Abstract
BACKGROUND
In vitro maturation of oocytes can, in some circumstances, provide an alternative approach to gonadotrophin-induced maturation in clinical settings. However, the consequences of these protocols on the long-term health of offspring are unknown. Here, the long-term health status and lifespans of offspring produced by in vitro maturation of mouse oocytes was compared with that of oocytes induced to mature in vivo using gonadotrophin treatment.
METHODS
Mouse oocytes were matured in vitro using both an established optimized system and in the absence of amino acids to produce a suboptimal condition for maturation. Oocytes induced to mature in vivo with gonadotrophins constituted the control group. All metaphase II oocytes were fertilized in vitro and transferred at the 2-cell stage to the oviducts of pseudo-pregnant foster mothers for development to term. Offspring were subjected to a wide variety of physiological and behavioral tests for the first year of life and natural lifespan determined.
RESULTS
There was no difference among the groups in lifespan or in most of the physiological and behavioral analyses. However, the pulse rate and cardiac output were slightly, but significantly, reduced in the optimized in vitro matured group compared with the in vivo matured group (P = 0.0119 and P = 0.0197, respectively). Surprisingly, these decreases were largely abrogated in the in vitro group matured without amino acids.
CONCLUSIONS
Evidence presented here using a mouse model suggests that the in vitro maturation of oocytes has minimal effects on the long-term health of offspring. However, a finding of slight reductions in pulse rate and cardiac output may focus future clinical attention.
Keywords: oocyte maturation, health of offspring, lifespan, cardiac function, in vitro maturation
Introduction
Pincus and Enzmann in the 1930s were the first to report the spontaneous gonadotrophin-independent meiotic maturation of mammalian oocytes in vitro (Pincus and Enzmann, 1935; Pincus and Saunders, 1939). This finding implicated follicular somatic components in maintaining mammalian oocytes at the immature germinal vesicle (GV) stage within antral follicles. Controversy followed regarding the normalcy of spontaneously matured oocytes since initial studies failed to produce eggs competent to undergo fertilization and embryogenesis. It was thus thought that spontaneous maturation in vitro involved just the meiotic, or nuclear, events of oocyte maturation and not a complete ‘cytoplasmic maturation’, processes in addition to meiotic maturation needed to produce a functional egg (Thibault, 1977; Leibfried and Bavister, 1983). Nevertheless, refinements in methodologies resulted in protocols that can produce eggs competent to undergo fertilization and embryogenesis, though the frequency of success is usually less than that resulting from hormone-induced oocyte maturation in vivo (Schroeder and Eppig, 1984). These developmental successes with animal models presented clinicians with the possibility to use oocytes matured in vitro when desirable to avoid hormonal stimulation of women or when considering financial issues. In fact, it has been suggested that more than 400 babies have now been born after in vitro oocyte maturation (Jurema and Nogueira, 2006).
A growing body of evidence has raised concerns about the long-term health effects of assisted reproduction technologies. The development of mammalian preimplantation embryos under less than optimal conditions clearly produces offspring that display either immediate or long-term health concerns using various animal models (Ecker et al., 2004; Fernandez-Gonzalez et al., 2004, 2007; Thompson et al., 2007; Watkins et al., 2008a, b). These deleterious effects may have epigenetic origins (Sinclair et al., 2007; Morgan et al., 2008). However, optimizing culture conditions using animal models has minimized, though probably not eliminated, potential deleterious effects in animals and, by extension, in human clinical IVF technology (Banwell and Thompson, 2008). It is still unclear, however, whether conditions of oocyte maturation can affect the long-term health of offspring. The finding that female mice fed a reduced protein diet specifically during the oocyte maturation period results in both physiological and behavioral aberrations in the long-term health of offspring (Watkins et al., 2008a, b) elevates concerns about in vitro oocyte maturation, particularly when maturation occurs under conditions that have not been optimized. A comparison of transcripts expressed by in vivo and in vitro matured oocytes has revealed significant differences that could constitute a basis for differences in developmental competence (Jones et al., 2008), indicating that further optimization of in vitro maturation conditions for human oocytes is warranted.
This study was undertaken to assess the long-term health of mice produced after in vitro maturation of oocytes isolated at the GV stage. The strategy was to compare mice born after hCG-induced oocyte maturation in vivo with mice born after maturation in vitro, using a broad spectrum of physiological and behavioral assays at various times up to 1 year of age. All eggs, derived from both in vivo and in vitro maturation, were fertilized in vitro and transferred to pseudo-pregnant foster mothers at the 2-cell stage. Therefore, the only difference between the groups was the mode of oocyte maturation. Oocytes were matured in vitro using two protocols: a standard well-described and optimized system (O'Brien et al., 2003) and the same system without amino acids. It was thought that the absence of amino acids might constitute a suboptimal system that could exacerbate any deleterious effects of maturation in vitro. The rationale for this approach was that reduced protein in the diet of females during the period of oocyte maturation in vivo has deleterious effects on the health of offspring (Watkins et al., 2008a, b) and, as shown here, the absence of amino acids during maturation in vitro results in decreased cleavage to the 2-cell stage. In order to avoid complications caused by possible differences in maternal environment, embryos from two groups were mixed before transfer, one group having been genetically marked to express green fluorescent protein (GFP) ubiquitously. Although small, but statistically significant, differences between in vivo and in vitro maturation were found in some physiological and behavioral tests, there were no major differences, and lifespan was unaffected.
Materials and Methods
Animals
Oocytes were obtained from 22- to 24-day-old B6SJLF1 females raised in the research colonies of the investigators at the Jackson Laboratory. GV-stage oocytes for in vitro maturation were isolated from large antral follicles 44 h after i.p. injection of 5 IU equine chorionic gonadotrophin (eCG). Superovulation was induced by first administering 5 IU of eCG and then, 44 h later, 5 IU of hCG. Metaphase II (MII) stage oocytes were isolated from oviducts of females 15 h after the administration of hCG. MII oocytes from both groups, identified by the presence of the first polar body, were fertilized with epididymal sperm obtained from either GFP homozygous or wild-type (non-GFP-carrying) males from the STOCK TgN(GFPU)5Nagy mouse line (stock 3115: JAX Mice and Services, Bar Harbor, ME, USA). All animal protocols were approved by the Administrative Panel on Laboratory Animal Care at The Jackson Laboratory, and all experiments were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
In vitro maturation, fertilization and cleavage
Oocyte–cumulus complexes with a tight unexpanded cumulus oophorus and an oocyte at the GV-stage were selected for in vitro maturation. In vitro maturation, fertilization and cleavage were carried out as described previously (Eppig and O'Brien, 1996; O'Brien et al., 2003). Briefly, oocyte–cumulus cell complexes were matured in minimum essential medium (MEM)α supplemented with 3 mg/ml bovine serum albumin, 1 mg/ml fetuin [purified by the Protein Chemistry Service of the Jackson Laboratory as described by Spiro (1960)], 0.23 mM pyruvic acid, 10 µg/ml streptomycin sulfate, 75 µg/ml penicillin G and 1 ng/ml epidermal growth factor (EGF, culture grade, Becton Dickinson Labware, Bedford, MA, USA). MEMα was prepared either with or without amino acids. After ∼17 h of maturation, IVF and cleavage to the 2-cell stage were carried out in MEM, supplemented as indicated above for MEMα with pyruvate and antibiotics, but without fetuin or EGF. MEM used for fertilization and first cleavage contained the normal complement of amino acids whether the MEMα used for maturation contained amino acids or not.
Embryo transfer
Groups to be compared were fertilized with either wild-type sperm or sperm carrying the GFP construct. Each group contained approximately equal numbers of offspring derived from eggs fertilized with each genotype of sperm. Two-cell stage embryos derived from two different experimental groups were mixed and introduced to oviducts of the same pseudo-pregnant foster mothers. For example, an in vivo matured group fertilized with GFP-carrying sperm was mixed with either of the two in vitro matured groups fertilized without the GFP marker, or the two in vitro matured groups (those matured either with or without amino acids) were mixed before transfer. This protocol eliminated any potential bias that could be introduced by either the genotype of sperm or the uterine environment, and enabled identification of the offspring at birth by presence or absence of green fluorescence.
Post-natal assessments
Animals were sexed and weighed at birth and earmarked for future identification. All assays from that point forward were carried out blind and the code was not broken until the death of each mouse. The Physiology and In-Vivo Imaging Service of the Jackson Laboratory carried out the metabolic, cardiopulmonary, neuromuscular and behavioral assays (see below) when animals reached 3, 6, 9,and 12 months of age.
Metabolic studies
Metabolic studies were carried out using a Comprehensive Lab Animal Monitoring System (CLAMS; Columbus Instruments, Columbus, OH, USA). These cages simultaneously determine calorimetric parameters (oxygen consumption, VO2, and carbon dioxide production, VCO2, both as ml/kg/h), food and drink consumption as well as ambulatory and rearing activity (through counting the number of breaks in a system of infrared beams) of individually housed animals over a 72 h period. Metabolic parameters calculated include the respiratory exchange ratio (VCO2/VO2) and metabolic heat (kcal/h). Since mice are nocturnal, data from the CLAMS were analyzed as daily values as well as for light and dark periods.
Cardiovascular studies
Cardiac electrical activity, the electrocardiogram, was assessed in isoflurane-anesthetized mice using TD-24 Tri-(silver-silver chloride) electrode strips (Discount Disposables, St Albans, VT, USA) attached to an ML136 Animal Bio Amp and ML750 PowerLab/4SP (ADInstruments, Colorado Springs, CO, USA). Animals were maintained on as low isoflurane concentration as possible to keep them still, while the electrical activity was recorded for up to 10 min, at which time the isoflurane was turned off and the animals allowed to recover from anesthesia. Up to 60 s of artifact-free tracing was analyzed for heart rate and rhythm as well as waveform characteristics such as height, duration and interval duration. The analysis was performed using Chart v4.1.1 for Mac and the SAECG (signal averaging) extension (ADInstruments).
Blood pressure was measured non-invasively using the BP2000 Blood Pressure Analysis System (Visitech Systems, Apex, NC, USA). Mice were tested for up to 30 min for 5 consecutive days; the first 10, of 30, measurements each day were discarded as acclimation cycles and the first 3 days were considered training days and the last 2 days as test days. Since this system is considered to approximate systolic blood pressure any recorded values that were below 60 mmHg were excluded as were any values that were then more than ±2SD from the subsequent calculated daily mean.
Echocardiography was performed using a Vevo 770 High-Frequency Ultrasound (Visualsonics, Toronto, Ontario, Canada). Routine examinations included B-mode parasternal short- and long-axis views for structural and functional features and M-mode examination of the same views for standard echocardiographic measurements and calculations such as septal and posterior wall thickness, ventricular volume, fractional shortening, ejection fraction and cardiac output (Collins et al., 2003).
Neuromuscular and neurobehavioral studies
Mice were tested for forelimb grip strength using a vertically oriented Chatillon-Ametek Digital Force Gauge, DFIS 2 (Columbus Instruments). The peak force, in kilograms, was averaged for three consecutive trials. The motor coordination and performance and balance of mice were also assessed using an Economex Accelerating Rota-Rod (Columbus Instruments). Mice were placed on the stationary rod for three consecutive trials with a 1 min cut-off. Each mouse was then placed on the rod rotating at a constant speed of 4.0 rpm for three consecutive trials, again, each limited to 1 min. Lastly, mice were re-positioned on the rotating rod (4.0 rpm) followed by an increase of rotational speed by 0.1 revolutions/s (i.e. 6 rpm) for up to 5 min also for consecutive trials. The latency to fall from the rod was recorded for each trial.
SHIRPA is a suite of standardized protocols for behavioral and functional assessment that provides sensitive measures for quantifying phenotype expression (Rogers et al., 1999). These tests identify defects in gait or posture, motor control and co-ordination, changes in excitability and aggression, salivation, lacrimation, piloerection, defecation, muscle tone and gross pain sensation.
Open-field/exploratory hole-board: the exploratory hole-board (Ugo Basile, Comerio, Italy) and open-field were used to assess locomotor activity, anxiety, exploratory activity, risk assessment and arousal in a novel environment (Ohl et al., 2001). The exploratory hole-board consists of a piece of gray Plexiglas, 24′ × 24′ in total area separated into a 4′ perimeter and 20′ open arena. The perimeter has lines on its surface to determine locomotor activity. The open arena has 16 1′ holes, each containing infrared photobeam detectors to count each episode of the mouse dunking its head into a hole.
Statistical analyses
For measurements generated from physical testing, metabolic testing, cardiopulmonary testing, neuromuscular testing and behavioral exploratory board testing, linear mixed models with repeated measures were employed. For measurements generated from behavioral SHIRPA testing, generalized linear mixed model analyses with repeated measures were performed. For each trait, a full model was fitted considering the three factors (conditions for oocyte maturation, sex and age) and all possible interactions. A backward model selection approach was used to achieve a final model, P-values from which were used for significance tests for factors of interest. After maturation in vitro, with or without amino acids, the percentages of MII eggs that cleaved to the 2-cell stage, the 2-cell stage oocytes that developed to blastocysts, and MII that developed to blastocysts were acrsin-square root transformed, and two-sample t-test was applied to the transformed data for each stage comparison. Chi-square (χ2) analyses were conducted to test for possible differences in birth rates. Survival analysis was performed for lifespan data. R2.4.1 (www.r-project.org) and SAS 9.1.3 (SAS institute, Cary, NC, USA) were used in the analyses. A P-value of <0.05 was considered statistically significant.
Results
Effect of amino acid deprivation during in vitro oocyte maturation on developmental competence
Oocytes were matured in vitro in medium with or without amino acids and then all groups were washed and fertilized in medium containing amino acids. After fertilization, all groups were cultured in KSOM/AA for preimplantation development. As shown in Table I, the percentage of eggs that cleaved to the 2-cell stage was significantly reduced (P = 0.0312) as a consequence of amino acid deprivation during oocyte maturation. However, development from the 2-cell stage to the blastocyst stage in vitro was unaffected (P = 0.3661). There were no differences in the number of cells/blastocyst among any of the groups (data not shown); in all cases, there were ∼70 cells/blastocysts.
Table I.
Developmental competence of mouse oocytes matured in vitro (IVM) in the presence (+) or absence (−) of amino acids (AA)
| % 2-Cell1 | % 2-Cell to blastocyst2 | % Total blastocyst3 | |
|---|---|---|---|
| IVM + AA | 75.5 ± 3.07 | 79 ± 1.35 | 59.7 ± 2.17 |
| IVM − AA | 57.7 ± 5.44 | 82.7 ± 3.71 | 47.5 ± 4.73 |
| P-value | 0.0312 | 0.3661 | 0.0575 |
1Mean percentage ± SEM of MII oocytes developing to the 2-cell stage after IVF.
2Mean percentage ± SEM of 2-cell stage embryos developing to the blastocyst stage.
3Mean percentage ± SEM of MII oocytes developing to the blastocyst stage.
Two-cell stage embryos were transferred to uteri of pseudo-pregnant foster mothers and allowed to develop to term. Offspring were identified at birth according to the presence or absence of total body green fluorescence. The percentages of pups born of the total number of 2-cell stage embryos transferred were: in vivo matured, 51.8% (29/56); in vitro matured 21.3% (26/122); amino-acid-deprived in vitro matured 33.8% (26/77). Groups were compared by χ2 analysis. The percentage of pups born after maturation in vivo was significantly greater than after maturation in vitro with (χ2 = 16.69, P = 0.0004) or without (χ2 = 4.34, P = 0.0372) amino acids. The difference between the two in vitro matured groups was marginally significant (χ2 = 3.79, P = 0.0514).
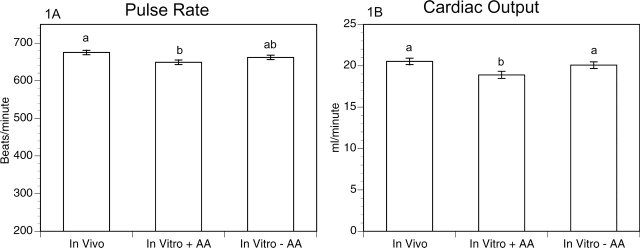
Offspring were subjected to a battery of physiological and behavioral tests at 3, 6, 9 and 12 months of age. The major tests employed are indicated on Table II. No consistent differences were detected in any of the tests, at any age, except for a reduction in pulse rate and cardiac output (P = 0.0119 and P = 0.0197, respectively, in optimized in vitro matured group versus in vivo matured group). These differences were seen across all age groups. However, they were surprisingly abrogated by in vitro maturation in the absence of amino acids (Fig. 1). For both these cardiac parameters, the pups born after in vitro maturation with amino acids had the lowest values. There were no differences in heart rate measured during either echocardiography or electrocardiography (data not shown). It should be noted that the pulse rate was determined by tail cuff of conscious restrained mice, whereas echocardiography and electrocardiography were conducted using isoflurane-anesthetized mice.
Table II.
Results of physiological and behavioral testing
| Category | Test | Measurements | In vivo versus IVM + AA |
|---|---|---|---|
| Physical | Weight | ND (dns) | |
| Metabolic | Comprehensive cage monitoring system | Volume of O2 consumed | ND (dns) |
| Volume of CO2 produced | ND (dns) | ||
| Respiratory exchange ratio | ND (dns) | ||
| Heat | ND (dns) | ||
| Accumulated feed | ND (dns) | ||
| Accumulated drink | ND (dns) | ||
| Ambulation | ND (dns) | ||
| Cardiopulmonary | Electrocardiography | Heart rate | ND (dns) |
| Echocardiography | Heart rate | ND (dns) | |
| Left ventricle % ejection fraction | ND (dns) | ||
| Left ventricle % fractional shortening | ND (dns) | ||
| Cardiac output | P = 0.0197 (Fig. 1B) | ||
| Blood pressure | Systolic pressure | ND (dns) | |
| Pulse rate | P = 0.0119 (Fig. 1A) | ||
| Neuromuscular | Grip-strength | Force | ND (dns) |
| Rotarod | Duration | ND (dns) | |
| Behavioral | SHIRPA | Aggression | ND (dns) |
| Startle response | ND (dns) | ||
| Transfer arousal | ND (dns) | ||
| Exploratory Board | Stretched attends | ND (dns) | |
| Hole visit | ND (dns) | ||
| Defecation | ND (dns) | ||
| Lifespan | Age at death | ND (Fig. 2) |
ND, not significantly different; dns, data not shown.
Figure 1.
Effect of in vitro maturation on (A) pulse rate and (B) cardiac output. The same letter above the bars indicates that the groups are not significantly different. Pulse rate P = 0.0119, cardiac output P = 0.0197. Bars indicate the mean ± SEM. AA, amino acids.
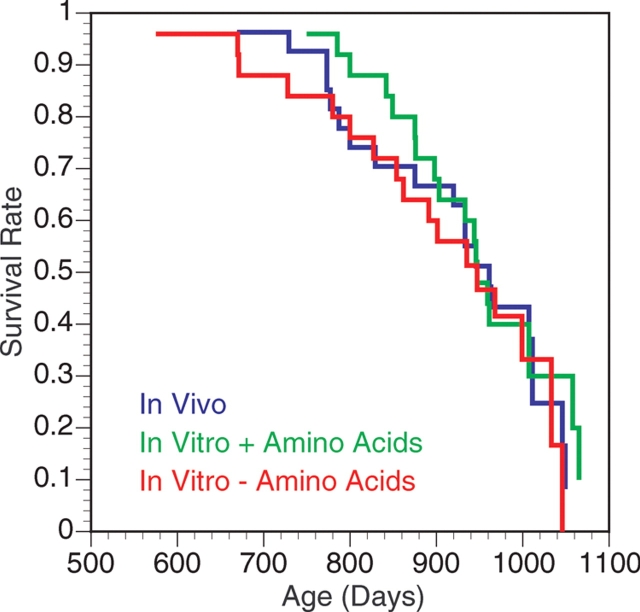
Mice were maintained until natural death or until institutional animal care protocols required euthanasia. The age at euthanasia was considered the same as the date of natural death. There was no difference (χ2 = 0.58, P = 0.7487) in the mean lifespans of mice derived from any of the maturation groups: ovulated 904.9 ± 24.1 days; in vitro matured 943.6 ± 20.0 days; in vitro matured without amino acids 896.9 ± 28.9 days (Fig. 2).
Figure 2.
Effect of in vitro maturation on lifespan of the offspring. No differences were observed.
Discussion
Analyses of offspring produced by in vitro maturation of human oocytes detected no adverse health consequences (Cha et al., 2005; Mikkelsen, 2005; Soderstrom-Anttila et al., 2006). However, these studies did not report health status beyond early childhood and it will be many years before potential long-term outcomes can be evaluated. Nevertheless, studies with animal models revealed late onset health problems in offspring, particularly behavioral problems and symptoms associated with obesity and hypertension, after maternal dietary restriction during specific times of oocyte maturation and preimplantation embryogenesis (Kwong et al., 2000; Watkins et al., 2008a, b). Therefore, the present study focused on comparing potential long-term health consequences to offspring produced by in vitro maturation of mouse oocytes with hCG-induced maturation. This comparison revealed equivalent lifespans of mice derived from all groups, but statistically significant decreases in pulse rate and cardiac output could indicate issues of concern for offspring derived from in vitro matured human oocytes.
The experimental design of these experiments was intended to further ‘handicap’ in vitro maturation by depriving the maturing oocytes of free amino acids. The absence of amino acids from the maturation culture medium only resulted in a significantly reduced development to the 2-cell stage after fertilization but did not affect the development of the 2-cell stage embryos to the blastocyst stage in vitro. Although a significantly lower percentage of offspring were born after oocyte maturation in vitro compared with in vivo maturation (51.8% versus 21.3%, respectively, P = 0.0004) as expected, those deprived of amino acids in the maturation medium surprisingly were born at a slightly higher frequency than were born after maturation in complete medium (33.8% versus 21.3%, respectively, P = 0.0514). Moreover, where a physiological difference was consistently observed between offspring derived from in vivo and in vitro oocyte maturation (the pulse rate and cardiac output), the difference was largely abrogated by deprivation of amino acids in the culture medium. Thus, although the deprivation of free amino acids during oocyte maturation was clearly deleterious to preimplantation development, this treatment seemed to have some beneficial post-natal effects. The origin of this apparent conundrum may be the transfer of all embryos at the 2-cell stage; i.e. those that successfully develop to the 2-cell stage after maturation in amino acid-free medium may have been hardiest—those able to cleave despite sub-optimal culture conditions for maturation. It could also be argued that maturation in the absence of free amino acids could benefit some aspects of developmental competence, such as those leading to birth, whereas at the same time impair other aspects, such as cleavage to the 2-cell stage.
As indicated above, the only consistent physiological differences observed in this study were pulse rate and cardiac output, seen at all ages studied (up to 12 months of age). Although these two parameters were measured under different conditions at different times, together they suggest a possible real effect upon cardiac function as a result of in vitro maturation, especially with amino acids. However, the amount of these differences was ∼4% for pulse rate and 8% for the cardiac output, in reality quite small values; differences apparently insufficient to cause differences in overall group lifespan. Furthermore, the cardiac output measurements were taken, while the animals were isoflurane-anesthetized rather than conscious but somewhat stressed from handling, as was the case for pulse rate. Isoflurane, even at low depths of anesthesia, affects cardiac function; compared with other inhalational anesthetics, it affects heart rate and blood pressure much more than cardiac output (Pagel et al., 2000). This effect is to reduce both these parameters and decrease the inherent variability of heart rate in particular and the animal's ability to respond to environmental influences. As a consequence, measurements of heart or pulse rates in conscious animals better reflect the true state and response of the animals than when collected during anesthesia. All of these effects are dose-dependent and animals were delivered as low a concentration as possible to just provide physical restraint during data collection. To test whether these differences were physiologically significant would have required testing of the mice under some metabolically stressful situation, such as a metabolic treadmill, which was not done. It is possible that there were significant effects in physiological parameters that were not assessed in this study. Nevertheless, there was no detectable effect of in vitro maturation on lifespan. Similarly, no effects on longevity were found in mouse offspring derived from preimplantation embryo culture under conditions considered to be optimal versus suboptimal (Sommovilla et al., 2005). The suboptimal conditions for embryo culture produced significant behavioral problems and imprinting differences, but adverse physiological outcomes were not reported (Doherty et al., 2000; Ecker et al., 2004).
An important concept emerging from studies on the development of culture systems for oocyte and preimplantation development is that the production of healthy offspring is the best outcome and that assessing only the ability to complete preimplantation development can be deceptive. As an example, although the maturation of mouse oocytes in a wide range of atmospheric oxygen concentrations produced no differences in the frequency of development to the blastocyst stage, or the implantation or birth rates, the proportion of cell types in the blastocysts and the fetal and placental weights were significantly affected by the oxygen concentration during oocyte maturation (Banwell et al., 2007). Although the frequency and condition of birth are crucial and informative end-points, confidence in culture protocols will be bolstered by further studies on adult health.
Studies to optimize culture conditions for oocyte and embryo development using animal models are limited only by the availability of resources. This is obviously not the case for the establishment of optimal conditions for the human oocytes and embryos where the use of post-natal end-points face serious ethical as well as resource problems. Thus, animal models must establish the foundation for clinical application. The studies reported here, in conjunction with preliminary clinical studies (Cha et al., 2005; Mikkelsen, 2005; Soderstrom-Anttila et al., 2006), suggest that in vitro maturation, under certain conditions, may be safe for clinical application. Certainly, clinical application of in vitro maturation would have been discouraged if we had found gross consequences to adult physiology, behavior or lifespan, using the mouse model, such as those produced by cloning by nuclear transfer (Yanagimachi, 2002; Tamashiro et al., 2007). But this was not our finding. Do these results therefore imply that adult humans derived from the application of this technology will experience no adverse health effects? Certainly not, but there is now some experimental basis for optimism. Nevertheless, the finding reported here of a slightly reduced cardiac output in the in vitro matured with amino acids group should raise a flag of caution.
Although studies on the consequences of in vitro oocyte maturation to adult health using the mouse model, as described here, are promising, there are differences between mouse and human oocytes that are sufficient to instill caution in drawing parallel conclusions. For example, there is a major difference in the time course of meiotic maturation, with mouse and rat oocytes requiring only 12–15 h compared with 30+ hours for human oocytes (Edwards, 1965). Furthermore, the studies reported here were conducted using mice with limited genetic variation, and there is no certainty that the same results would be obtained using any mouse strain or genotype. Obviously, clinical applications involve patients of diverse genotypes, which could potentially impact outcomes. Thus, studies using animal model oocytes more similar to those of the human, such as those of domestic agricultural species and non-human primates, would be immensely valuable, although such studies would require significant resources.
Funding
This research was funded by grant HD21970 from the NICHD to JJE.
Acknowledgements
We thank Drs Mary Ann Handel and Luanne Peters for their helpful suggestions in the preparation of this paper and Crystal Davis and Jennifer Ryan of the Physiology and In-Vivo Imaging Service of The Jackson Laboratory.
References
- Banwell KM, Thompson JG. In vitro maturation of mammalian oocytes: outcomes and consequences. Semin Reprod Med. 2008;26:162–174. doi: 10.1055/s-2008-1042955. [DOI] [PubMed] [Google Scholar]
- Banwell KM, Lane M, Russell DL, Kind KL, Thompson JG. Oxygen concentration during mouse oocyte in vitro maturation affects embryo and fetal development. Hum Reprod. 2007;22:2768–2775. doi: 10.1093/humrep/dem203. [DOI] [PubMed] [Google Scholar]
- Cha KY, Chung HM, Lee DR, Kwon H, Chung MK, Park LS, Choi DH, Yoon TK. Obstetric outcome of patients with polycystic ovary syndrome treated by in vitro maturation and in vitro fertilization-embryo transfer. Fertil Steril. 2005;83:1461–1465. doi: 10.1016/j.fertnstert.2004.11.044. [DOI] [PubMed] [Google Scholar]
- Collins KA, Korcarz CE, Lang RM. Use of echocardiography for the phenotypic assessment of genetically altered mice. Physiol Genomics. 2003;13:227–239. doi: 10.1152/physiolgenomics.00005.2003. [DOI] [PubMed] [Google Scholar]
- Doherty AS, Mann MRW, Tremblay KD, Bartolomei MS, Schultz RM. Differential effects of culture on imprinted H19 expression in the preimplantation mouse embryo. Biol Reprod. 2000;62:1526–1535. doi: 10.1095/biolreprod62.6.1526. [DOI] [PubMed] [Google Scholar]
- Ecker DJ, Stein P, Xu Z, Williams CJ, Kopf GS, Bilker WB, Abel T, Schultz RM. Long-term effects of culture of preimplantation mouse embryos on behavior. Proc Natl Acad Sci USA. 2004;101:1595–1600. doi: 10.1073/pnas.0306846101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RG. Maturation in vitro of mouse, sheep, cow, pig, rhesus monkey and human ovarian oocytes. Nature. 1965;208:349–351. doi: 10.1038/208349a0. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, O'Brien MJ. Development in vitro of mouse oocytes from primordial follicles. Biol Reprod. 1996;54:197–207. doi: 10.1095/biolreprod54.1.197. [DOI] [PubMed] [Google Scholar]
- Fernandez-Gonzalez R, Moreira P, Bilbao A, Jimenez A, Perez-Crespo M, Ramirez MA, Rodriguez De Fonseca F, Pintado B, Gutierrez-Adan A. Long-term effect of in vitro culture of mouse embryos with serum on mRNA expression of imprinting genes, development, and behavior. Proc Natl Acad Sci USA. 2004;101:5880–5885. doi: 10.1073/pnas.0308560101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Gonzalez R, Ramirez MA, Bilbao A, De Fonseca FR, Gutierrez-Adan A. Suboptimal in vitro culture conditions: an epigenetic origin of long-term health effects. Mol Reprod Dev. 2007;74:1149–1156. doi: 10.1002/mrd.20746. [DOI] [PubMed] [Google Scholar]
- Jones GM, Cram DS, Song B, Magli MC, Gianaroli L, Lacham-Kaplan O, Findlay JK, Jenkin G, Trounson AO. Gene expression profiling of human oocytes following in vivo or in vitro maturation. Hum Reprod. 2008;23:1138–1144. doi: 10.1093/humrep/den085. [DOI] [PubMed] [Google Scholar]
- Jurema MW, Nogueira D. In vitro maturation of human oocytes for assisted reproduction. Fertil Steril. 2006;86:1277–1291. doi: 10.1016/j.fertnstert.2006.02.126. [DOI] [PubMed] [Google Scholar]
- Kwong WY, Wild AE, Roberts P, Willis AC, Fleming TP. Maternal undernutrition during the preimplantation period of rat development causes blastocyst abnormalities and programming of postnatal hypertension. Development. 2000;127:4195–4202. doi: 10.1242/dev.127.19.4195. [DOI] [PubMed] [Google Scholar]
- Leibfried ML, Bavister BD. Fertilizability of in vitro matured oocytes from golden hamsters. J Exp Zool. 1983;226:481–485. doi: 10.1002/jez.1402260320. [DOI] [PubMed] [Google Scholar]
- Mikkelsen AL. Strategies in human in-vitro maturation and their clinical outcome. Reprod Biomed online. 2005;10:593–599. doi: 10.1016/s1472-6483(10)61666-5. [DOI] [PubMed] [Google Scholar]
- Morgan HD, Jin XL, Li A, Whitelaw E, O'Neill C. The culture of zygotes to the blastocyst stage changes the postnatal expression of an epigentically labile allele, agouti viable yellow, in mice. Biol Reprod. 2008;79:618–623. doi: 10.1095/biolreprod.108.068213. [DOI] [PubMed] [Google Scholar]
- O'Brien MJ, Pendola JK, Eppig JJ. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol Reprod. 2003;68:1682–1686. doi: 10.1095/biolreprod.102.013029. [DOI] [PubMed] [Google Scholar]
- Ohl F, Holsboer F, Landgraf R. The modified hole board as a differential screen for behavior in rodents. Behav Res Methods Instrum Comput. 2001;33:392–397. doi: 10.3758/bf03195393. [DOI] [PubMed] [Google Scholar]
- Pagel PS, Farber NE, Waritier DC. Cardiovascular pharmacology. In: Miller RD, editor. Anesthesia. Philadelphia: Churchill Livingstone; 2000. pp. 96–124. [Google Scholar]
- Pincus G, Enzmann EV. The comparative behavior of mammalian eggs in vivo and in vitro. I. The activation of ovarian eggs. J Exp Med. 1935;62:655–675. doi: 10.1084/jem.62.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus G, Saunders B. The comparative behavior of mammalian eggs in vivo and in vitro. IV. The maturation of human ovarian ova. Anat Rec. 1939;75:537–545. [Google Scholar]
- Rogers DC, Jones DN, Nelson PR, Jones CM, Quilter CA, Robinson TL, Hagan JJ. Use of SHIRPA and discriminant analysis to characterise marked differences in the behavioural phenotype of six inbred mouse strains. Behav Brain Res. 1999;105:207–217. doi: 10.1016/s0166-4328(99)00072-8. [DOI] [PubMed] [Google Scholar]
- Schroeder AC, Eppig JJ. The developmental capacity of mouse oocytes that matured spontaneously in vitro is normal. Dev Biol. 1984;102:493–497. doi: 10.1016/0012-1606(84)90215-x. [DOI] [PubMed] [Google Scholar]
- Sinclair KD, Allegrucci C, Singh R, Gardner DS, Sebastian S, Bispham J, Thurston A, Huntley JF, Rees WD, Maloney CA, et al. DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status. Proc Natl Acad Sci USA. 2007;104:19351–19356. doi: 10.1073/pnas.0707258104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderstrom-Anttila V, Salokorpi T, Pihlaja M, Serenius-Sirve S, Suikkari AM. Obstetric and perinatal outcome and preliminary results of development of children born after in vitro maturation of oocytes. Hum Reprod. 2006;21:1508–1513. doi: 10.1093/humrep/dei503. [DOI] [PubMed] [Google Scholar]
- Sommovilla J, Bilker WB, Abel T, Schultz RM. Embryo culture does not affect the longevity of offspring in mice. Reproduction. 2005;130:599–601. doi: 10.1530/rep.1.00872. [DOI] [PubMed] [Google Scholar]
- Spiro RG. Studies on fetuin, a glycoprotein of fetal serum I. Isolation, chemical composition, and physicochemical properties. J Biol Chem. 1960;235:2860–2869. [PubMed] [Google Scholar]
- Tamashiro KL, Sakai RR, Yamazaki Y, Wakayama T, Yanagimachi R. Developmental, behavioral, and physiological phenotype of cloned mice. Adv Exp Med Biol. 2007;591:72–83. doi: 10.1007/978-0-387-37754-4_5. [DOI] [PubMed] [Google Scholar]
- Thibault C. Are follicular maturation and oocyte maturation independent processes? J Reprod Fertil. 1977;51:1–15. doi: 10.1530/jrf.0.0510001. [DOI] [PubMed] [Google Scholar]
- Thompson JG, Mitchell M, Kind KL. Embryo culture and long-term consequences. Reprod Fertil Dev. 2007;19:43–52. doi: 10.1071/rd06129. [DOI] [PubMed] [Google Scholar]
- Watkins AJ, Ursell E, Panton R, Papenbrock T, Hollis L, Cunningham C, Wilkins A, Perry VH, Sheth B, Kwong WY, et al. Adaptive responses by mouse early embryos to maternal diet protect fetal growth but predispose to adult onset disease. Biol Reprod. 2008;a 78:299–306. doi: 10.1095/biolreprod.107.064220. [DOI] [PubMed] [Google Scholar]
- Watkins AJ, Wilkins A, Cunningham C, Perry VH, Seet MJ, Osmond C, Eckert JJ, Torrens C, Cagampang FR, Cleal J, et al. Low protein diet fed exclusively during mouse oocyte maturation leads to behavioural and cardiovascular abnormalities in offspring. J Physiol. 2008;b 586:2231–2244. doi: 10.1113/jphysiol.2007.149229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagimachi R. Cloning: experience from the mouse and other animals. Mol Cell Endocrinol. 2002;187:241–248. doi: 10.1016/s0303-7207(01)00697-9. [DOI] [PubMed] [Google Scholar]