Abstract
Aims
Prospective studies indicate that apolipoprotein measurements predict coronary heart disease (CHD) risk; however, evidence is conflicting, especially in the US. Our aim was to assess whether measurements of apolipoprotein B (apoB) and apolipoprotein A-I (apoA-I) can improve the ability to predict CHD death beyond what is possible based on traditional cardiovascular (CV) risk factors and clinical routine lipid measurements.
Methods and results
We analysed prospectively associations of apolipoprotein measurements, traditional CV risk factors, and clinical routine lipid measurements with CHD mortality in a multi-ethnic representative subset of 7594 US adults (mean age 45 years; 3881 men and 3713 women, median follow-up 124 person-months) from the Third National Health and Nutrition Examination Survey mortality study. Multiple Cox-proportional hazards regression was applied. There were 673 CV deaths of which 432 were from CHD. Concentrations of apoB [hazard ratio (HR) 1.98, 95% confidence interval (CI) 1.09–3.61], apoA-I (HR 0.48, 95% CI 0.27–0.85) and total cholesterol (TC) (HR 1.17, 95% CI 1.02–1.34) were significantly related to CHD death, whereas high density lipoprotein cholesterol (HDL-C) (HR 0.68, 95% CI 0.45–1.05) was borderline significant. Both the apoB/apoA-I ratio (HR 2.14, 95% CI 1.11–4.10) and the TC/HDL-C ratio (HR 1.10, 95% CI 1.04–1.16) were related to CHD death. Only apoB (HR 2.01, 95% CI 1.05–3.86) and the apoB/apoA-I ratio (HR 2.09, 95% CI 1.04–4.19) remained significantly associated with CHD death after adjusting for CV risk factors.
Conclusion
In the US population, apolipoprotein measurements significantly predict CHD death, independently of conventional lipids and other CV risk factors (smoking, dyslipidaemia, hypertension, obesity, diabetes and C-reactive protein). Furthermore, the predictive ability of apoB alone to detect CHD death was better than any of the routine clinical lipid measurements. Inclusion of apolipoprotein measurements in future clinical guidelines should not be discarded.
Keywords: Apolipoprotein A-I, Apolipoprotein B, ApoB/apoA-I ratio, Cardiovascular risk factors, Cardiovascular mortality, Coronary heart disease, HDL-cholesterol, Outcomes, NHANES
Introduction
Apolipoproteins are the protein components of plasma lipoproteins.1 Recent reports from prospective studies indicate that the apolipoprotein B/apolipoproteinA-I (apoB/apoA-I) ratio is a useful predictor of risk for myocardial infarction (MI) and cardiovascular (CV) disease.2 In several recent clinical studies, the apoB/ApoA-I ratio, which reflects the cholesterol balance between potentially atherogenic and antiatherogenic lipoprotein particles, has been reported to be a better predictor of CV risk than any of the cholesterol indices;2,3 however, data are conflicting, especially in the US.4
Recently, we reported that the apoB/apoA-I ratio was associated with the presence of the individual components of the metabolic syndrome in a representative sample of the US population.5 Furthermore, we reported that the apoB/apoA-I ratio was associated with insulin resistance in both men and women independently of traditional risk factors, metabolic syndrome components, and inflammatory markers, thus adding independent information to the prediction of increased CV risk in the metabolic cluster phenomenon.6,7 However, there is an ongoing debate, particularly in the US, as to whether the apolipoprotein measurements should be implemented in the clinical guidelines, a debate that is fuelled by contradicting evidence.4,8,9
The aim of the present study was to assess whether measurements of apoB and apoA-I can improve the ability to predict coronary heart disease (CHD) death beyond what is possible based on traditional CV risk factors and clinical routine lipid measurements – namely, total cholesterol (TC), high density lipoprotein cholesterol (HDL-C), and low density lipoprotein cholesterol (LDL-C). In particular, we posed the question of whether the apoB/apoA-I ratio can improve the prediction of CHD death. To accomplish these aims, we studied prospectively a well-characterized multi-ethnic representative sample of the US population.
Methods
Subjects
The Third National Health and Nutrition Examination Survey (NHANES III) is a representative sample of the US non-institutionalized civilian population from 1988 to 1994. It consists of a periodic survey conducted by the United States National Center for Health Statistics designed to provide an estimate of the health of the nation. Detailed methods used in NHANES III are available for public access on the internet: http://www.cdc.gov/nchs/nhanes.htm. Subjects underwent institutional review board approval and included written informed consent.
Out of a sample of 39 695 adults and children selected for the NHANES III, 33 994 were interviewed and 30 818 submitted to an examination by a physician at a mobile examination centre, including extensive anthropometric, physiological, and laboratory testing. NHANES information on life-style characteristics, previous and current medical conditions, and medication was obtained during an in-home interview followed by a medical evaluation and blood sample collection at a mobile examination centre. For the present study, the sample was restricted to adults aged 20–89 years (n = 16 881). We excluded participants who were pregnant and those missing data for the apoB/apoA-I ratio (n = 9285) and with missing follow-up (n = 2). Measurement of apolipoproteins in NHANES III was done after collecting all the blood samples and it involved a complex randomization process done by NHANES protocol to avoid any selection bias. This resulted in a final analytic sample of 7594 subjects (3881 men and 3713 women) that was weighted according to the NHANES III analytic guidelines to account for the complex stratified sample. Height and weight were obtained using standardized techniques and equipment. Blood pressure was measured with the participant in a seated position following 5 min of quiet rest by a board-eligible physician at the NHANES Mobile Examination Center.
Laboratory measurements
Lipids were measured enzymatically with commercially available reagents (Cholesterol/HP, cat. no. 816302, and Triglycerides/GPO, cat. no. 816370, both from Boehringer Mannheim). HDL-C was measured in the clear supernatant after precipitating the other lipoproteins with heparin and MnCl2 (1.3 g/L and 0.046 mol/L, respectively) and removing excess Mn2+ by precipitation with NaHCO3. The biases (coefficients of variation) averaged −0.3% (1.7%), −2.1% (3.9%), and 0.3% (3.4%) for cholesterol, triglycerides, and HDL-C, respectively. C-reactive protein (CRP) concentrations were measured by latex-enhanced nephelometry on a Behring Nephelometer (Dade Behring Diagnostics Inc., Somerville, NJ, USA).
Apolipoprotein analysis
Samples were thawed at room temperature and mixed thoroughly for 30 min on a blood-rotating device before analysis. ApoB and apoA-I were measured by radial immunodiffusion in the first 8.2% (1055 specimens) of the specimens during the first 5 months of the study and by rate immunonephelometry for the remaining specimens during the last 31 months. At the beginning of the survey there were no standardized reference materials on which to base the measurements. Over the past few years, the World Health Organization-International Federation of Clinical Chemistry (WHO-IFCC) First International Reference Materials for apoB and apoA-I became available. The Northwest Lipid Research Laboratories, Seattle, WA, served as the coordinating laboratory for the development of these materials. The results were used to transform the immunonephelometry values to equivalent WHO-IFCC International Reference Materials-based values, which are presented here. More detailed methodology and laboratory procedures of NHANES III are published elsewhere.10
Definition of cardiovascular risk factor variables
Subjects were considered to have dyslipidaemia if they reported current usage of medications to lower blood cholesterol, had a self-reported diagnosis of hypercholesterolemia, and/or LDL-C ≥4.10 mmol/L (160 mg/dL), and/or HDL-C <1.036 mmol/L (40 mg/dL) in men and <1.30 mmol/L (50 mg/dL) in women, and/or triglycerides ≥1.7 mmol/L (150 mg/dL).11,12 Subjects were considered to be hypertensive if they were taking antihypertensive medications, had a self-reported diagnosis of hypertension and/or if their systolic pressure was ≥140 mmHg or diastolic pressure was ≥90 mmHg.13 Subjects were considered to be in the smoking group if they were current, former or ever smokers (>100 cigarettes in their life). Subjects were considered to have diabetes if they reported current usage of antidiabetic medications (insulin and oral medications), self-reported diagnosis of diabetes, and/or if their fasting plasma glucose was ≥7.0 mmol/L (126 mg/dL).14 Obesity was defined as body mass index (BMI) ≥30 kg/m2 and/or waist circumference ≥102 cm in men and ≥88 cm in women. We decided to combine measures of total and central obesity since BMI alone might not be the best measure of obesity and/or metabolic risk.15,16 We defined ‘high CRP’ as the sex-specific highest quartile of CRP (mg/dL) (≥0.33 in men; ≥0.44 in women) when compared with the three lowest quartiles (used as reference).
Cardiovascular mortality assessment
NHANES III participants aged ≥20 years for whom data were available for matching were matched to the National Death Index to determine mortality status. The National Death Index was searched up to December 31, 2000, for follow-up. NHANES III and the National Death Index are linked by probabilistic matching in the NHANES III mortality study. The National Center for Health Statistics conducted the linkage and created scores for potential matches. For a selected sample of NHANES III records, the centre reviewed the death certificate record to verify correct matches. Overall, 20 024 adult NHANES III participants were eligible for mortality follow-up by linkage with the National Death Index, of whom 3384 were identified as deceased. A complete description of the methodology used to link NHANES III records to the National Death Index can be found elsewhere.10
We used the underlying cause-of-death information that had been recoded using a standard list of 113 causes of death from the NHANES public-use mortality file according to the corresponding International Classification of Diseases, Ninth Revision (ICD-9) and ICD-10 codes. We grouped deaths into CV disease (codes 53–75) and all other causes; we then subdivided deaths from CV disease into deaths from CHD (codes 58–63) and all CV deaths unrelated to CHD (codes 53–57, 64–75). Person-months of follow-up were calculated for each participant based on the end of follow-up (date of death for those assumed deceased or December 31, 2000, for those assumed alive) minus the date of the NHANES III examination. Total mortality at follow-up was ascertained for 99% of our sample.
Statistical methods
The analysis of the NHANES III data was conducted according to the guidelines in the ‘Analytic and Reporting Guidelines: The Third National Health and Nutrition Examination Survey, NHANES III (1988 to 1994)’. Data were summarized by calculating means and standard deviations (SDs) for quantitative variables and percentages for qualitative variables. All analyses were adjusted (weighted) to the general US population using weights calculated for that purpose by the National Center for Health Statistics.
Multiple Cox-proportional hazard regression was used to estimate adjusted relations between lipid and lipoprotein risk factors and CV mortality. Hazard ratios were calculated after adjusting for different variables per SD increment. Two-sided P-values of <0.05 were considered statistically significant. The assumption of proportional hazards was assessed by visual inspection of the log–log survival curves for the categorical variables. Continuous variables were categorized and a graphical approach was applied to verify the linearity assumption. We investigated apolipoproteins as potential predictors of risk, over and above TC, HDL-C, and LDL-C, with continuous measurements. We controlled for age, race, and sex as possible confounders. Additional adjustments were used for dyslipidaemia, high blood pressure, smoking, diabetes, obesity and high CRP. Also multiple Cox models were created to evaluate the predictive ability of the apoB/apoA-I ratio quartiles for CV mortality. To address whether the apoB/apoA-I ratio had incremental predictive utility over the TC/HDL-C ratio, we performed multi-variable Cox-proportional hazards regression to investigate the relations of the apoB/apoA-I ratio to CHD death adjusting for traditional risk factors and TC/HDL-C ratio. Crude Kaplan–Meier survival curves were created to evaluate the quartiles of apoB and the apoB/apoA-I ratio using the Log-rank test. All analyses were performed using the SAS version 9.1 and SUDAAN 9.0.3.
Results
Descriptive characteristics
There were 7594 subjects with apolipoprotein measurements and for whom CV mortality follow-up data were available. Mean age was 45 years and 50% of the subjects were females. Table 1 shows descriptive characteristics. There were 673 subjects with CV death of which 432 (64%) were from CHD. The median follow-up for this sample was 124 person-months (inter-quartile range 75−25% 114–134 person-months).
Table 1.
Descriptive characteristics of 7594 subjects with cardiovascular mortality follow-up
| Pooled mean ± SE | Men (n = 3881) mean ± SE | Women (n = 3713) mean ± SE | |
|---|---|---|---|
| Age (years) mean ± SD | 45 ± 19 | 44 ± 19 | 46 ± 19 |
| Females, n (%) | 3714 (50) | – | – |
| Race-ethnicity, n (%) | |||
| Non-hispanic white | 3442 (78) | 1778 (79) | 1664 (78) |
| Non-hispanic black | 1851 (10) | 909 (9) | 942 (11) |
| Mexican-American | 2060 (5) | 1072 (5) | 988 (5) |
| Other | 243 (7) | 123 (7) | 120 (6) |
| BMI (kg/m2) | 26 ± 5 | 26 ± 5 | 26 ± 5 |
| Waist circumference (cm) | 92 ± 14 | 95 ± 13 | 91 ± 15 |
| Systolic blood pressure (mmHg) | 124 ± 20 | 126 ± 19 | 122 ± 22 |
| Diastolic blood pressure (mmHg) | 75 ± 12 | 76 ± 12 | 73 ± 11 |
| CRP (mg/dL) | 0.39 ± 0.7 | 0.35 ± 0.7 | 0.44 ± 0.7 |
| TC (mmol/L) | 5.3 ± 1 | 5.3 ± 1 | 5.3 ± 1 |
| LDL-C (mmol/L) | 3.3 ± 1 | 3.4 ± 1 | 3.3 ± 1 |
| HDL-C (mmol/L) | 1.3 ± 0.4 | 1.2 ± 0.4 | 1.4 ± 0.4 |
| Triglycerides (mmol/L) | 1.6 ± 1 | 1.7 ± 1 | 1.4 ± 1 |
| TC/HDL-C ratio | 4.4 ± 2 | 4.7 ± 2 | 3.9 ± 2 |
| ApoB (g/L) | 1.04 ± 0.3 | 1.06 ± 0.3 | 1.02 ± 0.3 |
| ApoA-I (g/L) | 1.43 ± 0.3 | 1.35 ± 0.3 | 1.50 ± 0.3 |
| ApoB/apoA-I ratio | 0.75 ± 0.3 | 0.80 ± 0.3 | 0.70 ± 0.2 |
BMI, body mass index; CRP, C-reactive protein; TC, total cholesterol; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; ApoB, apolipoprotein B; ApoA-I, apolipoprotein A-I.
Coronary heart disease mortality in relation to apolipoproteins and clinical routine lipids
Concentrations of apoB (HR per SD increment, 1.98, 95% CI 1.09–3.61), apoA-I (HR 0.48, 95% CI 0.27–0.85) and TC (HR 1.17, 95% CI 1.02–1.34) were significantly related to CHD death, whereas the concentration of HDL-C (0.68, 95% CI 0.45–1.05) was of borderline significance (Table 2). Both the apoB/apoA-I ratio (HR 2.14, 95% CI 1.11–4.10) and the TC/HDL-C ratio (HR 1.10, 95% CI 1.04–1.16) were related to CHD death. When we substituted LDL-C for TC in the TC/HDL-C ratio, the prediction of CHD death was not improved (HR 0.81, 95% CI 0.96–1.24).
Table 2.
Hazards ratios per standard deviation increment with a 95% confidence interval for mortality with a median follow-up of 124 person-months
| Total (n = 7594) | Cause of deatha |
||||
|---|---|---|---|---|---|
| All death (n = 1465) | CV diseaseb (n = 673) | CHDc (n = 431) | Other CV diseased (n = 242) | CHDc,e (n = 431) | |
| TC (mmol/L) | 1.00 (0.93–1.07) | 1.08 (0.97–1.21) | 1.17 (1.02–1.34) | 1.18 (0.95–1.46) | 1.15 (0.98–1.34) |
| HDL-C (mmol/L) | 0.87 (0.72–1.04) | 0.76 (0.56–1.05) | 0.68 (0.45–1.05) | 1.15 (0.41–3.24) | 1.12 (0.57–2.18) |
| LDL-C (mmol/L) | 0.90 (0.81–1.01) | 1.03 (0.87–1.21) | 1.16 (0.95–1.41) | 0.83 (0.55–1.24) | 1.33 (0.91–1.96) |
| Apolipoprotein B (g/L) | 1.17 (0.83–1.64) | 1.63 (1.02–2.60) | 1.98 (1.09–3.61) | 1.46 (0.55–3.88) | 2.01 (1.05–3.86) |
| Apolipoprotein A-I (g/L) | 0.84 (0.65–1.09) | 0.61 (0.42–0.90) | 0.48 (0.27–0.85) | 1.26 (0.28–5.76) | 0.78 (0.31–1.94) |
| LDL-C/HDL-C ratio | 0.98 (0.88–1.08) | 0.95 (0.81–1.12) | 0.99 (0.82–1.19) | 0.81 (0.96–1.24) | 1.01 (0.62–1.65)) |
| TC/HDL-C ratio | 1.04 (0.98–1.08) | 1.08 (1.02–1.14) | 1.10 (1.04–1.16) | 1.09 (0.96–1.29) | 1.02 (0.91–1.14) |
| ApoB/apoA-I ratio | 1.35 (0.92–1.98) | 1.94 (1.14–3.27) | 2.14 (1.11–4.10) | 1.49 (0.40–5.54) | 2.09 (1.04–4.19) |
CV, cardiovascular; CHD, coronary heart disease; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; ApoB, apolipoprotein B; ApoA-I, apolipoprotein A-I; TC, total cholesterol.
aCorresponding numbers from 113-causes-of death list, based on Anderson et al.34
bCodes 53–75.
cCodes 58–63.
dCodes 55–57 and 64–74.
All variables adjusted for age, race and sex. eFurther adjusted for smoking, dyslipidaemia, hypertension, obesity, diabetes and high CRP.
When adjustments were made for traditional CV risk factors that included CRP, the TC/HDL-C ratio was no longer significant in the model (HR 1.02, 95% CI 0.91–1.14), whereas only the apoB/apoA-I ratio(HR 2.09, 95% CI 1.04–4.19) and apoB (HR 2.01, 95% CI 1.05–3.86) remained significant.
Predictive comparisons
The apoB/apoA-I ratio remained significantly associated with CHD death (HR 2.98, 95% CI 1.07–6.58), after adjusting for traditional CV risk factors and the TC/HDL-C ratio; in contrast, the TC/HDL-C ratio was not significant (HR 0.92, 95% CI 0.79–1.09), after adjustment for traditional CV risk factors and the apoB/apoA-I ratio. Nonetheless, when we tested the superiority of the apoB/apoA-I ratio vs. using apoB alone it was non-significant.
Similarly, subjects in the highest quartile of the apoB (HR 1.92, 95% CI 1.18–3.13) and the apoB/apoA-I ratio (HR 1.73, 95% CI 1.06–2.77) had significantly greater risk of CV mortality compared with those in the lowest quartile, whereas subjects in the highest quartile of the TC/HDL-C ratio did not (HR 1.35, 95% CI 0.77–2.36). Accordingly, the incidence of CV death in the highest quartile of apoB and the apoB/apoA-I ratio was greater than that in the lowest quartile (8.3% and 2.0%, respectively for apoB P < 0.001; 7.4% and 2.9%, respectively for the apoB/apoA-I ratio P < 0.001). The event-free rate for CV mortality according to quartiles of apoB and the TC/HDL-C ratio is shown in Figure 1.
Figure 1.
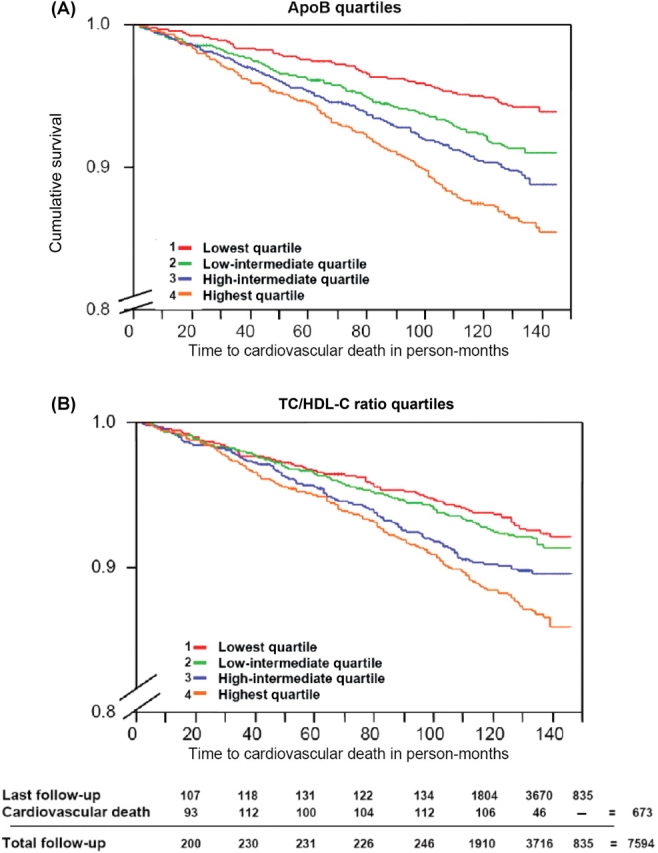
Crude Kaplan–Meier curves of quartiles of apolipoprotein B (A) and the total cholesterol/high density lipoprotein cholesterol ratio (B) for cardiovascular death during follow-up.
Stratification according to age
Stratification of the subjects above and <75 years of age revealed that the apoB/apoA-I ratio was a significant predictor of CHD death irrespective of age, whereas TC/HDL-C ratio was only significantly associated with CHD death in the subjects <75 years (Table 3).
Table 3.
Age specific hazards ratios per standard deviation increment with a 95% confidence interval for coronary heart disease deaths
| Age 20–74 years (CHD events n = 199) | Age 75–89 years (CHD events n = 232) | |
|---|---|---|
| TC/HDL-C ratio | 1.10 (1.03–1.16) | 1.06 (0.96–1.16) |
| ApoB/apoA-I ratio | 2.49 (1.05–5.88) | 2.13 (1.08–4.21) |
CHD, coronary heart disease; TC, total cholesterol; HDL-C, high density lipoprotein cholesterol; ApoB, apolipoprotein B; ApoA-I, apolipoprotein A-I; HR, hazards ratio.
All variables adjusted for age, race and sex.
Discussion
The novel findings of this prospective analysis of a representative multi-ethnic sample of the US civilian general population are three-fold. First, the apoB/apoA-I ratio was significantly associated with CHD death, independently of several established CV risk factors including CRP in the US population. Secondly, the predictive ability of apoB to detect CHD death was comparable with that of the apoB/apoA-I ratio. Thirdly, both the apoB/apoA-I ratio and the apoB were better predictors of CHD death than the TC/HDL-C ratio and other traditional CV risk factors. This suggests that the measurement of apolipoproteins has superior clinical utility over traditional risk markers such as the TC/HDL-C ratio in identifying subjects at risk for fatal CV disease.
Our results are strongly supported by the major findings of the INTERHEART17 study, a Case–Control study, which showed that in all 52 countries investigated, the apoB/apoA-I ratio was not only the strongest factor in explaining risk of MI, but that the ratio was also the most prevalent risk factor of all of the nine conventional risk factors investigated, irrespective of age, sex, race, and other lipids or lipid ratios. Moreover, recent reports from prospective risk studies, such as Apolipoprotein MOrtality RIsk Study,18 the European Prospective Investigation of Cancer-Norfolk study,19 Uppsala Longitudinal Study of Adult Men,20 the MONItoring of trends and determinants in CArdiovascular disease Augsburg/KOoperative Gesundheitsforschung in der Region Augsburg,21 as well as from other studies on diseases related to atherosclerosis2 indicate that the apoB/apoA-I ratio is a useful predictor of risk of both non-fatal and fatal MI. A meta-analysis of the apoB/apoA-I ratio also supports its use as a risk marker of future CV disease.3 Furthermore, in a cross-sectional analysis of the US population, LDL-C was not significantly correlated with history of atherosclerotic disease, suggesting that LDL-C is not the best target for lipid-lowering treatment strategies.22 Similarly, in the recently published data from the Framingham study, LDL-C was not found to be a significant risk factor of CHD.4
Recently, we published an association between the apoB/apoA-I ratio and the metabolic syndrome and its components5 and reported a strong association with insulin resistance beyond traditional risk factors, metabolic syndrome components, and inflammatory markers in US non-diabetic subjects.6 Therefore, the longitudinal results in the present analysis extend upon our previous findings and suggest that the apoB/apoA-I ratio is a powerful predictor of CV risk in the US population.
On the other hand, a recent study4 of a US population reported after 15 years median follow-up that the apoB/apoA-I ratio was not a better predictor of CHD than the TC/HDL-C ratio. However, the ‘composite endpoint’ used included a diverse selection of events covering a wide range of clinical severity (angina, unrecognized MI, recognized MI, coronary insufficiency, and CV death) and there was no mention of the number of CV deaths.8,9 Thus, our study shows that the apoB/apoA-I ratio does indeed improve the prediction of hard endpoints (i.e. CHD death) when compared with conventional lipid ratios, even after adjusting for CV risk factors including CRP. Of note, CRP concentrations were not adjusted for in the recent report from the Framingham study.4
It is worth noting that in our analysis, apoB was a strong predictor of CHD death by itself and far better than either LDL-C or HDL-C alone and as powerful as the apoB/apoA-I ratio. This raises the question of whether apoB is the primary force underlying the high predictive value of the apoB/apoA-I ratio, which demands further investigation. Interestingly, in our study non-HDL-C was not a significant predictor. Thus, apoB seems to be superior to non-HDL-C in predicting CV risk as often found and summarized.6 Regarding apoB, our results are in line with the Framingham Study,4 in which neither TC nor LDL-C were significant predictors. In that study, HDL-C was markedly superior to apoA-I, so much better as to account for the fact that the apoB/apoA-I and TC/HDL-C ratios were equal in predictive power. Thus, it is the outcome with respect to apoA-I and HDL-C that differs so much from study to study as reflected in the present NHANES materials. At least in our study, the measurement of apoA-I, although significant in univariate analysis, seems to contribute little to the prediction of CHD death up and above apoB when expressed as the apoB/apoA-I ratio and also controlled for a number of dyslipidaemic and other CV risk variables. A possible reason that could explain the lack of predictive power for apoA-I in this setting may be this multi-variate comparison as well as the fact that apoA-I was measured with two different assays adding some increase in the SD for these values. However, inclusion of also apoA-I into this ratio has previously been show to give additional information of risk since the apoB/apoA-I ratio can pick up risk also for other phenotypes in a population at risk than those dependent on apoB only.6
The reasons why clinical routine lipid measurements such as LDL-C, HDL-C, and/or the TC/HDL-C ratio were not independent predictors in our population could be that strict adjustments were made and that the additional information provided by any of these markers beyond traditional risk factors is very small. Since our adjustment strategy using dichotomized risk factor variables could be questioned, we also (when possible) adjusted for CV risk factors (such as BMI, blood pressure, and CRP) as continuous variables. The results remained unchanged, favouring apolipoprotein measurements over traditional clinical measurements. Other possible explanations for the lack of additional predictive ability for clinical routine lipid measurements could be that about half of our samples were non-fasting; however, when we did the same analysis in the subset with the fasting samples (n = 3338) the predictive information of apolipoproteins was significant for both fasting and non-fasting subjects, while the predictive information of the TC/HDL-C ratio was only predictive in the fasting state. This fact could well have attenuated the predictive power of routine clinical lipid measurements; on the other hand it also underscores the importance and usefulness of apolipoprotein measurements since they do not require fasting samples. Furthermore, our study shows that apolipoproteins provide clinical information regarding CHD death that could neither be obtained with traditional risk factors nor with clinical routine lipid measurements.
Limitations
This analysis could only be applied to a portion of the NHANES population, given that not all the adult population had measurements of the apolipoproteins. However, NHANES was designed with the aim that results may be generalized to apply to all persons in the US as a result of its stratified multi-stage probability sampling design, even when applying this to small subsets. Excluded patients appeared to be similar to those included in the analysis, on the basis of their age and gender, and when we compared the weighted incidence rates between the present sample and excluded subjects, they showed a similar incidence of CV death (5.1%, n = 7594 vs. 4.1%, n = 9287, P = NS). Another important limitation could be the use of death certificates, due to possible misclassification of endpoints. Despite the possibility of misclassification of death on the death certificate, death certificate data remain important and probably the only feasible option in such large epidemiologic cohorts as the NHANES III mortality study.23–25
Practical implications
Our study clearly indicates that apoB is equally predictive as the apoB/apoA-I ratio of CHD death and better than clinical routine lipid measurements, thus showing an advantage of using apolipoproteins as CV risk predictors in parallel with the metabolic cluster risk-phenomenon.7,26 This has important implications for primary CV prevention.27,28
ApoB can adequately measure the number of apoB-containing pro-atherogenic lipoprotein particles, including the small dense LDL particles, which is an advantage in patients with the metabolic syndrome.29–31 Furthermore, the methods can easily be automated, analyses are cheap, can be performed on previously frozen sera, and importantly, non-fasted samples can be used. Apolipoprotein measurements were recently published in guidelines for diabetic subjects in the US.32,33 Nonetheless, there is an ongoing controversy as to whether the apolipoproteins should be implemented in CV clinical guidelines, especially in the US.
Conclusion
In the US population, apolipoprotein measurements significantly predict CHD death, independently of CV risk factors. Furthermore, the predictive ability of apoB alone to detect CHD death was comparable with that of the apoB/apoA-I ratio and better than any of the routine clinical lipid measurements. Thus, apolipoprotein measurements are important for assessing CV risk in a multi-ethnic representative US population and their inclusion in future clinical guidelines should not be discarded.
Funding
J.S.-J. was supported in part by faculty funds from the Board of Post-Graduate Education of the Karolinska Institutet (KID Award), the European Foundation for the Study of Diabetes Lilly Research Fellowship and the Swedish Heart and Lung Foundation. Prof. A.H. was supported in part by the Swedish Heart and Lung Foundation and the Stockholm County Council. R.M.F. was supported in part by the Swedish Research Council (project 15352) and the Swedish Diabetes Association. A.R.-C. was supported by American Heart Association Award. Prof. V.K.S. was supported in part by NIH R01 HL73211. Prof. M.-L.H. was supported in part by the Swedish Heart and Lung Foundation, the Stockholm County Council and the Swedish Council for Working Life and Social Research. The funding bodies had no role in the study design, data collection, data analysis, data interpretation or writing the report.
Acknowledgements
The data reported here have been analysed using National Health and Nutrition Examination survey files available for public use. All the analyses, interpretations, and/or conclusions reached in this paper are work of the authors and not of the National Center for Health Statistics.
Conflict of interest: none declared.
References
- 1.Olofsson SO, Wiklund O, Boren J. Apolipoproteins A-I and B: biosynthesis, role in the development of atherosclerosis and targets for intervention against cardiovascular disease. Vasc Health Risk Manag. 2007;3:491–502. [PMC free article] [PubMed] [Google Scholar]
- 2.Walldius G, Jungner I. The apoB/apoA-I ratio: a strong, new risk factor for cardiovascular disease and a target for lipid-lowering therapy – a review of the evidence. J Intern Med. 2006;259:493–519. doi: 10.1111/j.1365-2796.2006.01643.x. [DOI] [PubMed] [Google Scholar]
- 3.Thompson A, Danesh J. Associations between apolipoprotein B, apolipoprotein AI, the apolipoprotein B/AI ratio and coronary heart disease: a literature-based meta-analysis of prospective studies. J Intern Med. 2006;259:481–492. doi: 10.1111/j.1365-2796.2006.01644.x. [DOI] [PubMed] [Google Scholar]
- 4.Ingelsson E, Schaefer EJ, Contois JH, McNamara JR, Sullivan L, Keyes MJ, Pencina MJ, Schoonmaker C, Wilson PW, D’Agostino RB, Vasan RS. Clinical utility of different lipid measures for prediction of coronary heart disease in men and women. JAMA. 2007;298:776–785. doi: 10.1001/jama.298.7.776. [DOI] [PubMed] [Google Scholar]
- 5.Sierra-Johnson J, Somers VK, Kuniyoshi FH, Garza CA, Isley WL, Gami AS, Lopez-Jimenez F. Comparison of apolipoprotein-B/apolipoprotein-AI in subjects with versus without the metabolic syndrome. Am J Cardiol. 2006;98:1369–1373. doi: 10.1016/j.amjcard.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 6.Sierra-Johnson J, Romero-Corral A, Somers VK, Lopez-Jimenez F, Walldius G, Hamsten A, Hellenius ML, Fisher RM. ApoB/apoA-I ratio: an independent predictor of insulin resistance in US non-diabetic subjects. Eur Heart J. 2007;28:2637–2643. doi: 10.1093/eurheartj/ehm360. [DOI] [PubMed] [Google Scholar]
- 7.Sniderman AD. The apoB/apoA-I ratio and insulin resistance: sorting out the metabolic syndrome. Eur Heart J. 2007;28:2563–2564. doi: 10.1093/eurheartj/ehm434. [DOI] [PubMed] [Google Scholar]
- 8.Sierra-Johnson J, Romero-Corral A, Lopez-Jimenez F. Clinical utility of different lipid measures. JAMA. 2008;299:35. doi: 10.1001/jama.2007.3. [DOI] [PubMed] [Google Scholar]
- 9.Sierra-Johnson J, Fisher RM. Is it time to discard the apolipoprotein B:apolipoprotein A-I ratio as a predictor of cardiovascular disease? Nat Clin Pract Cardiovasc Med. 2008;5:18–19. doi: 10.1038/ncpcardio1040. [DOI] [PubMed] [Google Scholar]
- 10.National Center for Health Statistics. Maryland: Hyattsville; Office of Analysis and Epidemiology, Public-use NHANES III Linked Mortality file, 2007. http://www.cdc.gov/nchs/r&d/nchs_datalinkage/nhanes3_data_linkage_mortality_activities.htm. (31 January 2008) [Google Scholar]
- 11.Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 12.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F American Heart Association; National Heart, Lung, Blood Institute. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 13.Chobanian A, Bakris G, Black H, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ Joint National Committee on Prevention, Detection, Evaluation, Treatment of High Blood Pressure; National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 14.American Diabetes Association. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 15.Romero-Corral A, Montori VM, Somers VK, Korinek J, Thomas RJ, Allison TG, Mookadam F, Lopez-Jimenez F. Association between body weight with mortality and with cardiovascular events in patients with coronary disease: a systematic review of cohort studies. Lancet. 2006;368:666–678. doi: 10.1016/S0140-6736(06)69251-9. [DOI] [PubMed] [Google Scholar]
- 16.Romero-Corral A, Somers VK, Sierra-Johnson J, Jensen MD, Thomas RJ, Squires RW, Allison TG, Korinek J, Lopez-Jimenez F. Diagnostic performance of body mass index to detect obesity in patients with coronary artery disease. Eur Heart J. 2007;28:2087–2093. doi: 10.1093/eurheartj/ehm243. [DOI] [PubMed] [Google Scholar]
- 17.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L INTERHEART Study Investigators. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 18.Walldius G, Jungner I, Holme I, Aastveit AH, Kolar W, Steiner E. High apolipoprotein B, low apolipoprotein A-I, and improvement in the prediction of fatal myocardial infarction (AMORIS study): a prospective study. Lancet. 2001;358:2026–2033. doi: 10.1016/S0140-6736(01)07098-2. [DOI] [PubMed] [Google Scholar]
- 19.Vaessen SF, Schaap FG, Kuivenhoven JA, Groen AK, Hutten BA, Boekholdt SM, Hattori H, Sandhu MS, Bingham SA, Luben R, Palmen JA, Wareham NJ, Humphries SE, Kastelein JJ, Talmud PJ, Khaw KT. Apolipoprotein A-V, triglycerides and risk of coronary artery disease: the prospective Epic-Norfolk Population Study. J Lipid Res. 2006;47:2064–2070. doi: 10.1194/jlr.M600233-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Dunder K, Lind L, Zethelius B, Berglund L, Lithell H. Evaluation of a scoring scheme, including proinsulin and the apolipoprotein B/apolipo-protein AI ratio, for the risk of acute coronary events in middle-aged men: Uppsala Longitudinal Study of Adult Men (ULSAM) Am Heart J. 2004;148:596–601. doi: 10.1016/j.ahj.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 21.Meisinger C, Loewel H, Mraz W, Koenig W. Prognostic value of apolipoprotein B and A-I in the prediction of myocardial infarction in middle-aged men and women: results from the MONICA/KORA Augsburg cohort study. Eur Heart J. 2005;26:271–278. doi: 10.1093/eurheartj/ehi003. [DOI] [PubMed] [Google Scholar]
- 22.Hsia SH, Pan D, Berookim P, Lee ML. A population-based, cross-sectional comparison of lipid-related indexes for symptoms of atherosclerotic disease. Am J Cardiol. 2006;98:1047–1052. doi: 10.1016/j.amjcard.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 23.Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298:2028–2037. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- 24.Saydah S, Graubard B, Ballard-Barbash R, Berrigan D. Insulin-like growth factors and subsequent risk of mortality in the United States. Am J Epidemiol. 2007;166:518. doi: 10.1093/aje/kwm124. [DOI] [PubMed] [Google Scholar]
- 25.Iribarren C, Crow RS, Hannon PJ, Jacobs DR, Jr, Luepker RV. Validation of death certificate diagnosis of out-of-hospital sudden cardiac death. Am J Cardiol. 1998;82:50–53. doi: 10.1016/s0002-9149(98)00240-9. [DOI] [PubMed] [Google Scholar]
- 26.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 27.Mudd JO, Borlaug BA, Johnston PV, Kral BG, Rouf R, Blumenthal RS, Kwiterovich PO., Jr Beyond low-density lipoprotein cholesterol: defining the role of low-density lipoprotein heterogeneity in coronary artery disease. J Am Coll Cardiol. 2007;18:1735–1741. doi: 10.1016/j.jacc.2007.07.045. [DOI] [PubMed] [Google Scholar]
- 28.Sniderman AD, Holme I, Aastveit A, Furberg C, Walldius G, Jungner I. Relation of age, the apolipoprotein B/apolipoprotein A-I ratio, and the risk of fatal myocardial infarction and implications for the primary prevention of cardiovascular disease. Am J Cardiol. 2007;100:217–221. doi: 10.1016/j.amjcard.2007.02.086. [DOI] [PubMed] [Google Scholar]
- 29.Walldius G, Jungner I. Rationale for using apolipoprotein B and apolipoprotein A-I as indicators of cardiac risk and as targets for lipid-lowering therapy. Eur Heart J. 2005;26:210–212. doi: 10.1093/eurheartj/ehi077. [DOI] [PubMed] [Google Scholar]
- 30.Walldius G, Jungner I. Apolipoprotein A-I versus HDL cholesterol in the prediction of risk for myocardial infarction and stroke. Curr Opin Cardiol. 2007;22:359–367. doi: 10.1097/HCO.0b013e3281bd8849. [DOI] [PubMed] [Google Scholar]
- 31.Walldius G, Jungner I, Aastveit AH, Holme I, Furberg CD, Sniderman AD. The apoB/apoA-I ratio is better than the cholesterol ratios to estimate the balance between plasma proatherogenic and antiatherogenic lipoproteins and to predict coronary risk. Clin Chem Lab Med. 2004;42:1355–1363. doi: 10.1515/CCLM.2004.254. [DOI] [PubMed] [Google Scholar]
- 32.Brunzell JD, Davidson M, Furberg CD, Goldberg RB, Howard BV, Stein JH, Witztum JL American Diabetes Association; American College of Cardiology Foundation. Lipoprotein management in patients with cardiometabolic risk: consensus statement from the American Diabetes Association and the American College of Cardiology Foundation. Diabetes Care. 2008;31:811–822. doi: 10.2337/dc08-9018. [DOI] [PubMed] [Google Scholar]
- 33.Brunzell JD, Davidson M, Furberg CD, Goldberg RB, Howard BV, Stein JH, Witztum JL. Lipoprotein management in patients with cardiometabolic risk: consensus conference report from the American Diabetes Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2008;51:1512–1524. doi: 10.1016/j.jacc.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 34.Anderson RN, Minino AM, Hoyert DL, Rosenberg HM. Comparability of cause of death between ICD-9 and ICD-10: preliminary estimates. Natl Vital Stat Rep. 2001;49:1–32. http://www.cdc.gov/nchs/data/nvsr/nvsr49/nvsr49_02.pdf. 31 January 2008. [PubMed] [Google Scholar]


