Abstract
Aims
Given the selectivity of clinical trial patients and meager representation of elderly in the major implantable cardioverter defibrillator (ICD) randomized trials (<25%), whether such data apply to elderly patients overall is unclear. The purpose of our study is to understand the influence of age on mortality after ICD implantation.
Methods and results
We performed a retrospective cohort study of 502 consecutive patients receiving ICDs from 1993 to 2003 at a single university hospital. The primary predictor was patient age, categorized as <65, 65–75, and >75 years at ICD implantation. The primary outcome was time to death. Mean follow-up was 4 years. Median survival after ICD implantation was 5.3 years among subjects >75 years, less than half that of the youngest group. After adjusting for potential confounders, compared with subjects <65 years of age, patients >75 years [hazard ratio (HR), 4.7; 95% confidence interval (CI), 2.8–7.9; P < 0.001] and those 65–75 years (HR, 2.8; 95% CI, 1.7–4.8; P < 0.001) were at greater risk of death. Increased age was associated with higher total, cardiac, and non-cardiac mortality (all P ≤ 0.001).
Conclusion
Age at ICD implantation is strongly and independently associated with mortality. Age should be considered among potential co-morbidities in anticipating survival of the elderly patient prior to ICD implantation.
Keywords: Implantable cardioverter defibrillator, Age, Mortality, Predictor, Elderly, Sudden cardiac death
Introduction
Multiple large clinical trials, most recently MADIT-II and SCD-HeFT, have demonstrated mortality benefit with implantable cardioverter defibrillator (ICD) implantation for increasingly broad indications.1–7 In 2005, the Center for Medicare and Medicaid Services estimated that half a million Medicare beneficiaries may be candidates for primary prevention ICD therapy in the United States.8 With this expansion of the population eligible for an ICD, resource limitations and discussions of cost-effectiveness have come to the forefront.9,10 Concomitantly, enthusiasm has grown for increased precision in predicting potential benefit of these devices, and multiple studies have strived to identify predictors of appropriate shocks and mortality.11–15
One predictor whose influence on subsequent mortality and role in patient selection has been much debated is patient age at ICD implantation. While a recent MADIT-II substudy found an equivalent reduction of mortality with ICD implantation in those ≥75 years as in those <75 years,16 no statistically significant ICD survival benefit was seen in those ≥65 years in SCD-HeFT.7 Less than 25% of the subjects included in the major clinical ICD trials are estimated to be >75 years based on published medians, standard deviations, and inter-quartile ranges.1–7 The average age of patients at the time of study enrolment was between 58 and 65 years in the secondary prevention trials1–3 and 58 and 66 years in the primary prevention trials.4–7 Further, those few older patients selected for admission into large clinical trials may not be representative of the elderly population as a whole. Though one cohort study observed longer survival in elderly ICD recipients than controls hospitalized for heart failure,17 whether the findings of the randomized trials may be generalized to the elderly population is unclear. Cohort studies of real-world populations that have compared survival between different age groups have generally been small and results have been inconsistent, with several showing significantly higher mortality among the more elderly ICD recipients compared with the younger ICD recipients18–20 and others demonstrating similar survival between groups.21–23 The aim of our study is to understand the influence of age on mortality after ICD implantation.
Methods
Study design and subjects
We performed a retrospective cohort study of consecutive patients who had a transvenous ICD implantation or revision at the University of California, San Francisco (UCSF) between 1 January 1993 and 31 December 2003. Patients who underwent multiple procedures during this time were included only once. Subjects were excluded if they were <18 years of age. The study was approved by the UCSF's Committee on Human Research and complied with the Declaration of Helsinki.
Measurements
Patient information including age, sex, medical history, and clinical indication was obtained from the UCSF hospital electronic medical record and a clinical database, into which patient parameters are recorded at the time of implant by the UCSF electrophysiology service. The primary predictor for this study was patient age at the time of initial ICD implantation. Patients were categorized as <65, 65–75, and >75 years of age, for comparison to previous studies. In addition to age, data were collected on 16 potential confounding variables, including indication for ICD implantation, symptomatic chronic heart failure, and left ventricular ejection fraction (LVEF). Patient variables that were not documented within 1 month of ICD implantation were treated as missing data. Data from the medical record were abstracted retrospectively by two trained, skilled research associates blinded to the outcome.
Outcomes
The primary outcome was time from implant to death. Mortality data, including cause of death where possible, were ascertained via hospital medical records and by a search of the national death index (NDI). Cause of death was as recorded on the death certificate, as reported by the NDI, and was grouped into cardiac, non-cardiac, and unknown cause. Patients not found in the NDI through 31 December 2003 were assumed to be alive and were censored on this date or on their last known date of clinical contact, whichever was later.
Statistical analysis
The primary data analysis evaluated the effect of age at ICD implantation on post-implantation mortality rate, with adjustment for possible confounders. Differences in baseline characteristics across age categories were compared by the χ2 or Fisher's exact test for categorical variables and analysis of variance for continuous variables. Cumulative survival was measured by the method of Kaplan–Meier, and unadjusted differences were compared using the log-rank test and Cox proportional hazard models. Analyses adjusting for potential confounders were performed using multivariable Cox proportional hazards models. Covariates were selected based on face validity as a potential confounding factor and/or an unadjusted association of P < 0.2 in univariate models with potential confounders. Because treating age as a three-level categorical variable in the Cox models required encoding two dichotomous predictor variables (<65 vs. 65–75 years and <65 vs. >75 years), the overall association of age with the outcomes was assessed by using Wald tests. The Cox model assumption of proportional hazards was found to be valid by using the log-minus-log curves and the Schoenfeld test.24,25 Age was also examined as a continuous predictor of mortality using Cox proportional hazard models. All comparisons were two-sided and a P-value of <0.05 was considered to indicate statistical significance. Statistical analyses were performed using STATA 9.0 (College Station, TX, USA) and were validated by a second, independent analyst.
Results
A total of 502 patients were included in the study (Table 1); 119 patients (24%) constituted the oldest age group of >75 years, 135 patients (27%) were between 65 and 75 years, and 248 patients (49%) were in the <65-year-old reference group. Groups were similar with regard to proportion reporting a primary prevention indication for ICD, an LVEF ≤35%, presence of end-stage renal disease, diabetes, and dyslipidaemia. Compared with the youngest group, the oldest group had a lower prevalence of prior resuscitated sudden cardiac arrest and a higher proportion of male subjects. The oldest group was also more likely to have a history of heart failure, ischaemic cardiomyopathy, coronary revascularization, chronic renal insufficiency, stroke, hypertension, malignancy, and chronic obstructive pulmonary disease (COPD). The 65- to 75-year-old group more closely resembled the older group than the younger group in most baseline characteristics (Table 1).
Table 1.
Baseline characteristics of subjects categorized by age
| Characteristic | Age at the time of ICD implantationa |
P-value | ||
|---|---|---|---|---|
| <65 years, mean = 50 years (n = 248) | 65–75 years, mean = 70 years (n = 135) | >75 years, mean = 80 years (n = 119) | ||
| Female sex | 80 (32) | 25 (19) | 21 (18) | 0.001 |
| History of cigarette smoking | 87 (51) | 54 (69) | 44 (54) | 0.001 |
| Indication for ICD implantation | ||||
| Prior sudden cardiac death | 72 (37) | 45 (42) | 24 (26) | 0.04 |
| Primary prevention of sudden death | 54 (22) | 25 (19) | 30 (25) | 0.43 |
| History of congestive heart failure | 155 (67) | 104 (85) | 102 (90) | <0.001 |
| Left ventricular ejection fraction ≤35% | 104 (76) | 68 (70) | 70 (74) | 0.55 |
| Coronary disease | ||||
| Ischaemic cardiomyopathy | 69 (45) | 76 (75) | 76 (77) | <0.001 |
| Prior revascularization | 56 (23) | 67 (50) | 58 (49) | <0.001 |
| Renal disease | ||||
| Chronic renal insufficiency | 4 (2) | 8 (8) | 10 (10) | 0.006 |
| End-stage renal disease | 2 (1) | 3 (3) | 3 (3) | 0.33 |
| Prior stroke | 6 (3) | 6 (6) | 12 (12) | 0.005 |
| Hypertension | 73 (34) | 65 (63) | 56 (55) | <0.001 |
| Diabetes mellitus | 32 (15) | 22 (22) | 23 (23) | 0.16 |
| Chronic obstructive pulmonary disease | 14 (7) | 13 (13) | 15 (15) | 0.04 |
| Malignancy | 14 (7) | 24 (24) | 15 (15) | <0.001 |
| Dyslipidaemia | 61 (28) | 43 (42) | 32 (32) | 0.06 |
aValues are given in number (%).
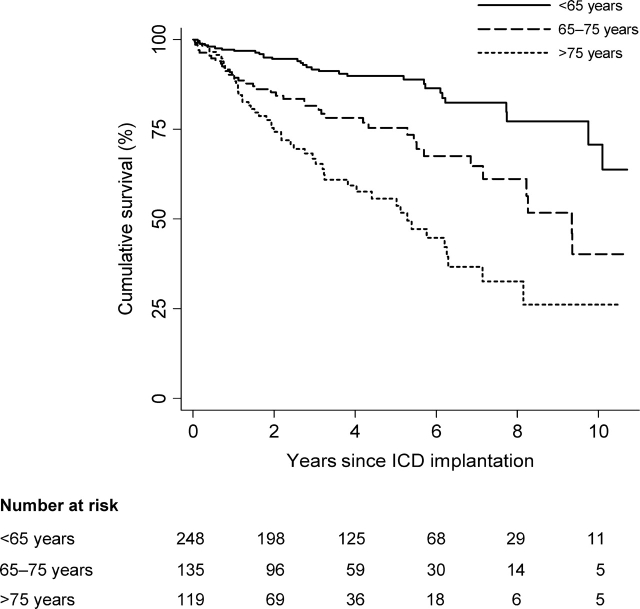
During a mean follow-up time of 4.0 ± 2.7 years, 24% of the patients (119/502) died (Table 2). Median survival decreased by age at implantation. The median survival for those >75 years at the time of ICD implant was 5.3 years, less than half that of the youngest group (Table 2). Figure 1 displays Kaplan–Meier estimates of survival categorized by age at ICD implant. All three curves diverge in the first 1–2 years and continue to separate throughout most of the follow-up period. Among the <65-year-old group, nearly two-thirds of all subjects not yet censored were still alive at the maximum follow-up of 12.4 years (Table 2).
Table 2.
Age at the time of ICD implantation and long-term mortality
| Age at the time of ICD implantation (years) | Mortality |
Unadjusted models |
Multivariable-adjusted modelsa |
||||
|---|---|---|---|---|---|---|---|
| n | Events/100 person-years (no. of events) | Median survival in years | Hazard ratiob (95% CI) | P-value | Hazard ratiob (95% CI) | P-value | |
| <65 | 248 | 2.9 (31) | >12.4 | Reference group | — | Reference group | — |
| 65–75 | 135 | 7.1 (38) | 9.3 | 2.5 (1.5–4.0) | <0.001 | 2.8 (1.7–4.8) | <0.001 |
| >75 | 119 | 13.6 (50) | 5.3 | 4.7 (3.0–7.4) | <0.001 | 4.7 (2.8–7.9) | <0.001 |
CI, confidence interval.
aAdjusted for sex, prior sudden cardiac death, ICD implantation indication, history of congestive heart failure, left ventricular ejection fraction ≤35%, ischaemic cardiomyopathy diagnosis, prior coronary revascularization, renal disease, stroke, hypertension, dyslipidaemia, history of cigarette smoking, history of malignancy, diabetes mellitus, and chronic obstructive pulmonary disease.
bUsing Wald tests to assess the overall associations, age was strongly associated with total mortality in both unadjusted (P < 0.001) and adjusted models (P < 0.001).
Figure 1.
Kaplan–Meier graph showing survival after ICD implantation by age categories. The three curves separate early and diverge further throughout the follow-up period (P < 0.001 by log-rank test).
Mortality increased more than four-fold from the youngest to the oldest group, with 13.6 deaths/100 person-years in the oldest group (Table 2). Age was significantly associated with increased total mortality, as demonstrated by univariate analysis (P < 0.001). There was a 5.2% increased risk of mortality for each additional year of age [95% confidence interval (CI), 3.5–6.8; P < 0.001]. Age remained significantly associated with death among those >75 years [hazard ratio (HR), 4.7; 95% CI, 2.8–7.9; P < 0.001] and those 65–75 years (HR, 2.8; 95% CI, 1.7–4.8; P < 0.001) after adjustment for sex, prior sudden cardiac death, ICD implantation indication, history of congestive heart failure, LVEF ≤35%, ischaemic cardiomyopathy diagnosis, prior coronary revascularization, renal disease, stroke, hypertension, dyslipidaemia, history of cigarette smoking, history of malignancy, diabetes mellitus, and COPD. Analysis stratified by primary vs. secondary prevention indication produced similar results.
Advanced age was associated with increased risk of both cardiac and non-cardiac mortality, with the oldest group displaying a five-fold risk of non-cardiac death as compared with the youngest group after adjusting for potential confounders (HR, 5.1; 95% CI, 2.0–12.8; P < 0.001) (Table 3). Compared with those subjects of age <65 years, there was a two-fold increase in the risk of non-cardiac mortality among those subjects aged 65–75 years, even after adjusting for confounders. Likely due to small numbers of events (10) in this stratum, however, this difference was not statistically significant; the CIs were wide and did not exclude 1.0 (HR, 1.9; 95% CI, 0.7–5.1; P = 0.22). After adjusting for confounders, age was strongly associated with total mortality (P < 0.001), cardiac mortality (P < 0.001), and non-cardiac mortality (P = 0.001).
Table 3.
Age at the time of ICD implantation and long-term mortality by cause of death
| Age at the time of ICD implantation (years) | n | Events/100 person-years (no. of events) | Unadjusted models |
Multivariable-adjusted modelsa |
||
|---|---|---|---|---|---|---|
| Hazard ratiob (95% CI) | P-value | Hazard ratiob (95% CI) | P-value | |||
| Cardiac mortality | ||||||
| <65 | 248 | 1.8 (19) | Reference group | — | Reference group | — |
| 65–75 | 135 | 4.7 (25) | 2.6 (1.5–4.8) | 0.001 | 3.1 (1.6–6.1) | 0.001 |
| >75 | 119 | 7.3 (27) | 4.1 (2.3–7.4) | <0.001 | 4.1 (2.1–8.0) | <0.001 |
| Non-cardiac mortality | ||||||
| <65 | 248 | 0.9 (10) | Reference group | — | Reference group | — |
| 65–75 | 135 | 1.9 (10) | 2.0 (0.8–4.9) | 0.11 | 1.9 (0.7–5.1) | 0.22 |
| >75 | 119 | 4.9 (18) | 5.3 (2.5–11.6) | <0.001 | 5.1 (2.0–12.8) | <0.001 |
CI, confidence interval.
aAdjusted for sex, prior sudden cardiac death, ICD implantation indication, history of congestive heart failure, left ventricular ejection fraction≤35%, ischaemic cardiomyopathy diagnosis, prior coronary revascularization, renal disease, stroke, hypertension, dyslipidaemia, history of cigarette smoking, history of malignancy, diabetes mellitus, and chronic obstructive pulmonary disease.
bUsing Wald tests to assess the overall associations, age was strongly associated with cardiac mortality in unadjusted (P < 0.001) and adjusted models (P < 0.001) and non-cardiac mortality in unadjusted (P < 0.001) and adjusted models (P = 0.001).
Discussion
This study quantifies the independent association of age on the length of survival following ICD implantation. Among all subjects >75 years at the time of ICD implantation, median survival was 5.3 years, about half that of the other two groups. After controlling for potential confounders and co-morbidities, age at ICD implantation remained strongly and significantly associated with subsequent total, cardiac, and non-cardiac mortality. Divergence in survival occurred early and continued to increase throughout the majority of follow-up time. While residual confounding could explain these results, most likely age itself does exert an effect on mortality independent of baseline co-morbidity status, via intrinsic effects of age on things such as risk of procedural complications and chance of future health events, items not controlled for by the baseline covariates included in our model. Given that age itself helps predict future longevity, a predictable but not previously consistent finding in past studies, we herein advocate for treating it as an additional co-morbidity to be considered when deeming appropriateness of ICD implantation.
These findings confirm and expand upon those reported by another single-centre retrospective study, which found a lower median survival after ICD implantation in octogenarians,20 and a Canadian study, which observed older age at ICD implantation to be associated with a higher risk of death.26 Our study included a longer follow-up period of comparisons across several age groups, and contained a more complete adjustment for confounding variables than was performed in either previous study. In particular, as the Canadian study aimed to identify the independent predictors of mortality, its authors pared down the covariates included in their final Cox model, rather than examining the association of age with mortality after fully adjusting for potential confounding factors. Additionally, our study showed that both cardiac and non-cardiac mortality risk increased with age.
In contrast to our results, several previous studies have shown comparable survival rates after ICD implantation in elderly and younger patient groups.21–23 Their elderly groups were younger (70 years or greater) when compared with those 75 years or greater in this study; of note is that in the present study, the 65- to 75-year-old group did display an increased risk of death when compared with those subjects <65 years of age. Two of these studies were performed outside the USA, where the patient selection process may be expected to be more stringent.21,23 The German study also had a significant difference between groups in the percent of subjects who received an ICD for secondary prevention and therefore were at lower risk of non-sudden cardiac death (78% of the elderly patient group vs. 63% of the younger group). The other two studies were small; the study by Geelen et al., 21 for example, included only 32 patient aged 70 years or greater. In the study by Quan et al., 22 the survival curves are seen to differentiate up to ∼4 years, after which time no further deaths were observed among the older group. No denominators are given for the Kaplan–Meier curves, but by extrapolation their conclusion of similar mortality rates appears to be based on ∼47 very robust elderly patients. Thus, healthier populations receiving ICDs, baseline demographic differences between age groups, and the small number of elderly patients in outside studies may all contribute to their finding of similar survival times between elderly and younger patients after ICD implantation.
Coincident with the survival benefit of ICD implantation among the elderly subset of MADIT-II, an increased mortality rate was seen in those ≥75 years when compared with those <75 years.16 In fact, while follow-up was insufficient to determine median survival, at 3.5 years, <60% of those highly selected elderly patients with ICDs were alive. As our median survival of 5.3 years among the elderly group echoes, ICD implantation may reduce mortality in the elderly, but it should be recognized to constitute a smaller absolute clinical benefit.
Our study results have potential implications for cost-effectiveness of ICDs in elderly patients. On the basis of data from eight large primary prevention trials, the incremental cost-effectiveness of prophylactic ICD therapy has been estimated to range from $34 000 to $70 200 per QALY added.9 Sensitivity analysis showed costs to remain less than $100 000 per QALY as long as mortality was reduced for ≥7 years, with estimated costs escalating quickly as the duration of efficacy was reduced, for example, to ∼$50 000–$125 000 per QALY gained if ICD efficacy was assumed to cease after the first 5 years. Perhaps, more applicable to the current study, if the time horizon was simply truncated at 5 years cost estimates varied even more widely from $90 000 to $250 000. As all the patients in our study received an ICD, we cannot calculate risk reduction due to the ICD, but the absolute maximum average benefit in the elderly group is 5.3 years, and likely substantially shorter. Applying the estimates from Sanders et al. to our elderly patient group, the estimated cost per QALY is at least $90 000 and likely much more. Though a single threshold for cost-effectiveness has not been established, values ranging from $50 000 to $120 000 per QALY have been proposed.27,28 Thus, among the patient population that we studied, ICD intervention among the elderly as a group may not be cost-effective. The large effect of the time horizon on estimates of cost-effectiveness supports careful consideration of age at the time of ICD implantation.
Our study has several limitations. This is a single-centre observational study and unidentified or residual confounding may be present. Additionally, we assessed and adjusted for only baseline co-morbidity status. While we hypothesize that age may exert some of its influence on mortality via predilection of those with increased age to have more health events, that is, progression of baseline co-morbidites and development of new diseases, it is beyond the scope of this study to address exactly how this may occur. Although the subjects comprising the study population were identified prospectively, the retrospective abstraction of the most of the data prohibited full resolution of all missing variables, which generally constituted 25% or less of the total population for any variable. Of note, we included in our models indicators for missing data status, and we did not find evidence of informative missingness. As cause of death was collected retrospectively, there may have been some error in differentiating primacy of cause, even crudely between cardiac and non-cardiac aetiologies, though any bias would likely be shared equally among groups. Further, the small number of non-cardiac deaths, particularly in the two younger age groups, should caution interpretation of these results. Primary prevention patients were represented only in the last few years of the study, concurrent with expanding indications for ICD implantation over the course of the study. Finally, as a cohort study of patients with defibrillators, we did not have a comparison group of elderly patients who did not receive ICDs.
In conclusion, in patients receiving ICDs, increased age is significantly and progressively associated with shorter survival times after ICD implant, after controlling for known confounders. As the co-morbidity burden of this population can have a meaningful impact on survival, we would advocate an individualized approach. We would not recommend a strict age cutoff for ICDs, but perhaps a recommendation to limit their application to those expected to live more than 5 years after implantation. Age and co-morbidities should be addressed in estimating the time horizon for the effectiveness of ICDs in future cost-effectiveness analyses.
Funding
American Heart Association Western States Affiliate to (0625065Y to C.N.P.); Veterans Affairs Health Services Research and Development Research Career Development Award (RCD 04-115-2 to P.V.).
Conflict of interest: B.L. has received research support from Medtronic and has received honoraria from Boston Scientific Corporation and St Jude Medical. P.D.V. has received honoraria for assisting with development of educational programs for Boston Scientific Corporation.
References
- 1.The Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N Engl J Med. 1997;337:1576–83. doi: 10.1056/NEJM199711273372202. [DOI] [PubMed] [Google Scholar]
- 2.Connolly SJ, Gent M, Roberts RS, Dorian P, Roy D, Sheldon RS, et al. Canadian implantable defibrillator study (CIDS): a randomized trial of the implantable cardioverter defibrillator against amiodarone. Circulation. 2000;101:1297–302. doi: 10.1161/01.cir.101.11.1297. [DOI] [PubMed] [Google Scholar]
- 3.Kuck KH, Cappato R, Siebels J, Ruppel R. Randomized comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest: the Cardiac Arrest Study Hamburg (CASH) Circulation. 2000;102:748–54. doi: 10.1161/01.cir.102.7.748. [DOI] [PubMed] [Google Scholar]
- 4.Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335:1933–40. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 5.Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 1999;341:1882–90. doi: 10.1056/NEJM199912163412503. [DOI] [PubMed] [Google Scholar]
- 6.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–83. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 7.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 8.McClellan MB, Tunis SR. Medicare coverage of ICDs. N Engl J Med. 2005;352:222–4. doi: 10.1056/NEJMp048354. [DOI] [PubMed] [Google Scholar]
- 9.Sanders GD, Hlatky MA, Owens DK. Cost-effectiveness of implantable cardioverter-defibrillators. N Engl J Med. 2005;353:1471–80. doi: 10.1056/NEJMsa051989. [DOI] [PubMed] [Google Scholar]
- 10.Zwanziger J, Hall WJ, Dick AW, Zhao H, Mushlin AI, Hahn RM, et al. The cost effectiveness of implantable cardioverter-defibrillators: results from the Multicenter Automatic Defibrillator Implantation Trial (MADIT)-II. J Am Coll Cardiol. 2006;47:2310–8. doi: 10.1016/j.jacc.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 11.Grimm W, Flores BT, Marchlinski FE. Shock occurrence and survival in 241 patients with implantable cardioverter-defibrillator therapy. Circulation. 1993;87:1880–8. doi: 10.1161/01.cir.87.6.1880. [DOI] [PubMed] [Google Scholar]
- 12.Anvari A, Gottsauner-Wolf M, Turel Z, Stix G, Podesser B, Mayer C, et al. Predictors of outcome in patients with implantable cardioverter defibrillators. Cardiology. 1998;90:180–6. doi: 10.1159/000006841. [DOI] [PubMed] [Google Scholar]
- 13.Exner DV, Sheldon RS, Pinski SL, Kron J, Hallstrom A. Do baseline characteristics accurately discriminate between patients likely versus unlikely to benefit from implantable defibrillator therapy? Evaluation of the Canadian implantable defibrillator study implantable cardioverter defibrillatory efficacy score in the antiarrhythmics versus implantable defibrillators trial. Am Heart J. 2001;141:99–104. doi: 10.1067/mhj.2001.111768. [DOI] [PubMed] [Google Scholar]
- 14.Bloomfield DM, Bigger JT, Steinman RC, Namerow PB, Parides MK, Curtis AB, et al. Microvolt T-wave alternans and the risk of death or sustained ventricular arrhythmias in patients with left ventricular dysfunction. J Am Coll Cardiol. 2006;47:456–63. doi: 10.1016/j.jacc.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 15.Goldenberg I, Vyas AK, Hall WJ, Moss AJ, Wang H, He H, et al. Risk stratification for primary implantation of a cardioverter-defibrillator in patients with ischemic left ventricular dysfunction. J Am Coll Cardiol. 2008;51:288–96. doi: 10.1016/j.jacc.2007.08.058. [DOI] [PubMed] [Google Scholar]
- 16.Huang DT, Sesselberg HW, McNitt S, Noyes K, Andrews ML, Hall WJ, et al. Improved survival associated with prophylactic implantable defibrillators in elderly patients with prior myocardial infarction and depressed ventricular function: a MADIT-II substudy. J Cardiovasc Electrophysiol. 2007;18:833–8. doi: 10.1111/j.1540-8167.2007.00857.x. [DOI] [PubMed] [Google Scholar]
- 17.Groeneveld PW, Farmer SA, Suh JJ, Matta MA, Yang F. Outcomes and costs of implantable cardioverter-defibrillators for primary prevention of sudden cardiac death among the elderly. Heart Rhythm. 2008;5:646–53. doi: 10.1016/j.hrthm.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 18.Panotopoulos PT, Axtell K, Anderson AJ, Sra J, Blanck Z, Deshpande S, et al. Efficacy of the implantable cardioverter-defibrillator in the elderly. J Am Coll Cardiol. 1997;29:556–60. doi: 10.1016/s0735-1097(96)00527-x. [DOI] [PubMed] [Google Scholar]
- 19.Trappe HJ, Pfitzner P, Achtelik M, Fieguth HG. Age dependent efficacy of implantable cardioverter-defibrillator treatment: observations in 450 patients over an 11 year period. Heart (British Cardiac Society) 1997;78:364–70. [PMC free article] [PubMed] [Google Scholar]
- 20.Koplan BA, Epstein LM, Albert CM, Stevenson WG. Survival in octogenarians receiving implantable defibrillators. Am Heart J. 2006;152:714–9. doi: 10.1016/j.ahj.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Geelen P, Lorga Filho A, Primo J, Wellens F, Brugada P. Experience with implantable cardioverter defibrillator therapy in elderly patients. Eur Heart J. 1997;18:1339–42. doi: 10.1093/oxfordjournals.eurheartj.a015447. [DOI] [PubMed] [Google Scholar]
- 22.Quan KJ, Lee JH, Costantini O, Konstantakos AK, Murrell HK, Carlson MD, et al. Favorable results of implantable cardioverter-defibrillator implantation in patients older than 70 years. Ann Thorac Surg. 1997;64:1713–7. doi: 10.1016/s0003-4975(97)00922-3. [DOI] [PubMed] [Google Scholar]
- 23.Duray G, Richter S, Manegold J, Israel CW, Gronefeld G, Hohnloser SH. Efficacy and safety of ICD therapy in a population of elderly patients treated with optimal background medication. J Interv Card Electrophysiol. 2005;14:169–73. doi: 10.1007/s10840-006-5200-y. [DOI] [PubMed] [Google Scholar]
- 24.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–26. [Google Scholar]
- 25.Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69:239–41. [Google Scholar]
- 26.Lee DS, Tu JV, Austin PC, Dorian P, Yee R, Chong A, et al. Effect of cardiac and noncardiac conditions on survival after defibrillator implantation. J Am Coll Cardiol. 2007;49:2408–15. doi: 10.1016/j.jacc.2007.02.058. [DOI] [PubMed] [Google Scholar]
- 27.Schackman BR, Gold HT, Stone PW, Neumann PJ. How often do sensitivity analyses for economic parameters change cost-utility analysis conclusions? Pharmacoeconomics. 2004;22:293–300. doi: 10.2165/00019053-200422050-00003. [DOI] [PubMed] [Google Scholar]
- 28.Hirth RA, Chernew ME, Miller E, Fendrick AM, Weissert WG. Willingness to pay for a quality-adjusted life year: in search of a standard. Med Decis Making. 2000;20:332–42. doi: 10.1177/0272989X0002000310. [DOI] [PubMed] [Google Scholar]