Abstract
BACKGROUND
According to conventional theory, the oocyte population is not renewed in mammalian ovaries after birth. A new hypothesis proposes that oocytes are generated continuously from haematopoietic progenitor cells. There is, however, no evidence that they can ovulate, although they may partially restore fertility by organizing ‘helper follicles’. The hypothesis that follicles can form de novo in adult ovaries has been tested in a transplant model.
METHODS
Ovaries from adult mice were transplanted under the kidney capsule or into the ovarian bursa of histocompatible, transgenic CAG::H2B-EGFP host animals. Some donors were sterilized before transplantation by X-irradiation to ensure ‘empty niches’ were available for repopulation. The phenotype of follicular oocytes at 2, 4 and 8 weeks post-transplantation was scored by epifluorescence.
RESULTS
A total of 819 oocytes were examined in 30 ovarian grafts. None expressed green fluorescence, as would be predicted if they had formed de novo from germ cell progenitors in the systemic circulation of the host. Furthermore, small follicles eliminated by irradiation were not replaced in transplanted ovaries, and the few growing follicles present were apparently survivors of the original population.
CONCLUSIONS
No evidence was found to support the hypothesis that progenitor cells from extra-ovarian sources can repopulate the adult ovary. The findings are consistent with the conventional view that a limited number of oocytes are formed before birth and declines with age. The study did not, however, rule out the possibility that germline stem cells may reside in the adult ovary.
Keywords: follicle, ovary, regeneration, transplantation, X-irradiation
Introduction
Male germ cells are generated throughout adult life from spermatogonial stem cells, but it has long been accepted that the population of oocytes is fixed before or shortly after birth, at least in mammalian species (Zuckerman, 1951). This theory has been one of the foundations of ovarian biology for over 50 years and was not seriously challenged until recently.
In 2004, Johnson et al. reported finding that oocytes are continuously replaced in adult mouse ovaries and observed candidate germline stem cells in the surface epithelium (Johnson et al., 2004). These findings were dramatically at odds with conventional theory because they implied that the ovary can continuously renew its germ cell reserve, reminiscent of spermatogenesis in the testes, and they appeared to confirm some early 20th century findings which had been largely discredited (e.g. Allen, 1923). The claims made by Johnson et al. essentially rested on three lines of experimental evidence: (i) follicular turnover appeared to exceed the available follicular store, (ii) molecular markers of early meiosis were expressed in adult ovaries and (iii) chimaeric follicles were sometimes observed in pairs of fused ovaries, suggesting they were newly formed. Collectively, these data implied that follicles were being renewed by resident germline stem cells in adult mouse ovaries, but the following year the same group published a revised hypothesis based on data showing that the germ cell progenitors were actually derived from the circulation and ultimately from bone marrow. Johnson et al. reported that bone marrow cells were able to generate new oocytes in ovaries that were genetically sterile or depleted by cytotoxins and they suggested, furthermore, that tandem variation in germline marker expression in bone marrow with stages of the oestrous cycle implied the existence of a feedback mechanism regulating production of new follicles (Johnson et al., 2005a).
These striking claims have been challenged by a number of groups (Byskov et al., 2005; Telfer et al., 2005; Bristol-Gould et al., 2006; Eggan et al., 2006; John et al., 2007) and countered, in turn, by the claimants (Johnson et al., 2005b; Lee et al., 2007; Tilly and Johnson, 2007). New evidence in favour of the conventional theory was obtained by Eggan et al. (2006), who found that when a bone marrow donor or one partner of a parabiotically joined pair of mice was transgenic for green fluorescent protein (GFP), and the respective wild-type recipient or contralateral partner never ovulated green fluorescent oocytes. The study design did not address the possibility that GFP-positive oocytes had nevertheless migrated to recipient ovaries but were never ovulated. Lee et al. (2007) confirmed that offspring are exclusively derived from host oocytes rather than bone marrow-derived progenitors, but they also reported that bone marrow transplantation soon after semi-sterilization with cyclophosphamide–busulphan treatment substantially improved fertility and partially restored the stock of ovarian follicles. They hypothesized that fertility benefited from bone marrow transplantation because of an indirect effect mediated by ‘helper follicles’ organizing around germ cell progenitors derived from circulating cells, as reported by Johnson et al. (2005a).
We have used ovarian transplantation in a mouse model to test whether the stock of oocytes in the ovary is replenished by germ cell progenitors in the circulation. Wild-type mouse ovaries were transplanted into histocompatible hosts expressing a nuclear-localized GFP marker and screened at intervals to ascertain whether they were repopulated with oocytes expressing GFP. Since ovaries lose at least half of their follicles from ischaemic damage after transplantation (Jones and Krohn, 1960), the procedure theoretically creates ‘empty niches’, such as those needed for germ cell turnover in Drosophila ovaries (Xie and Spradling, 2000), that may be required for new follicles to form in mammals. To further increase this capacity for folliculogenesis, some donor ovaries were irradiated prior to transplantation to completely eliminate small oocytes.
Materials and Methods
Animals
All mice were maintained under specific pathogen-free condition in AALAC accredited facilities of the College of Physicians and Surgeons of Columbia University. Four-week-old female 129/SvJ mice (The Jackson Laboratory, Bar Harbor, ME, USA) were used as ovary donors. Host animals were histocompatible 129-CAG::H2B-EGFP females (8–24 weeks of age) and transgenic for a histone 2B enhanced GFP fusion transgene controlled by a ubiquitous promoter. This marker is highly specific for chromatin (Hadjantonakis and Papaioannou, 2004).
Ovarian transplantation
In the first experiment, donor ovaries were isolated and bisected immediately prior to transplantation using aseptic techniques. A dorsolateral incision was made in the left flank and in the body wall of host animals to exteriorize the left kidney and ovary. Hemi-ovaries were transplanted into a pocket created under the kidney capsule, whereas the other half was inserted into the ovarian bursa after removing the resident gonad. The bursa was sutured with 10/0 nylon, and the skin incision was closed with wound clips. Ovarian grafts from both sites and the intact contralateral host ovary were harvested 2, 4 and 8 weeks later.
In a second experiment, wild-type donor mice received 0.5 gray whole-body irradiation at a dose rate of 0.0139 gray s−1 before being used as ovarian donors. At this dose, primordial follicles are completely eliminated within 24 h, but the more radio-resistant growing follicles take several weeks to clear by natural processes (Gosden, 1990). These donors were sacrificed 24 h later and their ovaries were bisected and transplanted under the kidney capsules of transgenic adult hosts, which were autopsied after 4 weeks. Ovaries were also harvested at 24 h, 2 and 4 weeks from unoperated, irradiated mice to serve as controls for verifying the sterilizing effects of irradiation.
Microscopical analysis of ovaries and ovarian grafts
Grafted and control ovaries were fixed in 4% paraformaldehyde at 4°C for 2 h, followed by 30% sucrose overnight and embedded in Tissue Tek O.C.T. compound (Sakura Finetechnical Co, Ltd, Tokyo, Japan). The specimens were stored at −80°C for up to 1 month until cryostat sections were cut at 10 µm from the frozen blocks. A few representative sections from grafts and irradiated controls were stained with haematoxylin and eosin for morphological study but the majority was examined with epifluorescence. The sections were stained with 4′,6′-diamidine-2-phenylindole dihydrochloride (DAPI) for DNA and mounted under a coverslip with Gel/Mount (Biomeda Corp., Foster City, CA, USA) before examination under a Zeiss Axiovert 200 M microscope. A Plan Apo 100×, 1.4NA oil immersion lens and filter sets for DAPI and GFP were used to distinguish nuclei in cells of donor or host origin. A total of 15–30 frozen sections from both orthotopic and heterotopic grafts were examined from five animals at each of the three time points. A minimum of 25 follicles were screened per graft, except for one orthotopic graft at the 2 week interval in which only nine follicles were found. In irradiated heterotopic grafts, 15–25 sections were examined using the same methods.
Follicles were examined for DAPI staining to verify the identity and stage of follicle and confirm the presence of the oocyte nucleus in the section before scoring for GFP. Observations were confirmed by a second observer before every oocyte was photographed using DAPI and GFP filter sets in turn. Primordial follicles were characterized by a small oocyte with mainly squamous pregranulosa cells, whereas growing follicles had a larger oocyte and one or more layers of cuboidal granulosa cells. Antral stages were follicles containing a fully grown oocyte with a large extracellular or ‘antral’ cavity. Chromatin in oocytes varies with stage of development, being denser and more evenly distributed in the nuclei of small oocytes than at antral stages in which it is diffuse except for a rim around the nucleolus (Mattson and Albertini, 1990).
To confirm GFP expression in oocytes of host mice, ovarian sections were stained with an anti-GFP antibody (Molecular Probes, Eugene, OR, USA) and an anti-murine Vasa homologue (MVH) antibody (Abcam Inc, Cambridge, MA, USA). Whole ovaries from 4-week-old 129-CAG::H2B-EGFP females were fixed in 4% paraformaldehyde at 4°C for 2 h, washed with phosphate-buffered saline, and treated with Triton X-100 (0.1%) for 3 h, stained with DAPI overnight and examined with laser scanning confocal microscopy under a Zeiss LSM510 NLO or processed for immunohistochemistry. Immunohistochemistry was performed using a standard avidin-biotin peroxidase staining method using peroxidase-conjugated goat anti-rabbit antibody (Jackson Immuno Research, West Grove, PA, USA) and 3,3′-diaminobenzidine as the substrate (Vector Laboratories Inc., Burlingame, CA, USA).
Results
Ovaries from CAG::H2B-EGFP transgenic mice expressed GFP in oocytes and most other nuclei, the notable exception being the granulosa cells where the expression was variable (Fig. 1A). Expression of GFP in oocyte chromatin was confirmed by immunostaining adjacent sections with anti-GFP and anti-MVH antibodies, a germ cell-specific marker (Fig. 1B and C).
Figure 1:
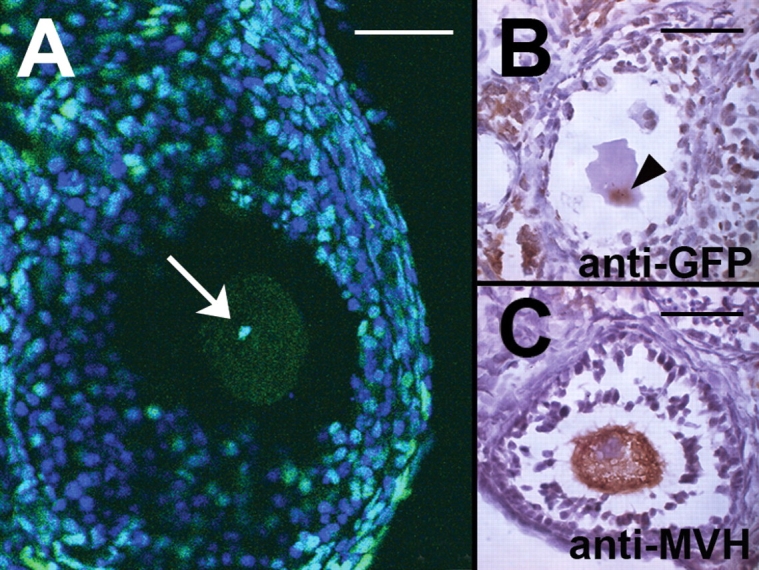
Detection of oocyte nuclei in CAG::H2B-EGFP transgenic mouse ovaries. (A) Laser scanning confocal fluorescence image of a 4′,6′-diamidine-2-phenylindole dihydrochloride (DAPI) stained ovary. Green fluorescence clearly marks the nucleus of the growing oocyte (arrow), whereas the granulosa cell population has both GFP-expressing and non-expressing (blue) nuclei. (B and C) Immunohistochemistry using anti-GFP (B) and anti-murine Vasa homologue (MVH) (C) antibodies mark the nucleus (arrowhead) and cytoplasm, respectively, of oocytes in growing follicles. Bars = 50 µm.
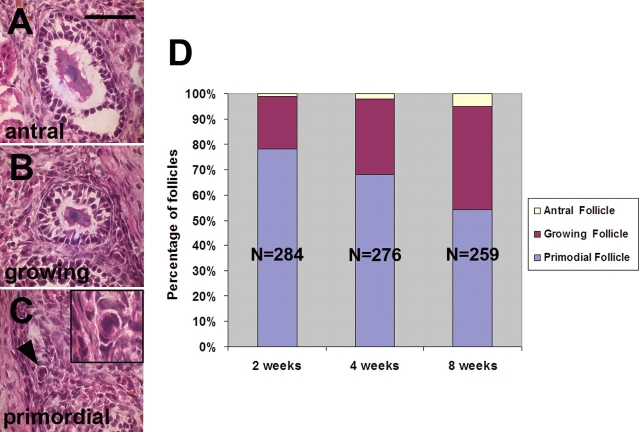
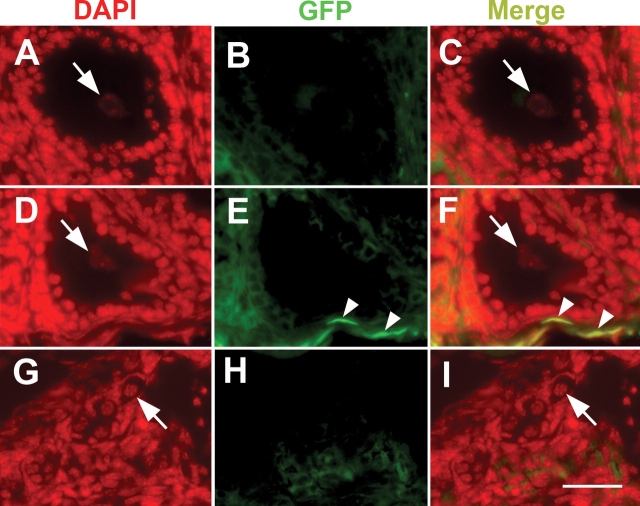
All ovarian grafts were informative insofar as they contained follicles and lacked any obvious pathological changes. There was an increase in the percentage of larger follicles in the older grafts indicating that ischaemic tissues had been revascularized and surviving follicles were developing normally (Table I and Fig. 2). GFP expression in host tissues was confirmed by green fluorescence in the nuclei of kidney tissue adjacent to heterotopic grafts and in contralateral ovary sections (not shown). Oocytes in grafted ovaries were scored for fluorescence individually using DAPI and GFP filters in turn (Fig. 3), including 422 oocytes from heterotopic grafts and 397 oocytes from orthotopic grafts. There were no obvious differences in follicle morphology between the two sites. A few cells with fluorescent nuclei, presumably of host origin, were observed in the stroma of grafts (Fig. 3E and F); however, none of the 819 oocytes in the 30 grafts expressed GFP in their nuclei. The majority of these observations were based on primordial follicles (67%), the most abundant stage in all specimens.
Table I.
Number of mouse oocytes in different types of follicles scored in five ovarian grafts from each transplantation site and time point as well as four irradiated ovarian grafts from the kidney capsule.
| Type of follicle | Two weeks |
Four weeks |
Eight weeks |
Total | Irradiated grafts (4 weeks) | |||
|---|---|---|---|---|---|---|---|---|
| Bursa | Kidney | Bursa | Kidney | Bursa | Kidney | |||
| Primordial | 90 | 133 | 97 | 92 | 73 | 66 | 551 | 1 |
| Growing | 27 | 33 | 50 | 32 | 53 | 53 | 248 | 44 |
| Antral | 0 | 1 | 1 | 4 | 6 | 8 | 20 | 3 |
| Total | 117 | 167 | 148 | 128 | 132 | 127 | 819 | 48 |
No oocyte nuclei were positive for GFP.
Figure 2:
The progression of folliculogenesis in ovary transplants. (A–C) Haematoxylin and eosin stained sections of ovarian grafts illustrating the morphology of antral (A), growing (B) and primordial (C) follicles. Inset in C is a higher magnification of the follicle indicated by the arrowhead. (D) Stacked histograms showing the proportion of primordial, growing and antral follicles observed in transplanted ovarian grafts recovered at the three time points indicated. The decreasing proportion of immature, primordial follicles with time reflects the progression of folliculogenesis. Each bar represents counts from 10 grafts; N, number of follicles scored. Bar = 50 µm.
Figure 3:
DAPI (A, D and G) and GFP (B, E and H) fluorescence images and merged images (C, F and I) (false coloured) of antral (A–C), growing (D–F) and primordial (G–I) follicles from wild-type ovarian grafts into GFP-positive hosts. The oocyte nuclei (arrows) are all negative for GFP. A few GFP-positive nuclei at the periphery of the ovary (arrowheads in E and F) represent host-derived, non-oocyte cells that have migrated into the graft, and serve as positive controls for nuclear GFP fluorescence. Bar = 50 µm.
Irradiated, control ovaries were examined at 24 h, 2 and 4 weeks. Primordial follicles were completely absent in these organs, although a few growing follicles persisted at all time points, consistent with published data (Gosden, 1990). Four of the five irradiated ovaries grafted to the kidney capsule survived and 15–25 sections were examined from each of the four. Only one primordial follicle was observed in the set and most of the growing follicles (48 in total) were apparently in the process of atresia since they exhibited pycnotic granulosa cells. Nevertheless, the nuclei of these oocytes could still be scored for DAPI fluorescence, although none of them expressed GFP (Table I).
Discussion
Ovarian transplantation has a long history in experimental endocrinology and is highly effective in restoring normal oestrous cycles and fertility to ovariectomized mice (Krohn, 1977; Gosden, 2008). This technique was used to test the hypothesis that circulating germ cells can regenerate the oocyte population in adult ovaries, but no supporting evidence was found after screening large numbers of oocytes in ovaries grafted from wild-type mice to hosts expressing GFP in their nuclei. A few GFP cells were observed in the stroma of grafts, possibly of vascular origin, such as the committed leucocytes described by Eggan et al. (2006) in their study of bone marrow transplantation.
This study was prompted by claims that folliculogenesis continues during adult life (Johnson et al., 2004), that oocytes are formed from progenitors in the circulation (Johnson et al., 2005b) and that fertility is partially restored by bone marrow transplantation after cytotoxic treatment, at least according to one small series (Lee et al., 2007). These observations could have enormous clinical significance for ovarian regeneration in young cancer patients receiving sterilizing chemotherapy and who are therefore losing the chance of genetic parenthood (Johnson et al., 2005b). However, no evidence has been published to date showing that oocytes derived from bone marrow cells after transplantation can be ovulated (Eggan et al., 2006; Lee et al., 2007). An apparent conundrum that bone marrow transplantation has beneficial effects on fertility without directly contributing to gametes for fertilization might be explained by systemic effects in animals whose health has been compromised by treatment with alkylating agents. On the other hand, Lee et al. (2007) demonstrated that oocytes of donor origin appeared in host ovaries and boosted numbers overall, although none of the follicles of donor origin progressed beyond the early pre-antral stage even 2 months post-transplantation. Since these follicles did not ovulate, yet fertility improved in the hosts, they might tentatively be regarded as ‘helper follicles’, which promote the maturation or survival of follicles remaining in an ovary otherwise compromised by cytotoxic treatment. There is some doubt, however, whether such a hypothesis is relevant, at least in the context of ovaries depleted by ageing, because the threshold number of follicles required to maintain spontaneous ovulation is very low (<100) (Jones and Krohn, 1961; Gosden et al., 1983). In other words, it is unlikely that supplementary factors provided by bone marrow transplantation will make any difference to the functional lifespan of the normal ovary.
In the present study, we have transplanted GFP-negative ovaries into GFP-positive transgenic hosts to test whether circulating germ cell progenitors can colonize the ovaries and organize new follicles. Detection of a GFP-positive cell in an otherwise GFP-negative tissue is a particularly sensitive assay for cells migrating into a graft, and the nuclear localization of the GFP allows positive identification of every oocyte. No support was found for oocyte replenishment after ascertaining that none of the germ cells in ovarian grafts was GFP positive, i.e. derived from the host. Moreover, even up to 8 weeks after transplantation, the majority of oocytes examined were in primordial follicles, which is the most immature stage and the first to be formed after neo-oogenesis. Since these oocytes are among the most radio-sensitive of all mammalian cells, it is unlikely that somatic cells required for the organ were destroyed by a minimal sterilizing dose of radiation. The lack of regeneration in these ovaries is a further denial of the hypothesis that circulating germ cell progenitors exist in adults, while irradiation had provided the maximum potential niches for repopulation. Although it is possible that it could take more that 8 weeks for regeneration to occur, Johnson et al. (2005a) reported all stages of follicles present within 8 weeks. Overall, we therefore find no support for oocyte regeneration in mouse ovaries, including indirect promotion of fertility through hypothetical ‘helper follicles’. Circulating cells appear not to offer any potential contribution to fertility preservation, although we cannot rule out the existence of germline stem cells in the ovary, except to point out the lack of evidence of activity in adult life, if they exist at all.
Funding
This work was funded by NIH grant HD33082 (V.E.P.).
References
- Allen E. Ovogenesis during sexual maturity. Am J Anat. 1923;31:439–581. [Google Scholar]
- Bristol-Gould SK, Kreeger PK, Selkirk CG, Kilen SM, Mayo KE, Shea LD, Woodruff TK. Fate of the initial follicle pool: empirical and mathematical evidence supporting its sufficiency for adult fertility. Dev Biol. 2006;298:149–154. doi: 10.1016/j.ydbio.2006.06.023. [DOI] [PubMed] [Google Scholar]
- Byskov AG, Faddy MJ, Lemmen JG, Anderson CY. Eggs forever? Differentiation. 2005;73:438–446. doi: 10.1111/j.1432-0436.2005.00045.x. [DOI] [PubMed] [Google Scholar]
- Eggan K, Jurga S, Gosden R, Min IM, Wagers AJ. Ovulated oocytes in adult mice derive from non-circulating germ cells. Nature. 2006;441:1109–1114. doi: 10.1038/nature04929. [DOI] [PubMed] [Google Scholar]
- Gosden RG. Restitution of fertility in sterilized mice by transferring primordial ovarian follicles. Hum Reprod. 1990;5:499–504. doi: 10.1093/oxfordjournals.humrep.a137132. [DOI] [PubMed] [Google Scholar]
- Gosden RG. Ovary and uterus transplantation. Reproduction. 2008 doi: 10.1530/REP-08-0099. in press. [DOI] [PubMed] [Google Scholar]
- Gosden RG, Laing SC, Felicio LS, Nelson JF, Finch CE. Imminent oocyte exhaustion and reduced follicular recruitment mark the transition to acyclicity in aging C57BL/6J mice. Biol Reprod. 1983;28:255–260. doi: 10.1095/biolreprod28.2.255. [DOI] [PubMed] [Google Scholar]
- Hadjantonakis A-K, Papaioannou VE. Dynamic in vivo imaging and cell tracking using a histone fluorescent protein fusion in mice. BMC Biotechnol. 2004;4:33. doi: 10.1186/1472-6750-4-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John GB, Shirley LJ, Gallardo TD, Castrillon DH. Specificity of the requirement for Foxo3 in primordial follicle activation. Reproduction. 2007;133:855–863. doi: 10.1530/REP-06-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J, Canning J, Kaneco T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428:145–150. doi: 10.1038/nature02316. [DOI] [PubMed] [Google Scholar]
- Johnson J, Bagley J, Skaznik-Wikiel M, Lee H-J, Adams GB, Niikura Y, Tschudy KS, Tilly JC, Cortes ML, Forkert R, et al. Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell. 2005;a 122:305–315. doi: 10.1016/j.cell.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Johnson J, Skaznik-Wikiel M, Lee H-J, Niikura Y, Tilly JC, Tilly JL. Setting the record straight on data supporting postnatal oogenesis in female mammals. Cell Cycle. 2005;b 4:1471–1477. doi: 10.4161/cc.4.11.2186. [DOI] [PubMed] [Google Scholar]
- Jones EC, Krohn PL. Orthotopic ovarian transplantation in mice. J Endocrinol. 1960;20:135–146. doi: 10.1677/joe.0.0200135. [DOI] [PubMed] [Google Scholar]
- Jones EC, Krohn PL. The relationships between age, numbers of oocytes and fertility in virgin and multiparous mice. J Endocrinol. 1961;21:469–495. doi: 10.1677/joe.0.0210469. [DOI] [PubMed] [Google Scholar]
- Krohn PL. Transplantation of the ovary. In: Zuckerman L, Weir BJ, editors. The Ovary, Vol. 2, Physiology. New York: Academic Press; 1977. [Google Scholar]
- Lee H-J, Selesniemi K, Niikura Y, Niikura T, Klein R, Dombkowski DM, Tilly JL. Bone marrow transplantation generates immature oocytes and rescues long term fertility in a preclinical mouse model of chemotherapy-induced premature ovarian failure. J Clin Oncol. 2007;25:3198–3204. doi: 10.1200/JCO.2006.10.3028. [DOI] [PubMed] [Google Scholar]
- Mattson BA, Albertini DF. Oogenesis: chromatin and microtubule dynamics during meiotic prophase. Mol Reprod Dev. 1990;25:374–383. doi: 10.1002/mrd.1080250411. [DOI] [PubMed] [Google Scholar]
- Telfer EE, Gosden RG, Byskov AG, Spears N, Albertini D, Anderson CY, Anderson R, Braw-Tal R, Clarke H, Gougeon A, et al. On regenerating the ovary and generating controversy. Cell. 2005;122:821–822. doi: 10.1016/j.cell.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Tilly JL, Johnson J. Recent arguments against germ cell renewal in the adult human ovary: is an absence of marker gene expression really acceptable evidence of an absence of oogenesis? Cell Cycle. 2007;6:879–883. doi: 10.4161/cc.6.8.4185. [DOI] [PubMed] [Google Scholar]
- Xie T, Spradling AC. A niche maintaining germ line stem cells in Drosophila ovary. Science. 2000;290:328–330. doi: 10.1126/science.290.5490.328. [DOI] [PubMed] [Google Scholar]
- Zuckerman S. The number of oocytes in the mature ovary. Recent Prog Horm Res. 1951;6:63–108. [Google Scholar]