Abstract
Aims
To determine whether an ultrasensitive assay can permit quantification of changes in circulating cardiac troponin (Tn) in the setting of stress test-induced myocardial ischaemia.
Methods and results
Blood samples were obtained before, immediately after, and 2 and 4 h after stress testing with nuclear perfusion imaging in 120 patients. Troponin was measured using commercial assays as well as with a novel, ultrasensitive cardiac TnI assay with a limit of detection of 0.2 pg/mL. Using the ultrasensitive assay, TnI was detectable in all patients before stress testing (median 4.4 pg/mL, interquartile range 3.1–8.6 pg/mL). By 4 h, troponin levels were unchanged in patients without ischaemia, whereas circulating levels had increased by a median of 1.4 pg/mL (24% increase) in patients with mild ischaemia (P = 0.002) and by 2.1 pg/mL (40% increase) in patients with moderate-to-severe ischaemia (P = 0.0006). In contrast, changes in troponin levels across patients in different ischaemic categories were indistinguishable using commercial troponin assays. When added to clinical factors, a >1.3 pg/mL increase in TnI using the ultrasensitive assay was an independent predictor of ischaemia (odds ratio 3.54, P = 0.007).
Conclusion
Transient stress test-induced myocardial ischaemia is associated with a quantifiable increase in circulating troponin that is detectable with a novel, ultrasensitive TnI assay.
Keywords: Troponin, Myocardial ischaemia, Stress test
Introduction
Release of cardiac troponins I and T into the circulation occurs with cardiomyocyte necrosis and serves as the basis for their use as the preferred diagnostic biomarkers for acute myocardial infarction (MI).1,2 Whether troponin is released from cardiomyocytes in the setting of transient myocardial ischaemia remains an area of continued debate.3,4 However, limitations to the analytical performance of current commercial troponin assays at very low concentrations5 have made it challenging to address adequately whether there is a quantifiable release of cardiac troponins in the clinical setting of myocardial ischaemia.
Recently, a cardiac troponin I assay has been developed that can quantify molecules of troponin in a microcapillary and has a limit of detection that is at least one order of magnitude lower than current commercial assays.6 Using this novel ultrasensitive assay, we investigated whether we could demonstrate a rise in circulating levels of troponin I in response to cardiac stress test-induced transient myocardial ischaemia.
Methods
Patients
A total of 155 patients undergoing stress testing with myocardial perfusion imaging were enrolled in the Protein Markers of Ischemia using Proteomic Testing (PROMPT)—TIMI 35 prospective cohort study.7 The study protocol was approved by the Human Research Committee, and all patients provided written informed consent. All patients who were referred for stress testing for the evaluation of possible myocardial ischaemia were eligible for participation. Patients with angina symptoms in the prior 48 h (n = 19), those who underwent an adenosine stress test (n = 15), and/or those in whom adequate perfusion images were not obtained (n = 3) were excluded, leaving a total of 120 patients included in these analyses.
Study protocol
Patients underwent stress testing using standard protocols.7 If the patient developed angina during the test, the timing, quality (typical vs. atypical), and effect on the test (limiting or non-limiting) were noted. The maximal horizontal or downsloping ST-segment changes were recorded in each ECG lead. A stress–rest imaging protocol was used, as described previously.7 A 20-segment myocardial model was used for semi-quantitative analysis, with a visual perfusion rating of 0–4 for each segment by nuclear cardiologists blinded to biomarker data.8 Quantitative analysis of myocardial perfusion was also performed using the CEqual method to calculate the per cent reversible and fixed perfusion defects.9 Patients were categorized as having no definitive (n = 50), mild (n = 31), or moderate-to-severe ischaemia (n = 39) without knowledge of biomarker levels.
Blood samples were obtained immediately before [n = 108, median 12 min, interquartile range (IQR) 8–19 min], immediately after (n = 90, 7 min, IQR 5–9 min), 2 h (n = 51, median 1.9 h, IQR 1.8–2.1 h), and 4 h (n = 108, median 3.9 h, IQR 3.3–4.4 h) after stress testing. Blood samples were not available at all times in all patients due to technical issues. Blood samples were placed on ice and processed within 60 min. Lithium heparinized plasma was stored at −80°C, and aliquots were thawed for these analyses.
Biomarkers
Cardiac troponin I was measured using the ultrasensitive Singulex Erenna System (at Singulex, Inc., Berkeley, CA, USA), based on capillary flow single molecule counting combined with microparticle immunoassay technology.6 The assay was standardized to National Institute of Standards and Technology Material and validated with a lower limit of detection of 0.0002 ng/mL or 0.20 pg/mL. The inter-assay coefficient of variation (CV) is 10% at 0.91 pg/mL, and the 99th percentile in a healthy control population is 9 pg/mL. In this study, 50 µL of plasma was used, and samples were measured in duplicate in a 96-well plate batch mode. The mean variation between duplicates was 12%. A total of 18 assay runs (2 h per assay) were performed over 4 days using a single lot of reagents. Cardiac troponin was measured in 106 subjects using two older assays at the TIMI Biomarker Core Laboratory (Boston, MA, USA): the ACS:180 Chemiluminescence cTnI Immunoassay (Bayer Diagnostics, Tarrytown, NY, USA), which has a limit of detection of 0.03 ng/mL and an inter-assay CV of 10% at 0.4 ng/mL, and the cardiac troponin T assay on the Elecsys 1010 (Roche Diagnostics, Indianapolis, IN, USA), which has a limit of detection of 0.01 ng/mL, an inter-assay CV of 10% at 0.03 ng/mL, and an established clinical decision limit of 0.1 ng/mL. Cardiac troponin was also measured in 60 subjects (due to exhaustion of samples) using the current generation commercial sensitive troponin I assay used at Brigham and Women's Hospital, the Tn-I Ultra assay (Siemens Healthcare Diagnostics, Deerfield, IL, USA), which has a limit of detection of 0.006 ng/mL and an inter-assay CV of 10% at 0.03 ng/mL. B-type natriuretic peptide (BNP) was measured in this study using an established sequential sandwich immunoassay (Biosite, Inc., San Diego, CA, USA). All assays were performed by personnel blinded to stress test results.
Statistical analyses
Patients were categorized for the primary analysis on the basis of the severity of ischaemia as determined by myocardial perfusion imaging. Troponin concentrations were compared across groups using a non-parametric test for trend across ordered groups (Jonckheere-Terpstra test). The differences between baseline and post-stress test troponin levels within groups were compared using signed rank tests. The optimal cutpoint for the rise in troponin post-stress-testing for discrimination of myocardial ischaemia by scintigraphy was determined by finding the value above the threshold for 10% CV that maximized accuracy (proportion of true positives plus true negatives). Bootstrap analyses were performed to validate the cutpoint and generate 95% confidence intervals (CIs). The independent value of rise in troponin beyond that cutpoint for predicting ischaemia was then calculated using a multivariable logistic regression model that contained rise in troponin plus the three traditional factors used and reported in exercise stress testing: stress test time, the presence of angina (non-limiting or limiting), and the magnitude of ST depression (0, 0.1, or 0.2 mV). All reported P-values are two-sided, and a significance level of <0.05 was used. The primary hypothesis tested was that the rise in cardiac troponin I values as measured by the ultrasensitive assay would be associated with the degree of inducible ischaemia; no adjustments were made to the significance threshold for other exploratory analyses. The authors had full access to the data, performed all analyses using SAS 9.1 (Cary, NC, USA), and take full responsibility for the data.
Results
Baseline characteristics and stress testing
The baseline characteristics of the 120 subjects are shown in Table 1. Mean stress test duration was 7.7 ± 3.0 min, maximum workload achieved was 8.6 ± 3.2 METs, per cent of maximum predicted heart rate was 81 ± 12%, and the peak rate×systolic blood pressure product was 21 844 ± 5652. Twenty-five per cent of the patients had non-limiting angina and 11% had stress-test limiting angina. Horizontal or downsloping ST depression of 0.1 mV developed in 17% of the patients and ≥0.2 mV in 14% of the patients. On nuclear perfusion imaging, 50 (41.7%) patients had no inducible ischaemia, 31 (25.8%) had mild inducible ischaemia, and 39 (32.5%) had moderate-to-severe inducible ischaemia based on nuclear perfusion imaging. Stress test parameters stratified by severity of inducible ischaemia are shown in Table 2. Compared with patients with no or only mild ischaemia, there were trends for those with moderate-to-severe ischaemia to have achieved lower peak workloads and to have experienced rate-limiting angina or developed ST deviation. By design, the extent of the reversible perfusion defect differed significantly between the three groups.
Table 1.
Patient baseline characteristics (n = 120)
| Demographics | |
| Age (years) | 65 ± 11 |
| Male | 82 (68.3) |
| White | 89 (74.2) |
| Cardiac risk factors | |
| Hypertension | 85 (70.8) |
| Diabetes | 31 (25.8) |
| Current or former smoker | 78 (65.0) |
| Hyperlipidaemia | 88 (74.0) |
| Cardiac disease | |
| Documented CAD | 66 (55.5) |
| Prior MI | 53 (44.2) |
| Prior PCI | 41 (34.2) |
| Prior CABG | 28 (23.3) |
Data are presented as mean ± SD or number (%) of patients.
CABG, coronary artery bypass graft; CAD, coronary artery disease; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Table 2.
Stress test results
| None (n = 50) | Mild (n = 31) | Moderate-to-severe (n = 39) | P-value | |
|---|---|---|---|---|
| Stress test performance | ||||
| Duration (min) | 7.8 ± 3.0 | 8.1 ± 3.1 | 7.2 ± 2.9 | 0.47 |
| Maximum workload (METs) | 8.7 ± 3.0 | 9.5 ± 3.3 | 7.7 ± 3.1 | 0.08 |
| Peak HR (b.p.m.) | 131 ± 19 | 125 ± 23 | 122 ± 22 | 0.17 |
| Per cent of maximal predicted HR (%) | 83 ± 11 | 81 ± 12 | 79 ± 12 | 0.17 |
| Peak SBP (mmHg) | 176 ± 27 | 168 ± 23 | 169 ± 26 | 0.25 |
| Double product (b.p.m. × mmHg, 1000 s) | 23 ± 5 | 21 ± 6 | 21 ± 5 | 0.12 |
| Angina | ||||
| None | 27 (62.8) | 22 (78.6) | 19 (54.3) | 0.13 |
| Non-limiting | 12 (27.9) | 4 (14.3) | 10 (28.6) | 0.33 |
| Limiting | 4 (9.3) | 2 (7.1) | 6 (17.1) | 0.40 |
| Duration (min) | 0 (0–3) | 0 (0–2) | 2 (0–6) | 0.09 |
| ST depression (mV) | ||||
| 0.0 | 36 (75.0) | 23 (76.7) | 21 (56.8) | 0.12 |
| 0.1 | 7 (14.6) | 4 (13.3) | 8 (21.6) | 0.59 |
| ≥0.2 | 5 (10.4) | 3 (10.0) | 8 (21.6) | 0.26 |
| Nuclear perfusion imaging | ||||
| Difference in perfusion score (points) | 0.2 ± 0.6 | 3.4 ± 1.1 | 9.0 ± 5.4 | <0.0001 |
| Reversible perfusion defect (%) | 0.1 ± 0.3 | 3.2 ± 2.1 | 10.9 ± 7.1 | <0.0001 |
Ultrasensitive cardiac troponin I assay
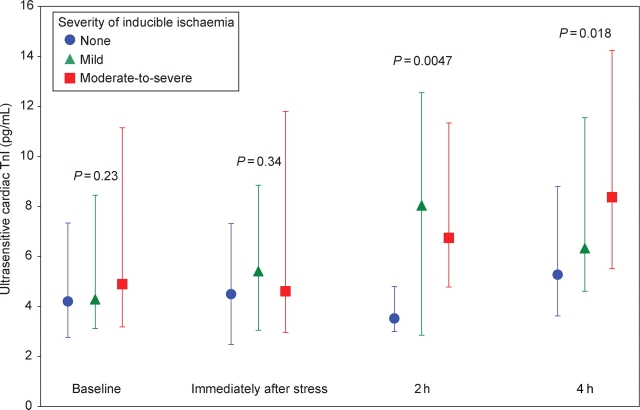
At baseline, cardiac troponin I could be detected in plasma samples from all patients using the ultrasensitive assay (median 4.4 pg/mL, IQR 3.1–8.6 pg/mL), with all values being above the assay's 10% CV threshold (0.91 pg/mL). At baseline and immediately after stress testing, cardiac troponin I levels were similar across the three pre-specified categories of ischaemia. Of note, in post hoc testing, the 16 patients who eventually manifested severe ischaemia did have slightly higher levels of baseline troponin (median 7.0 pg/mL, IQR 4.3–11.5) than did patients who manifested no ischaemia (median 4.2 pg/mL, IQR 2.8–7.3) (P = 0.04). At both 2 and 4 h after stress testing, cardiac troponin I levels were significantly higher in patients who had evidence of inducible myocardial ischaemia during stress testing (P = 0.0047 and 0.018, respectively) (Figure 1). Likewise, the increase in cardiac troponin I at 4 h was significantly associated with the degree of inducible ischaemia, ranging from a median of 0.6 pg/mL (11% increase) in patients with no ischaemia (P = 0.36 vs. baseline), to 1.4 pg/mL (24% increase) in patients with mild inducible ischaemia (P = 0.002), to 2.1 pg/mL (40% increase) in patients with moderate-to-severe inducible ischaemia (P = 0.0006) (P = 0.008 for trend, Figure 2). There was no significant heterogeneity in the rise in troponin in patients with ischaemia who either did or did not have a prior MI.
Figure 1.
Cardiac troponin I levels (median and interquartile range) measured using the ultrasensitive assay in patients with none (blue circles), mild (green triangles), and moderate-to-severe (red squares) ischaemia at baseline (samples available in 44, 30, and 34 patients, respectively), immediately after stress testing (37, 26, and 27 patients), 2 h after stress testing (20, 15, and 16 patients), and 4 h after stress testing (44, 28, and 36 patients). P-values are for trend across ischaemic categories at each timepoint.
Figure 2.
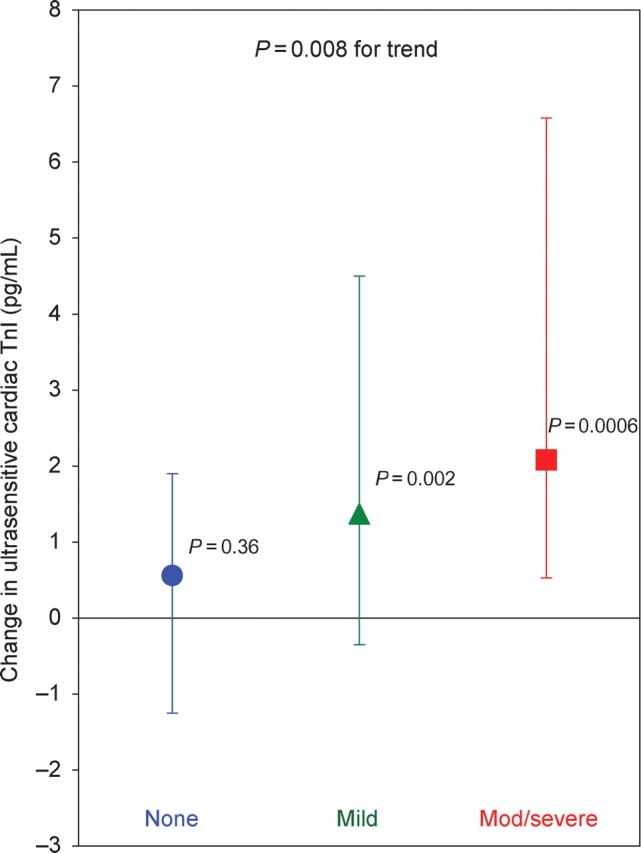
Change in cardiac troponin I levels (median and interquartile range) measured using the ultrasensitive assay from baseline to 4 h in patients with none (n = 39, blue circles), mild (n = 27, green triangles), and moderate-to-severe (n = 33, red squares) ischaemia. P-values by the point estimate symbols are for whether the difference is greater than 0, and the P-value at the top is for trend of differences across ischaemic categories.
In exploratory analyses, we examined the correlation between continuous measures of myocardial ischaemia, including the percentage of myocardium with scintigraphic reversible perfusion defects, the duration of angina during stress testing, and the sum of ST deviation, and the change in ultrasensitive cardiac troponin I levels at 2 and 4 h. The percentage of myocardium with scintigraphic reversible ischaemia was significantly and positively correlated with the change in cardiac troponin I levels at 4 h (r = 0.30, P = 0.003), but not at 2 h (r = 0.13, P = 0.38). The duration of angina during the stress test was significantly and positively correlated with the change in cardiac troponin I at 2 h (r = 0.37, P = 0.008), but not at 4 h (r = 0.15, P = 0.13). The sum of ST deviation was significantly and positively correlated with the change in cardiac troponin I levels at both 2 h (r = 0.33, P = 0.02) and 4 h (r = 0.27, P = 0.0066).
Conventional commercial troponin assays
Using older troponin I and T assays comparable with those used in prior studies investigating exercise-induced troponin release, troponin I levels were above the limit of detection (0.03 ng/mL) in 35.9% of the patients at baseline and in 23.6% of the patients at 4 h, but were not above the 10% CV threshold (0.4 ng/mL) in any patients at either timepoint. Troponin T levels were above the limit of detection (0.01 ng/mL) in only 4.7% of the patients at baseline and 7.6% of the patients at 4 h and were above the 10% CV threshold (0.03 ng/mL) in 2.8% of the patients at baseline and 1.0% at 4 h. The change in troponins I and T by 4 h was a median of 0.00 ng/mL in patients in each of the three myocardial ischaemia categories (P = 0.72 and 0.96 for comparison across ischaemic categories for troponins I and T, respectively).
We also measured troponin I using a current generation sensitive commercial assay. Troponin I levels were above the limit of detection (0.006 ng/mL) in 40% of the patients at baseline and 71% of the patients at 4 h; however, only 8.3% were above the 10% CV threshold (0.03 ng/mL) at baseline and 19.1% at 4 h. The change in troponin I by 4 h was approximately at or below the assay's limit of detection in the three myocardial ischaemia categories [0.003 ng/mL (IQR 0.000–0.005), 0.001 ng/mL (IQR 0.000–0.006), and 0.007 ng/mL (IQR 0.002–0.011), respectively], with no statistically significant differences across the three categories (P = 0.39). The absolute values of troponin I were correlated between the highly sensitive commercial assay and the ultrasensitive assay at baseline and 4 h (r = 0.72, P < 0.0001 for both), but less so for the change by 4 h (r = 0.45, P = 0.005).
Change in ultrasensitive cardiac troponin I assay levels to predict ischaemia
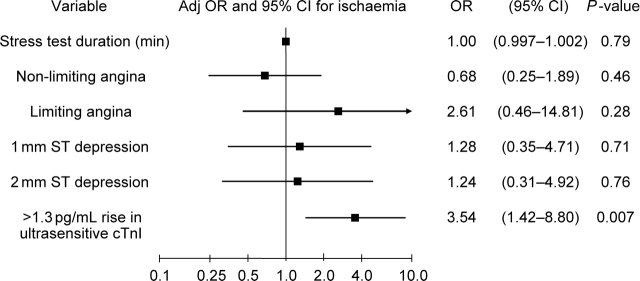
A cutpoint of >1.3 pg/mL for the rise in cardiac troponin I by 4 h using the ultrasensitive assay offered the best accuracy [sensitivity 60% (95% CI 47–72), specificity 69% (95% CI 52–83), accuracy 64% (95% CI 53–73)]. These performance characteristics compared favourably with traditional metrics such as ST deviation [sensitivity 34% (95% CI 23–47), specificity 75% (95% CI 60–86), accuracy 51% (95% CI 42–60)] and limiting angina [sensitivity 13% (95% CI 6–23), specificity 92% (95% CI 81–98), accuracy 46% (95% CI 37–55)]. Bootstrap analyses yielded an identical cutpoint (1.3 pg/mL) with an IQR of 1.1–1.4 pg/mL and a 95% CI of 1.0–2.6 pg/mL. We created a multivariable logistic regression model for inducible ischaemia that included duration of stress testing, presence and type of angina, magnitude of ST depression, and a rise in troponin I by 4 h of >1.3 pg/mL. As shown in Figure 3, an increase in troponin I of >1.3 pg/mL after stress testing was strongly associated with ischaemia, with an adjusted odds ratio of 3.54 (95% CI 1.42–8.80, P = 0.007). We have previously shown that an elevated (≥80 pg/mL) post-stress test BNP was associated with the presence of inducible myocardial ischaemia.7 Combining an increase in cTnI >1.3 pg/mL with a post-stress test BNP >80 pg/mL, the proportion with myocardial ischaemia was 36% if neither was positive, 61% if either was positive, and 90% if both were positive (P = 0.0003). When both biomarkers were added to a model containing the aforementioned clinical predictors, both troponin increase [odds ratio (OR) 4.47, 95% CI 1.59–12.5, P = 0.005] and an elevated BNP (OR 3.82, 95% CI 1.27–11.48, P = 0.017) remained significantly associated with myocardial ischaemia.
Figure 3.
Odds ratios and 95% confidence intervals for prediction of inducible myocardial ischaemia.
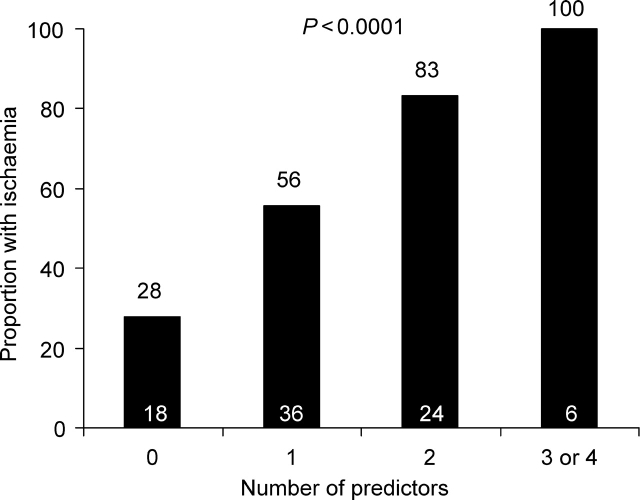
For the prediction of myocardial ischaemia, the combination of duration of stress testing, presence and type of angina, and magnitude of ST depression had a c-statistic of 0.62 (95% CI 0.50–0.74). Adding an elevated (>80 pg/mL) post-stress test BNP and ultrasensitive troponin I (rise >1.3 pg/mL) significantly improved the c-statistic to 0.76 [95% CI 0.66–0.87 (P = 0.01)]. When patients were categorized on the basis of how many of the four risk variables were present (limiting angina, ST depression >0.1 mV, post-stress test BNP ≥80 pg/mL, and a rise in troponin I >1.3 pg/mL), a stepwise and significant gradient of risk for inducible myocardial ischaemia was observed, ranging from 28 to 100% (Figure 4, P < 0.0001).
Figure 4.
Proportion with inducible ischaemia among patients categorized by the number of ischaemia predictors (limiting angina, ST depression >0.1 mV, >1.3 pg/mL rise in ultrasensitive cardiac troponin I by 4 h, and post-stress test BNP ≥80 pg/mL). Numbers within each bar represent the number of patients in that group.
Discussion
Using an ultrasensitive troponin I assay, we were able to demonstrate a quantifiable rise in circulating troponin levels in response to stress testing, and the magnitude of that rise was proportional to the degree of ischaemia on perfusion imaging. Furthermore, a rise in the level of troponin I after stress testing was significantly associated with inducible ischaemia even after adjusting for traditional parameters such as limiting angina and ST depression.
Cardiac troponins are the preferred diagnostic biomarker for MI.1 Using current commercial assays, most healthy individuals have levels below the limit of detection and certainly below the cutpoint at which the CV is <10%.5 Any elevation in troponin in the appropriate clinical setting has been considered indicative of myocardial necrosis rather than ischaemia.4 Previous attempts to quantify rises in troponin in the setting of transient myocardial ischaemia have been unrewarding. Specifically, in prior studies in individuals undergoing stress testing, troponin levels following stress testing have either been undetectable, or barely detectable but within the normal range, and changes in troponin were either undetectable, below the cutpoint for <10% CV, or not associated with the presence of ischaemia during the test.10–15 In three studies, an increase in troponin was demonstrable after stress testing, but in only a minority of patients with ischaemia and at levels close to the 10% CV cutpoint.16–18 Now, using a novel troponin assay that is one to two orders of magnitude more sensitive than commercial assays, we have been able to demonstrate that brief periods of stress-test induced myocardial ischaemia are associated with quantifiable rises in the concentration of circulating troponin.
Definitive delineation of the cellular events responsible for the rise in circulating troponin that we observed is beyond the scope of this clinical study. Specifically, whether cardiac troponin can be released in the setting of pure myocyte ischaemia without necrosis remains controversial. An immunohistochemistry study in animals subjected to coronary occlusion found staining for troponin in necrotic, but not in non-necrotic, areas of myocardium.19 Conversely, another animal study demonstrated elevated levels of circulating troponin without electron microscopic evidence of irreversible myocyte necrosis.20 Furthermore, cytosolic pools of troponin have been demonstrated, by some estimates accounting for ∼5% of total myocyte troponin.21 Troponin in these pools could egress from injured myocytes without the need for myofibril degradation. To that end, in specialized animal and human models, brief periods of myocardial ischaemia have been shown to lead to the release of troponin.22–24 Furthermore, in canine models, 15–20 min of sustained ischaemia is required to induce irreversible myocyte cell death;25 in contrast, the duration of myocardial oxygen supply–demand mismatch during stress testing was only a few minutes in our study, making necrosis unlikely. Nonetheless, although our findings support the concept that cardiac troponin may function as a biomarker of reversible myocardial injury, without pathological examination, the possibility of micronecrosis during stress testing cannot be excluded.
Our findings have several clinical implications. First, a more sensitive troponin assay that can detect transient myocardial injury would give clinicians the ability to seek biochemical data to support the diagnosis of unstable angina. Such objective evidence would be welcome in these cases, for which clinicians currently are forced to rely on a subjective history and transient ECG changes. Although the elevations seen in our study were subtle, and the range of values overlapped significantly between those with and without ischaemia, the duration and magnitude of ischaemia were, by design, brief. We would speculate that spontaneous ischaemia due to plaque rupture would generate more profound elevations in troponin. Clinicians might be able to use such elevations to diagnose ACS objectively. Of course, the prognostic implications and the utility in terms of guiding aggressiveness of care of such truly quantitatively minor elevations in patients will need to be studied.
Secondly, measuring biomarkers of myocardial injury (troponin) and wall stress (BNP) in patients undergoing stress testing with electrocardiography could potentially aid in the diagnosis of clinically significant epicardial coronary artery disease. Incorporation of measurement of these biomarkers along with assessment of traditional risk predictors could help obviate the need for initial myocardial perfusion imaging in a subset of patients. However, our goal in this proof-of-concept study was not to define the clinical utility of such measurements, but rather to study the release patterns of cardiac troponin in response to transient myocardial ischaemia. The ability of troponin or BNP levels to add to the diagnostic utility of stress testing needs to be confirmed in additional, larger prospective studies.
Thirdly, the clinical adoption of a troponin assay as sensitive as the one we used will likely necessitate a revised approach to reporting of troponin levels. No longer will individuals outside the setting of MI typically have undetectable levels. Rather, similar to most of the analytes clinicians measure, there will be a quantifiable reference range for the population. Using the current commercial troponin T assay, we have previously shown that elevated levels of troponin T are found in <1% of the population, but that those individuals typically have cardiovascular abnormalities including heart failure, diabetes mellitus, and left ventricular hypertrophy.26 In these patients, an elevated troponin may reflect chronic supply–demand mismatch. Using a more sensitive assay, it may be possible to better quantify and even track chronic myocardial ischaemia over time. To that end, using a troponin T assay 10-fold more sensitive than current commercial assays, elevated levels of troponin T were found in 92% of the chronic heart failure patients and higher levels were associated with a worse long-term prognosis.27 In accordance with that observation, in our study, we found that patients who manifested the most severe ischaemia on stress testing had slightly higher levels of troponin at baseline.
Our study has several limitations that must be considered. Not all subjects had blood samples successfully obtained at all times points; however, statistical comparisons of biomarker values between timepoints were performed by analysing the change in values within subjects, thereby guaranteeing a comparison across identical cohorts. We relied on nuclear imaging to quantify the degree of ischaemia. This remains an imperfect gold standard. Nonetheless, we think it superior to angiography, which can verify the presence of coronary atherosclerosis but cannot demonstrate inducible ischaemia. The magnitude in rise of troponin was relatively small, the IQRs wide, paired measurements were required to optimally discriminate between those who did and did not develop inducible ischaemia, and even then the sensitivity and specificity were modest. However, the accuracy was better than either ST deviation or limiting angina: two key parameters currently used to determine whether a stress test is indicative of ischaemia. Moreover, the duration of significant ischaemia during stress testing was brief, an average of only 2 min even in those with moderate-to-severe ischaemia. Furthermore, the cutpoint we used of a rise of 1.3 pg/mL corresponds to an increase of 30%, similar to the 25% threshold recommended when evaluating a patient with elevated levels of biomarkers at baseline.28 We applied a dichotomous cutpoint for the ultrasensitive troponin assay that was derived in the same data set and therefore is likely overly optimistic. Bootstrapping yielded an identical result with narrow IQR but wide 95% CI. Validation of this cutpoint, particularly in the setting of multivariable analysis, and consideration of modelling ultrasensitive troponin as a continuous variable in larger external data sets will be required. As is the case for any assay, before it can be adopted into routine clinical practice, it requires validation according to Clinical and Laboratory Standards Institute guidelines, including limit of quantitation across multiple lots of reagents.
In summary, by using a novel, ultrasensitive troponin assay that is at least an order of magnitude more sensitive than current commercial assays, we could detect changes in circulating troponin that were associated with the degree of transient stress-test induced myocardial ischaemia as detected using myocardial perfusion imaging. These findings pave the way for additional studies of the diagnostic and prognostic utility of troponin assays with markedly improved sensitivity.
Funding
Measurement of cardiac troponin I using the Singulex Erenna System was performed by Singulex, Inc. Measurement of B-type natriuretic peptide was performed by Biosite, Inc. M.S.S. and D.A.M. are supported in part by NIH grant U01 HL083-1341. M.S.S. and J.A.de.L. are supported in part by the Donald W. Reynolds Foundation. M.S.S. has received research grant support from Ortho-Clinical Diagnostics and Roche Diagnostics. D.A.M. has received honoraria for educational presentations from Bayer Diagnostics, Beckman-Coulter, Dade-Behring, Sanofi-Aventis, and Roche Diagnostics. He has served as a consultant for GlaxoSmithKline and Sanofi-Aventis and on advisory boards for Beckman-Coulter, Critical Diagnostics, Genentech, Ortho-Clinical Diagnostics, Roche Diagnositcs, and Siemens Medical Solutions. J.A.de.L. has received research grant support and/or honoraria from Biosite and Roche. P.J. has received honoraria from Siemens. E.B. has received research grant support from Beckman-Coulter and Roche.
Acknowledgements
The authors thank Quynh Anh Lu and John A. Todd of Singulex, Inc. for performance of troponin I measurements.
Conflict of interest: none declared.
References
- 1.Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. J Am Coll Cardiol. 2007;50:2173–2195. doi: 10.1016/j.jacc.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Apple FS, Jesse RL, Newby LK, Wu AH, Christenson RH. National Academy of Clinical Biochemistry and IFCC Committee for Standardization of Markers of Cardiac Damage Laboratory Medicine Practice Guidelines: analytical issues for biochemical markers of acute coronary syndromes. Circulation. 2007;115:e352–e355. doi: 10.1161/CIRCULATIONAHA.107.182881. [DOI] [PubMed] [Google Scholar]
- 3.Wu AH, Ford L. Release of cardiac troponin in acute coronary syndromes: ischemia or necrosis? Clin Chim Acta Int J Clin Chem. 1999;284:161–174. doi: 10.1016/s0009-8981(99)00078-9. [DOI] [PubMed] [Google Scholar]
- 4.Jaffe AS, Ravkilde J, Roberts R, Naslund U, Apple FS, Galvani M, Katus H. It's time for a change to a troponin standard. Circulation. 2000;102:1216–1220. doi: 10.1161/01.cir.102.11.1216. [DOI] [PubMed] [Google Scholar]
- 5.Panteghini M, Pagani F, Yeo KT, Apple FS, Christenson RH, Dati F, Mair J, Ravkilde J, Wu AH. Evaluation of imprecision for cardiac troponin assays at low-range concentrations. Clin Chem. 2004;50:327–332. doi: 10.1373/clinchem.2003.026815. [DOI] [PubMed] [Google Scholar]
- 6.Todd J, Freese B, Lu A, Held D, Morey J, Livingston R, Goix P. Ultrasensitive flow-based immunoassays using single-molecule counting. Clin Chem. 2007;53:1990–1995. doi: 10.1373/clinchem.2007.091181. [DOI] [PubMed] [Google Scholar]
- 7.Sabatine MS, Morrow DA, de Lemos JA, Omland T, Desai MY, Tanasijevic M, Hall C, McCabe CH, Braunwald E. Acute changes in circulating natriuretic peptide levels in relation to myocardial ischemia. J Am Coll Cardiol. 2004;44:1988–1995. doi: 10.1016/j.jacc.2004.07.057. [DOI] [PubMed] [Google Scholar]
- 8.Berman DS, Kiat H, Friedman JD, Wang FP, van Train K, Matzer L, Maddahi J, Germano G. Separate acquisition rest thallium-201/stress technetium-99m sestamibi dual-isotope myocardial perfusion single-photon emission computed tomography: a clinical validation study. J Am Coll Cardiol. 1993;22:1455–1464. doi: 10.1016/0735-1097(93)90557-h. [DOI] [PubMed] [Google Scholar]
- 9.Garcia EV, Cooke CD, Van Train KF, Folks R, Peifer J, DePuey EG, Maddahi J, Alazraki N, Galt J, Ezquerra N, Ziffer J, Areedab J, Berman DS. Technical aspects of myocardial SPECT imaging with technetium-99m sestamibi. Am J Cardiol. 1990;66:23E–31E. doi: 10.1016/0002-9149(90)90608-4. [DOI] [PubMed] [Google Scholar]
- 10.Ashmaig ME, Starkey BJ, Ziada AM, Amro AA, Sobki SH, Ferns GA. Changes in serum concentrations of markers of myocardial injury following treadmill exercise testing in patients with suspected ischaemic heart disease. Med Sci Monit. 2001;7:54–57. [PubMed] [Google Scholar]
- 11.Carlson RJ, Navone A, McConnell JP, Burritt M, Castle MC, Grill D, Jaffe AS. Effect of myocardial ischemia on cardiac troponin I and T. Am J Cardiol. 2002;89:224–226. doi: 10.1016/s0002-9149(01)02206-8. [DOI] [PubMed] [Google Scholar]
- 12.Choragudi NL, Aronow WS, Prakash A, Kurup SK, Chiaramida S, Lucariello R. Does the serum cardiac troponin I level increase with stress test-induced myocardial ischemia? Heart Dis (Hagerstown, MD) 2002;4:216–219. doi: 10.1097/00132580-200207000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Akdemir I, Aksoy N, Aksoy M, Davutoglu V, Dinckal H. Does exercise-induced severe ischaemia result in elevation of plasma troponin-T level in patients with chronic coronary artery disease? Acta Cardiol. 2002;57:13–18. doi: 10.2143/AC.57.1.2005373. [DOI] [PubMed] [Google Scholar]
- 14.Schulz O, Paul-Walter C, Lehmann M, Abraham K, Berghofer G, Schimke I, Jaffe AS. Usefulness of detectable levels of troponin, below the 99th percentile of the normal range, as a clue to the presence of underlying coronary artery disease. Am J Cardiol. 2007;100:764–769. doi: 10.1016/j.amjcard.2007.03.096. [DOI] [PubMed] [Google Scholar]
- 15.Kurz K, Giannitsis E, Zehelein J, Katus HA. Highly sensitive cardiac troponin T values remain constant after brief exercise- or pharmacologic-induced reversible myocardial ischemia. Clin Chem. 2008;54:1234–1238. doi: 10.1373/clinchem.2007.097865. [DOI] [PubMed] [Google Scholar]
- 16.Lee KT, Lai WT, Chen WR, Sheu SH. Serial changes of cardiac troponin-I in acute myoischemia induced by exercise treadmill test. Kaohsiung J Med Sci. 2001;17:239–244. [PubMed] [Google Scholar]
- 17.Pastor G, San Roman JA, Gonzalez-Sagrado M, Vega JL, Arranz ML, Serrador AM, Epureanu V, Palomino RT, Sanz O, Tejedor P, Fernandez-Aviles F. [Dobutamine stress echocardiography and troponin T as a marker of myocardial injury] Rev Esp Cardiol. 2002;55:469–473. doi: 10.1016/s0300-8932(02)76637-2. [DOI] [PubMed] [Google Scholar]
- 18.Eryol NK, Basar E, Ozdogru I, Cicek Y, Abaci A, Oguzhan A, Topsakal R, Cetin S. Should troponin-T be assessed during exercise stress testing in patients with stable angina pectoris? Anadolu Kardiyol Derg. 2002;2:132–137. [PubMed] [Google Scholar]
- 19.Fishbein MC, Wang T, Matijasevic M, Hong L, Apple FS. Myocardial tissue troponins T and I. An immunohistochemical study in experimental models of myocardial ischemia. Cardiovasc Pathol. 2003;12:65–71. doi: 10.1016/s1054-8807(02)00188-6. [DOI] [PubMed] [Google Scholar]
- 20.Feng YJ, Chen C, Fallon JT, Lai T, Chen L, Knibbs DR, Waters DD, Wu AH. Comparison of cardiac troponin I, creatine kinase-MB, and myoglobin for detection of acute ischemic myocardial injury in a swine model. Am J Clin Pathol. 1998;110:70–77. doi: 10.1093/ajcp/110.1.70. [DOI] [PubMed] [Google Scholar]
- 21.Katus HA, Remppis A, Scheffold T, Diederich KW, Kuebler W. Intracellular compartmentation of cardiac troponin T and its release kinetics in patients with reperfused and nonreperfused myocardial infarction. Am J Cardiol. 1991;67:1360–1367. doi: 10.1016/0002-9149(91)90466-x. [DOI] [PubMed] [Google Scholar]
- 22.Remppis A, Scheffold T, Greten J, Haass M, Greten T, Kubler W, Katus HA. Intracellular compartmentation of troponin T: release kinetics after global ischemia and calcium paradox in the isolated perfused rat heart. J Mol Cell Cardiol. 1995;27:793–803. doi: 10.1016/0022-2828(95)90086-1. [DOI] [PubMed] [Google Scholar]
- 23.Vikenes K, Westby J, Matre K, Kuiper KK, Farstad M, Nordrehaug JE. Release of cardiac troponin I after temporally graded acute coronary ischaemia with electrocardiographic ST depression. Int J Cardiol. 2002;85:243–251. doi: 10.1016/s0167-5273(02)00162-6. [DOI] [PubMed] [Google Scholar]
- 24.Suleiman MS, Lucchetti V, Caputo M, Angelini GD. Short periods of regional ischaemia and reperfusion provoke release of troponin I from the human hearts. Clin Chim Acta Int J Clin Chem. 1999;284:25–30. doi: 10.1016/s0009-8981(99)00056-x. [DOI] [PubMed] [Google Scholar]
- 25.Blumgart HL, Gilligan DR, Schlesinger MJ. Experimental studies of the effect of temporary occlusion of coronary arteries. Am Heart J. 1941;22:374–389. [Google Scholar]
- 26.Wallace TW, Abdullah SM, Drazner MH, Das SR, Khera A, McGuire DK, Wians F, Sabatine MS, Morrow DA, de Lemos JA. Prevalence and determinants of troponin T elevation in the general population. Circulation. 2006;113:1958–1965. doi: 10.1161/CIRCULATIONAHA.105.609974. [DOI] [PubMed] [Google Scholar]
- 27.Latini R, Masson S, Anand IS, Missov E, Carlson M, Vago T, Angelici L, Barlera S, Parrinello G, Maggioni AP, Tognoni G, Cohn JN. Prognostic value of very low plasma concentrations of troponin T in patients with stable chronic heart failure. Circulation. 2007;116:1242–1249. doi: 10.1161/CIRCULATIONAHA.106.655076. [DOI] [PubMed] [Google Scholar]
- 28.Apple FS, Wu AH, Jaffe AS. European Society of Cardiology and American College of Cardiology guidelines for redefinition of myocardial infarction: how to use existing assays clinically and for clinical trials. Am Heart J. 2002;144:981–986. doi: 10.1067/mhj.2002.124048. [DOI] [PubMed] [Google Scholar]