Abstract
Aims
Silent electrocardiographic ST change predicts future coronary events in patients with coronary heart disease (CHD), but the prognostic significance of asymptomatic ST-segment depression with respect to sudden cardiac death in subjects without apparent CHD is not well known.
Methods and results
We investigated the association between silent ST-segment depression during and after maximal symptom-limited exercise test and the risk of sudden cardiac death in a population-based sample of 1769 men without evident CHD. A total of 72 sudden cardiac death occurred during the median follow-up of 18 years. The risk of sudden cardiac death was increased among men with asymptomatic ST-segment depression during exercise [hazard ratio (HR) 2.1, 95% confidence interval (CI) 1.2–3.9] as well as among those with asymptomatic ST-segment depression during recovery period (HR 3.2, 95% CI 1.7–6.0). Asymptomatic ST-depression during exercise testing was a stronger predictor for the risk of sudden cardiac death especially among smokers as well as in hypercholesterolaemic and hypertensive men than in men without these risk factors.
Conclusion
Asymptomatic ST-segment depression was a very strong predictor of sudden cardiac death in men with any conventional risk factor but no previously diagnosed CHD, emphasizing the value of exercise testing to identify asymptomatic high-risk men who could benefit from preventive measures.
Keywords: Exercise electrocardiography, Sudden death, Risk factors
Introduction
It is known that coronary heart disease (CHD) may develop during the decades without typical symptoms and thus early recognization of vulnerable CHD would be very difficult. Clinical studies have been largely unsuccessful in identifying specific markers of sudden cardiac death risk in the general population.1,2 Although the fundamental role of CHD as an underlying cause has been described, the prediction of sudden cardiac death in population has been difficult. The common cardiovascular risk factors and some other risk markers play a role in the aetiology of sudden cardiac death.2 However, in asymptomatic population, many of these markers may be uncommon findings. Accordingly, one of the most vexing issues remains the early identification of individuals at highest risk, especially subset of patients with sudden cardiac death without overt signs, symptoms or previous evidence of heart disease.
It is suggested that exercise testing may be recommended among those with the presence of at least one conventional risk factor.3–5 Careful selection of the population for the finding of exercise-induced silent ischaemia is necessary to achieve its maximal clinical utility. Exercise-induced myocardial ischaemia is known to increase the risk of future coronary events not only in patients with CHD6,7 but also in individuals with no previously diagnosed CHD.5,8 However, there is lack of data on the prognostic importance of silent ischaemic ST-changes in totally asymptomatic subjects without history of CHD, although it is proposed that silent ischaemia negatively affects prognosis.9,10 Some studies11,12 have indicated that symptomatic ischaemia is a stronger risk factor for future CHD events, whereas other studies7,13,14 have suggested that asymptomatic ST-change has a similar prognostic value in these patients. There are no data showing that painless ischaemic ST-change during and after exercise would have prognostic significance with regard to the risk of sudden cardiac death in persons without evident CHD. The likelihood to detect myocardial ischaemia by exercise testing is known to increase with increasing pre-test probability of CHD.8,15–17 Nonetheless, little is known if asymptomatic ST-depression may be related to the risk of sudden cardiac death in high-risk individuals. We therefore investigated the prognostic significance of asymptomatic ST-depression during and after exercise with regard to the risk of sudden cardiac death in a population-based sample of middle-aged men without evident CHD.
Methods
Subjects
This study was designed to investigate risk factors for CHD in a population-based sample of men from eastern Finland. Subjects were randomly selected sample of 3433 men aged 42–60 years, who resided in the town of Kuoio or its surrounding rural communities, 198 were excluded because of death, serious disease, or migration. Of the remainder, 2682 (83%) participated in the study. Baseline examinations were conducted between March 1984 and December 1989.
Men who had symptoms suggestive of prevalent CHD at the baseline (n = 888) and for whom exercise stress test was not performed due to severe cardiovascular disease (n = 25) were excluded from the study. Prevalent CHD was defined as either a history of myocardial infarction or angina pectoris, angina pectoris on effort, the use of nitroglycerin for chest pain once a week or more frequently, or chest pain as a cause of stopping exercise stress test at baseline. Thus, the present study is based on 1769 men who had completed data on electrocardiographic recordings during and after exercise.
Exercise electrocardiography
A maximal symptom-limited exercise stress test was performed using an electrically braked bicycle ergometer between 8:00 and 12:00 A.M. Maximal oxygen uptake was measured directly using a respiratory gas analyser, as explained previously.18 Also, heart rate and blood pressure were registered during the exercise test.
Electrocardiography was recorded continuously with the Kone 620 electrocardiograph (Kone, Turku, Finland). Electrocardiography was printed every 30 s intervals during exercise and at least 5 min of recovery while the subject was sitting on the bicycle. Asymptomatic ST-depression during exercise and after 5 min of recovery was defined as ischaemia in the electrocardiography without typical chest pain indicating CHD. The criteria for ischaemia in electrocardiography during exercise and recovery were horizontal or down-sloping ST-depression 1.0 mm at 80 ms after J point or any ST depression of more than 1.0 mm at 80 ms after J point.5
Assessment of other risk factors
The collection of blood specimens19 and the measurement of serum lipids and lipoproteins20 have been described elsewhere. Assessment of smoking, alcohol consumption, and blood pressure was carried out as described previously.18,19 Serum C-reactive protein was measured with an immunometric assay (Immulite High Sensitivity C-reactive protein Assay, DPC, Los Angeles, CA, USA). Body mass index was computed as the ratio of weight in kilograms to the square of height in meters. Type II diabetes was defined as fasting serum glucose of over or equal to 6.1 mmol/L or a clinical diagnosis of diabetes with either dietary, oral or insulin treatment. The European Systematic Coronary Risk Evaluation (SCORE) was calculated as an estimation of 10 year risk of fatal cardiovascular event, on the basis of age, gender, total cholesterol concentration, systolic blood pressure, and current smoking status.
Classification of sudden cardiac death
All deaths that occurred by the end of 2005 were checked from the hospital documents and death certificates. There were no losses to follow-up. CHD deaths were coded using to the Ninth International Classification of Diseases codes (410–414 for non-sudden myocardial infarction death, for sudden cardiac death 798.1) or the Tenth International Classification of Diseases codes (I20–I25 for non-sudden myocardial infarction death, for sudden cardiac death I46). Data on sudden cardiac deaths and coronary events collected from hospitals and wards of health centres and classified the outcome events.21 The sources of information were interviews, hospital documents, death certificates, autopsy reports, and medico-legal reports. The diagnostic classification of coronary events was based on symptoms, electrocardiographic findings, cardiac enzyme elevations, autopsy findings (80%), and history of CHD. The assessment of outcomes was blinded to the results of the exercise test.
Sudden cardiac death was defined as sudden unexpected arrest of presumed cardiac origin without traumatic nature of death. The arrest should have occurred within 24 h after onset of any symptoms that could retrospectively be interpreted as being cardiac origin. Sudden death was defined as natural death occurring within 24 h onset of symptoms. Deaths that were occurred unwitnessed during the night-time, such as being found dead in bed, were classified as those whose death occurred 24 h from the symptoms. The deaths like aortic aneurysm rupture, cardiac rupture or tamponade, and pulmonary embolism were not included as sudden cardiac death.
Statistical methods
Differences in baseline variable between men with and without asymptomatic ST-depression during exercise were analysed using Student's t-test and the χ2 test. Cox proportional-hazard regression model was used to examine the association between asymptomatic ST-depression and the risk of sudden cardiac death. Forced Cox proportional-hazards models were adjusted for age and other risk factors, which were selected on the basis of their previously established role as a well-defined predictive factor on the basis of overall evidence and available data. Thus, risk factors or covariates were chosen based on their clinical relevance. If possible, confounding factors were entered uncategorized into the Cox models. The proportional hazards assumption was verified for all variables by inspection of the plots of Schoenfeld residual for covariates. The linearity assumption was satisfied for all continuous variables, and it was assessed with Martingale residuals for each continuous variable against survival time (or time to event). The cumulative incidence of sudden cardiac death by the presence of asymptomatic ST-segment depression was calculated using the Kaplan–Meier method. The modification of the prognostic value of asymptomatic ST-depression by the major CHD risk factors was analysed by comparing smokers and non-smokers, men with high (≥3.9 mmol/L, median) and low (<3.9 mmol/L) serum low-density lipoprotein cholesterol and men with high (≥132.3 mmHg, median) and low (<132.3 mmHg) systolic blood pressure. Additionally, the interaction term was included in the Cox model when analysing the modification between asymptomatic ST-depression and risk factor of interest. All tests for statistical significance were two-sided. A value of P < 0.05 was considered significant. All statistical analyses were performed using SPSS 14.0 for Windows.
Results
Baseline characteristics
Baseline characteristics in men with and without asymptomatic ST-segment depression during exercise are shown in Table 1. Serum low-density lipoprotein cholesterol, systolic blood pressure, and maximal heart rate were higher and body mass index was lower in men with asymptomatic ST-segment changes during exercise. Similar differences were observed in men with and without silent ST-segment changes from exercise to recovery.
Table 1.
Baseline characteristics of the study population
| Men with no ST-depression during exercise (n = 1580) | Men with silent ST-depression during exercise (n = 189) | P-value for statistical significance | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Age (years) | 52.2 (5.3) | 52.7 (5.2) | 0.17 |
| Body mass index (kg/m2) | 26.8 (3.4) | 26.0 (3.1) | 0.002 |
| Smokers (%) | 30.2 | 27.1 | 0.36 |
| Cigarette smoking (pack-years)a | 7.99 (16.2) | 5.91 (13.1) | 0.05 |
| Alcohol consumption (g/week) | 76.3 (119.9) | 63.9 (84.5) | 0.07 |
| Diabetes (%)b | 4.0 | 4.2 | 0.87 |
| Serum low-density lipoprotein cholesterol (mmol/L) | 3.99 (0.99) | 4.15 (0.94) | 0.02 |
| Serum high-density lipoprotein cholesterol (mmol/L) | 1.31 (0.30) | 1.30 (0.27) | 0.83 |
| Systolic blood pressure (mmHg) | 133.6 (16.0) | 137.3 (17.4) | 0.006 |
| Diastolic blood pressure (mmHg) | 89.1 (10.3) | 88.6 (10.3) | 0.60 |
| C-reactive protein (mmol/L) | 2.11 (3.32) | 2.10 (3.63) | 0.97 |
| Resting heart rate (beats/min) | 62 (11) | 62 (10) | 0.78 |
| Maximal heart rate during exercise test (b.p.m.) | 160 (21) | 165 (17) | 0.001 |
| Maximal oxygen uptake (mL/min)c | 2534.8 (585.4) | 2476.9 (585.4) | 0.20 |
| Exercise capacity (METs) | 9.2 (2.1) | 9.1 (2.0) | 0.67 |
aPack-years denotes the lifelong exposure to smoking that was estimated as the product of years smoked and the number of tobacco products smoked daily at the time of examination.
bDiabetes was defined as fasting blood glucose of ≥6.1 mmol/L or a clinical diagnosis of diabetes with dietary, oral, or insulin treatment.
cExercise capacity was measured directly during exercise test using a respiratory gas analyser.
The most common reasons for stopping the exercise test were leg fatigue (1021 men), exhaustion (298 men), breathlessness (245 men), and pain in the leg muscles, joints, or back (77 men). The test was discontinued because of cardiorespiratory symptoms or abnormalities in 120 men. These included ventricular arrhythmias (60 men), an abnormal change in systolic or diastolic blood pressure (44 men), dizziness (9 men), or ischaemic electrocardiographic changes (over 3 mm horizontal or down-sloping ST depression) (7 men). Exercise-induced ventricular conduction disorders were observed in 5 men, one of whom also had asymptomatic ST-depression during exercise but not during recovery.
Asymptomatic ST-changes during exercise testing
There were 189 (10.7%) men with asymptomatic ST-depression during exercise and 54 (3.1%) men with asymptomatic ST-changes after exercise. Painless ischaemic electrocardiographic ST-change during exercise was observed in 9.6% (n = 51) of smokers, in 12.3% (n = 110) of hypercholesterolaemic men, and in 12.4% (n = 107) of hypertensive men. Painless ST-depression after exercise was recorded in 2.8% (n = 15) of smokers, in 3.3% (n = 30) of hypercholesterolaemic men, and in 4.0% (n = 35) of hypertensive men.
Numbers of outcome events during follow-up
The median follow-up time to death or the end of follow-up was 18.7 years (interquartile range 17.1–20.3 years). There were 102 CHD deaths, 72 of which were due to sudden cardiac death within 24 h after onset of symptoms. A total of 63 sudden cardiac death (87.5%) occurred out-hospital conditions, and 42 (66.7%) of these deaths were due to documented ventricular tachycardia or fibrillation.
Thirteen men (6.9%) out of 189 men with asymptomatic ST-depression during exercise and 59 (3.7%) out of 1580 men without ischaemic ST-changes had a sudden cardiac death during follow-up. The respective numbers were eight (14.8%) out of 54 men with silent ST-depression during recovery, and 64 (3.7%) out of 1715 men without ischaemic ST-changes had a sudden cardiac death during follow-up.
Hazard ratios of sudden cardiac death in men with asymptomatic ST-depression
The strongest risk factors for sudden cardiac death were smoking, systolic blood pressure, body mass index, exercise capacity, and asymptomatic ST-depression when adjusted for other risk factors (Table 2).
Table 2.
Strongest risk factors for sudden cardiac death among asymptomatic men
| Hazard ratioa | 95% CI | P-value | |
|---|---|---|---|
| Cigarette smoking (per 10 pack-years)b | 1.31 | 1.18–1.46 | <0.001 |
| Systolic blood pressure (per 10 mmHg) | 1.24 | 1.10–1.40 | 0.001 |
| Body mass index (per 5 kg/m2) | 1.42 | 1.00–2.03 | 0.050 |
| Maximal oxygen uptake (per 100 mL/min)c | 0.95 | 0.90–1.00 | 0.052 |
| Asymptomatic ST-depression during exercise (yes vs. no) | 2.12 | 1.15–3.90 | 0.016 |
aHazard ratios are adjusted also for age, alcohol consumption, diabetes, C-reactive protein, and serum low- and high-density lipoprotein cholesterol in a forced multivariable Cox model. Sudden cardiac death was defined when the arrest have occurred within 24 h after onset of any symptoms.
bPack-years denotes the lifelong exposure to smoking that was estimated as the product of years smoked and the number of tobacco products smoked daily at the time of examination.
cExercise capacity was measured directly during exercise test using a respiratory gas analyser.
Men with asymptomatic ST-depression during exercise had a 2.1-fold risk of sudden cardiac death and a 2.5-fold risk of CHD death compared with men without silent ischaemia after adjusting for conventional risk factors (Table 3). Asymptomatic ST-depression was associated with a 3.2-fold risk of sudden cardiac death. The cumulative hazard curves for sudden cardiac death continued to diverge during the follow-up period (Figure 1). The adjusted risk of all-cause death was also increased among those with asymptomatic ST-depression during [hazard ratio (HR) 1.4, 95% confidence interval (CI) 1.0–1.9, P = 0.041] and after exercise (HR 1.5; 95% CI 1.1–2.1; P = 0.013).
Table 3.
Risk of sudden cardiac death according to presence of painless ST-segment depression in an exercise electrocardiogram in asymptomatic men
| Risk factor | Sudden cardiac death, 72 events |
Coronary Heart Disease Death, 102 events |
||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P-value | Hazard ratio (95% CI) | P-value | |
| Asymptomatic ST-depression during exercisea | 2.13 (1.15–3.89) | 0.015 | 2.47 (1.52–3.99) | <0.001 |
| Asymptomatic ST-depression during recoverya | 3.17 (1.68–5.96) | <0.001 | 4.01 (2.12–7.58) | <0.001 |
aEach variable was entered separately into a Cox model with age, cigarette smoking, systolic blood pressure, alcohol consumption, body mass index, maximal oxygen uptake, diabetes, C-reactive protein, and serum low- and high-density lipoprotein cholesterol.
Figure 1.
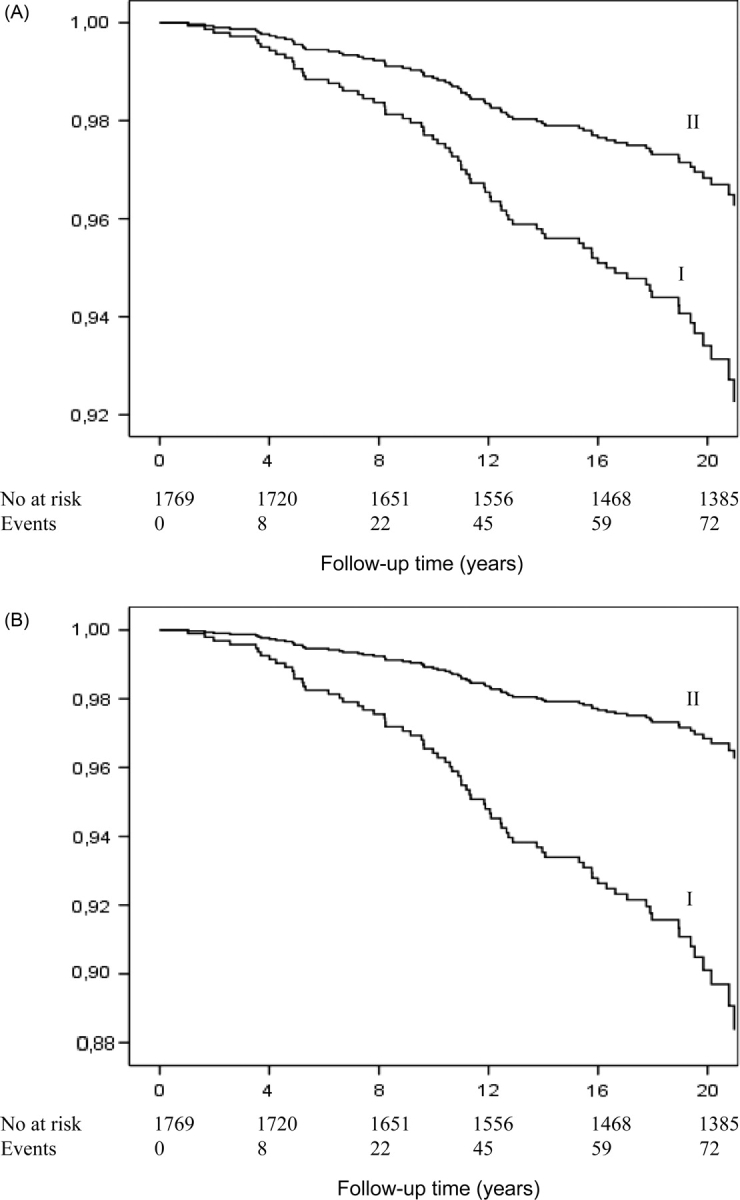
Cumulative survival for sudden cardiac death among men with asymptomatic ST-depression (A) during exercise and (B) after exercise. The cumulative survival curve I represents men with asymptomatic ST-depression and the curve II represents men without ischaemic ST-changes.
Interactions of asymptomatic ST-depression with conventional coronary risk factors and type II diabetes
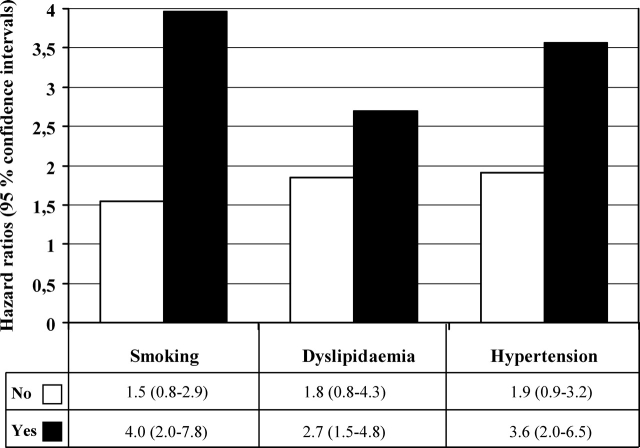
Asymptomatic ST-depression during exercise had a stronger association with the risk of sudden cardiac death in smokers and in hypertensive and hypercholestrolemic men than in men without such risk factors (Figure 2). Additionally, among those with type II diabetes with the presence of asymptomatic ST-depression during exercise had very high risk of sudden cardiac death (HR 8.2; 95% CI 2.4–28.3; P = 0.001). The interaction term between asymptomatic ST-depression during exercise and type II diabetes was significant (P = 0.026) as well as the interaction between asymptomatic ST-changes after exercise and smoking was significant (P = 0.028) when the interaction term was included in the Cox model with the risk factors of interest. Asymptomatic ischaemic ST-changes after exercise had a strong association with the risk of sudden cardiac death with the presence of risk factors (HR 10.9, 95% CI 4.4–27.3, P < 0.001 for smokers; HR 4.5, 95% CI 1.7–11.8, P = 0.003 for hypercholesterolaemic men; and HR 5.0, 95% CI 2.1–11.9, P < 0.001 for hypertensive men). All of these associations were statistically non-significant in men without any conventional risk factors.
Figure 2.
The hazard ratios (95% confidence intervals) of sudden cardiac death in men with asymptomatic ST-depression during exercise according to conventional risk factor levels. Men without ischaemic ST-change during exercise was a reference group. Cut-off for systolic blood pressure was 132 mmHg and for serum LDL-cholesterol 3.9 mmol/L. All hazard ratios are adjusted for age, alcohol consumption, body mass index, maximal oxygen uptake, diabetes, cigarette smoking, systolic blood pressure, C-reactive protein, and serum low- and high-density lipoprotein cholesterol, except the risk factor of interest.
The risk related to asymptomatic ST-segment depression during exercise was increased with the presence of conventional risk factors as defined by European risk SCORE method. Men with asymptomatic ST-depression during exercise with the presence of high SCORE of at least 5% had an elevated risk of CHD death (HR 3.5, 95% CI 1.4–8.8, P = 0.010) whereas among those with asymptomatic ST-depression with SCORE result indicating low risk (<5%) had also an increased risk CHD death (HR 1.9, 95% CI 1.9–3.3, P = 0.021).
Discussion
The present prospective study demonstrates that asymptomatic ST-segment depression in electrocardiography during and after exercise predicts unexpected sudden cardiac death in middle-aged men without evident CHD. A main finding was that exercise-induced ST-depression was a strong predictor of sudden cardiac death particularly in men with an unfavourable coronary risk factor profile including smoking, hypercholesterolemia, or hypertension.
Painless myocardial ischaemia is a not uncommon finding and increases the risk of future CHD death.22,23 Ischaemic ST-depression in the absence of pain has been more common than ST-depression with angina in daily life.24,25 In our study, we observed that painless ischaemic ST-depression during exercise was present in 10.7% of men. Some studies have shown that the more frequent or progressive the anginal symptoms are, the poorer the prognosis in patients with CHD.7,26 Other studies have indicated that symptomatic and silent ischaemic ST-changes are related to a similar prognosis in patients with documented CHD.8,10,11 Therefore, as patients become increasingly selected towards a higher a priori likelihood of developing CHD, there appears to be a tendency for chest pain to lose its significance as an additional predictive factor.26
The prognostic value of silent myocardial ischaemia, as indicated by exercise electrocardiographic findings, can vary most likely due to different selection criteria for the subjects;7,12,17,27 some studies have included only patients with CHD,8,10,11 whereas few studies have included persons without signs or symptoms suggesting CHD.5,17 It has been argued that the prognostic value of exercise electrocardiography is low in totally asymptomatic persons because of false-positive and -negative responders. However, in asymptomatic individuals with a high pre-test probability of CHD, e.g. in those with major coronary risk factors, the frequency of false-positive test responses for myocardial ischaemia is significantly lower than in those without coronary risk factors, which diminishes the bias associated with false-positive responders (the Bayes’ rule).12,28 This could be one explanation for our finding that the association between silent myocardial ischaemia with sudden cardiac death was stronger in high-risk groups.
It is not well known if ST-depression immediately after exercise would have an adverse prognostic value with regard to cardiac death in asymptomatic men.29–32 We found that asymptomatic electrocardiographic findings that prolonged or developed during recovery were associated with increased risk of sudden cardiac death. Interestingly, painless ST-depression during recovery was even more strongly related to sudden cardiac death than silent myocardial ischaemia during exercise. This suggests that asymptomatic ST-segment depression during post-exercise period could be of pivotal clinical importance, and the prognostic value of exercise testing can be improved by assessing ischaemic electrocardiographic changes during recovery. It has been suggested that risk assessment for primary CHD is enhanced by detection of abnormal exercise electrocardiographic findings only in those who had one or more conventional risk factor.12 In the present study, men with asymptomatic ST-depression during exercise testing had a substantially increased risk of sudden cardiac death, especially if they had any conventional risk factor. The risk was very high among men with silent ischaemic ST-change during recovery and in the presence of any of these risk factors. The finding was particularly evident among smokers.
Some previous studies have suggested that silent myocardial ischaemia is a pathophysiological mechanism through which exercise increases the occurrence of sudden cardiac death.15,16,33 One explanation for this could be that painless ischaemia increases the susceptibility to sudden plaque rupture, myocardial infarction, left ventricular dysfunction, and ultimately fatal ventricular arrhythmias.34 In our study, most of the men had documented ventricular tachycardia or fibrillation before the onset of sudden cardiac death, although the definition of ventricular arrhythmias is impossible for all out-hospitalized patients dying suddenly. The transient impairment of coronary flow during and after exercise may be caused by dynamic coronary stenosis as a result of epicardial coronary constriction, endothelial dysfunction, spasm, and thrombosis.24,25 Such brief episodes may be painless because the stimulus is either inadequate or the pain usually appears quite late after the onset of ischaemia.23,26 Furthermore, ischaemic ST-depression during recovery may be due to elevated levels of plasma catecholamines during the post-exercise period, which could enhance myocardial oxygen demand and fatal cardiac arrhythmias.31,35,36
One of the explanations for the stronger associations between asymptomatic ischaemic ST-depression and sudden cardiac death risk in men with conventional risk factors may be that ST-depression is more likely due to true ischaemia in these men than in those with no risk factors. Better quality of electrocardiographic recording after exercise may be one explanation for the strong association of asymptomatic ST-depression after exercise with sudden cardiac death. The use of a smaller ST-depression tends to attenuate the specificity of the ischaemic findings since also other factors than myocardial ischaemia, such as hyperventilation, electrolyte abnormalities, anaemia, ventricular hypertrophy, and increased sympathetic activity are known to cause ST-depression.17 One limitation of the present study is that we were able to study only men, and thus our findings may be not generalized to female populations. On the other hand, according to our study, a total of 87.5% of all sudden cardiac events (63 deaths) occurred out of hospital that makes the registration of asymptomatic ST-changes in the prediction of sudden cardiac death highly valuable. Despite recent advances in technology, survival from out-of-hospital sudden cardiac death remains low throughout the word at about 5%.37
On the basis of our results we suggest that exercise testing provides a possibility to detect those high-risk patients by using widely known non-invasive methods and the knowledge of common risk factors. The big question is that should we test all individuals with coronary risk factors who undergo exercise testing or only those asymptomatic men with high-risk occupation and those who start exercise training programme? Although the prognostic capability of screening exercise testing is established, its clinical value for improving long-term outcome is not well documented.9 However, studies have demonstrated impressive incremental relative hazards for the synergistic combination of the standard exercise test and risk factors.38,39 If screening could be performed in a logical way to decide therapies, then the results should be applied to preventive medicine policy. Evidence suggests that subjects at highest risk of severe myocardial ischaemia, even in the absence of symptoms, derive the greatest benefit from an aggressive diagnostic and therapeutic approach. It is suggested that anti-ischaemic drug therapy and aspirin among patients without CHD history but silent exercise ST-depression can be recommended.40 Secondly, a latest clinical trial suggests that optimal medical therapy combined with healthy life-style changes is useful a strategy as compared with invasive treatment in stable coronary artery disease.41 However, among patients with recent myocardial infarction and obstructive coronary artery disease, invasive therapy may me better than anti-ischaemic drug therapy reducing the long-term risk of major cardiac events.42 Finally, all risk factors should be carefully controlled and treated among those with documented silent ischaemic ST-changes.
Asymptomatic exercise-induced ST-segment depression was a common finding in middle-aged men with no prior CHD, and it was associated with greatly increased risk of sudden cardiac death, especially in smokers, hypercholesterolaemic and hypertensive men. The main clinical implication of our findings is that painless ischaemic ST-depression defined by exercise electrocardiography is of significant prognostic marker for sudden cardiac death when any conventional risk factors are present. This community-based study emphasizes the usefulness of identifying high-risk persons by exercise testing in greatest need of preventive measures.
Funding
The US National Heart, Lung, and Blood Institute (grant HL44199), Washington, DC; the Academy of Finland; and the Finnish Ministry of Education, Helsinki, Finland; City of Kuopio, Kuopio, Finland.
Conflict of interest: none declared.
Acknowledgements
We thank the staff of the Kuopio Research Institute of Exercise Medicine and the Research Institute of Public Health, and University of Kuopio, Kuopio, Finland, for data collection in the study.
References
- 1.Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med. 2001;345:1473–1482. doi: 10.1056/NEJMra000650. [DOI] [PubMed] [Google Scholar]
- 2.Muller D, Agrawal R, Hans-Richard A. How sudden is cardiac death? Circulation. 2006;114:1146–1150. doi: 10.1161/CIRCULATIONAHA.106.616318. [DOI] [PubMed] [Google Scholar]
- 3.Fowler-Brown A, Pignone M, Pletcher M, Tice JA, Sutton SF, Lohr KN. Exercise tolerance testing to screen for coronary heart disease: a systematic review for the technical support for the U.S. Preventive Services Task Force. Ann Intern Med. 2004;140:W9–W24. doi: 10.7326/0003-4819-140-7-200404060-w1. [DOI] [PubMed] [Google Scholar]
- 4.Fletcher GF, Balady GJ, Amsterdam EA, Chaitman B, Eckel R, Fleg J, Froelicher VF, Leon AS, Piña IL, Rodney R, Simons-Morton DA, Williams MA, Bazzarre T. Exercise standards for testing and training: a statement for healthcare professionals from the American Heart Association. Circulation. 2001;104:1694–1740. doi: 10.1161/hc3901.095960. [DOI] [PubMed] [Google Scholar]
- 5.Laukkanen JA, Kurl S, Lakka TA, Tuomainen TP, Rauramaa R, Salonen R, Eranen J, Salonen JT. Exercise-induced silent myocardial ischemia and coronary morbidity and mortality. J Am Coll Cardiol. 2001;38:72–79. doi: 10.1016/s0735-1097(01)01311-0. [DOI] [PubMed] [Google Scholar]
- 6.Detrano R, Gianrossi R, Mulvihill D, Lehmann K, Dubach P, Colombo A, Froelicher V. Exercise-induced ST segment depression in the diagnosis of multivessel coronary disease: a meta analysis. J Am Coll Cardiol. 1989;14:1501–1508. doi: 10.1016/0735-1097(89)90388-4. [DOI] [PubMed] [Google Scholar]
- 7.Weiner DA, Ryan TJ, McCabe CH, Ng G, Chaitman BR, Sheffield LT, Tristani FE, Fisher LD. Significance of silent myocardial ischemia during exercise testing in patients with coronary artery disease. Am J Cardiol. 1987;59:725–729. doi: 10.1016/0002-9149(87)91081-2. [DOI] [PubMed] [Google Scholar]
- 8.Bruce RA, Hossack KF, DeRouen TA, Hofer V. Enhanced risk assessment for primary coronary heart disease events by maximal exercise testing: 10 years’ experience of Seattle Heart Watch. J Am Coll Cardiol. 1983;2:563–573. doi: 10.1016/s0735-1097(83)80286-1. [DOI] [PubMed] [Google Scholar]
- 9.Lauer M, Froelicher ES, Williams M, Kligfield P. Exercise testing in asymptomatic adults: A statement for professionals from the American heart association council on clinical cardiology, subcommittee on exercise, cardiac rehabilitation, and prevention. Circulation. 2005;112:771–776. doi: 10.1161/CIRCULATIONAHA.105.166543. [DOI] [PubMed] [Google Scholar]
- 10.Almeda FQ, Kason TT, Nathan S, Kavinsky CJ. Silent myocardial ischemia: concepts and controversies. Am J Med. 2004;116:112–118. doi: 10.1016/j.amjmed.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 11.Mark DB, Hlatky MA, Califf RM, Morris JJ, Jr, Sisson SD, McCants CB, Lee KL, Harrell FE, Jr, Pryor DB. Painless exercise ST deviation on the treadmill: long term prognosis. J Am Coll Cardiol. 1989;14:885–892. doi: 10.1016/0735-1097(89)90459-2. [DOI] [PubMed] [Google Scholar]
- 12.Miranda CP, Lehmann KG, Lachterman B, Coodley EM, Froelicher VF. Comparison of silent and symptomatic ischemia during exercise testing in men. Ann Intern Med. 1991;114:645–656. doi: 10.7326/0003-4819-114-8-649. [DOI] [PubMed] [Google Scholar]
- 13.Falcone C, De Servi S, Poma E, Campana C, Sciré A, Montemartini C, Specchia G. Clinical significance of exercise-induced silent myocardial ischemia in patients with coronary artery disease. J Am Coll Cardiol. 1987;9:295–299. doi: 10.1016/s0735-1097(87)80378-9. [DOI] [PubMed] [Google Scholar]
- 14.Bonow RO, Bacharach SL, Green MV, LaFreniere RL, Epstein SE. Prognostic implications of symptomatic versus asymptomatic (silent) myocardial ischemia induced by exercise in mildly symptomatic and in asymptomatic patients with angiographically documented coronary artery disease. Am J Cardiol. 1987;60:778–783. doi: 10.1016/0002-9149(87)91022-8. [DOI] [PubMed] [Google Scholar]
- 15.Rautaharju PM, Prineas RJ, Eifler WJ, Furberg CD, Neaton JD, Crow RS, Stamler J, Cutler JA. Prognostic value of exercise electrocardiogram in men at high risk of future coronary heart disease: Multiple Risk Factor Interventional Trial experience. J Am Coll Cardiol. 1986;8:1–10. doi: 10.1016/s0735-1097(86)80084-5. [DOI] [PubMed] [Google Scholar]
- 16.Ekelund L-G, Suchindran CM, McMahon RP, Heiss G, Leon AS, Romhilt DW, Rubenstein CL, Probstfield JL, Ruwitch JF. Coronary heart disease morbidity and mortality in hypercholesterolemic men predicted from an exercise test: the Lipid Research Clinics Coronary Primary Prevention Trial. J Am Coll Cardiol. 1989;14:556–563. doi: 10.1016/0735-1097(89)90092-2. [DOI] [PubMed] [Google Scholar]
- 17.Fleg JL, Gerstenblith G, Zonderman AB, Becker LC, Weisfeldt ML, Costa PT, Jr, Lakatta EG. Prevalence and prognostic significance of exercise-induced silent myocardial ischemia detected by thallium scintigraphy and electrocardiography in asymptomatic volunteers. Circulation. 1990;81:428–436. doi: 10.1161/01.cir.81.2.428. [DOI] [PubMed] [Google Scholar]
- 18.Lakka TA, Venäläinen JT, Rauramaa R, Salonen R, Tuomilehto J, Salonen JT. Relation of leisure-time physical activity and cardiorespiratory fitness to the risk of acute myocardial infarction in men. N Engl J Med. 1994;330:1549–1554. doi: 10.1056/NEJM199406023302201. [DOI] [PubMed] [Google Scholar]
- 19.Salonen JT, Nyyssönen K, Korpela H, Tuomilehto J, Seppänen R, Salonen R. High stored iron levels are associated with excess risk of myocardial infarction in eastern Finnish men. Circulation. 1992;86:803–811. doi: 10.1161/01.cir.86.3.803. [DOI] [PubMed] [Google Scholar]
- 20.Salonen JT, Salonen R, Seppänen K, Rauramaa R, Tuomilehto J. HDL, HDL2, HDL3 subfractions, and the risk of acute myocardial infarction: a prospective population study in eastern Finnish men. Circulation. 1991;84:129–139. doi: 10.1161/01.cir.84.1.129. [DOI] [PubMed] [Google Scholar]
- 21.Tunstall-Pedoe H, Kuulasmaa K, Amoyel P, Arveiler D, Rajakangas AM, Pajak A. Myocardial infarction and coronary deaths in the World Health Organization MONICA Project: registration procedures, event rates and case fatality rates in 38 populations from 21 countries in 4 continents. Circulation. 1994;90:583–612. doi: 10.1161/01.cir.90.1.583. [DOI] [PubMed] [Google Scholar]
- 22.Kannel WB, Abbott RD. Incidence and prognosis of unrecognized myocardial infarction. An update on the Framingham Study. N Engl J Med. 1984;311:1144–1147. doi: 10.1056/NEJM198411013111802. [DOI] [PubMed] [Google Scholar]
- 23.Deanfield JE, Maseri A, Selwyn AP, Ribeiro P, Chierchia S, Krikler S, Morgan M. Myocardial ischaemia during daily life in patients with stable angina: its relation to symptoms and heart rate. Lancet. 1983;1:753–758. doi: 10.1016/s0140-6736(83)92295-x. [DOI] [PubMed] [Google Scholar]
- 24.Maseri A. Role of coronary artery spasm in symptomatic and silent myocardial ischemia. J Am Coll Cardiol. 1987;9:249–262. doi: 10.1016/s0735-1097(87)80372-8. [DOI] [PubMed] [Google Scholar]
- 25.Deedwania PC, Nelson JR. Pathophysiology of silent myocardial ischemia during daily life. Hemodynamic evaluation by simultaneous electrocardiographic and blood pressure monitoring. Circulation. 1990;82:1296–1304. doi: 10.1161/01.cir.82.4.1296. [DOI] [PubMed] [Google Scholar]
- 26.Klein J, Chao SY, Berman DS, Rozanski A. Is ‘silent’ myocardial ischemia as severe as symptomatic ischemia? The analytical effect of patient selection biases. Circulation. 1994;89:1958–1966. doi: 10.1161/01.cir.89.5.1958. [DOI] [PubMed] [Google Scholar]
- 27.Giagnoni E, Secchi MB, Wu SC, Morabito A, Oltrona L, Mancarella S, Volpin N, Fossa L, Bettazzi L, Arangio G. Prognostic value of exercise EKG testing in asymptomatic normotensive subjects. A prospective matched study. N Engl J Med. 1983;309:1085–1089. doi: 10.1056/NEJM198311033091803. [DOI] [PubMed] [Google Scholar]
- 28.Smith SC, Jr, Amsterdam E, Balady GJ, Bonow RO, Fletcher GF, Froelicher V, Heath G, Limacher MC, Maddahi J, Pryor D, Redberg RF, Roccella E, Ryan T, Smaha L, Wenger K. Prevention conference V. Beyond secondary prevention: identifying the high-risk patient for primary prevention: tests for silent and inducible ischemia. AHA scientific statement. Circulation. 2000;101:e12–e16. doi: 10.1161/01.cir.101.1.e12. [DOI] [PubMed] [Google Scholar]
- 29.Karnegis JN, Matts J, Tuna N, Amplatz K. Comparison of exercise-positive with recovery-positive treadmill graded exercise test. Am J Cardiol. 1987;60:544–547. doi: 10.1016/0002-9149(87)90302-x. [DOI] [PubMed] [Google Scholar]
- 30.Savage MP, Squires LS, Hopkins JT, Raichlen JS, Park CH, Chung EK. Usefulness of ST-segment depression as a sign of coronary artery disease when confined to the postexercise recovery period. Am J Cardiol. 1987;60:1405–1406. doi: 10.1016/0002-9149(87)90632-1. [DOI] [PubMed] [Google Scholar]
- 31.Lachterman B, Lehmann KG, Abrahamson D, Froelicher VF. ‘Recovery only’ ST segment depression predictive accuracy of the exercise test. Ann Intern Med. 1990;112:11–16. doi: 10.7326/0003-4819-112-1-11. [DOI] [PubMed] [Google Scholar]
- 32.Rywik TM, Zink RC, Gittings NS, Khan AA, Wright JG, O'Connor FC, Fleg JL. Independent prognostic significance of ischemic ST-segment response limited to recovery from treadmill exercise in asymptomatic subjects. Circulation. 1998;97:2117–2122. doi: 10.1161/01.cir.97.21.2117. [DOI] [PubMed] [Google Scholar]
- 33.Sharma B, Asinger R, Francis GS, Hodges M, Wyeth RP. Demonstration of exercise-induced painless ischemia in out-of-hospital ventricular fibrillation. Am J Cardiol. 1987;59:740–745. doi: 10.1016/0002-9149(87)91084-8. [DOI] [PubMed] [Google Scholar]
- 34.Barsky AJ, Hochstrasser B, Coles NA, Zisfein J, O'Donnell C, Eagle KA. Silent myocardial ischemia. Is the person or the event silent? JAMA. 1990;264:1132–1135. doi: 10.1001/jama.264.9.1132. [DOI] [PubMed] [Google Scholar]
- 35.Dimsdale JE, Hartley LH, Guiney T, Ruskin JN, Greenblatt D. Postexercise peril: plasma catecholamines and exercise. JAMA. 1984;251:630–632. doi: 10.1001/jama.251.5.630. [DOI] [PubMed] [Google Scholar]
- 36.Fleg JL, Tzankoff SP, Lakatta EG. Age-related augmentation of plasma catecholamines during dynamic exercise in healthy males. J Appl Physiol. 1985;59:1033–1039. doi: 10.1152/jappl.1985.59.4.1033. [DOI] [PubMed] [Google Scholar]
- 37.Josephson M, Wellens HJJ. Implantable defibrillators and sudden cardiac death. Circulation. 2004;109:2685–2691. doi: 10.1161/01.CIR.0000129322.97266.F3. [DOI] [PubMed] [Google Scholar]
- 38.Froelicher VF. Screening with the exercise test: time for a guideline change? Eur Heart J. 2005;26:1353–1354. doi: 10.1093/eurheartj/ehi303. [DOI] [PubMed] [Google Scholar]
- 39.Erikssen G, Bodegard J, Bjornholt JV, Liestol K, Thelle SD, Erikssen J. Exercise testing of healthy men in a new perspective: from diagnosis to prognosis. Eur Heart J. 2004;25:978–986. doi: 10.1016/j.ehj.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 40.Erne P, Schoenenberger AW, Zuber M, Burckhardt D, Kiowski W, Dubach P, Resink T, Pfisterer M. Effects of anti-ischaemic drug therapy in silent myocardial ischaemia type I: the Swiss Interventional Study on Silent Ischaemia type I (SWISSI I): a randomized, controlled pilot study. Eur Heart J. 2007;28:2110–2117. doi: 10.1093/eurheartj/ehm273. [DOI] [PubMed] [Google Scholar]
- 41.Boden WE, O'Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, Chaitman BR, Shaw L, Gosselin G, Nawaz S, Title LM, Gau G, Blaustein AS, Booth DC, Bates ER, Spertus JA, Berman DS, Mancini GB, Weintraub WS COURAGE Trial Research Group. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–1516. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 42.Erne P, Schoenenberger AW, Burckhardt D, Zuber M, Kiowski W, Buser PT. Effects of percutaneous coronary interventions in silent ischemia after myocardial infarction: the SWISSI II randomized controlled trial. JAMA. 2007;297:1985–1991. doi: 10.1001/jama.297.18.1985. [DOI] [PubMed] [Google Scholar]