Abstract
Aims
Growth factors play an important role in regulating vascular function. Data are limited regarding clinical and genetic correlates of endothelial growth factors and their associations with vascular function.
Methods and results
We evaluated clinical and genetic correlates of circulating vascular endothelial growth factor A (VEGF), its soluble receptor sFlt-1, and hepatocyte growth factor (HGF) in 3754 Framingham Study participants. We also related the growth factors to measures of brachial artery function. Serum VEGF and HGF were higher and sFLt-1 was lower in women and smokers. VEGF and HGF were associated positively with body mass index; both displayed strong positive associations with the metabolic syndrome (P < 0.001) and its components. The heritabilities of VEGF, sFlt-1, and HGF were 78, 13, and 38%, respectively. VEGF and HGF were related positively to baseline brachial diameter (P < 0.01) and to baseline mean flow velocity (P < 0.001) in age- and sex-adjusted models, but the multivariable models failed to reach significance. None of the growth factors were related to flow-mediated dilation.
Conclusion
In our community-based sample, circulating VEGF and HGF demonstrated high heritabilities and a sexual dimorphism. Increased angiogenesis and greater endothelial cell turnover may underlie associations of these growth factors with risk factors including smoking.
Keywords: Vascular growth factors, VEGF, SFlt-1, HGF, Vascular function, Heritability, Metabolic syndrome
Introduction
Cardiovascular disease (CVD) is a major cause of morbidity and mortality in the United States.1 Substantial scientific evidence identified progressive structural and functional changes in the vessel wall—termed as vascular remodelling2—as hallmarks of atherosclerosis,3 which precedes overt CVD by decades. As a consequence, investigating the determinants of vascular remodelling and identifying biomarkers of the process are critical.
Vascular endothelial growth factor (VEGF) modulates physiological and pathophysiological vascular development.4 VEGF stimulates the production of nitric oxide (NO) and prostacyclin by endothelial cells,5,6 increases vascular permeability,7 stimulates growth,8 and prevents apoptosis of endothelial cells. Elevated circulating VEGF has been observed in hypertension,9 coronary disease,10 myocardial infarction,11 peripheral arterial disease,10 and heart failure.12 VEGF binds two receptors, VEGFR1 and VEGFR2.13 The soluble isoform of VEGFR1 (sFlt-1) is found in circulation where it inhibits VEGF by direct sequestration.13
Hepatocyte growth factor (HGF) is another angiogenic growth factor expressed by multiple cell types, including endothelial and vascular smooth muscle cells.14 Increased circulating HGF has been reported in hypertension,15 obesity,16 myocardial infarction,17 ventricular hypertrophy,18 and heart failure.19 HGF is also positively associated with arterial stiffness and with reactive hyperaemia in hypertension,20 and has been related to carotid artery remodelling.21
Although VEGF, sFlt-1, and HGF play a fundamental role in vascular remodelling, the clinical correlates of circulating VEGF, sFlt-1, and HGF have not been comprehensively assessed. We related VEGF, sFlt-1, and HGF levels to CVD risk factors and to measures of brachial artery (BA) function, and estimated the heritabilities of these biomarkers. We hypothesized that: a higher burden of CVD risk factors is associated with higher VEGF and HGF but lower sFlt-1; these biomarkers are heritable; VEGF and HGF are associated with better endothelial function but sFlt-1 is associated with endothelial dysfunction.
Methods
Study sample
The design of the Framingham Offspring Study has been described elsewhere.22 Starting in 2002, 4095 participants with at least one parent in the Offspring cohort were enrolled in the Generation 3 cohort (Examination 1).23 We excluded 341 attendees for reasons detailed in the Supplementary material online. After exclusions, 3754 participants remained eligible. At their first examination, Generation 3 participants have been comprehensively phenotyped as detailed in the Supplementary material online. All participants provided informed consent and the study complies with the Declaration of Helsinki and was approved by the Institutional Review Board at the Boston University Medical Center.
Laboratory measurements of vascular endothelial growth factor, sFlt-1, and hepatocyte growth factor
Blood was drawn after an overnight fast, immediately centrifuged and stored at −80°C until biomarkers were assayed. Serum VEGF, sFlt-1, and HGF were measured with commercial assays (R&D Inc.) as detailed in the Supplementary material online.
Flow-mediated dilation and reactive hyperaemia measurements
Baseline and hyperaemic measures of BA structure and function have been determined using a Toshiba SSH-140A ultrasound system as described previously24 and detailed in the Supplementary material online.
Statistical analyses
Additional details on the analyses performed are displayed in the Supplementary material online.
Clinical correlates
VEGF, sFlt-1, and HGF were natural log-transformed to normalize their distributions. To assess the association of each biomarker with clinical covariates [age, sex, systolic and diastolic BP, anti-hypertensive medication, diabetes, total cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, smoking, body mass index (BMI), alcohol consumption, estimated glomerular filtration rate (eGFR)], we first identified predictors for each biomarker separately using stepwise forward multivariable linear regression (P ≤ 0.1 for model entry). The associations of each biomarker with variables that were significant in the stepwise forward regression analysis (P < 0.05) were then examined using the generalized estimating equations (GEE; using the Compound Symmetry Correlation Matrix) to account for relatedness among participants.
In secondary analyses, we related biomarkers to select risk factors that are categorical and frequently used in clinical practice: Hypertension, BP ≥140/90 mmHg or anti-hypertensive treatment; obesity, BMI ≥30 kg/m2; abdominal obesity, waist circumference ≥102 cm (men) or ≥89 cm (women); low HDL cholesterol, <40 mg/dL (men) or <50 mg/dL (women); high triglycerides, ≥150 mg/dL or lipid-lowering treatment. Age and eGFR were modelled as continuous variables, all other significant continuous traits were replaced by their binary counterpart in secondary analyses. We also related VEGF and HGF to prevalence of the metabolic syndrome (MetS), adjusting for age, sex, and smoking. MetS was defined by the presence of ≥3 of: increased waist circumference; elevated BP [≥130 (systolic) or ≥85 mmHg (diastolic), or anti-hypertensive treatment]; hyperglycaemia (fasting glucose ≥100 mg/dL or treatment for elevated glucose); hypertriglyceridaemia (≥150 mg/dL or lipid-lowering treatment); low HDL cholesterol [<40 (men), <50 mg/dL (women)].25 A two-sided P-value below 0.05 was considered statistically significant.
Heritability estimates
As detailed in the Supplementary material online, heritability for each log-biomarker was estimated using variance components analysis. The analyses were performed on residuals from models adjusting for (1) age and sex; (2) age, sex, and all other covariates that were significantly associated with the respective biomarker in our prior analyses (clinical correlates).
Relations to vascular function
Log-biomarker concentrations were related to four vascular function measures using GEE: baseline BA diameter, baseline BA mean flow velocity, flow-mediated dilation (FMD), and hyperaemic mean flow velocity. Model 1 adjusted for age and sex; model 2 adjusted for age, sex, mean arterial pressure, pulse pressure, heart rate, BMI, total/HDL cholesterol, fasting glucose, diabetes, smoking within 6 h prior to the procedure, prevalent CVD, hormone replacement therapy, hypertension, lipid-lowering medication, and walk test. These covariates correlate with BA vascular function in our cohort.24
Results
Information on the number of siblings per family in the study sample is provided in Supplementary material online, Table S1. The characteristics of our study sample are shown in Table 1. After adjusting for age and sex, LogVEGF correlated weakly with LogsFlt-1 (r = 0.024) and moderately with LogHGF (r = 0.18). The age- and sex-adjusted correlation between LogsFlt-1 and LogHGF was low (r = 0.14).
Table 1.
Characteristics of the study sample
| Variables | Women (n = 2002) | Men (n = 1752) | |
|---|---|---|---|
| Clinical features | |||
| Age, years | 40 ± 9 | 40 ± 9 | |
| Systolic BP, mmHg | 113 ± 14 | 121 ± 12 | |
| Diastolic BP, mmHg | 73 ± 9 | 78 ± 9 | |
| BMI, kg/m2 | 25.9 ± 6 | 27.8 ± 4.6 | |
| Waist circumference, inches | 34.8 ± 6.1 | 38.6 ± 5 | |
| Smoking, % | 14 | 16 | |
| Alcohol consumption, ounces per month | 5.8 ± 7.9 | 13.6 ± 17.9 | |
| eGFR, mL/min/1.73 m2 | 99 ± 19 | 100 ± 17 | |
| Hypertension, % | 12 | 20 | |
| Diabetes, % | 2 | 3 | |
| UACR, mg/g | 9.2 ± 24.8 | 5.9 ± 12.6 | |
| Biochemical features | |||
| Total cholesterol, mg/dL | 185 ± 34 | 193 ± 37 | |
| HDL cholesterol, mg/dL | 61 ± 16 | 47 ± 12 | |
| Triglycerides, mg/dL | 97 ± 63 | 134 ± 105 | |
| VEGF, ng/mL | 285 (159,471) | 274 (157,437) | |
| sFlt-1, ng/mL | 136 (100,184) | 149 (112,194) | |
| HGF, ng/mL | 823 (696,970) | 814 (700,965) | |
| Metabolic syndrome, % | 14 | 26.6 | |
| Vascular function | N = 1860 | N = 1674 | |
| Baseline brachial diameter, mm | 3.50 ± 0.44 | 4.76 ± 0.61 | |
| FMD, % | 7.12 ± 3.81 | 4.57 ± 2.94 | |
| Baseline mean flow velocity, cm/s | 6.76 ± 3.58 | 7.95 ± 4.71 | |
| Hyperaemic mean flow velocity, cm/s | 65.9 ± 18.0 | 57.7 ± 17.6 | |
Values represent mean±SD for continuous variables and % for discrete. Values for VEGF, sFlt-1, and HGF represent median (25th percentile, 75th percentile).
BMI, body mass index; BP, blood pressure; SD, standard deviation; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; HGF, hepatocyte growth factor; sFlt-1, soluble fms-like tyrosine kinase-1; UACR, urinary albumin to creatinine ratio; FMD, flow-mediated dilation; VEGF, vascular endothelial growth factor.
Clinical correlates of vascular endothelial growth factor, sFlt-1, and hepatocyte growth factor
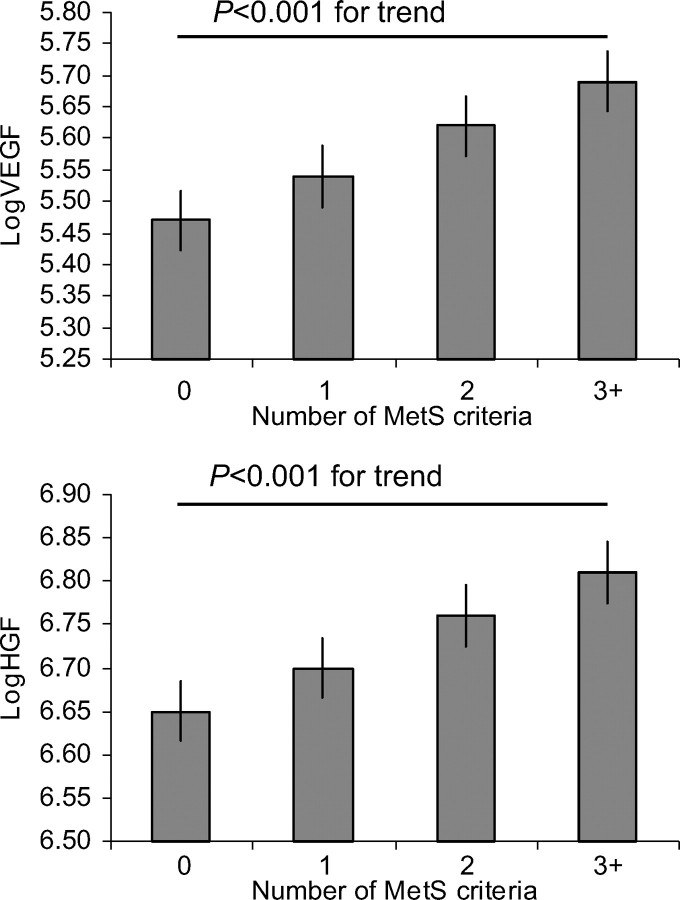
In multivariable analyses, VEGF had significant positive associations with female sex, systolic BP, smoking, and BMI (Table 2). Replacing all significantly associated continuous variables by their categorical counterparts, VEGF was positively associated with abdominal obesity (P <0.001), hypertension (P = 0.02), and MetS (P <0.001). Consistent with this finding, VEGF increased with the number of components of MetS (Figure 1). In secondary analyses, VEGF concentrations did not differ between pre- and post-menopausal women, nor between women in the follicular vs. luteal phases of their menstrual cycle (data not shown). Serum sFlt-1 levels were positively associated with male sex and age and negatively associated with smoking and renal function (Table 2).
Table 2.
Clinical correlates of VEGF, sFlt-1, and HGF
| Estimate | 95% CI | P-value | |
|---|---|---|---|
| Dependent: VEGF | |||
| Age, years | 0.023 | (−0.005,0.051) | 0.10 |
| Sex, m vs. f | −0.120 | (−0.168,−0.073) | <0.001 |
| Smoking, yes vs. no | 0.127 | (0.065,0.190) | <0.001 |
| Systolic BP, mmHg | 0.033 | (0.007,0.060) | 0.014 |
| Triglycerides, mg/dL | 0.026 | (−0.0004,0.052) | 0.054 |
| BMI, kg/m2 | 0.074 | (0.048,0.100) | <0.001 |
| Dependent: sFlt-1 | |||
| Age, years | 0.022 | (0.001,0.043) | 0.041 |
| Sex, m vs. f | 0.110 | (0.074,0.147) | <0.001 |
| Smoking, yes vs. no | −0.103 | (−0.161,−0.045) | <0.001 |
| eGFR, mg/g | −0.027 | (−0.049,−0.005) | 0.017 |
| Dependent: HGF | |||
| Age, years | 0.027 | (0.018,0.036) | <0.001 |
| Sex, m vs. f | −0.041 | (−0.059,−0.023) | <0.001 |
| Diastolic BP, mmHg | 0.013 | (0.004,0.022) | 0.004 |
| Anti-hypertensive medication, yes vs. no | 0.044 | (0.013,0.075) | 0.005 |
| Diabetes, yes vs. no | 0.090 | (0.039,0.140) | <0.001 |
| High-density lipoprotein cholesterol, mg/dL | −0.01 | (−0.019,−0.001) | 0.039 |
| Triglycerides, mg/dL | 0.01 | (0.001,0.019) | 0.039 |
| Smoking, yes vs. no | 0.115 | (0.092,0.139) | <0.001 |
| BMI, kg/m2 | 0.053 | (0.044,0.063) | <0.001 |
Estimates reflect increase in log-biomarker level per one-SD increase of the continuous traits.
BMI, body mass index; BP, blood pressure; SD, standard deviation; SE, standard error; eGFR, estimated glomerular filtration rate; HGF, hepatocyte growth factor; sFlt-1, soluble fms-like tyrosine kinase-1; VEGF, vascular endothelial growth factor; CI, confidence interval of estimate.
Figure 1.
Multivariable adjusted means for log-transformed vascular endothelial growth factor (logVEGF) and hepatocyte growth factor (logHGF) stratified by the number of criteria for the metabolic syndrome (MetS).
HGF displayed positive associations with age, female sex, diastolic BP, anti-hypertensive treatment, diabetes, triglycerides, smoking, and BMI, and an inverse association with HDL cholesterol (Table 2). In secondary analyses, replacing significant continuous traits by binary variables, HGF was positively associated with hypertension (P <0.001), diabetes (P <0.001), high triglycerides (P <0.001), smoking (P <0.001), abdominal obesity (P <0.001), and the MetS (P <0.001). HGF levels increased gradually with the number of components of the MetS (P < 0.001; Figure 1).
Heritability estimates for vascular endothelial growth factor, sFlt-1, and hepatocyte growth factor
For VEGF, the estimated heritability ranged from 0.77 to 0.78. For sFlt-1, the heritabilities were lower at 0.12–0.13. The heritability for HGF was 0.37–0.38 (Table 3).
Table 3.
Heritability estimates for VEGF, sFlt-1, and HGF
| Biomarker | Model | Heritability | 95% CI | P-value |
|---|---|---|---|---|
| HGF | Age- and sex-adjusted | 0.37 | (0.29,0.45) | <0.001 |
| Multi-variable adjusted | 0.38 | (0.29,0.46) | <0.001 | |
| sFlt-1 | Age- and sex-adjusted | 0.12 | (0.05,0.19) | <0.001 |
| Multi-variable adjusted | 0.13 | (0.05,0.20) | <0.001 | |
| VEGF | Age- and sex-adjusted | 0.77 | (0.71,0.84) | <0.001 |
| Multi-variable adjusted | 0.78 | (0.71,0.85) | <0.001 |
Multivariable models were adjusted for those variables, which were significant for the respective biomarkers in Table 2.
HGF, hepatocyte growth factor; sFlt-1, soluble fms-like tyrosine kinase-1; VEGF, vascular endothelial growth factor; CI, confidence interval.
Association of vascular endothelial growth factor, sFlt-1, and hepatocyte growth factor with vascular function
In age- and sex-adjusted models, VEGF and HGF were associated positively with baseline BA diameter and with baseline BA mean flow velocity. These associations were no longer significant after adjusting for covariates (Table 4). Adding BMI alone or heart rate and BMI to the models was sufficient to render statistically non-significant the associations of growth factors with vascular function (noted in age- and sex-adjusted models) in most instances. sFlt-1 was not associated with any measure of vascular structure or function (Table 4).
Table 4.
Association of VEGF, sFlt-1, and HGF with measures of vascular function
| Beta | 95% CI | P-value | Beta | 95% CI | P-value | |
|---|---|---|---|---|---|---|
| Baseline brachial diameter | Flow-mediated dilation | |||||
| Age- and sex-adjusted | ||||||
| VEGF | 0.03 | (0.01,0.05) | 0.0049 | −0.05 | (−0.20,0.09) | 0.47 |
| HGF | 0.11 | (0.05,0.18) | 0.001 | −0.002 | (−0.44,0.44) | 0.99 |
| sFlt-1 | 0.03 | (−0.004,0.06) | 0.09 | 0.09 | (−0.11,0.29) | 0.38 |
| Multivariable-adjusted | ||||||
| VEGF | 0.01 | (−0.01,0.03) | 0.29 | −0.05 | (−0.19,0.10) | 0.52 |
| HGF | −0.02 | (−0.09,0.04) | 0.50 | 0.01 | (−0.45,0.46) | 0.98 |
| sFlt1-1 | 0.02 | (−0.005,0.05) | 0.11 | 0.05 | (−0.14,0.25) | 0.59 |
| Baseline mean flow velocity | Hyperaemic mean flow velocity | |||||
| Age- and sex-adjusted | ||||||
| VEGF | 0.35 | (0.17,0.53) | <0.001 | 0.07 | (−0.68,0.83) | 0.85 |
| HGF | 2.17 | (1.64,2.70) | <0.001 | 0.31 | (−1.98,2.59) | 0.79 |
| sFlt-1 | −0.05 | (−0.29,0.19) | 0.68 | −0.51 | (−1.55,0.53) | 0.33 |
| Multivariable-adjusted | ||||||
| VEGF | 0.02 | (−0.14,0.19) | 0.81 | −0.24 | (−1.00,0.53) | 0.55 |
| HGF | 0.07 | (−0.45,0.59) | 0.80 | −1.84 | (−4.27,0.58) | 0.14 |
| sFlt1-1 | −0.09 | (−0.31,0.13) | 0.42 | −0.64 | (−1.67,0.40) | 0.23 |
Beta: regression coefficient. Regression coefficient indicates increase of baseline brachial diameter in millimetres, of flow-mediated dilation in %, baseline and hyperaemic mean flow in cm/s per one-unit increase in log biomarker. For example, a 2.72-fold (2.72=e1) increase in VEGF is associated with an increase of 0.03 mm in baseline brachial diameter.
HGF, hepatocyte growth factor; sFlt-1, soluble fms-like tyrosine kinase-1; VEGF, vascular endothelial growth factor; confidence interval.
Discussion
Principal findings
First, we observed a sexual dimorphism in circulating biomarkers: VEGF and HGF concentrations were higher in women, whereas sFlt-1 was lower. Second, both VEGF and HGF were highly significantly and positively associated with select risk factors, i.e. smoking, BMI, and obesity, and with the constellation identifying MetS. sFlt-1 was inversely related to smoking. Third, we observed very high heritability for VEGF, moderately high heritability for HGF, and modest heritability for sFlt-1. Finally, VEGF and HGF were associated with baseline brachial diameter and baseline mean flow velocity in age- and sex-adjusted models. However, adjustment for clinical covariates rendered these associations non-significant, suggesting that the associations of growth factors with baseline measures of vascular structure and function may be mediated via their relations to other risk factors.
Comparison with the literature
Sexual dimorphism of circulating vascular endothelial growth factor, hepatocyte growth factor, and sFlt-1
Sexual dimorphism for vascular growth factors has previously been reported in a relatively small case–control study of obese vs. non-obese individuals.26 In that study, circulating VEGF-C, VEGF-D, and angiopoetin-2 were significantly higher in women.26 Yamamoto et al.21 observed no association of plasma HGF with sex. Recently, one study reported higher VEGF in men,27 whereas another observed increased concentrations in women.28 Our results are in agreement with the latter findings. We observed significantly higher VEGF and HGF and lower sFlt-1 in women.
The mechanisms for these sex-related differences in circulating growth factors are not clear, but experimental studies suggest that sex hormones may modify vascular growth factor levels. Endogenous estrogens have vasculo-protective effects, in part by regulating VEGF expression.29 For example, estrogen modulates VEGF and sFlt-1 expression in the endometrium,30 in cancer cells,31 and in vascular smooth muscle cells in vitro.32 Estrogens also increased HGF production.33 It is conceivable, therefore, that higher endogenous estrogen contributes to increased VEGF and HGF in women.
Association of vascular endothelial growth factor, sFlt-1, and hepatocyte growth factor with cardiovascular disease risk factors
The strong positive associations of VEGF and HGF concentrations with obesity and presence of the MetS are consistent with prior reports.16,34 Statistically significant correlations have been reported between VEGF or HGF and lipid levels/dyslipidaemia in some27,34,35 but not in other studies.21 Importantly, most prior studies had relatively small samples, limiting their statistical power to detect modest associations. In our large community-based sample, we noticed a borderline significant positive association of VEGF with triglycerides, a significant positive association of HGF with triglycerides, and a significant negative association of HGF with HDL cholesterol. We also observed strong associations between biomarkers and smoking. VEGF and HGF were higher in smokers, whereas sFlt-1 levels were lower. These results are in agreement with most27,36 but not all previous studies.37,38
Previous clinical studies reported higher growth factors in hypertensives.9,15 In agreement with these results, we observed positive associations of VEGF and HGF concentrations with hypertension, and with BP variables modelled as continuous traits.
Finally, we observed a positive association of HGF and sFlt-1 levels with age. This is consistent with a prior report by Yamamoto et al.21
Although the different biomarkers were in part related to the same clinical correlates (e. g. BP and BMI), the correlation between the biomarkers themselves was rather low. This might be explained by the fact that traits like BP or BMI are influenced by multiple mechanisms.
Furthermore, the lack of correlation between serum levels of the three biomarkers is intriguing, but it does not negate potential correlations among the markers at the tissue level.
Possible mechanisms for association of vascular endothelial growth factor and hepatocyte growth factor with cardiovascular disease risk factors
Increasing evidence links vascular growth factors to CV risk factors, subclinical, and overt atherosclerotic disease. Clinical studies observed elevated circulating VEGF and HGF in patients with various forms of CVD.10–12,17,19 Furthermore, growth factor levels have been associated with subclinical atherosclerotic disease.18,21 It is controversial, however, whether vascular growth factors have a proatherogenic or a vasculoprotective net effect.39
HGF and VEGF and their receptors are found in atherosclerotic lesions,40–42 and VEGF promoted plaque progression in animal models.43 Other studies support a vasculoprotective role of VEGF44 and VEGF gene transfer has been discussed as a new treatment option for ischaemic heart disease.45
Our data indicate that circulating growth factors are related to several CVD risk factors and thus underscore that these biomarkers might play an important role in atherosclerotic diseases, either as disease markers or as contributors to the disease process. In particular, we found a prominent positive association of circulating HGF and VEGF with obesity and the MetS. Production of both biomarkers within the adipose tissue16,46 may explain this finding. An alternative possibility is that vascular growth factors may play a key role in the regulation of adipogenesis.47
We also observed a positive association of HGF and VEGF with hypertension. Impaired vasculogenesis has been proposed as a contributing factor to hypertension and VEGF application has been reported to induce hypotension in animal models48 and in clinical trials.49 Furthermore, hypertension is a known side-effect of VEGF-pathway inhibitors, e. g. in the setting of different cancer therapies.50,51 Overall, these data are consistent with the notion that VEGF may be a marker for hypertension, with higher circulating levels representing a response to high systemic BP. The observed association between all three biomarkers and smoking may be because smoking promotes atherosclerosis/inflammation, also known to increase growth factor expression.52 Of note, ageing is associated with modifications in the vessel wall, which are in many ways mechanistically comparable to changes in atherosclerosis.53 This might explain the associations of sFlt-1 and HGF levels with age.
Heritability of vascular endothelial growth factor, sFlt-1, and hepatocyte growth factor
To the best of our knowledge, this is the first study to report heritability estimates for sFlt-1 and HGF. The heritability for sFlt-1 was relatively low, that of HGF was moderate. In contrast, VEGF displayed substantial heritability. Consistent with our data, two previous studies reported very high heritability for VEGF.54,55 Overall, these data are consistent with the notion that angiogenesis/collateral formation is a heritable phenotype.
Association of vascular endothelial growth factor, sFlt1, and hepatocyte growth factor with vascular function
Data on the association of vascular growth factors with vascular function are relatively limited. Flow-mediated dilation was impaired in a small series of hypertensive patients and correlated negatively with circulating VEGF.56 Schmidt-Lucke et al.,38 similarly found an inverse correlation between circulating VEGF and endothelial function.
To our knowledge, relations of circulating VEGF, sFlt-1, and HGF to indices of vascular function in the community have not been reported. We observed an association of VEGF and HGF with baseline measures of vascular structure (BA diameter) and function (BA mean flow velocity). Interestingly, these associations were attenuated upon adjustment for other clinical covariates, suggesting that the association between VEGF and HGF and vascular structure and function may be mediated via these risk factors.
Limitations
Limitations of our investigation include the cross-sectional design. Also, the sample was predominantly white, which limits the generalizability of our findings. Given that platelets are an important source of VEGF, it would be desirable to adjust levels for platelet counts. We were unable to do so because platelet counts were not measured in our samples. Each biomarker was measured only on a single occasion in each participant, which might have led to some random misclassification biasing our results towards the null hypothesis. Due to the exploratory character of the present analyses, we did not adjust for multiple testing and acknowledge that our findings require confirmation in other cohorts.
Conclusions
In our investigation of a large community-based sample, we observed that VEGF, its soluble receptor sFlt-1, and HGF are heritable. VEGF and HGF display associations with established vascular risk factors, including the cluster defining the MetS. Additional studies are warranted to confirm these findings.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported through NIH/NHLBI contract N01-HC-25195, RO1 HL 077477, and HL K24 04334 (R.S.V.) and RO1 HL70100 (E.J.B.).
Conflict of interest: G.F.M. is owner of Cardiovascular Engineering Inc., a company that designs and manufactures devices that measure vascular stiffness.
Supplementary Material
References
- 1.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y. Heart disease and stroke statistics–2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Gibbons GH, Dzau VJ. The emerging concept of vascular remodeling. N Engl J Med. 1994;330:1431–1438. doi: 10.1056/NEJM199405193302008. [DOI] [PubMed] [Google Scholar]
- 3.Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 4.Yla-Herttuala S, Rissanen TT, Vajanto I, Hartikainen J. Vascular endothelial growth factors: biology and current status of clinical applications in cardiovascular medicine. J Am Coll Cardiol. 2007;49:1015–1026. doi: 10.1016/j.jacc.2006.09.053. [DOI] [PubMed] [Google Scholar]
- 5.Hood JD, Meininger CJ, Ziche M, Granger HJ. VEGF upregulates ecNOS message, protein, and NO production in human endothelial cells. Am J Physiol. 1998;274:H1054–H1058. doi: 10.1152/ajpheart.1998.274.3.H1054. [DOI] [PubMed] [Google Scholar]
- 6.Neagoe PE, Lemieux C, Sirois MG. Vascular endothelial growth factor (VEGF)-A165-induced prostacyclin synthesis requires the activation of VEGF receptor-1 and -2 heterodimer. J Biol Chem. 2005;280:9904–9912. doi: 10.1074/jbc.M412017200. [DOI] [PubMed] [Google Scholar]
- 7.Bates DO, Hillman NJ, Williams B, Neal CR, Pocock TM. Regulation of microvascular permeability by vascular endothelial growth factors. J Anat. 2002;200:581–597. doi: 10.1046/j.1469-7580.2002.00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 9.Belgore FM, Blann AD, Li-Saw-Hee FL, Beevers DG, Lip GY. Plasma levels of vascular endothelial growth factor and its soluble receptor (SFlt-1) in essential hypertension. Am J Cardiol. 2001;87:805–807. doi: 10.1016/s0002-9149(00)01512-5. A9. [DOI] [PubMed] [Google Scholar]
- 10.Blann AD, Belgore FM, McCollum CN, Silverman S, Lip PL, Lip GY. Vascular endothelial growth factor and its receptor, Flt-1, in the plasma of patients with coronary or peripheral atherosclerosis, or Type II diabetes. Clin Sci (Lond) 2002;102:187–194. [PubMed] [Google Scholar]
- 11.Hojo Y, Ikeda U, Zhu Y, Okada M, Ueno S, Arakawa H, Fujikawa H, Katsuki T, Shimada K. Expression of vascular endothelial growth factor in patients with acute myocardial infarction. J Am Coll Cardiol. 2000;35:968–973. doi: 10.1016/s0735-1097(99)00632-4. [DOI] [PubMed] [Google Scholar]
- 12.Chin BS, Chung NA, Gibbs CR, Blann AD, Lip GY. Vascular endothelial growth factor and soluble P-selectin in acute and chronic congestive heart failure. Am J Cardiol. 2002;90:1258–1260. doi: 10.1016/s0002-9149(02)02848-5. [DOI] [PubMed] [Google Scholar]
- 13.Shibuya M. Vascular endothelial growth factor receptor-1 (VEGFR-1/Flt-1): a dual regulator for angiogenesis. Angiogenesis. 2006;9:225–230. doi: 10.1007/s10456-006-9055-8. [DOI] [PubMed] [Google Scholar]
- 14.Zarnegar R, Michalopoulos GK. The many faces of hepatocyte growth factor: from hepatopoiesis to hematopoiesis. J Cell Biol. 1995;129:1177–1180. doi: 10.1083/jcb.129.5.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morishita R, Moriguchi A, Higaki J, Ogihara T. Hepatocyte growth factor (HGF) as a potential index of severity of hypertension. Hypertens Res. 1999;22:161–167. doi: 10.1291/hypres.22.161. [DOI] [PubMed] [Google Scholar]
- 16.Rehman J, Considine RV, Bovenkerk JE, Li J, Slavens CA, Jones RM, March KL. Obesity is associated with increased levels of circulating hepatocyte growth factor. J Am Coll Cardiol. 2003;41:1408–1413. doi: 10.1016/s0735-1097(03)00231-6. [DOI] [PubMed] [Google Scholar]
- 17.Soeki T, Tamura Y, Shinohara H, Tanaka H, Bando K, Fukuda N. Role of circulating vascular endothelial growth factor and hepatocyte growth factor in patients with coronary artery disease. Heart Vessels. 2000;15:105–111. doi: 10.1007/pl00007263. [DOI] [PubMed] [Google Scholar]
- 18.Malatino LS, Cataliotti A, Benedetto FA, Stancanelli B, Bellanuova I, Belluardo P, Bonaiuto L, Tripepi G, Mallamaci F, Castellino P, Zoccali C. Hepatocyte growth factor and left ventricular geometry in end-stage renal disease. Hypertension. 2003;41:88–92. doi: 10.1161/01.hyp.0000046919.41112.4b. [DOI] [PubMed] [Google Scholar]
- 19.Lamblin N, Susen S, Dagorn J, Mouquet F, Jude B, Van Belle E, Bauters C, de Groote P. Prognostic significance of circulating levels of angiogenic cytokines in patients with congestive heart failure. Am Heart J. 2005;150:137–143. doi: 10.1016/j.ahj.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 20.Komai N, Ohishi M, Morishita R, Moriguchi A, Kaibe M, Matsumoto K, Rakugi H, Higaki J, Ogihara T. Serum hepatocyte growth factor concentration is correlated with the forearm vasodilator response in hypertensive patients. Am J Hypertens. 2002;15:499–506. doi: 10.1016/s0895-7061(02)02274-4. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto Y, Kohara K, Tabara Y, Miki T. Association between carotid arterial remodeling and plasma concentration of circulating hepatocyte growth factor. J Hypertens. 2001;19:1975–1979. doi: 10.1097/00004872-200111000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 23.Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D'Agostino RB, Sr, Fox CS, Larson MG, Murabito JM, O'Donnell CJ, Vasan RS, Wolf PA, Levy D. The Third Generation Cohort of the National Heart, Lung, and Blood Institute's Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- 24.Kathiresan S, Gona P, Larson MG, Vita JA, Mitchell GF, Tofler GH, Levy D, Newton-Cheh C, Wang TJ, Benjamin EJ, Vasan RS. Cross-sectional relations of multiple biomarkers from distinct biological pathways to brachial artery endothelial function. Circulation. 2006;113:938–945. doi: 10.1161/CIRCULATIONAHA.105.580233. [DOI] [PubMed] [Google Scholar]
- 25.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 26.Silha JV, Krsek M, Sucharda P, Murphy LJ. Angiogenic factors are elevated in overweight and obese individuals. Int J Obes (Lond) 2005;29:1308–1314. doi: 10.1038/sj.ijo.0802987. [DOI] [PubMed] [Google Scholar]
- 27.Kimura K, Hashiguchi T, Deguchi T, Horinouchi S, Uto T, Oku H, Setoyama S, Maruyama I, Osame M, Arimura K. Serum VEGF–as a prognostic factor of atherosclerosis. Atherosclerosis. 2007;194:182–188. doi: 10.1016/j.atherosclerosis.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 28.Malamitsi-Puchner A, Tziotis J, Tsonou A, Protonotariou E, Sarandakou A, Creatsas G. Changes in serum levels of vascular endothelial growth factor in males and females throughout life. J Soc Gynecol Investig. 2000;7:309–312. [PubMed] [Google Scholar]
- 29.Rubanyi GM, Johns A, Kauser K. Effect of estrogen on endothelial function and angiogenesis. Vascul Pharmacol. 2002;38:89–98. doi: 10.1016/s0306-3623(02)00131-3. [DOI] [PubMed] [Google Scholar]
- 30.Albrecht ED, Babischkin JS, Lidor Y, Anderson LD, Udoff LC, Pepe GJ. Effect of estrogen on angiogenesis in co-cultures of human endometrial cells and microvascular endothelial cells. Hum Reprod. 2003;18:2039–2047. doi: 10.1093/humrep/deg415. [DOI] [PubMed] [Google Scholar]
- 31.Garvin S, Nilsson UW, Dabrosin C. Effects of oestradiol and tamoxifen on VEGF, soluble VEGFR-1, and VEGFR-2 in breast cancer and endothelial cells. Br J Cancer. 2005;93:1005–1010. doi: 10.1038/sj.bjc.6602824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang DH, Yu ES, Yoon KI, Johnson R. The impact of gender on progression of renal disease: potential role of estrogen-mediated vascular endothelial growth factor regulation and vascular protection. Am J Pathol. 2004;164:679–688. doi: 10.1016/S0002-9440(10)63155-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang HZ, Bennett JM, Smith KT, Sunil N, Haslam SZ. Estrogen mediates mammary epithelial cell proliferation in serum-free culture indirectly via mammary stroma-derived hepatocyte growth factor. Endocrinology. 2002;143:3427–3434. doi: 10.1210/en.2002-220007. [DOI] [PubMed] [Google Scholar]
- 34.Hiratsuka A, Adachi H, Fujiura Y, Yamagishi S, Hirai Y, Enomoto M, Satoh A, Hino A, Furuki K, Imaizumi T. Strong association between serum hepatocyte growth factor and metabolic syndrome. J Clin Endocrinol Metab. 2005;90:2927–2931. doi: 10.1210/jc.2004-1588. [DOI] [PubMed] [Google Scholar]
- 35.Blann AD, Belgore FM, Constans J, Conri C, Lip GY. Plasma vascular endothelial growth factor and its receptor Flt-1 in patients with hyperlipidemia and atherosclerosis and the effects of fluvastatin or fenofibrate. Am J Cardiol. 2001;87:1160–1163. doi: 10.1016/s0002-9149(01)01486-2. [DOI] [PubMed] [Google Scholar]
- 36.Yamanouchi H, Fujita J, Yoshinouchi T, Hojo S, Kamei T, Yamadori I, Ohtsuki Y, Ueda N, Takahara J. Measurement of hepatocyte growth factor in serum and bronchoalveolar lavage fluid in patients with pulmonary fibrosis. Respir Med. 1998;92:273–278. doi: 10.1016/s0954-6111(98)90108-1. [DOI] [PubMed] [Google Scholar]
- 37.Belgore FM, Lip GY, Blann AD. Vascular endothelial growth factor and its receptor, Flt-1, in smokers and non-smokers. Br J Biomed Sci. 2000;57:207–213. [PubMed] [Google Scholar]
- 38.Schmidt-Lucke C, Belgore F, Reinhold D, Ansorge S, Klein HU, Schmidt-Lucke JA, Lip GY. Soluble vascular endothelial growth factor, soluble VEGF receptor Flt-1 and endothelial function in healthy smokers. Int J Cardiol. 2005;100:207–212. doi: 10.1016/j.ijcard.2004.05.046. [DOI] [PubMed] [Google Scholar]
- 39.Khurana R, Simons M, Martin JF, Zachary IC. Role of angiogenesis in cardiovascular disease: a critical appraisal. Circulation. 2005;112:1813–1824. doi: 10.1161/CIRCULATIONAHA.105.535294. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, Wilkinson FL, Kirton JP, Jeziorska M, Iizasa H, Sai Y, Nakashima E, Heagerty AM, Canfield AE, Alexander MY. Hepatocyte growth factor and c-Met expression in pericytes: implications for atherosclerotic plaque development. J Pathol. 2007;212:12–19. doi: 10.1002/path.2155. [DOI] [PubMed] [Google Scholar]
- 41.Morsi WG, Shaker OG, Ismail EF, Ahmed HH, El Serafi TI, Maklady FA, Abdel-Aziz MT, El Asmar MF, Atta HM. HO-1 and VGEF gene expression in human arteries with advanced atherosclerosis. Clin Biochem. 2006;39:1057–1062. doi: 10.1016/j.clinbiochem.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 42.Inoue M, Itoh H, Ueda M, Naruko T, Kojima A, Komatsu R, Doi K, Ogawa Y, Tamura N, Takaya K, Igaki T, Yamashita J, Chun TH, Masatsugu K, Becker AE, Nakao K. Vascular endothelial growth factor (VEGF) expression in human coronary atherosclerotic lesions: possible pathophysiological significance of VEGF in progression of atherosclerosis. Circulation. 1998;98:2108–2116. doi: 10.1161/01.cir.98.20.2108. [DOI] [PubMed] [Google Scholar]
- 43.Celletti FL, Waugh JM, Amabile PG, Brendolan A, Hilfiker PR, Dake MD. Vascular endothelial growth factor enhances atherosclerotic plaque progression. Nat Med. 2001;7:425–429. doi: 10.1038/86490. [DOI] [PubMed] [Google Scholar]
- 44.Zachary I, Mathur A, Yla-Herttuala S, Martin J. Vascular protection: A novel nonangiogenic cardiovascular role for vascular endothelial growth factor. Arterioscler Thromb Vasc Biol. 2000;20:1512–1520. doi: 10.1161/01.atv.20.6.1512. [DOI] [PubMed] [Google Scholar]
- 45.Losordo DW, Vale PR, Symes JF, Dunnington CH, Esakof DD, Maysky M, Ashare AB, Lathi K, Isner JM. Gene therapy for myocardial angiogenesis: initial clinical results with direct myocardial injection of phVEGF165 as sole therapy for myocardial ischemia. Circulation. 1998;98:2800–2804. doi: 10.1161/01.cir.98.25.2800. [DOI] [PubMed] [Google Scholar]
- 46.Zhang QX, Magovern CJ, Mack CA, Budenbender KT, Ko W, Rosengart TK. Vascular endothelial growth factor is the major angiogenic factor in omentum: mechanism of the omentum-mediated angiogenesis. J Surg Res. 1997;67:147–154. doi: 10.1006/jsre.1996.4983. [DOI] [PubMed] [Google Scholar]
- 47.Cao Y. Angiogenesis modulates adipogenesis and obesity. J Clin Invest. 2007;117:2362–2368. doi: 10.1172/JCI32239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li B, Ogasawara AK, Yang R, Wei W, He GW, Zioncheck TF, Bunting S, de Vos AM, Jin H. KDR (VEGF receptor 2) is the major mediator for the hypotensive effect of VEGF. Hypertension. 2002;39:1095–1100. doi: 10.1161/01.hyp.0000018588.56950.7a. [DOI] [PubMed] [Google Scholar]
- 49.Henry TD, Annex BH, McKendall GR, Azrin MA, Lopez JJ, Giordano FJ, Shah PK, Willerson JT, Benza RL, Berman DS, Gibson CM, Bajamonde A, Rundle AC, Fine J, McCluskey ER. The VIVA trial: Vascular endothelial growth factor in Ischemia for Vascular Angiogenesis. Circulation. 2003;107:1359–1365. doi: 10.1161/01.cir.0000061911.47710.8a. [DOI] [PubMed] [Google Scholar]
- 50.Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX, Rosenberg SA. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prat A, Casado E, Cortes J. New approaches in angiogenic targeting for colorectal cancer. World J Gastroenterol. 2007;13:5857–5866. doi: 10.3748/wjg.v13.i44.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hatakeyama M, Imaizumi T, Sakaki H, Yoshida H, Tanaka H, Kimura H, Fukuda I, Satoh K. Interleukin-1 induces the expression of vascular endothelial growth factor in human pericardial mesothelial cells. Heart Vessels. 2007;22:123–127. doi: 10.1007/s00380-006-0942-0. [DOI] [PubMed] [Google Scholar]
- 53.Ferrari AU, Radaelli A, Centola M. Invited review: aging and the cardiovascular system. J Appl Physiol. 2003;95:2591–2597. doi: 10.1152/japplphysiol.00601.2003. [DOI] [PubMed] [Google Scholar]
- 54.Pantsulaia I, Trofimov S, Kobyliansky E, Livshits G. Heritability of circulating growth factors involved in the angiogenesis in healthy human population. Cytokine. 2004;27:152–158. doi: 10.1016/j.cyto.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 55.Berrahmoune H, Herbeth B, Lamont JV, Masson C, Fitzgerald PS, Visvikis-Siest S. Heritability for plasma VEGF concentration in the Stanislas family study. Ann Hum Genet. 2007;71:54–63. doi: 10.1111/j.1469-1809.2006.00298.x. [DOI] [PubMed] [Google Scholar]
- 56.Tsai WC, Li YH, Huang YY, Lin CC, Chao TH, Chen JH. Plasma vascular endothelial growth factor as a marker for early vascular damage in hypertension. Clin Sci (Lond) 2005;109:39–43. doi: 10.1042/CS20040307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.