior to controls (P < 0.05). Benzethonium chloride/essential oils was more active at 24 h than polymyxin B/gramicidin versus all four strains (P < 0.05) and more active than neomycin/polymyxin B versus three of four strains (P < 0.05).
Conclusions
These topical agents demonstrated variable activity against the four strains tested. Benzethonium chloride/essential oils was more rapidly and completely active than the other agents tested.
Keywords: MRSA, community-acquired infections, antimicrobial activity, skin infections
Introduction
Community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) is a virulent organism that has emerged as a primary pathogen in skin and soft tissue infections.1,2 The USA 300 clone has been most commonly implicated, with USA 400 strains playing a minor role.2
Topical anti-infectives have been widely utilized for the prevention of skin and soft tissue infections.3 Testing of the antimicrobial activity of topical wound care products is performed with the final marketed product, rather than the active components, using the Food and Drug Administration (FDA) Bactericidal Assay in the USA.4 Data regarding the comparative in vitro activity of topical anti-infective wound care products against CA-MRSA USA 300 and USA 400 strains are lacking. We compared the activity of three commercially available wound care products against CA-MRSA using a modified, time–kill version of the FDA Bactericidal Assay methodology.
Materials and methods
Bacterial strains
Four CA-MRSA isolates were tested. Isolates 1139 and 10841 are distinct USA 300 clones that were obtained from JMI Laboratories, North Liberty, IA, USA. An additional USA 300 clone (NRS384) and a USA 400 clone (NRS123) were obtained through the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA) programme.
Medium
All susceptibility testing was performed using Mueller–Hinton agar (Difco Laboratories, Detroit, MI, USA). Enumeration of colony counts in time–kill experiments was performed using Dey–Engley agar (DEA; Difco).5
Antimicrobial agents
Three commercially available topical wound care products were tested: (i) benzethonium chloride 0.2%, tea tree oil and white thyme oil (StaphAseptic, Tec Laboratories Inc., Albany, OR, USA); (ii) neomycin 3.5 mg/g and polymyxin B sulphate 10 000 U/g (Maximum Strength Antibiotic Cream, Rite Aid Corp., Harrisburg, PA, USA); and (iii) polymyxin B sulphate 10 000 U/g and gramicidin 0.25 mg/g (Polysporin Cream, Pfizer Inc., Markham, ON, USA).
Susceptibility testing
MICs were determined using the Etest methodology (AB Biodisk, Solna, Sweden) with an inoculum of 5 × 105 cfu/mL, according to the CLSI guidelines.6 MICs of a variety of antimicrobials were determined in order to establish antibiogram profiles for each CA-MRSA isolate.
Confirmation of inactivation by DEA
In order to confirm that DEA would inactivate the topical products, each product was diluted 1:10 with DEA. Saline (0.9%) was used as a control. A known bacterial inoculum (prepared in sterile 0.9% sodium chloride) was added to each sample. All samples were serially diluted and plated on DEA. Colony counts were compared between the inactivated samples and the saline controls. Inactivation tests were performed for each bacterium included in this work.
Time–kill analysis
Time–kill studies were performed using a modified FDA Bactericidal Assay technique.4 The primary modification to the test was the inclusion of multiple time point analyses, as only a 10 min sample is required by the FDA. Bacterial inocula were prepared in 0.9% sodium chloride and adjusted to a turbidity equivalent to that of a 3 McFarland standard using a Vitek colorimeter (bioMérieux Inc., Durham, NC, USA). In a 50 mL conical tube, 0.5 mL of this inoculum was combined with 0.5 mL of fetal bovine serum (HyClone, Logan, UT, USA) and 4 mL of each topical agent, manually mixed, vortexed and placed in an incubator at 35°C, 0.5% CO2. Fetal bovine serum is included as an interfering substance to mimic the presence of organic materials in a wound.4 Normal saline was used as a control. The final tested volume of 5 mL was modified from the FDA‐recommended 10 mL volume in a proportional manner to minimize the total drug quantities required. Samples were collected at 0, 1, 5, 10, 20 and 45 min, and at 1, 2, 4, 6, 8 and 24 h. At each time point, samples were inactivated with a 1:10 addition of Dey–Engley broth (DEB) in order to quench further antibacterial activity.5 Samples were then serially diluted in cold DEB. Bacterial quantification was performed by plating triplicate 20 µL aliquots of each diluted sample on DEA. All samples were diluted at least 10-fold in order to minimize antibiotic carryover. Plated samples were incubated at 35°C in 0.5% CO2 for 24 h, and colony counts (log10 cfu/mL) were enumerated manually (limit of detection 50 cfu/mL). Time–kill curves were created by plotting mean colony counts (log10 cfu/mL) versus time. Bactericidal activity was defined as a sustained 3 log10 reduction in cfu/mL. All time–kill analyses were performed in duplicate.
Statistical analysis
Reductions in colony counts (log10 cfu/mL) from initial inocula at 10 min and 24 h were compared between agents using analysis of variance with Tukey’s test for multiple comparisons. For all experiments, a P value of ≤0.05 was considered indicative of statistical significance. All statistical analyses were performed using SPSS (version 10; SPSS, Inc., Chicago, IL, USA).
Results
Susceptibility testing
All isolates were susceptible to vancomycin, linezolid, quinupristin/dalfopristin, clindamycin and trimethoprim/sulfamethoxazole. Isolates 10841 and NRS384 were intermediate to ciprofloxacin. Only NRS123 was susceptible to erythromycin.
Confirmation of inactivation by DEA
After inactivation with DEA, no difference in bacterial recovery was observed for all three topical products when compared with the saline control (data not shown).
Time–kill analysis
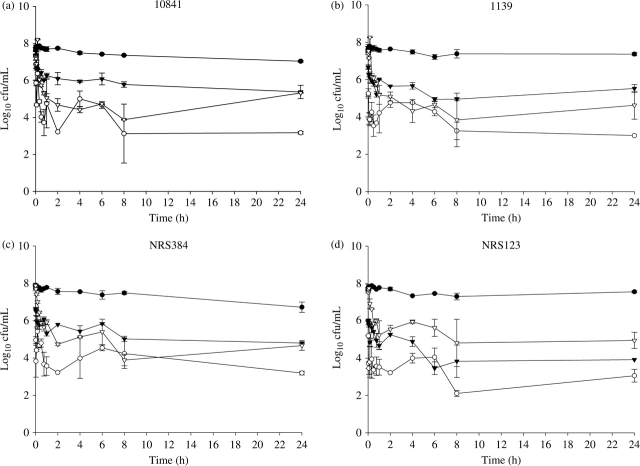
Time–kill curves are shown in Figure 1(a–d). At 10 min, the reduction (mean ± SD) in log10 cfu/mL for all strains was 2.87 ± 1.22, 1.86 ± 0.76 and 0.143 ± 0.82 for benzethonium chloride/essential oils, neomycin/polymyxin B and polymyxin B/gramicidin, respectively. Both benzethonium chloride/essential oils (P < 0.01) and neomycin/polymyxin B (P < 0.05) demonstrated a significant reduction in cfu/mL at 10 min versus the control against all strains. Polymyxin B/gramicidin did not demonstrate a significant reduction in cfu/mL versus the control at 10 min against any strain (P = not significant). Benzethonium chloride/essential oils was significantly more active in reducing cfu/mL at 10 min than neomycin/polymyxin B against two strains (10841 and 1139, P < 0.05) and polymyxin B/gramicidin against all four strains (P < 0.05). Neomycin/polymyxin B was significantly more active than polymyxin B/gramicidin at 10 min against three strains (10841 and 1139, P < 0.01; NRS123, P < 0.05).
Figure 1.
Activity of benzethonium chloride/essential oils (open circles), neomycin/polymyxin B (filled triangles) and polymyxin B/gramicidin (open triangles) against three CA-MRSA USA 300 strains, 10841 (a), 1139 (b) and NRS384 (c), and one USA 400 strain NRS123 (d). Growth control curves are represented by the filled circles.
Bactericidal activity was observed against two strains each for neomycin/polymyxin B (at 24 h against NRS384 and at 6 h against NRS123) and polymyxin B/gramicidin (at 4 h against 1139 and at 8 h against NRS384). Benzethonium chloride/essential oils was bactericidal against all CA-MRSA, with sustained bactericidal activity achieved at 6 h against strains 1139 and 10841, at 20 min against NRS384 and at 2 min against NRS123.
At 24 h, all three agents were superior to controls in reducing cfu/mL (P < 0.05). Benzethonium chloride/essential oils was more active at 24 h than polymyxin B/gramicidin against all four strains (P < 0.05), and more active than neomycin/polymyxin B against strains 10841 (P < 0.01), 1139 (P < 0.05) and NRS384 (P < 0.01). No significant differences in cfu/mL reduction at 24 h were observed between neomycin/polymyxin B and polymyxin B/gramicidin (P = not significant).
Discussion
Little published data exist regarding the relative activity of over-the-counter topical anti-infectives commonly used in wound care, and none has examined activity against CA-MRSA strains. Published studies of other topical products typically use broth microdilution with the active ingredients of each formulation.7 Because the drug vehicles can influence the effectiveness of these products, the FDA approves these compounds based upon the antimicrobial activity of the final commercial formulation.4 Similar suspension techniques (EN 1276 and EN 12054) using the final topical product are recommended by the governing bodies in the European Union.8 Bactericidal activity at a single time point after 10 min of exposure is the FDA standard for evaluation of activity. As many topical agents are applied to the skin multiple times daily and infrequently washed from the skin, the current analysis evaluated activity of the commercially available products over a 24 h period. The current analysis also investigated earlier activity.
The topical products evaluated in the current study were chosen based upon their common usage, availability as over-the-counter products and their preparation in water-miscible vehicles. This allowed the same time–kill techniques to be applied to all of the agents and reduced the likelihood that disparate handling of these finished topical agents would affect the results. One limitation of the last criterion is the fact that we were unable to evaluate the common petrolatum-based triple antibiotic ointment containing neomycin/polymyxin B/bacitracin, although a water-miscible formulation of the double antibiotic agent neomycin/polymyxin B was available and was included in this study. Bacitracin has displayed minimal activity (susceptibility of <1% tested isolates) against MRSA in a traditional susceptibility study,7 so it is unlikely that different results would be observed if a bacitracin-containing product was used in the current study.
Benzethonium chloride is a commonly used antiseptic. The commercially available product evaluated in the current work combines benzethonium chloride with tea tree and white thyme oils, both of which have been studied as anti-infectives.9,10 The role of these additional essential oils in the activity of the commercial product is not currently known, but they may play a role in its antibacterial activity.
The present study suggests that there are differences among a variety of available topical anti-infective products that may be important when considering the management of wound care in the setting of CA-MRSA. As it was most active against all tested CA-MRSA and more rapidly bactericidal, further evaluation of the novel product containing benzethonium chloride/essential oils is warranted.
Funding
This work was supported by an unrestricted grant from Tec Laboratories Inc., Albany, OR, USA.
Transparency declarations
J. M. C. serves as a consultant to Tec Laboratories Inc. D. T. B. and G. P. A. have no conflicts to declare.
Acknowledgements
A portion of this work was presented at the Forty-second Midyear Clinical Meeting of the American Society of Health-System Pharmacists, Las Vegas, NV, USA, December 2007. Isolates 1139 and 10841 were acquired from Thomas R. Fritsche, MD, JMI Laboratories, North Liberty, IA, USA. Isolates NRS384 and NRS123 were obtained through the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA) programme, supported under NIAID, NIH Contract No. N01-AI-95359.
References
- 1.Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. J Am Med Assoc. 2007;298:1763–71. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 2.Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666–74. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura Y, Daya M. Use of appropriate antimicrobials in wound management. Emerg Med Clin North Am. 2007;25:159–76. doi: 10.1016/j.emc.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Topical antimicrobial drug products for over-the-counter human use; tentative final monograph for first aid antiseptic drug products. United States Federal Register Notice. 1991;56:33644–80. [Google Scholar]
- 5.Dey BP, Engley FB. Methodology for recovery of chemically treated Staphylococcus aureus with neutralizing medium. Appl Environ Microbiol. 1983;45:1533–7. doi: 10.1128/aem.45.5.1533-1537.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically—Seventh Edition: Approved Standard M7-A7. Wayne, PA, USA: CLSI; 2006. [Google Scholar]
- 7.Jones RN, Li Q, Kohut B, et al. Contemporary antimicrobial activity of triple antibiotic ointment: a multiphased study of recent clinical isolates in the United States and Australia. Diagn Microbiol Infect Dis. 2006;54:63–71. doi: 10.1016/j.diagmicrobio.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Messager S, Hammer KA, Carson CF, et al. Assessment of the antibacterial activity of tea tree oil using the European EN 1276 and EN 12054 standard suspension tests. J Hosp Infect. 2005;59:113–25. doi: 10.1016/j.jhin.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Caelli M, Porteous J, Carson CF, et al. Tea tree oil as an alternative topical decolonization agent for methicillin-resistant Staphylococcus aureus. J Hosp Infect. 2000;46:236–7. doi: 10.1053/jhin.2000.0830. [DOI] [PubMed] [Google Scholar]
- 10.Inouye S, Yamaguchi H, Takizawa T. Screening of the antibacterial effects of a variety of essential oils on respiratory tract pathogens, using a modified dilution assay method. J Infect Chemother. 2001;7:251–4. doi: 10.1007/s101560170022. [DOI] [PubMed] [Google Scholar]