Abstract
BACKGROUND
High isoflavone intake has been related to decreased fertility in animal studies, but data in humans are scarce. Thus, we examined the association of soy foods and isoflavones intake with semen quality parameters.
METHODS
The intake of 15 soy-based foods in the previous 3 months was assessed for 99 male partners of subfertile couples who presented for semen analyses to the Massachusetts General Hospital Fertility Center. Linear and quantile regression were used to determine the association of soy foods and isoflavones intake with semen quality parameters while adjusting for personal characteristics.
RESULTS
There was an inverse association between soy food intake and sperm concentration that remained significant after accounting for age, abstinence time, body mass index, caffeine and alcohol intake and smoking. In the multivariate-adjusted analyses, men in the highest category of soy food intake had 41 million sperm/ml less than men who did not consume soy foods (95% confidence interval = –74, –8; P, trend = 0.02). Results for individual soy isoflavones were similar to the results for soy foods and were strongest for glycitein, but did not reach statistical significance. The inverse relation between soy food intake and sperm concentration was more pronounced in the high end of the distribution (90th and 75th percentile) and among overweight or obese men. Soy food and soy isoflavone intake were unrelated to sperm motility, sperm morphology or ejaculate volume.
CONCLUSIONS
These data suggest that higher intake of soy foods and soy isoflavones is associated with lower sperm concentration.
Keywords: soy, isoflavones, semen analysis, sperm concentration, infertility
Introduction
Xenoestrogens have been suggested to play a role in a variety of male reproductive disorders including possible declines in sperm concentration (Sharpe, 2001; Skakkebaek et al., 2001). Isoflavones are plant-derived polyphenolic compounds with estrogenic activity and are found mainly in soy beans and soy-derived products. They are generally considered to have a weak estrogenic activity, being able to bind estrogen receptor (ER) α with an affinity 100–1000 times lower than estradiol (Miksicek, 1994; Kuiper et al., 1998; Song et al., 1999; Matthews et al., 2000; Branham et al., 2002; Harris et al., 2002). Nevertheless, isoflavones have also been found to bind strongly to membrane ERs (Thomas and Dong, 2006) and to exert non-genomic actions potentially deleterious to male fertility (Fraser et al., 2006). In addition, isoflavones have been related to male reproductive disorders in mammals, including impaired development of reproductive organs, especially following intrauterine exposure (Atanassova et al., 2000). Data on humans are scarce, however, and often inconsistent with the preponderance of animal data. Thus, whether consuming soy foods during adulthood could affect fertility in men is still an unresolved question. Here, we present a cross-sectional analysis relating soy food and isoflavone intake to semen quality parameters among men presenting for semen analysis at an infertility clinic in an academic medical center.
Materials and Methods
Male partners in subfertile couples who presented for evaluation at the Massachusetts General Hospital Fertility Center between 2000 and 2006 were invited to participate in an ongoing study of environmental factors and fertility (Hauser et al., 2006). Approximately 60% of eligible men agreed to participate. Men presenting for post-vasectomy semen analysis were not invited to participate. The study was approved by the Human Subject Committees of the Harvard School of Public Health and the Massachusetts General Hospital, and informed consent was obtained from all participants.
A semen sample was produced on-site by masturbation into a sterile plastic specimen cup. After collection, the sample was liquefied at 37°C for 20 min before analysis. Men were instructed to abstain from ejaculation for 48 h before producing the semen sample. All semen samples were analyzed for sperm concentration and motion parameters by CASA (Hamilton-Thorn Version 10HTM-IVOS) as previously described (Duty et al., 2003, 2004). Sperm morphology was determined using the strict criteria described by Kruger et al. (1988). Results were expressed as percent normal spermatozoa.
Height and weight were measured on-site by trained personnel. In addition, men were asked to complete a questionnaire to report the length of sexual abstinence prior to providing the semen sample and to collect information on medical history and lifestyle factors. The questionnaire contained a reduced food frequency questionnaire that included 15 soy-based foods (Appendix 1). Men were asked to report how often, on average, they consumed each of these 15 foods during the preceding 3 months and to describe the usual serving size for each food in relation to a specified ‘medium’ serving size. There were nine possible frequencies of intake ranging from never or less than once per month to twice or more per day, and three possible usual serving sizes: medium (the specified serving size), small (less than specified) and large (more than specified). The isoflavone content of each food and specified portion size was obtained from a database developed by the United States Department of Agriculture (United States Department of Agriculture, 2007). Intakes of total and specific isoflavones (daidzein, genistein and glycitein) were estimated by summing the isoflavone contribution of all food items in the questionnaire.
Statistical analysis
Of the 598 men enrolled in the main study, 140 provided information about their intake of soy foods, corresponding to all men enrolled since the introduction of the soy food intake questionnaire into the study. Among these 140 men, 40 men were excluded from the statistical analysis because they did not provide a semen sample. One azoospermic man was also excluded to prevent undue influence from extreme sperm counts and because the mechanism responsible for azoospermia may be related to obstructive or genetic causes rather than environmental influences. This left 99 men with complete dietary and semen analysis data available for analyses. These men were divided into four groups according to their soy food and isoflavone intake. The reference group included men without any intake of soy or each of the isoflavones examined. Men with any consumption of soy foods or specific isoflavones were divided into three groups according to tertiles of intake. To examine the association between soy food and isoflavone intake, we first calculated means and standard deviations of semen analysis parameters (ejaculate volume, total sperm count, sperm concentration, sperm motility and sperm morphology) for each of the four intake categories of soy foods and isoflavones. We then used linear regression to estimate the mean difference in semen analysis parameters between men who did not consume soy or specific isoflavones and men consuming increasing amounts of these products, while accounting for differences in age, abstinence time, body mass index (BMI), smoking status and intakes of caffeine and alcohol. Robust estimators of the variance were used in the computation of 95% confidence intervals (CI) around the mean to account for potential differences in the variance across intake groups (White, 1980). Tests for trend were conducted using a variable with the median intakes in each category as a continuous variable in the linear regression models.
To examine the possibility that the relationship between soy food or isoflavone intake may affect men with high sperm counts differently than men with low sperm counts, we used quantile regression (Koenker and Bassett, 1978) to model the relationship between soy food intake and specific percentiles of the sperm concentration distribution (10th, 25th, 50th, 75th and 90th) while accounting for differences in personal characteristics. We examined the possibility that the relationship of soy food intake and sperm concentration differed according to BMI and age by introducing cross-product terms between soy food intake and the variables of interest. All analyses were conducted using Statistical Analysis Software (SAS) version 9.1 (SAS Institute Inc., Cary, NC, USA).
Results
Men were primarily Caucasian (90%) with a mean (SD) age of 36.4 (5.0) years. The majority of men were overweight or obese (72%) defined as a BMI ≥25 kg/m2. Most of the men had never smoked (74%) and only four were current smokers. Overall, 42% of men had a normal semen analysis whereas 10% had a sperm concentration below 20 million/ml, 55% of men had <50% motile sperm and 26% of men had <4% normal morphology sperm. The mean intake of isoflavones was 5.4 mg/day. There were no appreciable differences in age, BMI, height, abstinence time, smoking status or intakes of caffeine or alcohol across levels of soy food intake (Table I). As expected, intake of specific isoflavones increased with increasing intake of soy foods.
Table I.
Characteristics of the study population by soy foods intake (N = 99).
| Range of intake frequency | Total soy foods intake |
|||
|---|---|---|---|---|
| Never | <2/month | 2/month to 2/week | ≥2/week | |
| N | 39 | 18 | 22 | 20 |
| Age, years | 36.3 (4.2) | 35.3 (4.1) | 36.7 (5.8) | 37.2 (6.7) |
| Body mass index, kg/m2 | 28.0 (5.0) | 25.0 (2.3) | 28.2 (5.8) | 26.1 (3.7) |
| Height, cm | 181.7 (6.7) | 182.3 (7.1) | 182.1 (7.2) | 181.7 (6.9) |
| Abstinence time, days | 3.2 (1.6) | 3.8 (2.8) | 4.2 (3.7) | 3.2 (1.0) |
| Caffeine, mg/day | 134 (151) | 192 (159) | 121 (96) | 193 (140) |
| Alcohol, drinks/day | 0.56 (0.73) | 0.72 (0.55) | 0.51 (0.65) | 0.45 (0.40) |
| Ever smoker, % | 31 | 44 | 14 | 15 |
| Daidzein, mg/day | 0 | 0.31 (0.14) | 1.39 (0.98) | 8.80 (8.70) |
| Genistein, mg/day | 0 | 0.43 (0.19) | 2.07 (1.50) | 13.0 (13.1) |
| Glycitein, mg/day | 0 | 0.03 (0.03) | 0.25 (0.26) | 1.30 (1.55) |
In univariate analyses, soy food and isoflavone intakes were inversely related to sperm concentration (Table II). This association was strongest for soy foods. Men in the highest intake level of soy foods had, on average, 35 million sperm/ml less than men who did not consume soy foods (95% CI: −67, −3), and there was a statistically significant trend toward decreasing sperm concentration with increasing soy foods intake (P, trend = 0.03). The results for individual isoflavones mirrored the results for soy foods and were strongest for glycitein, but did not reach statistical significance. Men in the highest intake level of glycitein had, on average, 33 million sperm/ml less than men without any glycitein intake (95% CI: −68, 2) with a suggestion of a linear trend (P, trend = 0.08). Soy food and isoflavone intakes were unrelated to total sperm count, ejaculate volume, sperm motility or sperm morphology in these analyses.
Table II.
Semen quality parameters [mean (SD)] by levels of soy isoflavones soy foods intake.
| Intake range [median] | N | Total sperm count (millions) | Ejaculate volume (ml) | Sperm concentration (millions/ml) | Sperm motility (% motile) | Sperm morphology (% normal) |
|---|---|---|---|---|---|---|
| Daidzein (mg/day) | ||||||
| 0 [0] | 39 | 297 (245) | 3.5 (1.9) | 106 (82) | 47 (22) | 7.5 (5.0) |
| 0.01–0.47 [0.34] | 20 | 266 (162) | 3.3 (1.7) | 94 (66) | 44 (21) | 6.7 (4.1) |
| 0.48–2.15 [1.22] | 20 | 330 (240) | 3.8 (1.6) | 97 (82) | 49 (18) | 6.6 (3.9) |
| ≥2.16 [5.15] | 20 | 266 (209) | 4.1 (2.2) | 78 (60) | 48 (24) | 6.2 (3.3) |
| P, trend | 0.66 | 0.26 | 0.13 | 0.71 | 0.26 | |
| Genistein (mg/day) | ||||||
| 0 [0] | 39 | 297 (245) | 3.5 (1.9) | 106 (82) | 47 (22) | 7.5 (5.0) |
| 0.01–0.75 [0.46] | 21 | 259 (162) | 3.4 (1.8) | 90 (66) | 45 (20) | 6.6 (4.1) |
| 0.76–2.96 [1.80] | 19 | 341 (240) | 3.8 (1.6) | 101 (83) | 48 (19) | 6.7 (4.0) |
| ≥2.97 [7.48] | 20 | 266 (209) | 4.1 (2.2) | 78 (60) | 48 (24) | 6.2 (3.3) |
| P, trend | 0.70 | 0.27 | 0.15 | 0.73 | 0.27 | |
| Glycitein (mg/day) | ||||||
| 0 [0] | 46 | 300 (244) | 3.4 (1.9) | 106 (79) | 47 (22) | 7.3 (4.8) |
| 0.01–0.08 [0.05] | 16 | 270 (157) | 3.5 (1.6) | 91 (65) | 46 (18) | 6.6 (4.3) |
| 0.09–0.28 [0.23] | 19 | 341 (239) | 4.0 (1.7) | 100 (84) | 48 (22) | 7.2 (4.0) |
| ≥0.28 [0.91] | 18 | 236 (184) | 3.9 (2.3) | 73 (59) | 45 (24) | 5.8 (3.1) |
| P, trend | 0.28 | 0.46 | 0.08 | 0.79 | 0.16 | |
| Soy foods (serv/day) | ||||||
| 0 [0] | 39 | 297 (245) | 3.5 (1.9) | 106 (82) | 47 (22) | 7.5 (5.0) |
| 0.01–0.07 [0.04] | 18 | 261 (171) | 3.4 (1.8) | 92 (69) | 49 (19) | 6.5 (3.9) |
| 0.08–0.29 [0.16] | 22 | 331 (242) | 3.7 (1.6) | 104 (86) | 42 (22) | 7.0 (4.2) |
| ≥0.30 [0.54] | 20 | 264 (191) | 4.1 (2.1) | 72 (45) | 50 (22) | 5.9 (3.1) |
| P, trend | 0.65 | 0.24 | 0.03 | 0.59 | 0.14 |
Statistical adjustment for age, abstinence time, BMI, caffeine and alcohol intakes and smoking did not change most of the associations and made most of them slightly stronger (Table III). In these multivariate analyses, men in the highest intake category of soy foods had, on average, 41 million sperm/ml less than men who did not eat soy foods (P = 0.02). The association between isoflavones and sperm concentration was similar but did not reach statistical significance in these analyses either. As was the case in the univariate analyses, there were no associations between soy foods or isoflavones and total sperm count, ejaculate volume, sperm motility or morphology in the multivariate analyses.
Table III.
Adjusted* difference (95% CI) in sperm concentration by levels of soy foods intake.
| Intake range [median] | Total sperm count (millions) | Ejaculate volume (ml) | Sperm concentration (millions/ml) | Sperm motility (% motile) | Sperm morphology (% normal) |
|---|---|---|---|---|---|
| Daidzein (mg/day) | |||||
| 0 [0] | Ref. | Ref. | Ref. | Ref. | Ref. |
| 0.01–0.47 [0.34] | −81 (−201, 37) | −0.3 (−1.3, 0.6) | −21 (−63, 21) | −4 (−14, 5) | −0.6 (−2.8, 1.6) |
| 0.48–2.15 [1.22] | −9 (−125, 107) | 0.2 (−0.7, 1.1) | −16 (−59, 27) | 3 (−8, 13) | −0.7 (−2.8, 1.5) |
| ≥2.16 [5.15] | −27 (−135, 82) | 0.8 (−0.3, 1.8) | −32 (−69, 5) | 5 (−8, 17) | −0.2 (−2.3, 1.8) |
| P, trend | 0.84 | 0.12 | 0.11 | 0.34 | 0.92 |
| Genistein (mg/day) | |||||
| 0 [0] | Ref. | Ref. | Ref. | Ref. | Ref. |
| 0.01–0.75 [0.46] | −84 (−200, 31) | −0.2 (−1.2, 0.7) | −24 (−64, 17) | −4 (−13, 6) | −0.9 (−3.1, 1.3) |
| 0.76–2.96 [1.80] | −2 (−123, 118) | 0.1 (−0.8, 1.0) | −14 (−58, 31) | 2 (−8, 13) | −0.4 (−2.6, 1.7) |
| ≥2.97 [7.48] | −26 (−135, 83) | 0.8 (−0.3, 1.8) | −32 (−69, 5) | 5 (−8, 17) | −0.2 (−2.3, 1.8) |
| P, trend | 0.86 | 0.12 | 0.12 | 0.35 | 0.98 |
| Glycitein (mg/day) | |||||
| 0 [0] | Ref. | Ref. | Ref. | Ref. | Ref. |
| 0.01–0.08 [0.05] | −50 (−162, 63) | 0.1 (−0.8, 1.0) | −20 (−60, 21) | −2 (−12, 8) | −0.7 (−3.2, 1.7) |
| 0.09–0.28 [0.23] | −7 (−108, 121) | 0.5 (−0.3, 1.4) | −13 (−56, 29) | 2 (−10, 14) | 0.2 (−1.9, 2.4) |
| ≥0.28 [0.91] | −48 (−160, 65) | 0.8 (−0.4, 2.0) | −35 (−73, 2) | 2 (−10, 14) | −0.4 (−2.3, 1.5) |
| P, trend | 0.48 | 0.18 | 0.07 | 0.69 | 0.79 |
| Soy foods (serv/day) | |||||
| 0 [0] | Ref. | Ref. | Ref. | Ref. | Ref. |
| 0.01–0.07 [0.04] | −84 (−200, 32) | −0.3 (−1.3, 0.7) | −24 (−67, 19) | 0 (−11, 11) | −1.2 (−3.6, 1.9) |
| 0.08–0.29 [0.16] | −1 (−118, 115) | 0.2 (−0.7, 1.1) | −8 (−52, 36) | −3 (−14, 8) | 0 (−2.2, 2.1) |
| ≥0.30 [0.54] | −41 (−147, 65) | 0.7 (−0.3, 1.8) | −41 (−74, −8) | 7 (−4, 19) | −0.5 (−2.5, 1.5) |
| P, trend | 0.65 | 0.13 | 0.02 | 0.19 | 0.80 |
*Adjusted for age, abstinence time, BMI, caffeine and alcohol intake, and smoking status.
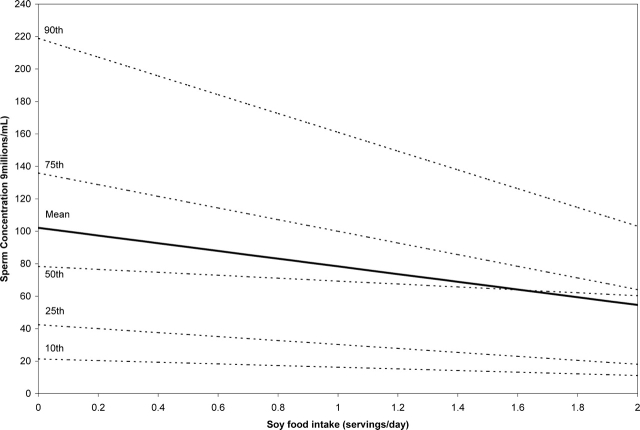
To evaluate whether the association between soy food intake and sperm concentration was constant across the sperm concentration distribution, we modeled the relationship between soy food intake and specific quantiles (10th, 25th, 50th, 75th and 90th) of the sperm concentration distribution using quantile regression adjusting for age, abstinence time, BMI, caffeine and alcohol intakes and smoking. Although soy food intake had little impact on sperm concentration on the lower end of the distribution, there was a stronger inverse relation between soy food intake and sperm concentration at the higher end of the distribution (Fig. 1).
Figure 1:
Predicted sperm concentration values according to soy food intake*.
*Values are predicted from separate multivariate linear or quantile regression models for non-smoking men with 2 days of abstinence at the median age (36 year), median BMI (26 kg/m2), median caffeine intake (111 mg/day) and median alcohol intake (0.29 drinks/day).
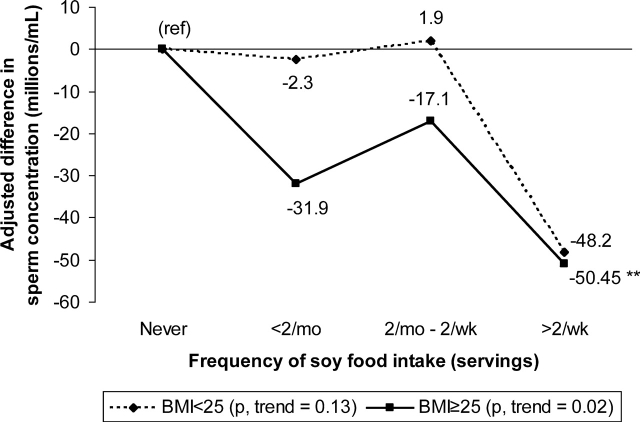
Lastly, we evaluated whether the association between soy food or isoflavone intake and sperm concentration differed according to age or BMI. There was no evidence of effect modification by age. There was, however, a suggestion that the association between soy food intake and sperm concentration was more pronounced among overweight and obese men than among lean men (Fig. 2) (P, interaction = 0.10).
Figure 2:
Adjusted* difference in sperm concentration by levels of soy foods intake among normal weight and overweight or obese men.
*Adjusted for age, abstinence time, BMI, caffeine and alcohol intake, and smoking status. **P < 0.05 compared with men without soy food intake in the respective BMI category.
Discussion
In this cross-sectional study, dietary intake of soy food and isoflavones was inversely related to sperm concentration after accounting for multiple potential confounders. This association was stronger at the higher end of the sperm concentration distribution suggesting that soy food intake may have stronger associations among men with normal or high sperm concentrations than among men with low sperm concentration. Also, soy food intake was more strongly inversely related to sperm concentration among overweight and obese men. Intake of soy foods or isoflavones was unrelated to the remaining semen analysis parameters examined.
Only two studies have previously examined the relation between soy food or isoflavone intake and semen quality parameters in humans. Mitchell et al. (2001) evaluated the reproductive effects of daily supplementation with 40 mg of isoflavones for 2 months among 14 young men. There were no appreciable changes in semen quality parameters or reproductive hormone levels compared with pre-supplementation levels (Mitchell et al., 2001). However, the lack of a control group and the small size of the study make difficult the interpretation of their findings. In a study with a design similar to ours, Song et al. investigated the relationship between isoflavone intake and semen quality in a group of 48 men with abnormal semen parameters and 10 men with normal semen parameters. In contrast with our results, they found that isoflavone intake was positively related to sperm count and motility and inversely related to sperm DNA damage (Song et al., 2006).
The role of perinatal exposure to phytoestrogen on male reproductive health has been thoroughly evaluated in animal models. In rodents, exposure to phytoestrogens in utero or during early post-natal life through diet or subcutaneous injection results in multiple reproductive abnormalities during adult life, including decreased testicular weight or size (Atanassova et al., 2000; Nagao et al., 2001; Wisnieswki et al., 2003; West et al., 2005), decreased spermatogenesis (Atanassova et al., 2000; West et al., 2005), lower testosterone (Wisnieswki et al., 2003), DHT (Yi et al., 2002) and FSH levels (Atanassova et al., 2000), decreased testicular expression of steroid hormone receptors (Shibayama et al., 2001), decreased ano-genital distance (Wisnieswki et al., 2003, 2005) and alterations of reproductive and aggressive behavior (Wisnieswki et al., 2003, 2005). However, these changes are not always consistent across studies. Moreover, in marmoset monkeys, soy formula feeding starting at 3 days of age resulted in suppression of the neonatal testosterone surge and increased Leydig cell number lasting into adulthood, but no adverse effects on pubertal progression or fertility were documented (Sharpe et al., 2002; Tan et al., 2006).
Animal data regarding adult exposure to phytoestrogens are not as extensive and have yielded less consistent results. A study in rats found that dietary phytoestrogens led to a transient decrease in fertility accompanied by changes in the expression patterns of ERα and AR in the epididymis without any appreciable changes in conventional semen quality parameters (Glover and Assinder, 2006). Similarly, phytoestrogens decreased circulating testosterone levels in rats (Weber et al., 2001). However, others have found no effects of phytoestrogen-rich diets on testicular weight or spermatogeneis in adult rats (Faqi et al., 2004). On the other hand, a study in rabbits found that a phytoestrogen-rich diet increased libido and improved all conventional semen quality parameters (Yousef et al., 2004). A study in macaques found no changes in testicular weight or semen quality in response to different dietary doses of phytoestrogens (Perry et al., 2007). Among sheep feeding on phytoestrogen-rich pastures, intact males do not show evidence of reproductive morbidity whereas castrated males present development of mammary glands, lactation and squamous metaplasia of the prostate and other accessory glands accompanied by enlargement of Cowper’s gland (Bennetts et al., 1946). The lack of consistent results across species suggests the possibility of species-specific susceptibility and highlights the importance of conducting further studies in humans.
At least two arguments are raised against the possibility that phytoestrogens may have deleterious effects on male fertility. First, it has been implied that the low in vitro affinity of individual phytoestrogens to ERα, ∼100–1000 times lower than estradiol (Miksicek, 1994; Kuiper et al., 1998; Song et al., 1999; Matthews et al., 2000; Branham et al., 2002; Harris et al., 2002), makes it unlikely that phytoestrogens can exert significant estrogenic activity to result in major altered reproductive function. However, phytoestrogens found in soy foods can induce transcriptional activity through ERα at levels comparable and even higher than estradiol under certain conditions (Kuiper et al., 1998). In addition, this argument ignores the fact that estrogens (endogenous and xenoestrogens) can induce responses not mediated by nuclear ERs (Watson et al., 2007). Phytoestrogens can bind membrane ERs with greater affinity than they bind nuclear receptors and, through the membrane receptors, induce transcriptional activity to the same extent estradiol does (Thomas and Dong, 2006). Moreover, in mouse sperm, phytoestrogens can exert actions, such as inducing capacitation and premature acrosome reaction, at concentrations 100–1000 times lower than estradiol (Adeoya-Osiguwa et al., 2003). This finding may have particular importance to humans as human sperm appears to be more sensitive than mouse sperm to the actions of specific isoflavones (Fraser et al., 2006). Whether phytoestrogens could affect spermatogenesis by acting through membrane ERs or other mechanisms needs to be examined further.
A second argument is that Asian diets include high amounts of phytoestrogens from soy foods without any apparent deleterious effect on fertility. However, one small study of autopsy specimens found that Asian men had lower testicular weight, germ cell number and Sertoli cell function compared with Caucasian and Hispanic men (Johnson et al., 1988). Similarly, testicular volume and sperm concentration were slightly lower in Asian men than in non-Asian men in larger studies, although the statistical significance of these findings was not reported (World Health Organization, 1996). Whether these differences are real and attributable to differences in diet is not known. Also, although it is true that Asian men consume 5–10 times more phytoestrogens than men in our study (Yamamoto et al., 2001; Lee et al., 2007), there may be other factors that could make Western men more susceptible to phytoestrogens. One possibility is that excess body weight modifies the relation between phytoestrogen intake and semen quality as our data suggest. While increasing at alarming rates (Dearth-Wesley et al., 2007; Wang et al., 2007; Tuan et al., 2008), the prevalence of overweight and obesity is still much lower in Asia than in the USA. In China, a country with one of the steepest increases in overweight and obesity in the region, 26% of adult men have a BMI over 25 (Dearth-Wesley et al., 2007) which languishes in comparison with the 71% of overweight or obese men in the general US population (Ogden et al., 2006) and the 72% prevalence in our study. It is possible that exposure to high endogenous estrogen levels arising from conversion of androgens to estrogens in the adipose tissue makes reproductive organs more sensitive to the action of environmental estrogens. While this hypothesis is consistent with our data, it needs to be examined in future studies in Western and Asian populations.
Strengths of our study include our ability to account for multiple potential confounders which had not been the case in previous studies. In addition, this is, to our knowledge, the largest study in humans so far examining the relationship between phytoestrogens and semen quality. The most important limitation of the study is the fact that it is a cross-sectional and observational study which limits our ability to determine causality. A second limitation is that we limited our assessment of isoflavone intake to soy-based foods. Although soy foods are the most important source of isoflavones in Western populations (Horn-Ross et al., 2000; Ritchie et al., 2006), we could not assess intake of isoflavones from other sources, most importantly bakery products containing soy flour. However, not assessing these foods would result in random misclassification of isoflavone intake and likely bias the results toward the null hypothesis, thus attenuating the reported associations. An additional difficulty of our dietary assessment is that it has not been validated. However, food frequency questionnaires with much less detailed soy intake information have been previously shown to validly estimate usual isoflavone intake in Western populations (Heald et al., 2006; Horn-Ross et al., 2006).
In conclusion, we found an inverse association between consumption of soy foods and sperm concentration which was more pronounced at the higher end of the sperm concentration distribution and among overweight or obese men. The clinical significance of these findings remains to be determined. Owing to the scarcity of human data in this area, it is very important that this issue is examined further, ideally in randomized trials.
Funding
Supported by National Institute of Environmental Health Sciences (NIEHS) grants ES09718 and ES00002, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) training grant T32 DK07703 and the Yerby Postdoctoral Fellowship Program.
Appendix 1: list of soy foods assessed
| 1. Tofu (all types), including low-fat, flavored, marinated, smoked |
| 2. Tempeh, all types |
| 3. Tofu or soy breakfast sausage, bacon, cold cuts, hot dogs or other deli meat substitutes |
| 4. Veggie soy or tofu burger, ground meat substitute (TVP), soy or tofu chicken or turkey |
| 5. Packaged mixed dishes with soy or tofu, such as lasagna, burritos or stir fry |
| 6. Miso soup |
| 7. Soymilk (regular or low-fat), plain or flavored |
| 8. Soy cheese, including foods made with soy cheese |
| 9. Soy yogurt, all types |
| 10. Soy ice cream, tofutti or other soy desserts |
| 11. Cooked soybeans or edamame (green soybeans) |
| 12. Roasted soy nuts |
| 13. Liquid nutrition drinks with soy or soy protein, such as Odwalla Future Shake, Ensure Plus |
| 14. Soy protein powders, such as performance or body builder powders |
| 15. High energy bars or diet bars containing soy or soy protein |
References
- Adeoya-Osiguwa SA, Markoulaki S, Pocock V, Milligan SR, Fraser LR. 17β-estradiol and environmental estrogens significantly affect mammalian sperm function. Hum Reprod. 2003;18:100–107. doi: 10.1093/humrep/deg037. [DOI] [PubMed] [Google Scholar]
- Atanassova N, McKinnell C, Turner KJ, Walker M, Fisher JS, Morley M, Millar MR, Groome NP, Sharpe RM. Comparative effects of neonatal exposure of male rats to potent and weak (environmental) estrogens on spermatogenesis at puberty and the relationship to adult testis size and fertility: evidence for stimulatory effects of low estrogen levels. Endocrinology. 2000;141:3898–3907. doi: 10.1210/endo.141.10.7723. [DOI] [PubMed] [Google Scholar]
- Bennetts HW, Underwood EJ, Shier FL. A specific breeding problem of sheep on subterranean clover pastures in western Australia. Aust Vet J. 1946;22:2–12. doi: 10.1111/j.1751-0813.1946.tb15473.x. [DOI] [PubMed] [Google Scholar]
- Branham WS, Dial SL, Moland CL, Hass BS, Blair RM, Fang H, Shi L, Tong W, Perkins RG, Sheehan DM. Phytoestrogens and mycoestrogens bind to the rat uterine estrogen receptor. J Nutr. 2002;132:658–664. doi: 10.1093/jn/132.4.658. [DOI] [PubMed] [Google Scholar]
- Dearth-Wesley T, Wang H, Popkin BM. Under- and overnutrition dynamics in Chinese children and adults (1991–2004) Eur J Clin Nutr. 2007 doi: 10.1038/sj.ejcn.1602853. July 18 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Duty SM, Silva MJ, Barr DB, Brock JW, Ryan L, Chen Z, Herrick RF, Christiani DC, Hauser R. Phthalate exposure and human semen parameters. Epidemiology. 2003;14:269–277. [PubMed] [Google Scholar]
- Duty SM, Calafat AM, Silva MJ, Brock JW, Ryan L, Chen Z, Overstreet J, Hauser R. The relationship between environmental exposure to phthalates and computer-aided sperm analysis motion parameters. J Androl. 2004;25:293–302. doi: 10.1002/j.1939-4640.2004.tb02790.x. [DOI] [PubMed] [Google Scholar]
- Faqi AS, Johnosn WD, Morrissey RL, McCormick DL. Reproductive toxicity assessment of chronic dietary exposure to soy isoflavones in male rats. Reprod Toxicol. 2004;18:605–611. doi: 10.1016/j.reprotox.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Fraser LR, Beyret E, Milligan SR, Adeoya-Osiguwa SA. Effects of estrogenic xenobiotics on human and mouse spermatozoa. Hum Reprod. 2006;21:1184–1193. doi: 10.1093/humrep/dei486. [DOI] [PubMed] [Google Scholar]
- Glover A, Assinder SJ. Acute exposure of adult male rats to dietary phytoestrogens reduces fecundity and alters epididymal steroid hormone receptor expression. J Endocrinol. 2006;189:565–573. doi: 10.1677/joe.1.06709. [DOI] [PubMed] [Google Scholar]
- Harris HA, Bapat AR, Gonder DS, Frail DE. The ligand binding profiles of estrogen receptors α and β are species dependent. Steroids. 2002;67:379–384. doi: 10.1016/s0039-128x(01)00194-5. [DOI] [PubMed] [Google Scholar]
- Hauser R, Meeker JD, Duty S, Silva MJ, Calafat AM. Altered semen quality in relation to urinary concentrations of phtalate monoester and oxidative metabolites. Epidemiology. 2006;17:682–691. doi: 10.1097/01.ede.0000235996.89953.d7. [DOI] [PubMed] [Google Scholar]
- Heald CL, Bolton-Smith C, Ritchie MR, Morton MS, Alexander FE. Phyto-oestrogen intake in Scottish men: use of serum to validate a self-administered food-frequency questionnaire. Eur J Clin Nutr. 2006;60:129–135. doi: 10.1038/sj.ejcn.1602277. [DOI] [PubMed] [Google Scholar]
- Horn-Ross PL, Lee M, John EM, Koo J. Sources of phytoestrogen exposure among non-Asian women in California, USA. Cancer Causes Control. 2000;11:299–302. doi: 10.1023/a:1008968003575. [DOI] [PubMed] [Google Scholar]
- Horn-Ross PL, Barnes S, Lee VS, Collins CN, Reynolds P, Lee MM, Stewart SL, Canchola AJ, Wilson L, Jones K. Reliability and validity of an assessment of usual phytoestrogen consumption (United States) Cancer Causes Control. 2006;17:85–93. doi: 10.1007/s10552-005-0391-6. [DOI] [PubMed] [Google Scholar]
- Johnson L, Barnard JJ, Rodriguez L, Smith E, Swerdloff RS, Wang XH, Wang C. Ethnic differences in testicular structure and spermatogenic potential may predispose testes of Asian men to a heightened sensitivity to steroidal contraceptives. J Androl. 1988;19:348–357. [PubMed] [Google Scholar]
- Koenker R, Bassett GW. Regression quantiles. Econometrica. 1978;46:33–50. [Google Scholar]
- Kruger TF, Acosta AA, Simmons KF, Swanson RJ, Matta JF, Oehninger S. Predictive value of abnormal sperm morphology in in vitro fertilization. Fertil Steril. 1988;49:112–117. doi: 10.1016/s0015-0282(16)59660-5. [DOI] [PubMed] [Google Scholar]
- Kuiper GGJM, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson J-A. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptorβ. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Lee S-A, Wen W, Xiang Y-B, Barnes S, Liu D, Cai Q, Zheng W, Shu XO. Assessment of dietary isoflavone intake among middle-aged Chinese men. J Nutr. 2007;137:1011–1016. doi: 10.1093/jn/137.4.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews J, Celius T, Halgren R, Zacharewski T. Differential estrogen receptor binding of estrogenic substances: a species comparison. J Steroid Biochem Mol Biol. 2000;74:223–234. doi: 10.1016/s0960-0760(00)00126-6. [DOI] [PubMed] [Google Scholar]
- Miksicek RJ. Interaction of naturally occurring nonsteroidal estrogens with expressed recombinant human estrogen receptor. J Steroid Biochem Mol Biol. 1994;49:153–160. doi: 10.1016/0960-0760(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Mitchell JH, Cawood E, Kinniburgh D, Provan A, Collins AR, Irvine DS. Effect of a phytoestrogen food supplement on reproductive health in normal males. Clin Sci (Lond) 2001;100:613–618. [PubMed] [Google Scholar]
- Nagao T, Yoshimura S, Saito Y, Nakagomi M, Usumi K, Ono H. Reproductive effects in male and female rats of neonatal exposure to genistein. Reprod Toxicol. 2001;15:399–411. doi: 10.1016/s0890-6238(01)00141-1. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- Perry DL, Spedick JM, McCoy TP, Adams MR, Franke AA, Cline JM. Dietary soy protein containing isoflavonoids does not adversely affect the reproductive tract of male cynomolgus macaques (Macaca fascicularis) J Nutr. 2007;137:1390–1394. doi: 10.1093/jn/137.6.1309. [DOI] [PubMed] [Google Scholar]
- Ritchie MR, Cummings JH, Morton MS, Steel CM, Bolton-Smith C. A newly constructed and validated isoflavone database for the assessment of total genistein and daidzein intake. Br J Nutr. 2006;95:204–213. doi: 10.1079/bjn20051603. [DOI] [PubMed] [Google Scholar]
- Sharpe RM. Hormones and testis development and the possible adverse effects of environmental chemicals. Toxicol Lett. 2001;120:221–232. doi: 10.1016/s0378-4274(01)00298-3. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, Martin B, Morris K, Greig I, McKinnell C, McNeilly AS, Walker M. Infant feeding with soy formula milk: effects on the testis and on blood testosterone levels in marmoset monkeys during the period of neonatal testicular activity. Hum Reprod. 2002;17:1692–1703. doi: 10.1093/humrep/17.7.1692. [DOI] [PubMed] [Google Scholar]
- Shibayama T, Fukata H, Sakurai K, Adachi T, Komiyama M, Iguchi T, Mori C. Neonatal exposure to genistein reduces expression of estrogen receptor alpha and androgen receptor in testes of adult mice. Endocr J. 2001;48:655–663. doi: 10.1507/endocrj.48.655. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE, Rajpert De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod. 2001;16:972–978. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- Song TT, Hendrich S, Murphy PA. Estrogenic activity of glycitein, a soy isoflavone. J Agric Food Chem. 1999;47:1607–1610. doi: 10.1021/jf981054j. [DOI] [PubMed] [Google Scholar]
- Song G, Kochman L, Andolina E, Herko RC, Brewer KJ, Lewis V. O-115: beneficial effects of dietary intake of plant phytoestrogens on semen parameters and sperm DNA integrity in infertile men. Fertil Steril. 2006;86:S49. [Google Scholar]
- Tan KAL, Walker M, Morris K, Greig I, Mason JI, Sharpe RM. Infant feeding with soy formula: effects on puberty progression, reproductive function and testicular cell numbers in marmoset monkeys in adulthood. Hum Reprod. 2006;21:896–904. doi: 10.1093/humrep/dei421. [DOI] [PubMed] [Google Scholar]
- Thomas P, Dong J. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: a potential novel mechanism of endocrine disruption. J Steroid Biochem Mol Biol. 2006;102:175–179. doi: 10.1016/j.jsbmb.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Tuan NT, Tuong PD, Popkin BM. Body mass index (BMI) dynamics in Vietnam. Eur J Clin Nutr. 2008;62:78–86. doi: 10.1038/sj.ejcn.1602675. [DOI] [PubMed] [Google Scholar]
- United States Department of Agriculture. USDA-Iowa State University database on the isoflavone content of foods, release 1.4-2007. 2007 www.ars.usda.gov/nutrientdata. (date last accessed March 2007) [Google Scholar]
- Wang Y, Mi J, Shan X, Wang QJ, Ge K. Is China facing an obesity epidemic and the consequences? The trends in obesity and chronic disease in China. Int J Obes. 2007;31:177–188. doi: 10.1038/sj.ijo.0803354. [DOI] [PubMed] [Google Scholar]
- Watson CS, Alyea RA, Jeng YL, Kochokov MY. Nongenomic actions of low concentration estrogens and xenoestrogens on multiple tissues. Mol Cell Endocrinol. 2007;274:1–7. doi: 10.1016/j.mce.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber KS, Setchell KDR, Stocco DM, Lephart ED. Dietary soy-phytoestrogens decrease testosterone levels and prostate weight without altering LH, prostate 5α-reductase or testicular steroidogenic acute regulatory peptide levels in adult male Sprague–Dawley rats. J Endocrinol. 2001;170:591–599. doi: 10.1677/joe.0.1700591. [DOI] [PubMed] [Google Scholar]
- West MCL, Anderson L, McClure N, Lewis SEM. Dietary oestrogens and male fertility potential. Hum Fertil (Camb) 2005;8:197–207. doi: 10.1080/14647270500030266. [DOI] [PubMed] [Google Scholar]
- White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980;48:817–830. [Google Scholar]
- Wisnieswki AB, Klein SL, Lakshmanan Y, Gerhart JP. Exposure to genistein during gestation and lactation demasculinies the reproductive system in rats. J Urol. 2003;169:1582–1586. doi: 10.1097/01.ju.0000046780.23389.e0. [DOI] [PubMed] [Google Scholar]
- Wisnieswki AB, Cernetich A, Gerhart JP, Klein SL. Perinatal exposure to genistein alters reproductive development and aggressive behavior in male mice. Physiol Behav. 2005;84:327–334. doi: 10.1016/j.physbeh.2004.12.008. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Contraceptive efficacy of testosterone-induced azoospermia and oligozoospermia in normal men. Fertil Steril. 1996;65:821–829. [PubMed] [Google Scholar]
- Yamamoto S, Sobue T, Sasaki S, Kobayashi M, Arai Y, Uehara M, Adlercreutz H, Watanabe S, Takashabi T, Iotoi Y, et al. Validity and reproducibility of a self-administered food-frequency questionnaire to assess isoflavone intake in a Japanese population in comparison to dietary records and urine isoflavones. J Nutr. 2001;131:2741–2747. doi: 10.1093/jn/131.10.2741. [DOI] [PubMed] [Google Scholar]
- Yi MA, Son HM, Lee JS, Kwon CS, Lim JK, Yeo YK, Park YS, Kim JS. Regulation of male sex hormone levels by soy isoflavones in rats. Nutr Cancer. 2002;42:206–210. doi: 10.1207/S15327914NC422_9. [DOI] [PubMed] [Google Scholar]
- Yousef MI, Esmail AM, Baghdadi HH. Effect of isoflavones on reproductive performance, testosterone levels, lipid peroxidation and seminal plasma biochemistry of male rabbits. J Environ Sci Health B. 2004;39:819–833. [PubMed] [Google Scholar]