Abstract
Aims
Two-dimensional echocardiographic (2DE) continuity-equation derived aortic valve area (AVA) in aortic stenosis (AS) relies on non-simultaneous measurement of left ventricular outflow tract (LVOT) velocity and geometric assumptions of LVOT area, which can amplify error, especially in upper septal hypertrophy (USH). We hypothesized that real-time three-dimensional echocardiography (RT3DE) can improve accuracy of AVA by directly measuring LVOT stroke volume (SV) in one window.
Methods and results
RT3DE colour Doppler and 2DE were acquired in 68 AS patients (74 ± 12 yrs) prospectively. SV was derived from flow obtained from a sampling curve placed orthogonal to LVOT (Tomtec Imaging). Agreement between continuity-equation derived AVA by RT3DE (AVA3D-SV) and 2DE (AVA2D) and predictors of discrepancies were analysed. Validation of LVOT SV was performed by aortic flow probe in a sheep model with balloon inflation of septum to mimic USH. There was only modest correlation between AVA2D and AVA3D-SV (r = 0.71, difference 0.11 ± 0.23 cm2). The degree of USH was significantly associated with difference in AVA calculation (r = 0.4, P = 0.005). In experimentally distorted LVOT geometry in sheep, RT3DE correlated better with flow probe assessment (r = 0.96, P < 0.001) than 2DE (r = 0.71, P = 0.006).
Conclusion
RT3DE colour Doppler-derived LVOT SV in the calculation of AVA by continuity equation is more accurate than 2D, including in situations such as USH, common in the elderly, which modify LVOT geometry.
Keywords: Aortic stenosis, Real-time three-dimensional echocardiography, Colour Doppler, Valvular heart disease, Continuity equation
Introduction
Aortic stenosis is assessed routinely using the continuity equation which, based on the principle of conservation of mass, equates stroke volume (SV) proximal to the aortic valve (left ventricular outflow tract, LVOT) to SV through the stenotic aortic valve orifice.1 Using conventional two-dimensional echocardiography (2DE),2–4 the SV at the LVOT is obtained by measuring the diameter across the LVOT (to calculate its cross-sectional area, assuming it is circular) and stroke distance (from time velocity integral by pulse-wave Doppler). The validation and accuracy of LVOT SV calculation of aortic valve area (AVA) using continuity equation is most subjected to assumptions on geometry and uniform velocity, unlike the relatively simple continuous wave Doppler measurement used for the SV measurement at the level of the aortic valve. Hence, LVOT SV calculation is also most susceptible to error. 2D continuity method makes two important assumptions regarding flow across the LVOT. One, it assumes that flow is of uniform velocity in the LVOT and second, the LVOT geometry is assumed to be circular. These assumptions may not be valid where LVOT geometry is irregular secondary to upper septal hypertrophy (USH) or prominent annular calcification, which extends into the LVOT. In addition, uniform flow velocities are not present in settings of increased and non-laminar velocities such as might occur with hyperdynamic function or anatomic obstruction in the LVOT. Because the diameter of the LVOT is squared to determine area, small errors in measuring this linear dimension are amplified and are an important source of inaccuracy. Factors contributing to errors include image quality, annular calcification, non-circular annulus, failure to measure true diameter and the presence of USH. Furthermore, simultaneous measurements, in time and sometimes in location, of the LVOT flow and its area are not obtained by 2DE method.
The development of real-time three-dimensional echocardiography (RT3DE) can circumvent the limitations of 2DE by directly measuring LVOT SV in one imaging window without the need for geometric assumptions.5,6 We hypothesized that RT3DE improves accuracy of the AVA calculation in AS by directly measuring LVOT SV in one window.
Methods
Study population
We prospectively recruited adult patients with varying degrees of AS and USH. Exclusion criteria included poor echocardiographic imaging windows and presence of subvalvular or supravalvular aortic stenosis. The protocol was approved by the hospital Institutional Review Board.
Echocardiography
Transthoracic echocardiography was performed by experienced sonographers using Sonos IE33 machines (Philips Medical Systems, Andover, MA, USA). 2DE was done using an s3 probe and 3DE was done using x3 probe.
In addition to 2DE examination, peak and mean aortic velocities were obtained by continuous-wave Doppler echocardiography from a multiwindow approach and corresponding pressure gradients calculated using the modified Bernoulli equation. AVA was determined by continuity equation (AVA2D). Concomitant significant pathologies including aortic or mitral regurgitation (categorized into grades I–IV) and left ventricular systolic dysfunction were documented. The ratio of upper septal wall thickness to posterior wall thickness in diastole was measured to determine degree of USH. USH was considered present if the ratio was ≥ 1.3.
Apical 3DE colour Doppler volumes incorporating the LVOT and aortic valve were acquired with electrocardiographic gating and suspended respiration (over four beats). Nyquist limit and frame rate were maximized and depth setting adjusted to a minimum but keeping the aortic valve in view.
In addition, RT3D full volume datasets of the aortic valve in the parasternal window was obtained in a subgroup of patients and the pyramidal dataset cropped to determine the anatomical orifice.
Animal model validation
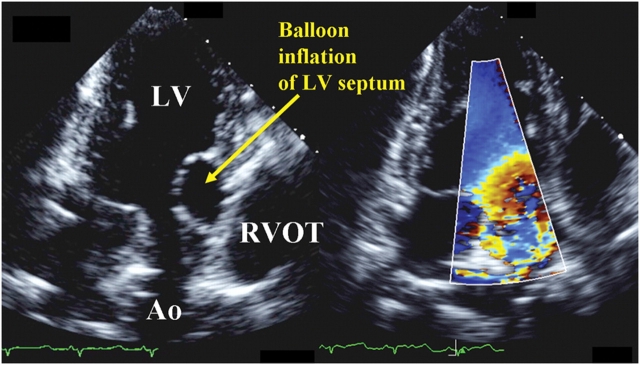
Three Dorsett sheep were intubated, ventilated, and studied under open-chested conditions. Anaesthesia was maintained with 2% isofluorane and oxygen. A custom-made balloon attached to syringe and inflatable to different size with saline was surgically inserted through the epicardium into the myocardium of the upper ventricular septum, as guided by echocardiography.7 This was performed to vary the LVOT geometry, mimicking the clinical spectrum of USH (Figure 1). Cardiac output (CO) was measured by placing an electromagnetic flow probe around the aortic root5 to provide mean flow rate on a beat-to-beat basis. A CO value (L/min) was recorded for each 2DE and 3DE acquisition. Echocardiographic windows at apical long axis were acquired with epicardial probes. Different CO was achieved with changing preload (by intravenous fluid challenges and inferior vena cava clamping) and pharmacologically. The institutional animal care and use committee approved all procedures.
Figure 1.
Epicardial echocardiograms of a sheep at the apical three-chamber window showing balloon inflation of the left ventricular (LV) septum at the LV outflow tract (OT), mimicking clinical upper septal hypertrophy and distorting the LVOT geometry (left panel). Colour Doppler revealed non-uniform flow across the LVOT (right panel). RV, right ventricle; Ao, Aorta.
Flow volume calculation
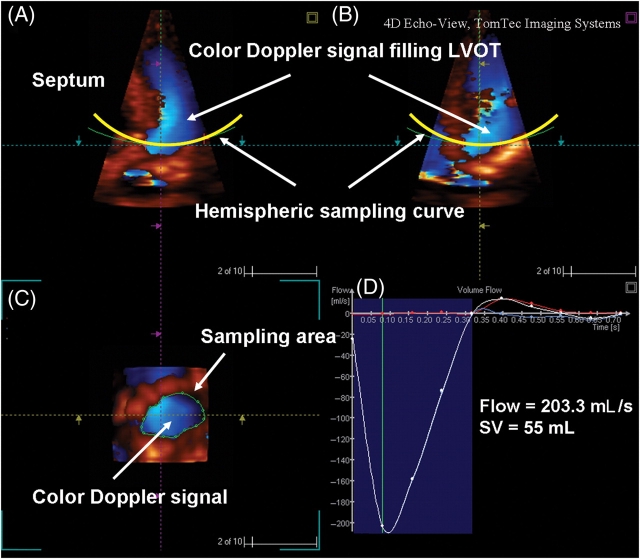
3D Doppler data were transferred to a laptop for flow volume computation using 4D Echo-View, TomTec Imaging Systems, Unterschleissheim, Germany and validation of this system has been previously described.5,6 Briefly, the cut plane of the 3D volume was moved in two perpendicular longitudinal B-planes, rotated and sliced to ensure the LVOT, its colour Doppler and the aortic valve were clearly seen. A hemispheric Gaussian sampling curve was placed on these orthogonal images proximal to the aortic valve and perpendicular to the direction of flow (Figure 2A and B).
Figure 2.
(A and B) Three-dimensional projections showing colour Doppler filling the LVOT. Sampling curve captured velocity vectors perpendicular to the hemispheric concave surface. (C) Region of interest was traced and the Tomtek software automatically calculated the forward flow in systole over time. (D) Derivation of stroke volume (SV) was obtained by integration of area under the flow-vs.-time curve. Cardiac output was calculated from the product of SV and heart rate.
The tissue/colour Doppler display priority of the dataset was set so that colour Doppler signal filled the LVOT without colour bleed into the tissues. For Doppler aliasing in the LVOT, baseline was shifted so that these velocities were included in the volume calculation. The software then projected the velocity data for each frame to give a colour image of the LVOT at the level of the sampling curve. The hemispheric umbrella captured colour velocities within and perpendicular to the LVOT, with its concavity towards the LV cavity. A sampling area was manually traced around the LVOT ensuring colour signal was included for each frame of the cardiac cycle (Figure 2C). Often, the sampling area was drawn just once for each measurement. This was because all the colour velocities were encompassed within the initial sampling area. Otherwise the region of interest could be adjusted as necessary. The LVOT cross-sectional shape was classified as circular, oval, or irregular (shapes that did not fit into circular or oval geometry). The software automatically read the retained velocity assignments in DICOM data within the identified region of interest and computed the flow velocity profile, SV, and CO over the projected Gaussian surface (Figure 2D).
Real-time three-dimensional echocardiographic aortic valve area measurement
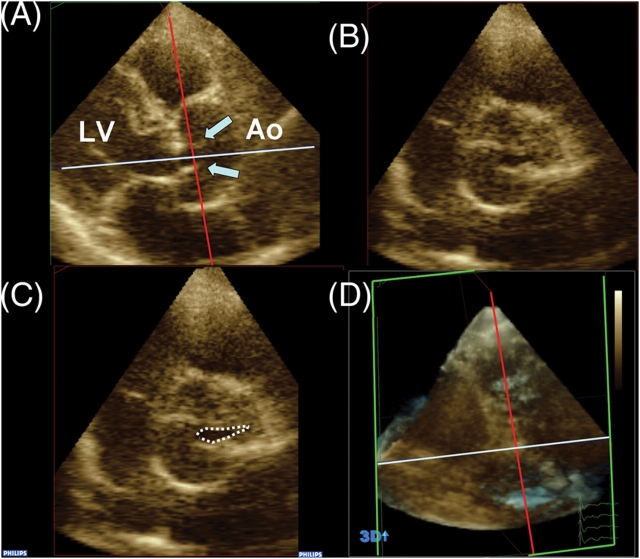
Continuity-equation derived AVA using SV from 3DE (AVA3D-SV) was obtained by dividing by the time-velocity integral of continuous wave Doppler profile. Directly planimetered values (AVA3D-Pl) were measured using cropping methods (Qlab, Philips Medical systems, Andover, MA, USA) to align short-axis plane along the narrowest orifice in systole, as determined in the orthogonal long-axis plane (Figure 3).
Figure 3.
Real-time three-dimensional echocardiographic aortic valve area (AVA) by planimetry. Cropping allows alignment of short-axis plane (B and C) at the narrowest orifice as visualized in long axis plane (A). Arrows point to the aortic valve leaflets in systole. Cropping planes are shown in 3D set for reference (D). LV, left ventricle; Ao, Ascending Aorta.
Statistical analysis
Normality of the distribution for each numerical variable was checked with the Shapiro–Wilk test. Continuous variables were presented as mean ± SD for normally distributed data and median with interquartile range if variables were non-normally distributed. Correlations between echocardiographic variables were assessed using Pearson’s correlation coefficient r. Agreements between methods (AVA2D vs. AVA3D-Pl; AVA3D-SV vs. AVA3D-Pl; 2DE vs. flow probe CO; 3DE vs. flow probe CO) were tested by Bland–Altman analyses8 and plotted with lines representing mean ± 2SD. Discrepancies, i.e. differences between AVA2D and AVA3D-SV were correlated to clinical predictors using Pearson’s r when they were continuous variables and analysed using one-way ANOVAs or Kruskal–Wallis tests for categorical variables. A two-sided P-value of < 0.05 was considered significant. SPSS for Windows (version 13.0, SPSS Inc., Chicago, IL, USA) was used.
Intra-observer and inter-observer variability
The datasets from 10 randomly selected patients were analysed by the first operator (K.K.P.) 3 months after the first analyses and by second operator (J.S.) who was blinded to the results of the first operator and other echocardiographic or clinical data. Intra- and inter-observer variability were assessed by intra-class correlation coefficients9 with 95% confidence intervals using SPSS reliability analyses.
Results
Seventy-three patients were initially assessed for inclusion: 5 were ineligible due to poor imaging windows or decline consent and 68 were included. Three-dimensional colour Doppler acquisitions were obtained in all 68 patients, whereas RT3DE full volume acquisitions were obtained in 47 random patients. Of these 47 cases, 38 were technically adequate for 3D cropping. Baseline demographics and selected echocardiographic characteristics were listed in Table 1. The mean LVOT SV by RT3DE was 68 ± 21 mL while the mean AVA3D-SV and AVA2D was 0.88 ± 0.30 and 0.99 ± 0.30 cm2, respectively. The cross-sectional shape of LVOT as viewed enface (obtained by cropping the RT3DE dataset), appeared circular, oval, and irregular in 29 (43%), 26 (38%), and 13 (19%) of the cases, respectively.
Table 1.
Clinical and selected echocardiographic variables in 68 patients with variable degrees of aortic valve stenosis
| Variable | n (%) or mean ± SD |
|---|---|
| Age (years) | 74 ± 12 |
| Male gender | 47 (69%) |
| Regular heart rhythm | 57 (84%) |
| Heart rate (b.p.m.) | 69 ± 16 |
| Left ventricular ejection fraction (%) | 60 ± 17 |
| Degenerative aortic valve stenosis (tri-leaflet) | 60 (88%) |
| Aortic valve area by 2D echocardiography (cm2) | 0.99 ± 0.30 |
| Peak pressure gradient across aortic valve (mmHg) | 53 ± 27 |
| Mean pressure gradient across aortic valve (mmHg) | 31 ± 16 |
| Septal wall: posterior wall ratio | 1.2 ± 0.2 |
| Concomitant aortic regurgitation | |
| Grade I (none/trivial) | 38 (56%) |
| Grade II (mild) | 20 (44%) |
| Grade III (moderate) | 10 (15%) |
| Grade IV (severe) | 0 (0%) |
| Concomitant mitral regurgitation | |
| Grade I (none/trivial) | 24 (35%) |
| Grade II (mild) | 24 (35%) |
| Grade III (moderate) | 17 (25%) |
| Grade IV (severe) | 3 (5%) |
Correlations between AVA3D-SV, AVA3D-Pl, and AVA2D
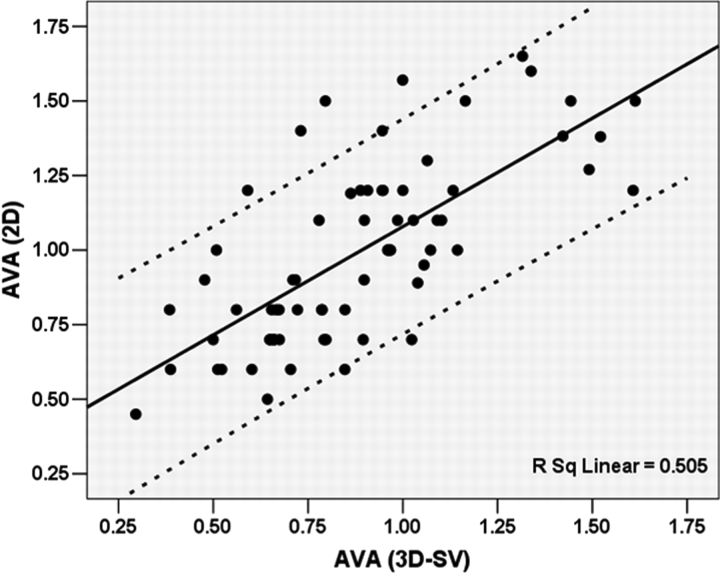
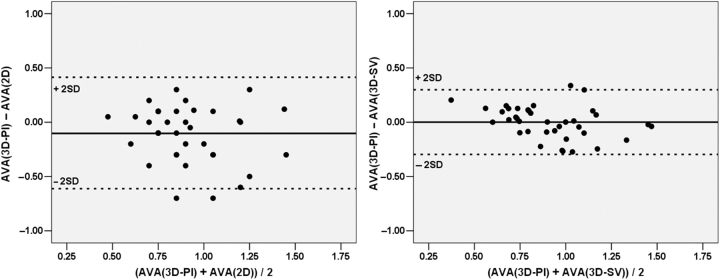
Correlation between AVA2D and AVA3D-SV for the entire cohort was modest (r = 0.71, P < 0.001, Figure 4; mean difference 0.11 ± 0.23 cm2). In a subgroup of 38 cases, AVA3D-Pl was compared to AVA2D and AVA3D-SV. AVA3D-SV correlated better with AVA3D-Pl than AVA2D (r = 0.81 vs. 0.51, z = −2.4, P = 0.018). In addition, AVA3D-SV agreed better with AVA3D-Pl than AVA2D (Figure 5; mean difference = −0.001 ± 0.15 vs. −0.10 ± 0.26 cm2, P = 0.038).
Figure 4.
Scatter plot of correlation between aortic valve area calculated from two-dimensional echocardiography derived continuity equation [AVA(2D)] and that obtained from real-time three-dimensional stroke volume quantification [AVA(3D-SV)]. Dashed lines represent 95% prediction band of the regression line.
Figure 5.
Bland–Altman analysis showing better agreement between aortic valve area calculated from three-dimensional continuity [AVA(3D-SV)] and three-dimensional planimetry (AVA(3D-Pl) (on the right panel) than that between aortic valve area from two-dimensional continuity [AVA(2D)] and planimetered area (left panel). Dashed lines represent ±2SD from the mean.
Discrepancies between AVA3D-SV and AVA2D
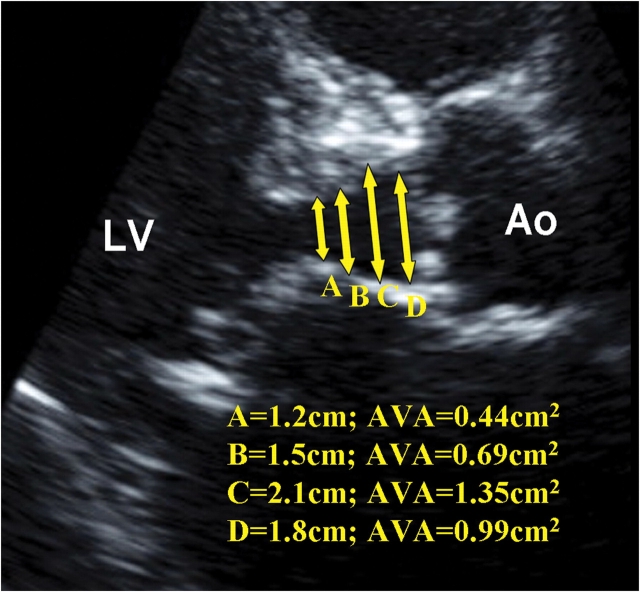
There was mild but significant correlation of the differences between AVA2D and AVA3D-SV with the ratio of upper septal wall thickness to posterior wall thickness in diastole (r = 0.36, P = 0.005) but not with age, heart rate, LV ejection fraction, and indices of aortic valve severity (all P > 0.14) (Table 2). Figure 6 illustrated an example of how USH may affect measurement of LVOT diameter resulting in erroneous calculation of AVA2D. We found significant association between the absolute differences of AVA3D-SV and AVA2D and LVOT shape (P = 0.008, Kruskal–Wallis test), being less in circular LVOT shapes [median (interquartile range) 0.07 (0.04–0.23) cm2] than in oval [0.15 (0.09–0.24) cm2] and irregular shapes [0.26 (0.15–0.43) cm2]. In the presence of USH, mean difference of AVA2D and AVA3D-SV was 0.22 ± 0.27 compared to 0.05 ± 0.18 cm2 when septal:posterior wall <1.3 (P = 0.002). Mean difference between AVA2D and AVA3D-SV did not appear to be predicted by concomitant mitral regurgitation grade, aortic regurgitant grade; presence of bicuspid vs. tricuspid aortic stenosis or regular vs. irregular rhythm (Table 2).
Table 2.
Pearson correlates and univariate predictors of discrepancies between AVA2D and AVA3D-SV
| AVA2D − AVA3D-SV |
||
|---|---|---|
| r | P-value | |
| Age | 0.03 | 0.81 |
| Heart rate | −0.18 | 0.14 |
| Left ventricular ejection fraction | 0.16 | 0.20 |
| Left ventricular septal: posterior wall ratio | 0.36 | 0.005 |
| Aortic valve Doppler | ||
| Peak pressure gradient | −0.02 | 0.86 |
| Mean pressure gradient | −0.02 | 0.86 |
| Mean ± SD | P-value | |
| Mitral regurgitation grade | ||
| Grade I (none/trivial) | 0.16 ± 0.27 | 0.31 |
| Grade II (mild) | 0.08 ± 0.17 | |
| Grade III (moderate) | 0.07 ± 0.20 | |
| Grade IV (severe) | 0.25 ± 0.37 | |
| Aortic regurgitation grade | ||
| Grade I (none/trivial) | 0.10 ± 0.23 | 0.90 |
| Grade II (mild) | 0.11 ± 0.25 | |
| Grade III (moderate) | 0.14 ± 0.16 | |
| Rhythm | ||
| Regular | 0.11 ± 0.23 | 0.95 |
| Irregular | 0.12 ± 0.22 | |
| Upper septal hypertrophy (USH, Septal:Posterior wall ≥1.3) | ||
| Absence of USH | 0.05 ± 0.18 | 0.002 |
| Presence of USH | 0.22 ± 0.27 | |
| Valve morphology | ||
| Bicuspid | 0.14 ± 0.24 | 0.72 |
| Tricuspid | 0.11 ± 0.23 | |
Figure 6.
Two-dimensional echocardiography at the parasternal long-axis view illustrating how upper septal hypertrophy distorting the left ventricular outflow tract geometry might lead to erroneous and wide variation of aortic valve area (AVA) calculation by two-dimensional continuity equation. LV, left ventricle; Ao, Aorta.
Validation of measurement of 3DE colour Doppler derived SV/CO in setting of experimentally distorted LVOT geometry
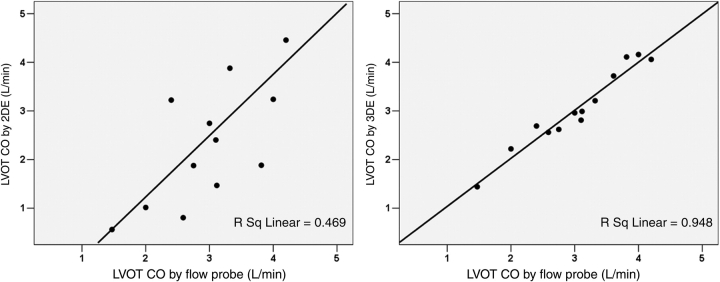
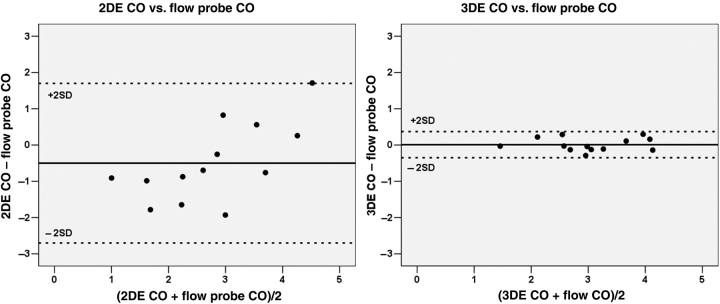
Using flow probe assessment as the referenced standard in sheep with experimentally produced variation in LVOT geometry, CO from colour Doppler RT3DE correlated better with flow probe CO (r = 0.96, P < 0.001) than 2DE derived CO with flow probe measurements (r = 0.71, P = 0.006) (Figure 7). The differences of flow probe data with 2DE and 3DE were −0.50 ± 1.1 and 0.01 ± 0.18 L/min, respectively (Figure 8).
Figure 7.
Correlations between the left ventricular outflow tract (LVOT) cardiac output (CO) derived from two-dimensional echocardiography (2DE) Doppler (left panel) and three-dimensional echocardiography (3DE) colour Doppler (right panel) vs. the referenced flow probe measurements. These were obtained from sheep models with distorted LVOT geometry.
Figure 8.
Bland–Altman plots of the cardiac output (CO) measurements by two-dimensional echocardiography (2DE) with flow probe measurements (left panel) and that of three-dimensional echocardiography (3DE) with flow probe CO (right panel). Dashed lines represent ±2SD from the mean.
Intra-observer and inter-observer differences
The intra-observer variabilities as assessed by the intra-class correlation coefficient (r) were 0.92 (95% CI 0.71–0.98) for 3DE colour Doppler SV and 0.80 (95% CI 0.41–0.95) for 3DE planimetry of the AVA. The inter-observer variabilities on these measurements were 0.88 (95% CI 0.62–0.97) and 0.87 (95% CI 0.57–0.97), respectively.
Discussion
We found that AVA derived from 2D continuity equation correlates only modestly with that derived from 3D colour Doppler and that significant discrepancies between both methods are predicted by presence of USH, representing distorted LVOT geometry. We demonstrate that RT3DE measurement of LVOT SV agrees better with the gold standard of aortic flow probe measurement in an animal model of varying LVOT geometry than 2DE. In addition, there was a better agreement of RT3DE derived AVA using colour Doppler with an independent anatomical standard, AVA guided by RT3DE planimetry.
Two-dimensional Doppler continuity equation
2DE derived continuity equation is still the current most utilized method in obtaining AVA since it is non-invasive, practical, and easily employed.1 However, its pitfalls are well known due to the inherent assumptions and simplifications. For instance, the LVOT cross-sectional area calculation assumes a circular shape for the LVOT and errors in measuring the LVOT diameter are squared in the computation of AVA. In addition, the determination of LVOT velocity by 2DE is performed using another echocardiographic window and so differs in timing and location as the LVOT area measurement. Flow velocity varies within the LVOT, having a non-uniform pattern with lower velocities at the vessel periphery, higher velocities at the centre especially in the septal and posterior area.10–14 Values obtained are thus dependent upon the position of pulse wave Doppler sampling area. In addition, significant USH may be associated with mild LVOT obstruction and introduce error. Pulse wave velocity determination may also be angle-dependent,15 inaccurate especially in low-output states10 and large sampling volumes may be required for reliable estimates of mean velocity.11,12
Geometric assumptions may not be valid in asymmetric LVOT shapes such as that seen with USH. Indeed, in our experimental studies where USH was simulated, the 2DE derived LVOT SV correlated poorly to flow probe compared to 3DE colour Doppler.
Three-dimensional Doppler continuity equation
Using RT3DE to measure AVA, these shortfalls can be circumvented altogether. RT3D colour Doppler can overcome inaccuracies of spectral Doppler for SV calculation.16 By directly measuring the LVOT SV, 3D acquisitions overcome geometric assumptions that the LVOT cross-sectional area is circular. Indeed, we found that in more than half of our cases, the LVOT is either oval or irregular in shape. This is consistent with other studies which found the majority of LVOT to be oval.17 Furthermore, with a 3D hemispheric sampling curve capturing velocities within the LVOT, varying velocities within the entire systole will be sampled and angle-dependency is less important in contrast to a planar sampling area, since velocities will project perpendicularly onto the hemispheres. Previous work has also shown less dependency with 3D methods of flow volume computation compared to 2D Doppler, though this was not performed in real time.18 Depending on whether the longer or shorter diameter of the oval cross-section was measured on 2DE, over- or under-estimation of the SV may result, compared to 3D Doppler. As the actual flow across the LVOT is directly quantitated, non-simultaneous separated measurements of stroke distance and cross-sectional area are therefore not necessary, reducing error.
When discrepancies between 2D and 3D continuity equation derived areas were analysed, the sole predictor was the degree of USH. In patients with no significant LVOT USH, discrepancies were lower and calculation of AVA2D may be sufficient. However, with USH, clinical differences between AVA3D-SV and AVA2D appeared determined by the LVOT geometry.
Recently, the use of live 3D colour Doppler in the assessment of LVOT CO has been shown to correlate well with flow probe (r2 = 0.93 or r2 = 0.99 after averaging the measurements) in normal juvenile pigs.5 The same group also showed good correlation between 3DE SV calculation and 2DE pulsed wave SV estimation in humans aged 28 ± 20.5 yrs with normal LVOT and aortic valve (r2 = 0.90).6 In a patient study, using thermodilution as the gold standard for comparison, 3D Doppler derived CO correlated better, with smaller bias and narrower limits of agreement compared to 2D Doppler derived CO.19 These studies demonstrated the validity of SV calculation using 3DE. They are consistent with our data which showed similarly excellent correlation of r2 = 0.95 even in the setting of USH.
Stroke volume calculation
Besides using 2D or 3D Doppler to assess left ventricular SV, other non-invasive echocardiographic methods include left ventricular volume calculations from 2D end-diastolic and end-systolic frames, by the summation of disks. These are prone to errors due to difficulties in defining the LV borders20 or abnormal ventricular geometry. 3D assessment of chamber volumes provides a better alternative without geometric assumption.21 However, it is more time-consuming and less direct than 3D Doppler assessments. Earlier studies have used older 3D-based methods relying on reconstruction of multiple 2D slices obtained with electrocardiographic gating. These are now superseded by real-time 3D techniques. Notwithstanding this, calculation of SV using end-diastolic and end-systolic LV volumes would also be inaccurate in the presence of valvular regurgitations or intracardiac shunts.
Three-dimensional planimetry
Unlike 2D transthoracic aortic valve planimetry, 3D planimetry allows for alignment of cropping planes to measure the smallest anatomical orifice in systole at the time point when the valve is maximally opened. We have reported that 3D planimetry of the aortic valve correlated and agreed better with AVA derived from 3D colour Doppler than from 2D continuity equation. This further supports the utility of 3D Doppler measurements in AS patients. We found that in about 20% of our full volume 3D dataset, planimetry of the aortic valve was suboptimal, mainly due to poor resolution, lower anatomical visualization, and heavy valvular calcification. However, even in these patients, we are able to obtain adequate Doppler signals to fill the LVOT area. The Doppler analyses should be independent of ability to visualize the aortic valve though this was not verified systematically.
Recently, cardiac magnetic resonance22 and computer tomography23 have also been used to evaluate AVA. Previous small studies have also highlighted the use of 3D planimetry of the aortic valve in AS by transesophageal24,25 reconstruction and live transthoracic echocardiograms.26 Traditionally, the Gorlin-derived AVA has been used as a ‘gold standard’. However, the Gorlin-derived AVA also has inherent limitations.27,28 In clinical practice, to obtain the Gorlin equation derived area, it is difficult to place the catheter in the vena contracta as this is not visualized during catheterization and the jet displaces the catheter. Consequently, catheter pressures are limited by measurements in the ascending aorta after pressure recovery has occurred.28 It is expected that continuity equation derived effective AVA will be smaller than AVA by planimetry because of the contraction phenomenon. At the same time, effective orifice area may be dynamic during the cardiac cycle and changes in effective orifice area may be flow-related.29 This is demonstrated to be related mainly to the formation of vortices at low flow rates which can result in an even smaller effective area.30,31 Indeed, beyond planimetered orifice area, other factors such as valve shape32 may be important in determining impact of AS on patient haemodynamics.
Limitations
Though 3D acquisitions are real-time, off-line SV computation and cropping for 3D full volume are still necessary. However, these processes are not lengthy and do not require extensive experience. High flow velocities over the Nyquist limit may result in inaccurate flow-volume computation. This can be compensated by baseline shift of the velocities to incorporate aliased velocities into the flow profile. In addition, one can adjust the tissue colour Doppler display priority so that colour Doppler signals fill the LVOT without excessive bleeding into the tissue. Inter-observer and intra-observer variability from our results showed reasonable consistency in this study.
In our animal validation model, balloon inflation of the upper septum to simulate USH may result in ‘non-physiologic’ shapes of the septum. However, it achieved our aim of distorting the upper septum and allowed variable changes in LVOT geometry.
In our clinical studies, we have included patients with irregular cardiac rhythm such as atrial fibrillation. This constituted a small subgroup of studies (6%) and does not predict discrepancies between 2D and 3D quantification. RT3DE cropping method to assess AVA by planimetry may have limited applications as some of the aortic valves are heavily calcified and hence have limited resolution of the valve orifice. AVA determination using cardiac magnetic resonance or computed tomography may be employed to obtain an independent parameter. However, these are not validated and have similar limitations.
It can be difficult to align planimetry plane to the narrowest aortic valve orifice using transesophageal echocardiograms. Since we analysed a wide range of aortic valve stenosis, only a proportion of patients underwent aortic valve replacement subsequently. Surgical correlation was therefore not available though this in itself may not be optimal since surgical specimens will be friable and may not represent the real-life opening of the valve subjected to haemodynamic stress. Indeed, a significant limitation of this study is the absence of an optimal gold standard.
Conclusions
RT3DE colour Doppler-derived LVOT SV in the calculation of AVA by continuity equation has potential advantages compared to 2D continuity. We demonstrated improved correlation and agreement with planimetry in AS patients and with in vivo flow probe. This is especially important in irregular LVOT geometry such as USH. RT3DE colour Doppler measurement is less variable and may be a better method for serial evaluation of AS.
Conflict of interest: none declared.
Funding
This work was supported in part by NIH NIBIB R21 EB005294 (J.H.) and NIH NHLBI RO1 038176 (R.A.L.) and a fellowship award from the National Medical Research Council, Singapore (K.K.P.).
References
- 1.Bonow RO, Carabello B, de Leon AC, Edmunds LH, Jr, Fedderly BJ, Freed MD, Gaasch WH, McKay CR, Nishimura RA, O'Gara PT, O'Rourke RA, Rahimtoola SH, Ritchie JL, Cheitlin MD, Eagle KA, Gardner TJ, Garson A, Jr, Gibbons RJ, Russell RO, Ryan TJ, Smith SC., Jr ACC/AHA Guidelines for the Management of Patients With Valvular Heart Disease. Executive Summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients With Valvular Heart Disease) J Heart Valve Dis. 1998;7:672–707. [PubMed] [Google Scholar]
- 2.Skjaerpe T, Hegrenaes L, Hatle L. Noninvasive estimation of valve area in patients with aortic stenosis by Doppler ultrasound and two-dimensional echocardiography. Circulation. 1985;72:810–818. doi: 10.1161/01.cir.72.4.810. [DOI] [PubMed] [Google Scholar]
- 3.Otto CM, Pearlman AS, Comess KA, Reamer RP, Janko CL, Huntsman LL. Determination of the stenotic aortic valve area in adults using Doppler echocardiography. J Am Coll Cardiol. 1986;7:509–517. doi: 10.1016/s0735-1097(86)80460-0. [DOI] [PubMed] [Google Scholar]
- 4.Zoghbi WA, Farmer KL, Soto JG, Nelson JG, Quinones MA. Accurate noninvasive quantification of stenotic aortic valve area by Doppler echocardiography. Circulation. 1986;73:452–459. doi: 10.1161/01.cir.73.3.452. [DOI] [PubMed] [Google Scholar]
- 5.Pemberton J, Li X, Karamlou T, Sandquist CA, Thiele K, Shen I, Ungerleider RM, Kenny A, Sahn DJ. The use of live three-dimensional Doppler echocardiography in the measurement of cardiac output: an in vivo animal study. J Am Coll Cardiol. 2005;45:433–438. doi: 10.1016/j.jacc.2004.10.046. [DOI] [PubMed] [Google Scholar]
- 6.Pemberton J, Li X, Kenny A, Davies CH, Minette MS, Sahn DJ. Real-time 3-dimensional Doppler echocardiography for the assessment of stroke volume: an in vivo human study compared with standard 2-dimensional echocardiography. J Am Soc Echocardiogr. 2005;18:1030–1036. doi: 10.1016/j.echo.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Levine RA, Lefebvre X, Guerrero JL, Vlahakes GJ, Cape EG, He S, Yoganathan AP, Weyman AE. Unifying concepts of mitral valve function and disease: SAM, prolapse and ischemic mitral regurgitation. J Cardiol. 1994;24(Suppl. 38):15–27. [Google Scholar]
- 8.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 9.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;2:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 10.Marshall SA, Weyman AE. Principles and Practice of Echocardiography. Lippincott Williams & Wilkins, Philadelphia;: 1994. Doppler estimation of volumetric flow; pp. 955–978. [Google Scholar]
- 11.Kvitting P, Hessevik I, Matre K, Segadal L. Three-dimensional cross-sectional velocity distribution in the ascending aorta in cardiac patients. Clin Physiol. 1996;16:239–258. doi: 10.1111/j.1475-097x.1996.tb00572.x. [DOI] [PubMed] [Google Scholar]
- 12.Haugen BO, Berg S, Brecke KM, Torp H, Slordahl SA, Skaerpe T, et al. Blood flow velocity profiles in the aortic annulus: a 3-dimensional freehand color flow Doppler imaging study. J Am Soc Echocardiogr. 2002;15:328–333. doi: 10.1067/mje.2002.117292. [DOI] [PubMed] [Google Scholar]
- 13.Wiseth R, Samstad S, Rossvoll O, Torp HG, Skjaerpe T, Hatle L. Cross-sectional left ventricular outflow tract velocities before and after aortic valve replacement: a comparative study with two-dimensional Doppler ultrasound. J Am Soc Echocardiogr. 1993;6:279–285. doi: 10.1016/s0894-7317(14)80064-3. [DOI] [PubMed] [Google Scholar]
- 14.Rossvoll O, Samstad S, Torp HG, Linker DT, Skjaerpe T, Angelsen BA, Hatle L. The velocity distribution in the aortic anulus in normal subjects: a quantitative analysis of two-dimensional Doppler flow maps. J Am Soc Echocardiogr. 1991;4:367–378. doi: 10.1016/s0894-7317(14)80447-1. [DOI] [PubMed] [Google Scholar]
- 15.Quinones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA. Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15:167–184. doi: 10.1067/mje.2002.120202. [DOI] [PubMed] [Google Scholar]
- 16.Pemberton J, Ge S, Thiele K, Jerosch-Herold M, Sahn DJ. Real-time three-dimensional color Doppler echocardiography overcomes the inaccuracies of spectral Doppler for stroke volume calculation. J Am Soc Echocardiogr. 2006;19:1403–1410. doi: 10.1016/j.echo.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 17.Baumgartner H, Kratzer H, Helmreich G, Kuehn P. Determination of aortic valve area by Doppler echocardiography using the continuity equation: a critical evaluation. Cardiology. 1990;77:101–111. doi: 10.1159/000174590. [DOI] [PubMed] [Google Scholar]
- 18.Rusk RA, Li XN, Irvine T, Mori Y, Wanitkun S, Li XK, Kenny A, Sahn DJ. Surface integration of velocity vectors from 3D digital colour Doppler: an angle independent method for laminar flow measurements. Eur J Echocardiogr. 2002;3:177–184. doi: 10.1053/euje.2002.0145. [DOI] [PubMed] [Google Scholar]
- 19.Lodato JA, Weinert L, Baumann R, Coon P, Anderson A, Kim A, Fedson S, Sugeng L, Lang RM. Use of 3-dimensional color Doppler echocardiography to measure stroke volume in human beings: comparison with thermodilution. J Am Soc Echocardiogr. 2007;20:103–112. doi: 10.1016/j.echo.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 20.Axler O, Megarbane B, Lentschener C, Fernandez H. Comparison of cardiac output measured with echocardiographic volumes and aortic Doppler methods during mechanical ventilation. Intensive Care Med. 2003;29:208–217. doi: 10.1007/s00134-002-1582-1. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt MA, Ohazama CJ, Agyeman KO, Freidlin RZ, Jones M, Laurienzo JM, Brenneman CL, Arai AE, von Ramm OT, Panza JA. Real-time three-dimensional echocardiography for measurement of left ventricular volumes. Am J Cardiol. 1999;84:1434–1439. doi: 10.1016/s0002-9149(99)00591-3. [DOI] [PubMed] [Google Scholar]
- 22.Reant P, Lederlin M, Lafitte S, Serri K, Montaudon M, Corneloup O, Roudaut R, Laurent F. Absolute assessment of aortic valve stenosis by planimetry using cardiovascular magnetic resonance imaging: comparison with transesophageal echocardiography, transthoracic echocardiography, and cardiac catheterisation. Eur J Radiol. 2006;59:276–283. doi: 10.1016/j.ejrad.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 23.Bouvier E, Logeart D, Sablayrolles JL, Feignoux J, Scheuble C, Touche T, Thabut G, Cohen-Solal A. Diagnosis of aortic valvular stenosis by multislice cardiac computed tomography. Eur Heart J. 2006;27:3033–3038. doi: 10.1093/eurheartj/ehl273. [DOI] [PubMed] [Google Scholar]
- 24.Ge S, Warner JG, Jr, Abraham TP, Kon ND, Brooker RF, Nomeir AM, Fowle KM, Burgess P, Kitzman DW. Three-dimensional surface area of the aortic valve orifice by three-dimensional echocardiography: clinical validation of a novel index for assessment of aortic stenosis. Am Heart J. 1998;136:1042–1050. doi: 10.1016/s0002-8703(98)70161-9. [DOI] [PubMed] [Google Scholar]
- 25.Menzel T, Mohr-Kahaly S, Kolsch B, Kupferwasser I, Kopp H, Spiecker M, Wagner S, Meinert R, Pagnia F, Meyer J. Quantitative assessment of aortic stenosis by three-dimensional echocardiography. J Am Soc Echocardiogr. 1997;10:215–223. doi: 10.1016/s0894-7317(97)70057-9. [DOI] [PubMed] [Google Scholar]
- 26.Vengala S, Nanda NC, S H, Singh V, Agrawal G, Sinha A, Khanna D, Upendram SK, Chockalingam A, McGiffin DC, Kirklin JK, Pacifico AD. Images in geriatric cardiology. Usefulness of live three-dimensional transthoracic echocardiography in aortic valve stenosis evaluation. Am J Geriatr Cardiol. 2004;13:279–284. doi: 10.1111/j.1076-7460.2004.02710.x. [DOI] [PubMed] [Google Scholar]
- 27.Garcia D, Kadem L. What do you mean by aortic valve area: geometric orifice area, effective orifice area, or gorlin area? J Heart Valve Dis. 2006;15:601–608. [PubMed] [Google Scholar]
- 28.Weyman AE, Scherrer-Crosbie M. Aortic stenosis: physics and physiology—what do the numbers really mean? Rev Cardiovasc Med. 2005;6:23–32. [PubMed] [Google Scholar]
- 29.DeGroff CG, Shandas R, Valdes-Cruz L. Analysis of the effect of flow rate on the Doppler continuity equation for stenotic orifice area calculations: a numerical study. Circulation. 1998;97:1597–1605. doi: 10.1161/01.cir.97.16.1597. [DOI] [PubMed] [Google Scholar]
- 30.Kadem L, Rieu R, Dumesnil JG, Durand LG, Pibarot P. Flow-dependent changes in Doppler-derived aortic valve effective orifice area are real and not due to artifact. J Am Coll Cardiol. 2006;47:131–137. doi: 10.1016/j.jacc.2005.05.100. [DOI] [PubMed] [Google Scholar]
- 31.Baumgartner H. Hemodynamic assessment of aortic stenosis: are there still lessons to learn? J Am Coll Cardiol. 2006;47:138–140. doi: 10.1016/j.jacc.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Gilon D, Cape EG, Handschumacher MD, Song JK, Solheim J, VanAuker M, King ME, Levine RA. Effect of three-dimensional valve shape on the hemodynamics of aortic stenosis: three-dimensional echocardiographic stereolithography and patient studies. J Am Coll Cardiol. 2002;40:1479–1486. doi: 10.1016/s0735-1097(02)02269-6. [DOI] [PubMed] [Google Scholar]