Abstract
BACKGROUND
The glycan cell surface molecules, stage-specific embryonic antigen (SSEA)-1, -3 and -4 and tumor-rejection antigen (TRA)-1-60 and -1-81, are expressed in specific combinations by undifferentiated pluripotent cells, i.e. embryonic stem cells, induced pluripotent stem cells, embryonal carcinoma cells, primordial germ cells and embryonic germ cells. Upon differentiation of the cells, these markers vanish. Recently, it has been shown that also neonatal and adult mouse testes contain pluripotent cells. Here, we aimed at identifying in situ possibly pluripotent cells in the adult primate testis.
METHODS
Monoclonal antibodies raised against the glyco-epitopes SSEA-1, -3 and -4 and TRA-1-60 and -1-81, respectively, were tested to detect cells expressing the antigens, by immunohistochemistry on Bouin's-fixed and paraffin-embedded adult primate testes. Man, the new-world monkey, Callithrix jacchus (common marmoset), and the old-world monkey species, Macaca mulatta (Rhesus macaque) and Macaca silenus (Lion-tailed macaque), were included. The percentage of SSEA-4-positive cells in three adult marmoset testes was determined using flow cytometry.
RESULTS
Spermatogonia in the testes of C. jacchus were labeled by SSEA-4, TRA-1-60 and -1-81-antibodies. In the macaques, spermatogonia were detected by SSEA-4 and TRA-1-81-antibodies. TRA-1-61 did not bind to macaque spermatogonia. Also, SSEA-1 and -3 did not bind to spermatogonia in any species. In human testes, we never obtained any clear staining. The total percentage of SSEA-4-positive cells in marmoset testes was 8.6 ± 1.61%.
CONCLUSIONS
SSEA-4 and TRA-1-81-antibodies may be very well suited for the identification and isolation of spermatogonia, and possibly also germline stem cells, in the non-human primate testis.
Keywords: stem cell, stage-specific embryonic antigen (SSEA), tumor-rejection antigen (TRA), spermatogonia, testis
Introduction
Spermatogonia are the descendants of primordial germ cells (PGC), which are the first cells in mammalian embryogenesis exclusively constricted to the germline. Importantly, human PGC can be cultured in vitro giving rise to immortal embryonic germ cell (EGC) lines, which are pluripotent (Shamblott et al., 1998), i.e. they can form derivatives of all three embryonic germ layers. In that, EGCs are very similar to embryonic stem cells (ESC) (Zwaka and Thomson, 2005), which can be considered as the prototype of pluripotent cells, as they can be established already from the embryonal morula stage. ESC, PGC and EGC can all form teratomas when injected into immuno-deficient mice and share the expression of many key molecules considered specific for pluripotent cells. For instance, they express the transcription factors Oct4 and Nanog (Boyer et al., 2005). Also, several surface antigens consisting of glycans, namely stage-specific embryonic antigens (SSEA)-1, -3 and -4 as well as the tumor-rejection antigens (TRA)-1-60 and 1-81, are specific and useful markers of pluripotent stem cells (reviewed in Lanctot et al., 2007). Glycans form a dense glycocalyx on the cell surface and often represent lineage-specific signatures specifically indicating the differentiation state of a cell. Therefore, cell type-specificity and strong antigenicity make the above mentioned antigens very valuable tools to identify stem cells in, and isolating such cells from, large cell populations. The antigens investigated here (SSEA-1, -3 and -4 and TRA-1-60 and 1-81) are not all expressed by all types of pluripotent cells; each stem-cell type has a signature depending on the species and its origin. Undifferentiated human (Thomson et al., 1998), rhesus macaque (Thomson et al., 1995) and common marmoset ESC (Thomson et al., 1996; Sasaki et al., 2005) express SSEA-3 and SSEA-4, but not SSEA-1. The same applies to human induced pluripotent stem (iPS) cells (Takahashi et al., 2007). In contrast, human EGC express SSEA-3 only very weakly and inconsistently but strongly express SSEA-1 and SSEA-4 (Shamblott et al., 1998). With regard to the presence of TRA-1-60 and -1-81 in primate ESC, PGC and EGC, there are also interesting differences: human (Thomson et al., 1998) and non-human primate (Thomson et al., 1995, 1996; Sasaki et al., 2005) ESC, human iPS cells (Takahashi et al., 2007) and cultured human EGC (Shamblott et al., 1998) express TRA-1-60 and -1-81, but, surprisingly, PGC in the early developing human male gonad (7–15 weeks post-fertilization) are TRA-1-60 and -1-81-negative (Kerr et al., 2007). Thus, it appears that there are differences in marker expression of germline cells depending on the source of the cells and on the cellular microenvironment (PGC in situ versus EGC in vitro).
Recently, pluripotent cells have also been isolated from the postnatal mouse testis (Kanatsu-Shinohara et al., 2004a; Guan et al., 2006; Seandel et al., 2007). However, to date, there are no reports on the isolation of pluripotent cell lines from adult primate testes. This might be, at least in part, due to the fact that the rodent and primate testes are differently organized (Ehmcke and Schlatt, 2006). In rodents, a very small population of Asingle-spermatogonia persist throughout life. This small population appears to be the target population for the derivation of pluripotent cells in the rodent. The enormous numbers of germ cells in the rodent testis are established through extended mitotic expansion during spermatogenesis. This is different in primates where mitotic expansion is limited. A much larger population of spermatogonia exists, which is subdivided into mitotically quiescent reserve stem cells and self-renewing progenitors (Ehmcke and Schlatt, 2006). We have shown that renewal and differentiation of spermatogonia in monkeys occurs via clonal expansion (Ehmcke et al., 2005a,b). Based on this, we have recently proposed a new model for spermatogonial stem-cell populations and their kinetics in monkey and man. We postulate that the adult primate testis contains no Asingle-like cells as seen in rodents, but that most of the spermatogonia in the pool of self-renewing progenitors have reached a more differentiated stage. We hypothesized that it is therefore more difficult to isolate developmentally primitive primate germ cells, which are potentially pluripotent.
Here, we investigated the expression of the cell surface glycan markers SSEA-1, -3 and -4 and TRA-1-60 and 1-81 in the adult testes of a new world-primate species (Callithrix jacchus) and in the testes of the two old world-primate species Macaca mulatta and Macaca silenus and found that SSEA-4 and TRA-1-60 and 1-81 are expressed by spermatogonia in C. jacchus. In the Macaque testes, we could detect only SSEA-4 and TRA-1-81, but not TRA-1-60. In human testes, we were not able to clearly demonstrate expression of any of these marker molecules.
Materials and Methods
Human testis tissue
Human testis tissue was obtained from the Health Sciences Tissue Bank of the UPMC Shadyside-Presbyterian Hospital under the license of the University of Pittsburgh IRB #0506140 (Exempt). Three normal tissue samples were obtained from adult (one person at the age of 21 years and two persons of unknown age) accident victims. Two other tissue samples showing normal spermatogenesis were non-tumor parts of adult testes from two patients with testicular cancer. In total, five human testicular samples were analyzed.
Animals
Animal husbandry was in accordance with the German Federal Law on the Care and Use of Laboratory Animals. The monkeys had access to species-specific pelleted food and unlimited access to tap water, and monkey chow was supplemented daily with fresh fruit. Animals were killed by an overdose of anesthetic, and the testes were surgically removed and immediately processed for further analyses. At the time of organ recovery, all animals were adult and without any pharmacological or other treatment. The rhesus monkeys used in this study are seasonal breeders (Herndon et al., 1996) and retain the seasonal pattern of reproduction of the colony from which they originally stem for several years (Kaup, Head of primate husbandry and infection pathology of the German Primate Center, personal communication). The first rhesus macaque (born in Germany in the German Primate Center's breeding colony) was killed on 31 May 2007 at the age of 3 years and 1 month (body weight 4.76 kg). The second rhesus monkey was imported from a Chinese breeding colony and killed on 20 July 2007 at the age of 8 years and 8 months (10.71 kg). The testes were recovered during the period of testicular recrudescence. Although there is seasonality in reproductive functions and behavior in rhesus monkeys, there is ongoing spermatogenesis in parts of the seminiferous tubules at any time during the seasonal changes (Kaup, personal communication). The testes used in this study showed variable states of the seminiferous tubules with regard to the diameter and the thickness and composition of the germinal epithelium. However, all seminiferous tubules exhibited postmeiotic germ cells with a significant portion of the tubules showing qualitatively and quantitatively normal spermatogenesis. The lion-tailed macaque was 19 years and 9 months (10.96 kg) and exhibited full spermatogenesis in all tubules. The marmosets were killed at the ages of 21, 22, 35 months and 15 years and 6 months, respectively. Body weights ranged from 245 to 403 g. All marmosets exhibited full spermatogenesis in all tubules.
Immunohistochemistry
Testicular tissue was fixed by immersion in Bouin's fixative for at least 12 h immediately after recovery of the organs and embedded in paraffin using standard techniques. For immunohistological evaluation, Bouin's-fixed and paraffin-embedded specimens were sectioned at 5 µm. Sections were rehydrated in decreasing ethanol concentration. Endogenous peroxidase was inhibited by incubation with peroxidase blocking reagent (DakoCytomation Carpinteria, CA, USA, LSAB+ system-HRP, K0679). Unspecific binding of the first antibody was blocked by a 30 min incubation step in 5% (w/v) BSA in 0.05 mol/l Tris–HCl, 0.15 mol/l NaCl pH 7.6 (TBS). Antibodies used were anti-SSEA-1 (clone MC-480; MAB4301), anti-SSEA-3 (clone MC-631; MAB4303), anti-SSEA-4 (clone MC-813-70; MAB4304), TRA-1-60 (clone TRA-1-60; MAB4360) and TRA-1-81 (clone TRA-1-81; MAB4381), all used at a 1:50 dilution in 5% BSA in TBS. All primary antibodies were purchased from Millipore (previously Chemicon; Schwalbach, Germany). All incubation steps were done in a humid chamber and incubations with the primary antibodies were performed over night at 4°C. DakoCytomation Universal LSAB Plus-kit (K0679) including biotinylated second antibody polymer directed against the monoclonal antibodies generated in mouse was used in case of SSEA-1, SSEA-4, TRA-1-60 and TRA-1-81. To detect SSEA-3 generated in rat, we used a biotinylated polyclonal rabbit anti-rat antibody (1:200; DakoCytomation, K0467). All antibodies were visualized using horseradish peroxidase (HRP) conjugated streptavidin. 3,3′-Diaminobenzidine (DAB) chromogen was used as substrate for the HRP and Mayer's hematoxylin as counterstain. Control stains were carried out omitting the primary antibodies.
Sample analysis
The number of stained cells in immunohistochemistry was determined based on the tissue samples given in Table I. Approximate staging of the seminiferous epithelium of the marmoset was done according to the scheme proposed by Millar et al. (2000).
Table I.
Tissue samples used and analyzed in this study.
| Species | Number of animals | Number of testes investigated | Tubules SSEA-41 tubules TRA-1-812 | SSEA-4-positive cells/tubule | TRA-1-81-positive cells/tubule |
|---|---|---|---|---|---|
| Callithrix jacchus | 4 | 5 | 58/10 | 12.6 ± 3.5 | 5.0 ± 2.16 |
| 55/10 | |||||
| Macaca mulatta | 2 | 3 | 77/20 | 2.66 ± 1.65 | 1.36 ± 1.18 |
| 69/20 | |||||
| Macaca silenus | 1 | 2 | Not evaluated | n.d. | n.d. |
1Number of roundish tubules evaluated for SSEA-4 expression (total/min per testis). 2Number of roundish tubules evaluated for TRA-1-81 expression (total/min per testis). n.d., not determined.
Flow cytometry
Flow cytometry, using the SSEA-4 antibody which was also used for immunohistochemical staining, was applied to three adult marmoset testes in three independent experiments. Testis tissue was manually dissociated in PBS using a scalpel and further dissociated by collagenase IV digestion (30 min, 37°C). The cells were washed two times in PBS and incubated with primary antibody (1:100) for 30 min at room temperature. The primary antibody was removed by washing with PBS two times, and the cells were incubated with A488 conjugated secondary antibody (1:50) for 30 min in the dark. After two washes with PBS, the cells were transferred into PBS containing propium iodine (1:10 000). For each experiment, 20 000 cells were measured by a BDLSR II FACS DIVA device. As controls and to set the gates, unstained cells and cells lacking primary antibody were used.
Results
Stage-specific embryonic antigens in C. jacchus testes
Staining for SSEA-4 resulted in strong staining of spermatogonial cells in C. jacchus (Fig. 1a–c). In contrast, SSEA-1 and -3 could not be detected in the common marmoset testis (not shown). SSEA-4-labeled spermatogonia were always in contact with the basal membrane (Fig. 1b and c (inset)). Single cells and short chains of up to four cells were stained in the two-dimensional tissue section. The stain was always localized on the cell membrane (Fig. 1c, red arrows) and in the cytoplasm, where it appeared to be evenly distributed (Fig. 1c, inset). Nuclei were never stained. There were 12.6 ± 3.5 (range 7–21, n = 58 tubules) cells, which were SSEA-4-positive per roundish tubular cross-section. Expression of SSEA-4 could be seen in all stages of the cycle of the seminiferous epithelium.
Figure 1:
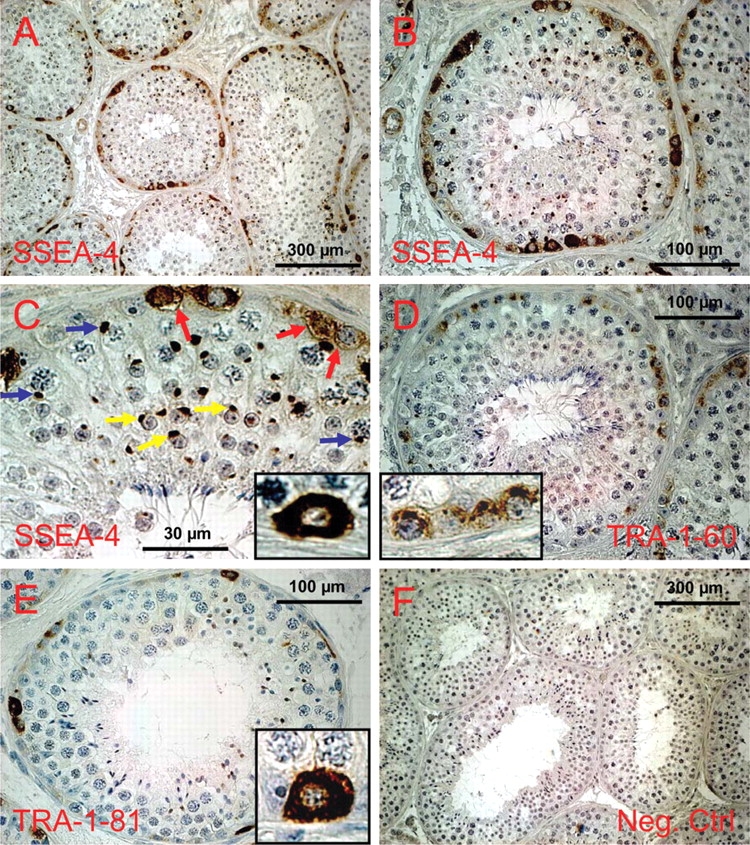
Expression of glycan stem-cell markers in the testis of C. jacchus as revealed by immunohistochemistry.
(a) SSEA-4 expression, overview. (b) SSEA-4 expression in a roundish cross-section of a seminiferous tubule. All labeled cells are in contact with the basal lamina. (c) Higher magnification of the upper part of the tubule shown in (b). Spermatocytes show a strongly stained perinuclear dot (blue arrows) which most likely represents the XY body. In spermatids the acrosome is stained (yellow arrows). Spermatogonia exhibit homogenous cytoplasmic and membrane staining (red arrows and inset), while the nuclei are devoid of stain. (d) A tubule showing TRA-1-60 positive spermatogonia, which are also invariably in contact with the basal membrane. However, subcellular distribution of the stain is clearly different from that of the other glycan markers. TRA-1-60 antigen is concentrated in the apical part of the spermatogonia (inset). Decreased stain was also detected in the lateral parts of the cells, while no stain was found in the cytoplasmic and membrane compartment between the nucleus and the basal membrane (inset). (e) Expression of TRA-1-81. Fewer cells are positive compared to the other glycan markers and those cells labeled are almost exclusively single cells. Subcellular distribution of the stain resembles SSEA-4, i.e. the cytoplasm is homogenously stained, but the nucleus is free of stain (inset). (f) Negative control for all antibodies (all from mouse) used.
In addition to spermatogonial cells, the SSEA-4 antibody also labeled in the marmoset testis subcellular compartments in spermatocytes and spermatids (Fig. 1c). Higher magnification revealed that the stained structure in spermatocytes appears as a solid round structure at the periphery of the nucleus and is always in contact with the nuclear envelope (Fig. 1c, blue arrows) indicating that this structure is likely the XY body, in which the X- and Y-chromosomes are secluded during certain phases of meiosis. In spermatids, the SSEA-4 antibody binds to the developing acrosomal vesicle of round spermatids (Fig. 1c, yellow arrows).
Weak staining (compared with that of spermatogonia) was also observed on endothelial cells of blood vessels (Fig. 1b, leftmost).
TRAs in C. jacchus
TRA-1-60 and -1-81 are both expressed by spermatogonia in the marmoset testis. TRA-1-60 exhibited only weak signals compared with SSEA-4 and TRA-1-81 (Fig. 1d). Positive cells were not detected in all seminiferous tubules. Light microscopic distribution of TRA-1-60 in spermatogonia was rather heterogenous with mostly apically localized signals (Fig. 1d, inset). The basal parts of the labeled spermatogonia were usually devoid of stain. TRA-1-81 was also expressed by spermatogonial cells and its labeling was much stronger than that of TRA-1-60 (Fig. 1e). Like SSEA-4, TRA-1-81 could be strongly detected in the cytoplasm and on the cell membrane. The nuclei were not stained. One to 10 and on average 5.0 (±2.16; n = 55 tubules) cells were TRA-1-81-positive in each tubule and these were mostly single spermatogonia and rarely pairs of spermatogonia.
Stage-specific embryonic antigens in macaque testes
In the testes of M. silenus and M. mulatta, we were also not able to detect either SSEA-1 or SSEA-3. However, SSEA-4 was clearly expressed in both macaque species by spermatogonia (Fig. 2b, c and e). In the macaques, mostly single spermatogonia and sometimes pairs of spermatogonia were stained. In the rhesus monkey, 2.66 (±1.65, range 0–7; n = 77 tubules) SSEA-4-positive spermatogonia were seen per roundish tubular cross-section. Localization of SSEA-4 in the macaque spermatogonia was also cytoplasmic and on the membrane. Also in the macaques, SSEA-4 expression could be observed during the whole seminiferous epithelial cycle. We also observed no relation between SSEA-4 expression and the state of the seminiferous tubules during recrudescence of the rhesus monkey testes. No binding of the antibody was observed in macaque spermatocytes and spermatids (Fig. 2c and e) in contrast to the findings in the marmoset testis (Fig. 1c).
Figure 2:
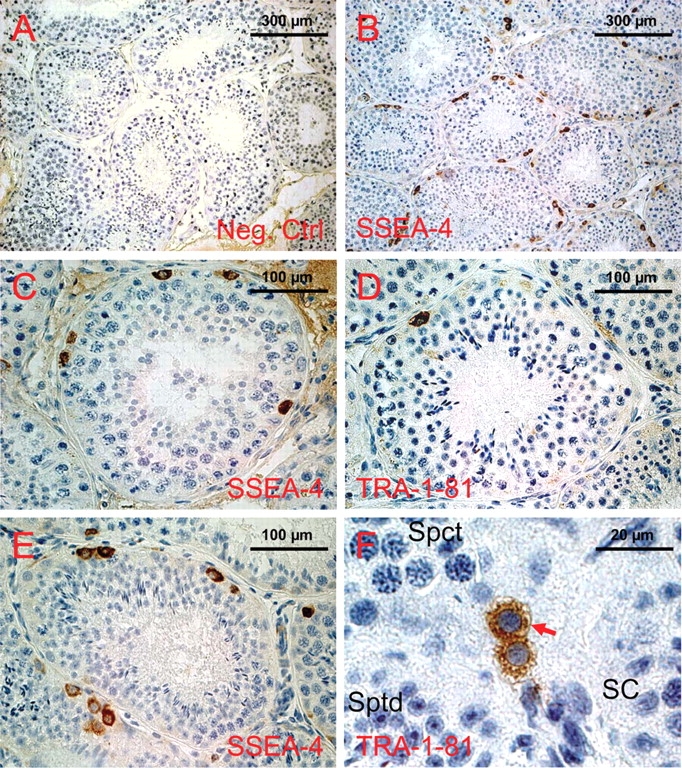
Expression of glycan stem-cell markers in the testes of the Lion-tailed macaque (Macaca silenus) and the Rhesus monkey (Macaca mulatta) as revealed by immunohistochemistry.
(a) Negative control valid for all stainings shown in this figure. (b) SSEA-4 expression in the testis of the Lion-tailed macaque, overview. (c) A representative roundish tubule showing clearly stained spermatogonia, while all other cells are completely devoid of stain. Like in the marmoset testis (see Fig. 1), the cells' membranes and cytoplasm are stained. The nucleus is free of stain. (d) Expression of TRA-1-81 in the Lion-tailed macaque's testis. Very few spermatogonial cells per tubule are labeled. Subcellular localization of TRA-1-81 is (like in the marmoset testis, see Fig. 1) at the light microscopical level indistinguishable from that of SSEA-4. (e) SSEA-4 expression in the Rhesus macaque testis was comparable to (c). (f) Para-sagittal section through a seminferous tubule in the Rhesus monkey testis showing a pair of TRA-1-81 positive cells. It is clearly visible that the cytoplasm and the cell membrane (red arrow heads) are stained. The nuclei of the spermatogonia, as well as all other cell types such as spermatocytes (Spct), Spermatids (Sptd) and Sertoli cells (SC), are totally devoid of stain.
TRAs in macaque testes
We detected TRA-1-81 in the testes of both macaque species (Fig. 2d and f). Single cells and rarely pairs of cells were labeled (in rhesus macaque average 1.36 ± 1.18 cells/tubule, range 0–4; n = 69 tubules). Fig. 2d shows a typical example of a single stained spermatogonial cell in a cross-section of a seminiferous tubule of the Lion-tailed macaque. Fig. 2f shows one of the rare pairs of TRA-1-81-positive spermatogonia in a para-sagittal section through a seminiferous tubule in the rhesus monkey. The cytoplasm and the membrane of the spermatogonia are clearly stained. In contrast, the nuclei of the spermatogonia, as well as all other cell types in this section such as spermatocytes, spermatids and Sertoli cells, are devoid of any stain.
Glycan stem-cell markers in human testes
We failed clearly to stain spermatogonia in the human testis with any of the antibodies used in this study. All antibodies caused a strong background stain over the whole germinal epithelium in the human testes. Potential staining of spermatogonia was only very weakly above background staining (not shown) and therefore not obvious and convincing. No improvement of staining was obtained by different variations of the protocol for immunohistochemistry such as antigen retrieval by cooking the sections in different buffers in the microwave oven.
Flow cytometric analysis of SSEA-4-positive cells in adult marmoset testes
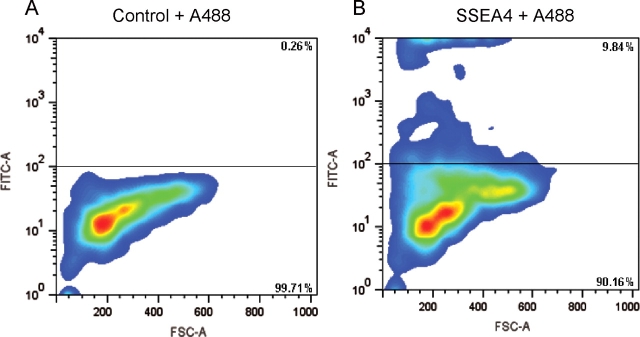
Using flow cytometry, we detected on average 8.6 ± 1.61% SSEA-4-positive cells in adult marmoset testes (n = 3). The data from Experiment 2 are shown as representative example in Fig. 3. As shown in the right panel of Fig. 3, there are two clearly distinct major populations of cells considered SSEA-4-positive. The slightly SSEA-4-positive population comprises 5.60 ± 1.25%. Additionally, we obtained a distinct population of more intensely labeled SSEA-4 positive cells, which accounts for 3.0 ± 0.47% of all cells analyzed.
Figure 3:
Flow cytometric determination of the percentage of SSEA-4-positive cells in the adult marmoset testis.
(a) Negative control omitting the SSEA-4 antibody but including the fluorescent A488 secondary antibody. Only 0.26% of all cells are above the set threshold level, above which cells were considered SSEA4-positive. (b) After incubation of the cells with SSEA-4 antibody 9.84% of all cells are above the threshold. Note that there are two distinct major populations of cells above the threshold level.
Discussion
The integrity of a stem cell depends on appropriate signaling and interaction with its stem-cell niche. For example, Notch, Wnt, Bmp, Shh and GDNF signals play important roles to maintain the stem-cell pools in different organs (Jones and Wagers, 2008). Glycans exist on the surface of all cells and are involved in modulating the function of several signaling molecules (Haltiwanger, 2002), including Notch, Wnt and FGF2. In that, glycans are also involved in the interaction and communication of a stem cell with its niche. Although not being essential for human ESC pluripotency (Brimble et al., 2007), SSEA-3 and -4 as well as the other glycans investigated in this study are well-established markers for stem cells. Here, we show that SSEA-4, TRA-1-60 and -1-81 are also expressed by adult non-human primate spermatogonia and speculate that these glycans modulate spermatogonial stem-cell properties or are even essential for their function in non-human primates.
During the last years several immunological, genetic and selective dye-dependent approaches have been established for the enrichment and isolation of spermatogonial stem cells from the mouse testis (von Schonfeldt et al., 1999; Shinohara et al., 2000; Giuili et al., 2002; Falciatori et al., 2004; Kanatsu-Shinohara et al., 2004b; Lo et al., 2005; Guan et al., 2006). However, to our knowledge, there were no successful attempts to isolate and cultivate spermatogonial stem cells from the adult non-human primate or human testis. This might be due to limited access to the tissue and to the fact that genetic labeling of primate germ cells has so far not been successful. Here, we show according to our knowledge for the first time that SSEA-4 and TRA-1-81 might be useful glycan surface markers for the enrichment and isolation of spermatogonia, and possibly of spermatogonial stem cells, from the adult non-human primate testis in all three non-human primate species tested. Previous studies failed to detect the glycan markers SSEA-1 and SSEA-3 (Damjanov et al., 1982) and TRA-1-60 (Giwercman et al., 1993) in adult human testes, while all antigens were found on germ cells in the fetal human gonad (Damjanov et al., 1982; Giwercman et al., 1993). In contrast to the human (Giwercman et al., 1993) and old-world primate testis (this study), in C. Jacchus, TRA-1-60 was expressed in spermatogonia in the adult testis and might also represent a useful testicular stem-cell marker in this species. Interestingly, PGC have recently been enriched from the early human fetal testis using SSEA-1-based magnetic cell sorting (MACS) (Kerr et al., 2007), demonstrating the great potential of using glycan surface markers for isolating cells in primates where many valuable genetic tools proved useful in rodents are missing. Application of MACS with the antibodies used in this study to the adult non-human primate will substantially promote germline stem-cell research in non-human primates.
Using flow cytometry, we detected 8.6 ± 1.61% SSEA-4-positive cells. However, there were two clearly distinct cell populations (Fig. 3). The population that was strongly positive for SSEA-4 accounted for 3.0 ± 0.47% of all cells. We assume that this population represents the spermatogonia since (i) on the tissue sections there was no cell type that showed stronger SSEA-4 staining than spermatogonia and (ii) the portion of strongly labeled cells in the flow cytometry and in immunohistochemistry is in good agreement. In average, we detected 12.6 ± 3.5 labeled cells per tubular cross-section. In five randomly chosen cross-sections (5 µm) of hematoxylin/eosin-stained seminiferous tubules from the marmoset, we counted between 266 and 350 cell nuclei. Thus, around 4% of all cells in a 5 µm section of the seminiferous tubule were SSEA-4-positive. We speculate that the remaining SSEA-4-positive fraction of cells showing weaker signals comprises endothelial cells of the blood vessels. These cells are also weakly SSEA-4-positive in tissue sections.
The biological significance of our findings beyond the methodological usefulness will be investigated in future studies by systematic analysis of the developmental capacities of enriched cells. In the future, these primate testicular stem cells might become a valuable source of pluripotent cells which can be used after differentiation in (pre-)clinical cell replacement therapy approaches. Currently, we speculate that the expression of the pluripotency markers as shown in this study reflects a rather primitive, undifferentiated developmental stage of the primate spermatogonial stem cell belonging to the reserve stem-cell pool. Especially, TRA-1-81 mostly labels only single cells likely indicating that this marker is expressed by true stem cells from the reserve pool undergoing only infrequent cell divisions, which is a characteristic of reserve stem cells.
In contrast to the macaques, in C. jacchus, SSEA-4 as well as TRA-1-60 usually tag clones of cells and not single cells. In fact, it has to be considered that the chains of stained cell that can be seen in the tissue sections most likely represent clones of up to 16 cells in the three-dimensional germinal epithelium. In general, TRA-1-81 appears to be a more specific marker for spermatogonial stem cells than SSEA-4 since in the marmoset as well as in the macaques less cells are TRA-1-81-positive than SSEA-4. Moreover, in the macaques, there are less SSEA-4 and TRA-1-81-positive cells per tubule than in the marmoset. This may reflect different forms of organization of the germinal epithelium in macaques and Callithrichidae or simply a decreased degradation rate of the glycan antigens after cell division in the marmoset testis compared with the macaque testes so that the glycans persist on the daughter cells.
We also tested the expression of the markers in the human testis according to our current protocol and also by modified methods including antigen retrieval variations. However, so far we failed to clearly identify spermatogonia in the human testis by staining for SSEA-4 or TRA-1-81. This failure might be due to technical problems and might not reflect a basic biological difference between human and non-human primate spermatogonia. We assume that this might be a preservation or accessibility problem of the antigens on the human tissue. Stainings of alternatively fixed and treated human testes tissues will reveal whether the glycan stem-cell markers can also be demonstrated on human spermatogonia. Indeed, a previous study reported SSEA-4 on spermatogonia in the adult human testis after paraformaldehyde fixation and subsequent cryopreservation (Tokuyama et al., 2003). However, the stainings presented by Tokuyama et al. (2003) appear not to be restricted to cells in contact with the basement membrane. Therefore, the question remains whether all cells labeled by the SSEA-4 antibody in this previous study are really spermatogonia.
In summary, we show here for the first time that spermatogonia in the adult non-human primate testis can be clearly identified by the use of the monoclonal antibodies recognizing the glycan epitopes, SSEA-4 and TRA-1-81.
Funding
This study was supported by an in-house grant from the German Primate Center and by NICHD/NIH through cooperative agreement (U54 08 160; project 1) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research.
Acknowledgement
We appreciate the excellent technical assistance of Marion Niebeling and the support of Dr Jens Ehmcke.
References
- Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimble SN, Sherrer ES, Uhl EW, Wang E, Kelly S, Merrill AH, Jr, Robins AJ, Schulz TC. The cell surface glycosphingolipids SSEA-3 and SSEA-4 are not essential for human ESC pluripotency. Stem Cells. 2007;25:54–62. doi: 10.1634/stemcells.2006-0232. [DOI] [PubMed] [Google Scholar]
- Damjanov I, Fox N, Knowles BB, Solter D, Lange PH, Fraley EE. Immunohistochemical localization of murine stage-specific embryonic antigens in human testicular germ cell tumors. Am J Pathol. 1982;108:225–230. [PMC free article] [PubMed] [Google Scholar]
- Ehmcke J, Schlatt S. A revised model for spermatogonial expansion in man: lessons from non-human primates. Reproduction. 2006;132:673–680. doi: 10.1530/rep.1.01081. [DOI] [PubMed] [Google Scholar]
- Ehmcke J, Luetjens CM, Schlatt S. Clonal organization of proliferating spermatogonial stem cells in adult males of two species of non-human primates, Macaca mulatta and Callithrix jacchus. Biol Reprod. 2005;a 72:293–300. doi: 10.1095/biolreprod.104.033092. [DOI] [PubMed] [Google Scholar]
- Ehmcke J, Simorangkir DR, Schlatt S. Identification of the starting point for spermatogenesis and characterization of the testicular stem cell in adult male rhesus monkeys. Hum Reprod. 2005;b 20:1185–1193. doi: 10.1093/humrep/deh766. [DOI] [PubMed] [Google Scholar]
- Falciatori I, Borsellino G, Haliassos N, Boitani C, Corallini S, Battistini L, Bernardi G, Stefanini M, Vicini E. Identification and enrichment of spermatogonial stem cells displaying side-population phenotype in immature mouse testis. FASEB J. 2004;18:376–378. doi: 10.1096/fj.03-0744fje. [DOI] [PubMed] [Google Scholar]
- Giuili G, Tomljenovic A, Labrecque N, Oulad-Abdelghani M, Rassoulzadegan M, Cuzin F. Murine spermatogonial stem cells: targeted transgene expression and purification in an active state. EMBO Rep. 2002;3:753–759. doi: 10.1093/embo-reports/kvf149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giwercman A, Andrews PW, Jorgensen N, Muller J, Graem N, Skakkebaek NE. Immunohistochemical expression of embryonal marker TRA-1–60 in carcinoma in situ and germ cell tumors of the testis. Cancer. 1993;72:1308–1314. doi: 10.1002/1097-0142(19930815)72:4<1308::aid-cncr2820720426>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Guan K, Nayernia K, Maier LS, Wagner S, Dressel R, Lee JH, Nolte J, Wolf F, Li M, Engel W, et al. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature. 2006;440:1199–1203. doi: 10.1038/nature04697. [DOI] [PubMed] [Google Scholar]
- Haltiwanger RS. Regulation of signal transduction pathways in development by glycosylation. Curr Opin Struct Biol. 2002;12:593–598. doi: 10.1016/s0959-440x(02)00371-8. [DOI] [PubMed] [Google Scholar]
- Herndon JG, Bein ML, Nordmeyer DL, Turner JJ. Seasonal testicular function in male rhesus monkeys. Horm Behav. 1996;30:266–271. doi: 10.1006/hbeh.1996.0032. [DOI] [PubMed] [Google Scholar]
- Jones DL, Wagers AJ. No place like home: anatomy and function of the stem cell niche. Nat Rev Mol Cell Biol. 2008;9:11–21. doi: 10.1038/nrm2319. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Inoue K, Lee J, Yoshimoto M, Ogonuki N, Miki H, Baba S, Kato T, Kazuki Y, Toyokuni S, et al. Generation of pluripotent stem cells from neonatal mouse testis. Cell. 2004;a 119:1001–1012. doi: 10.1016/j.cell.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Toyokuni S, Shinohara T. CD9 is a surface marker on mouse and rat male germline stem cells. Biol Reprod. 2004;b 70:70–75. doi: 10.1095/biolreprod.103.020867. [DOI] [PubMed] [Google Scholar]
- Kerr CL, Hill CM, Blumenthal PD, Gearhart JD. Expression of Pluripotent Stem Cell Markers in the Human Fetal Testis. Stem Cells. 2008;26:412–421. doi: 10.1634/stemcells.2007-0605. [DOI] [PubMed] [Google Scholar]
- Lanctot PM, Gage FH, Varki AP. The glycans of stem cells. Curr Opin Chem Biol. 2007;11:373–380. doi: 10.1016/j.cbpa.2007.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo KC, Brugh VM, 3rd, Parker M, Lamb DJ. Isolation and enrichment of murine spermatogonial stem cells using rhodamine 123 mitochondrial dye. Biol Reprod. 2005;72:767–771. doi: 10.1095/biolreprod.104.033464. [DOI] [PubMed] [Google Scholar]
- Millar MR, Sharpe RM, Weinbauer GF, Fraser HM, Saunders PT. Marmoset spermatogenesis: organizational similarities to the human. Int J Androl. 2000;23:266–277. doi: 10.1046/j.1365-2605.2000.00236.x. [DOI] [PubMed] [Google Scholar]
- Sasaki E, Hanazawa K, Kurita R, Akatsuka A, Yoshizaki T, Ishii H, Tanioka Y, Ohnishi Y, Suemizu H, Sugawara A, et al. Establishment of novel embryonic stem cell lines derived from the common marmoset (Callithrix jacchus) Stem Cells. 2005;23:1304–1313. doi: 10.1634/stemcells.2004-0366. [DOI] [PubMed] [Google Scholar]
- Seandel M, James D, Shmelkov SV, Falciatori I, Kim J, Chavala S, Scherr DS, Zhang F, Torres R, Gale NW, et al. Generation of functional multipotent adult stem cells from GPR125+ germline progenitors. Nature. 2007;449:346–350. doi: 10.1038/nature06129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamblott MJ, Axelman J, Wang S, Bugg EM, Littlefield JW, Donovan PJ, Blumenthal PD, Huggins GR, Gearhart JD. Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc Natl Acad Sci USA. 1998;95:13726–13731. doi: 10.1073/pnas.95.23.13726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara T, Orwig KE, Avarbock MR, Brinster RL. Spermatogonial stem cell enrichment by multiparameter selection of mouse testis cells. Proc Natl Acad Sci USA. 2000;97:8346–8351. doi: 10.1073/pnas.97.15.8346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Kalishman J, Golos TG, Durning M, Harris CP, Becker RA, Hearn JP. Isolation of a primate embryonic stem cell line. Proc Natl Acad Sci USA. 1995;92:7844–7848. doi: 10.1073/pnas.92.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JA, Kalishman J, Golos TG, Durning M, Harris CP, Hearn JP. Pluripotent cell lines derived from common marmoset (Callithrix jacchus) blastocysts. Biol Reprod. 1996;55:254–259. doi: 10.1095/biolreprod55.2.254. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Tokuyama S, Saito S, Takahashi T, Ohyama C, Ito A, Kanto S, Satoh M, Hoshi S, Endoh M, Arai Y. Immunostaining of stage-specific embryonic antigen-4 in intratubular germ cell neoplasia unclassified and in testicular germ-cell tumors. Oncol Rep. 2003;10:1097–1104. [PubMed] [Google Scholar]
- von Schonfeldt V, Krishnamurthy H, Foppiani L, Schlatt S. Magnetic cell sorting is a fast and effective method of enriching viable spermatogonia from Djungarian hamster, mouse, and marmoset monkey testes. Biol Reprod. 1999;61:582–589. doi: 10.1095/biolreprod61.3.582. [DOI] [PubMed] [Google Scholar]
- Zwaka TP, Thomson JA. A germ cell origin of embryonic stem cells? Development. 2005;132:227–233. doi: 10.1242/dev.01586. [DOI] [PubMed] [Google Scholar]