Abstract
Although elucidation of the medicinal chemistry of agonists and antagonists of the P2Y receptors has lagged behind that of many other members of group A G protein-coupled receptors, detailed qualitative and quantitative structure–activity relationships (SARs) were recently constructed for several of the subtypes. Agonists selective for P2Y1, P2Y2, and P2Y6 receptors and nucleotide antagonists selective for P2Y1 and P2Y12 receptors are now known. Selective nonnucleotide antagonists were reported for P2Y1, P2Y2, P2Y6, P2Y11, P2Y12, and P2Y13 receptors. At the P2Y1 and P2Y12 receptors, nucleotide agonists (5′-diphosphate derivatives) were converted into antagonists of nanomolar affinity by altering the phosphate moieties, with a focus particularly on the ribose conformation and substitution pattern. Nucleotide analogues with conformationally constrained ribose-like rings were introduced as selective receptor probes for P2Y1 and P2Y6 receptors. Screening chemically diverse compound libraries has begun to yield new lead compounds for the development of P2Y receptor antagonists, such as competitive P2Y12 receptor antagonists with antithrombotic activity. Selective agonists for the P2Y4, P2Y11, and P2Y13 receptors and selective antagonists for P2Y4 and P2Y14 receptors have not yet been identified. The P2Y14 receptor appears to be the most restrictive of the class with respect to modification of the nucleobase, ribose, and phosphate moieties. The continuing process of ligand design for the P2Y receptors will aid in the identification of new clinical targets.
Keywords: Nucleotide, Purine, Pyrimidine, G protein-coupled receptor, Structure activity relationship
Introduction
The P2Y receptors are 7TM (containing seven transmembrane domains) receptors that couple to G protein-dependent and -independent signaling pathways, including ion channels [1]. Eight human subtypes of the P2Y receptor family have been defined. According to a dendrogram relating sequence homology, the P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11 receptors form a cluster of preferentially Gq-coupled receptors, and the P2Y12, P2Y13, and P2Y14 receptors form a cluster of preferentially Gi-coupled receptors [2, 3].
Elucidation of the medicinal chemistry of agonists and antagonists of the P2Y receptors has lagged behind characterization of many other members of the group A G protein-coupled receptors (GPCRs) [4–8]. Many of the early ligands suffered from promiscuity, affecting not only multiple P2Y metabotropic receptors but also P2X nucleotide-gated ion channels, various enzymes involved in controlling levels of extracellular nucleotides and nucleosides, and intracellular signaling pathways. However, the armamentarium of P2Y receptor ligands available has recently expanded greatly in both quantity and quality. Qualitative and quantitative structure–activity relationships (SARs) were recently constructed in detail for nucleotides acting at P2Y1 and P2Y12 receptors and with less detail for the P2Y2 and P2Y6 receptors. Molecular modeling of these receptors and docked ligands with a rhodopsin-based template has aided in ligand design [2, 3]. Studies of radioligand binding have been successful at only a few of the P2Y receptors. In this review, we identify the most selective molecular probes reported for the P2Y receptors, which have enabled pharmacological studies in the nucleotide field. The availability of pharmacological tools for the various subtypes (along with genetically modified mouse strains) is also enabling biological studies.
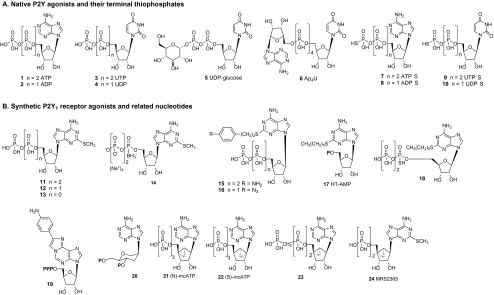
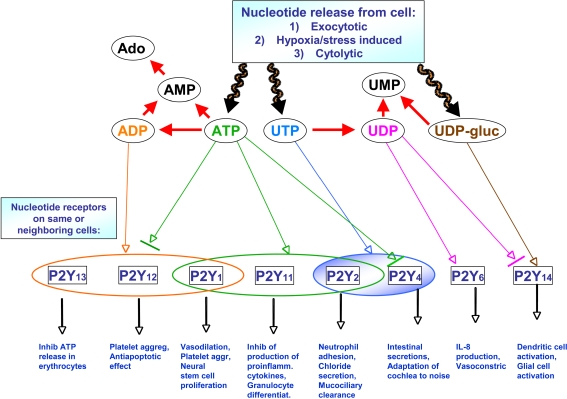
The five major native ligands of the P2Y receptors (Fig. 1) are adenosine 5′-triphosphate (ATP) 1, adenosine 5′-diphosphate (ADP) 2, uridine 5′-triphosphate (UTP) 3, uridine 5′-diphosphate (UDP) 4, and UDP-glucose 5 (or other UDP sugars). Dinucleotides such as Ap4U 6 are also naturally occurring P2Y agonists [9]. Adenosine monophosphate (AMP) and uridine monophosphate (UMP) are inactive at P2Y receptors. Figure 2 shows the correspondence of these nucleotides and the subtypes that they activate or antagonize, along with the diversity of representative biological effects induced by P2Y receptor activation. Some single nucleotides activate multiple subtypes (e.g., ATP and UTP) or activate one subtype and antagonize another (e.g., ATP and UDP). UDP acts as a competitive antagonist at the human but not rat P2Y14 receptor [10].
Fig. 1.
a Naturally occurring P2Y receptor agonists and terminally thiophosphate-substituted analogues. b Structures of adenine-derived nucleotide agonists of P2Y1, P2Y12, and P2Y13 receptors
Fig. 2.
Correspondence of the five principal native ligands of the human P2Y receptors and the subtypes they activate. Note that various dinucleotides also activate P2Y receptors. For example, dinucleoside triphosphates activate the P2Y6 receptor
Pharmacological studies of P2Y receptors are complicated by the presence of ectonucleotidases that degrade the native agonist and antagonist nucleotides. Products of the enzymatic hydrolysis might either be inactive or have activity at other P2Y receptor subtypes (e.g., 5′-triphosphate derivatives are converted to diphosphates) or at adenosine receptors (when adenosine is produced by the action of CD73/5′-nucleotidase on AMP [11]). Alternately, the 5′-diphosphates might be phosphorylated to biologically active 5′-triphosphates by the action of nucleoside diphosphokinase [12]. Thus, the state of activation of multiple P2Y receptors in a given tissue is a complex function of the kinetics of release, interconversion, and inactivation of the family of receptor-interactive nucleotides.
Various phosphate modifications, such as a terminal thiophosphate substitution, increase stability toward nucleotidases. Thus, ATPγS 7, ADPβS 8, UTPγS 9, and UDPβS 10 have been used to activate P2Y receptors, as noted in Table 1. The terminal thiophosphates are generally well tolerated in the nucleotide-binding sites of the P2Y receptors. However, these compounds are susceptible to oxidation and are difficult to synthesize on a large scale. Thiophosphate substitutions at nonterminal phosphate groups are better tolerated at the P2Y1 than at the P2Y2 and P2Y4 subtypes [15, 16]. Another type of modification that increases the stability of such nucleotide derivatives is replacement of a bridging oxygen of the phosphate chain with methylene or dihalomethylene; however, this modification tends to reduce potency at the P2Y receptors [17].
Table 1.
Potencies and selectivities of natural and synthetic ligands at the human P2Y receptors (references in brackets)
| Subfamily and Pharmacologic Characteristics | Receptor Subtype | Ligands (potency and selectivity) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Native agonist | pEC50 (ref) | Synthetic agonist | pEC50 (ref) | Cross reactivity | Synthetic (or native) antagonist | pEC50 (ref) | Cross reactivity | ||
| P2Y1-like, Gq-coupled | P2Y1 | ADP 2 | 5.09 [6] | MeSADP 12 | 8.22 [6] | P2Y12,13 | A3P5P 25 | 6.08 [35] | none |
| ADP-β-Sa 8 | 7.02 [6] | P2Y12,13 | MRS2179 26 | 6.48 [36] | none | ||||
| MRS2365 24 | 9.40 [34] | none | MRS2279 29 | 7.28 [6] | none | ||||
| MRS2500 30 | 9.02 [39] | none | |||||||
| P2Y2 | UTP 3 | 8.10 [4] | UTP-γ-Sa9 | 6.62 [4] | none | Suramin 48a | 4.32 [6] | P2Y11 | |
| ATP 1 | 7.07 [4] | NS365 68c | 7.00 [4] | P2Y4 | AR-C126313 52 | 6 [4,74] | |||
| INS37217 69 | 6.66 [4] | P2Y4 | MRS2576b57 | 4.04 [81] | P2Y1,4,6 | ||||
| MRS269863b | 8.10 [64] | none | |||||||
| P2Y4 | UTP 4 | 5.60 [6] | 2'-azido-dUTP 64 | 7.14 [12] | P2Y4 | PPADS 49 | < 5.00 [6] | P2Y2 | |
| ATP 1 | 4.37 [6] | various (ago), P2Y12 | |||||||
| MRS2577b56 | 4.01 [81] | P2Y6 | |||||||
| P2Y6 | UDP 4 | 6.52 [6] | UDP-β-S 10 | 7.33 [4] | none | MRS2578b55 | 7.43 [4] | none | |
| INS48823 71 | 6.90 [4] | none | |||||||
| MRS2633 67 | 6.64 [68] | none | |||||||
| MRS2693 66 | 7.83 [68] | None | |||||||
| P2Y11 | ATP 1 | 4.77 [6] | AR-C67085 35 | 5.05 [50] | P2Y12,13 (ant) | Suramin 48a | 4.79 [6] | P2Y2 | |
| ATP-γ-Sa7 | 5.52 [6] | P2Y1,2,12 | AMP-α-Sa18 | partial agonist [50] | none | ||||
| P2Y1-like, Gi-coupled | P2Y12 | ADP 2 | 7.22 [1] | MeSADP 12 | 7.85 [1] | P2Y1,13 | MeSAMP 13 | 4.00 [82] | none |
| ADP-β-Sa8 | 6.72 [1] | P2Y1,13 | ATP 1 | 3.60 [40] | various (ago), P2Y4 | ||||
| AR-C67085 36 | 4.52 [45] | P2Y11 (ago), P2Y13 | |||||||
| AR-C69931MX 37 | 9.40 [40] | P2Y11 (ago), P2Y13 | |||||||
| AZD6140 38 | 7.90 [45] | none | |||||||
| INS50589 40 | 7.80 [41] | none | |||||||
| Clopidogrelc41 | 5.74 [42] | none | |||||||
| P2Y13 | ADP 2 | 7.94 [84] | MeSADP 12 | 7.85 [84] | P2Y1,12 | MRS2211 50 | 5.97 [83] | none | |
| AR-C67085 36 | 6.67 [50] | P2Y11 (ago), P2Y12 | |||||||
| AR-C69931MX 37 | 8.40 [83] | P2Y11 (ago), P2Y13 | |||||||
| P2Y14 | UDP-glucose 75 | 6.45 [71] | MRS2690 78 | 7.31 [71] | none | UDP 4 | 7.28 [10] | P2Y6 (ago) | |
| UDP-galactose 76 | 6.17 [71] | ||||||||
| UDP-glucosamine 77 | 5.36 [71] | ||||||||
a, unstable to oxidation.
b, an insurmountable antagonist, which is hydrophobic and reactive toward nucleophiles and aqueous medium.
c, active only in vivo, through a thiol-reactive metabolite.
ago, agonist; ant, antagonist
The P2Y receptor agonists are nearly exclusively nucleotide derivatives, which presents barriers to drug development because of their instability, low bioavailability, nonspecific binding to biological membranes, and tedious synthesis, purification, and structural verification. Screening of small-molecule ligands of diverse structure has not yet been carried out extensively for the P2Y family. Radioligand binding is effective for ligand screening in many other GPCRs, but biological assays at the P2Y receptors usually exploit functional endpoints, e.g., typically Gq-stimulated phospholipase C-β for the P2Y1-like subfamily (Table 1). Suitable radioligand-binding methods are only available for the P2Y1 and P2Y12 receptors [18, 19]; those methods were developed after many attempts to use radiolabeled nucleotides were reported in the literature and later proved unsatisfactory.
Adenine nucleotide-responsive P2Y receptors
SAR of P2Y1, P2Y12, and P2Y13 receptors for 5′-diphosphates
The most prominent regions of distribution of these ADP-responsive receptors are P2Y1 (platelets, endothelial cells, brain), P2Y12 (platelets, brain), and P2Y13 (immune system, dendritic cells). There is no striking sequence homology between the P2Y1 receptor and P2Y12 or P2Y13 receptors. The sequence identity of the TM domains of the human P2Y1 is 26.9% and 28.0% for the P2Y12 and P2Y13 receptors, respectively. In contrast, the sequence identity within the TM domains of the P2Y12 and P2Y13 receptors is 57.0%.
Modification of the phosphate moiety ADP 2 is the principal endogenous agonist at the P2Y1, P2Y12, and P2Y13 receptors. ATP 1 interacts with less affinity and efficacy than ADP at the P2Y1 and P2Y12 receptors. At P2Y12 receptors, the loss of efficacy is pronounced, such that ATP and other 5′-triphosphate derivatives act as antagonists. At P2Y13 receptors, ADP and ATP both act as full agonists.Modifications of the di- and triphosphate moieties of the nucleotide ligands have been probed for effects on P2Y receptor activity (Fig. 1). For example, when an ionizable oxygen of the α-phosphate of the triphosphate moiety of adenine nucleotide derivatives is substituted with a BH2 moiety, it favors P2Y1 receptor potency [20]. Thus, the P2Y1 receptor can be activated by a 5′-(1-boranotriphosphate) derivative 14 of 2-methylthio-ATP 11. Separation of two stable isomers of 14 demonstrated stereoselectivity in activation of the rat P2Y1 receptor (EC50 = 2.6 nM, for the more potent R-isomer of 14).
Modification of the adenine moiety The SAR around the adenine moiety of the nucleotides has been extensively explored at the P2Y1 and P2Y12 receptors. Broad freedom of substitution has been observed at the C2 position, and sterically bulky groups and extended chains at this position are often tolerated in receptor binding.A small hydrophobic pocket in the receptor-binding site surrounds the N6-position of adenine nucleotides acting as P2Y1 agonists and antagonists [21], and the P2Y12 receptor has a considerably larger pocket [22]. Amino, ether, and thioether derivatives at the 2-position of the adenine ring of ATP were compared in P2Y1 potency; thioethers, in general, are the most potent [23].2-Alkylthio and 2-(arylalkylthio) ethers of ADP are highly potent agonists at these subtypes (Fig. 1b) [24, 25]. 2-Methylthio-ADP 12 is a potent agonist (EC50 in nM) at human P2Y1 (3), rat P2Y12 (1), and human P2Y13 (1) receptors [2, 7, 26]. 2-Methylthio (2-MeS)-ATP 11 is less potent (EC50 = 8 nM) than 12 at the P2Y1 receptor and also less selective because certain P2X receptors are activated [26]. A 2-[2-(4-aminophenylethyl)] 5′-triphosphate derivative 15 exhibits an EC50 of 1 nM at the P2Y1 receptor, and a related azido 5′-diphosphate derivative 16 serves as a photoaffinity label of P2Y receptors in platelets [27]. 2-Alkynyl substituents on adenine nucleotide analogues provide high potency as either P2Y1/12 receptor agonists or antagonists [28].Although AMP is inactive at the P2Y1 receptor, adding a 2-thioether substituent as a receptor “anchor” allows adenosine 5′-monophosphate analogues to bind to and activate the P2Y1 receptor. Among these derivatives, 2-(hexylthio) (HT)-AMP 17 is especially potent, with an EC50 of 59 nM at the turkey P2Y1 receptor [29]. 2-MeS-AMP 13 weakly activates the P2Y1 receptor but also acts as a weak antagonist of the P2Y12 receptor [30]. α-Thio ATP derivatives, such as compound 18, are potent P2Y1 receptor agonists (EC50 = 17 nM). The corresponding α-thio AMP derivatives are weak as P2Y receptor agonists, but the simultaneous inhibition of ATP diphosphohydrolase [ecto-apyrase nucleoside triphosphate diphosphohydrolase-1 (NTPDase1) or CD39, present in blood, neuronal, endothelial, pancreatic, and smooth muscle cells [31]] complicates their use as pharmacological probes [15].Limited alteration of the heterocyclic ring of P2Y-active nucleotides is possible. 8-Aza and 1-deaza modifications are generally tolerated at P2Y1 and P2Y12 receptors [22, 34, 87], but other modifications, such as N1, N6-etheno, result in inactivity at P2Y receptors [32]. A fluorescent derivative of ATP 19 containing a tricyclic nucleobase is strikingly potent in activation of the P2Y1 receptor [33].
Modification of the ribose moiety Replacement of the ribose 2′ and/or 3′ hydroxyl groups with H in adenine nucleotide agonists greatly reduces the potency at P2Y1 receptors. A 1,5-anhydrohexitol bisphosphate derivative 20 activates the P2Y1 receptor (EC50 = 6.29 μM at turkey P2Y1 receptor) [34] (Fig. 1b).
Simple carbocyclic (cyclopentyl) analogues of ATP enhance antagonist affinity at the P2Y12 receptor [22]. Among the more successful examples of the use of carbocyclic or sterically constrained carbocyclic substitution of the ribose moiety for P2Y receptor interactions are the bicyclic “methanocarba” analogues [35, 36]. Two isomeric forms of the methanocarba (bicyclo[3.1.0]hexane) ring system have been used as ribose replacements in ATP, representing a North (N), 2′-exo envelope 21 or South (S), 2′-endo envelope 22 conformation. The addition of a 2-MeS group to 21 to form 24 provides a highly potent and selective P2Y1 agonist, MRS2365 (EC50 = 0.40 nM) [37]. Unlike 2MeS-ADP, this compound does not activate P2Y12 or P2Y13 receptors [38]. (N)-methanocarba derivative 23 is a full agonist at the P2Y1 receptor (EC50 = 158 nM); the corresponding 9-riboside, β,γ-methylene-ATP, is a partial weak agonist at that subtype.
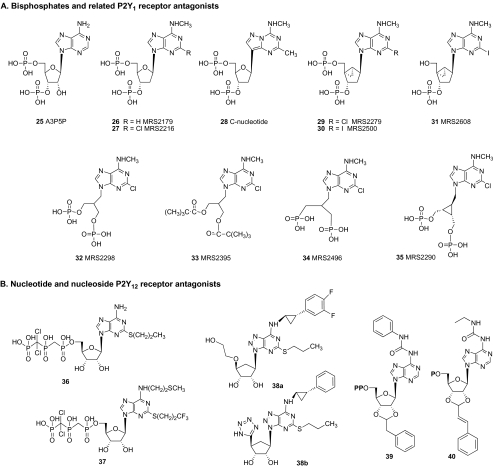
A successful approach to designing potent and selective P2Y1 receptor antagonists became possible with the observation by Boyer et al. that naturally occurring adenosine bisphosphate derivatives such as A3P5P 25 (Fig. 3a) act as partial agonists or antagonists of the receptor (EC50 = 0.83 μM) [39]. This has led to improved 2′-deoxyribose 3′,5′-bisphosphate derivatives MRS2179 26 (EC50 = 0.33 μM) and MRS2216 27 (EC50 = 0.21 μM), which are potent and selective P2Y1 receptor antagonists [34]. A C-nucleotide-based antagonist 28 of the P2Y1 receptor is patterned after MRS2179 [40]. (N)-methanocarba substitution within the family of bisphosphate antagonists yields MRS2279 29 and MRS2500 30, which display nanomolar potency at the P2Y1 receptor (EC50 = 52 nM and 0.95 nM, respectively) [18]. Moderate antagonist activity at the P2Y1 receptor is retained after removal of the 5′-phosphate group of MRS2500 in 31 (EC50 = 1560 nM) [41].
Fig. 3.
Structures of nucleotide-based antagonists of P2Y1 and P2Y12 receptors
Acyclic bisphosphate antagonists of the P2Y1 receptor and related derivatives 32–35 have been characterized [42, 43]. Short alkyl chains bearing two phosphate groups attached at the adenine 9-position are favored over long chains. The bisphosphate derivative MRS2298 32 is a potent antagonist of the P2Y1 receptor (binding Ki = 29.6 nM, human). MRS2496 34 is a bisphosphonate derivative, which remains tolerated in the P2Y1 receptor binding site (binding Ki = 76 nM, human). Compound 35, which contains a cycloproyl ring within the 9-alkyl subsituent, is a P2Y1 antagonist with micromolar affinity. Although various phosphate derivatives of the adenine 9-ribosides (cyclic) may be either agonists or antagonists of the P2Y1 receptor, only antagonism has been achieved in the acyclic series.
The observation that ATP analogues inhibit platelet aggregation by antagonism of the P2Y12 receptor enabled development of the 5′-triphosphate derivatives AR-C67085 (EC50 = 30 μM) 36 and AR-C69931MX 37 (Cangrelor, EC50 = 0.4 nM) as antithrombotic agents, which were in clinical testing [44] (Fig. 3b). Other nucleoside–nucleotide derivatives were investigated for P2Y12 receptor antagonism [42, 45]. For example, AZD6140 38a is an uncharged nucleoside-based antagonist of the P2Y12 receptor of high potency (pIC50 = 7.9) that has been in clinical trials [22, 89]. A similar carbocyclic derivative 38b containing a 1H-tetrazol-5-yl group was recently reported to bind to the P2Y12 receptor with an IC50 value of 2 nM [87]. The (1R,2S)-2-phenylcyclopropyl derivative 38b was found to be 30-fold more potent than the corresponding (1S,2R) isomer. The uncharged nucleoside derivative MRS2395 33 [2,2-dimethyl-propionic acid 3-(2-chloro-6-methylaminopurin-9-yl)-2-(2,2-dimethyl-propionyloxymethyl)-propyl ester] is a weak antagonist of the P2Y12 receptor [5]. The ADP derivative INS49266 39 (EC50 = 0.052 μM) and the AMP derivative INS50589 40 (EC50 = 0.011 μM) act as potent competitive antagonists of the P2Y12 receptor [46].
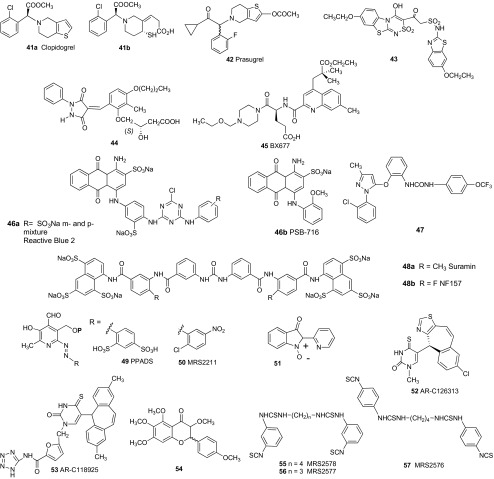
Nonnucleotide antagonists of P2 receptors have also been identified and modified to achieve P2Y12 receptor selectivity (Fig. 4). The clinical success of the thienopyridine clopidogel 41 as an antithrombotic agent has stimulated the development of other P2Y12 antagonists. The thienopyridines act as liver-activated prodrugs that are irreversible inhibitors of the P2Y12 receptor [47]. Thiol 41b is reported to be the active metabolite of clopidogrel. Prasugrel 42 is a P2Y12 antagonist with similar action, which shows clinical promise [48].
Fig. 4.
Structures of nonnucleotide antagonists of P2Y receptors
Competitive antagonists of P2Y12 receptors that do not require chemical conversion to an active species in vivo and that are nonnucleotides, i.e., not highly charged phosphate derivatives, are also sought as antithrombotic agents [45]. Screening of compound libraries has aided hit-and-lead identification in this effort. Compound 43 is a tricyclic benzothiazolo[2,3-c]thiadiazine antagonist of the P2Y12 receptor (EC50 = 0.18 μM) [19]. Compound 44 is a competitive P2Y12 receptor antagonist [49]. The directly acting (i.e., not requiring chemical transformation in vivo prior to interacting with the receptor) P2Y12 antagonist BX677 45 exhibits a wider therapeutic index than does clopidogrel in experimental models of thrombosis [50]. Other nonnucleotide heterocyclic derivatives have been discovered that act as selective P2Y1 receptor antagonists [51]. Recently, PRT128 (structure not disclosed) was introduced as an orally active direct-acting and reversible P2Y12 receptor antagonist [88].
Other classes of nonnucleotide antagonists of P2 receptors have also been identified and modified to achieve P2Y receptor selectivity (Fig. 4). Derivatives of pyridoxal phosphate, e.g., pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonate (PPADS) 49, are generally relatively nonselective antagonists of the P2Y1 receptor and other subtypes [4]. The SAR of P2 receptor antagonists derived from pyridoxal phosphate was reviewed by Lambrecht and coworkers [52]. Pyridyl isatogen 51 antagonizes the P2Y1 receptor.
Recently, a nonnucleotide antagonist of the P2Y1 receptor 47 has been reported through chemical optimization of a library hit [86]. This compound has a Ki value of 90 nM at the human P2Y1 receptor and is proposed for use as an antithrombotic agent. It is orally bioavailable with a half life in rats of 2.8 h.
A moderately selective antagonist of the P2Y13 receptor, MRS2211 50, has been identified [53]. Nucleotide derivatives AR-C67085 36 (EC50 = 4 nM) and AR-C69931MX 37 (EC50 = 213 nM) potently antagonize the P2Y13 receptor.
SAR of P2Y11 and P2Y2 receptors for 5′-triphosphates
P2Y11 receptor agonists
Ten years ago, the human P2Y11 receptor was cloned from human placenta [54]. This P2Y receptor subtype is also found on lymphocytes, kidney, and pancreatic-duct epithelial cells [55, 56]. The P2Y11 receptor exhibits moderate amino acid identity with other P2Y receptors. The P2Y1 receptor is the closest homologue of the P2Y11 receptor subtype, with 37.8% identity in the TM domains. The amino acid identity of the TM domains of the P2Y11 receptor with the P2Y2 receptor is 35.2%. The P2Y11 receptor demonstrates the least homology (20%–22%) with the P2Y12-like receptor subfamily.
As with the P2Y1 and P2Y2 receptors, the P2Y11 receptor can be activated by adenosine 5′-triphosphate (ATP) 1, which is its native agonist. ATP-induced activation of the P2Y11 receptor stably expressed in CHO-K1 cells and in 1321N1 astrocytoma cells results in accumulation of both cyclic AMP and IP3. These findings indicate that the P2Y11 receptor is coupled to both the adenylyl cyclase (via Gs) and the phosphoinositide (via Gq) pathways [54, 57].
It was suggested that nonadenine triphosphates, including UTP 3, guanosine 5′-triphosphate (GTP), cytidine 5′-triphosphate (CTP), thymidine 5′-triphosphate (TTP), inosine 5′-triphosphate (ITP), and UDP 4 are inactive at the P2Y11 receptor [54, 57]. Another report characterized UTP as a Ca2+-mobilizing agonist in P2Y11 receptor-expressing 1321N1 astrocytoma cells, whose potency and maximal response are similar to ATP. However, the production of IP3 does not increase during activation of the P2Y11 receptor by UTP [58].
Dinucleotide polyphosphates activate various subtypes of P2Y receptors [59] (Fig. 5b). At the P2Y1 receptor, diadenosine pentaphosphate (Ap5A 70c, EC50 = 0.32 μM) is more potent than ATP (EC50 = 0.65 μM), and Ap3A 70a (EC50 = 0.011 μM) is equipotent to ADP (EC50 = 0.014 μM) [60]. However, none of the diadenosine polyphosphates activate the P2Y11 receptor, with the exception of Ap4A 70b, which increases the level of intracellular [Ca2+] at a concentration of 1 mM [54, 57, 61].
Fig. 5.
Structures of additional uracil-derived nucleotide agonists of P2Y2, P2Y4, P2Y6, and P2Y14 receptors
Experiments performed on human P2Y11 receptor-infected 1321N1 astrocytoma cells indicated increased intracellular production of IP3 and cyclic AMP followed by elevation of Ca2+ induced by nicotinamide adenine dinucleotide 72 (β-NAD+) in a concentration range of 1–100 μM [62]. Thus, extracellular β-NAD+ appears to be an agonist of the P2Y11 receptor [12]. Moreover, nicotinic acid adenine dinucleotide phosphate 73 (NAADP+) also activates the P2Y11 receptor [63].
Various analogues of ATP 1 have been examined as potential agonists of the P2Y11 receptor (Fig. 1). The rank order of agonist potency is ATP > ADP >> AMP = adenosine in erythroleukemic K562 cells and in acute monocytic leukemia U937 cells stably transfected with the P2Y11 receptor (K11 and U11 cells, respectively) [64]. NB4 promyelocytic cells behave similarly [16]. ATP-γ-S 7 is a more potent P2Y11 receptor agonist than ATP in 1321N1, CHO-K1, U11, K11, and NB4 cells. Also in U11 and K11 cells, ATP-α-S and ADP-β-S 8 are more potent than ATP. Interestingly, ADP-β-S produces no agonist effect at the P2Y11 receptor in NB4 cells [65]. Adenosine 5′-thiomonophosphate (α-thio-AMP) is almost inactive at the P2Y11 receptor [64, 65].
BzATP (2′ or 3′-O-(4-benzoyl)benzoyl-ATP) is equipotent to ATP-γ-S in 1321N1, CHO-K1, U11, and NB4 cell lines. In contrast, in K11 cells, BzATP (EC50 = 63 μM) is one of the weakest P2Y11 receptor agonists. Interestingly, 2′-dATP is a more potent agonist of this receptor than ATP in all these cell lines [54, 57, 64].
Replacement of the α,β- or β,γ-oxygen atom by a methylene group decreases the potency of the ligand in comparison with ATP [64, 65]. Also, 2-MeS-ATP 11 is weaker than ATP at the P2Y11 receptor subtype expressed in 1321N1, CHO-K1, and U11 cells but not in K11 cells. In addition, 2-Cl-ATP is a weak P2Y11 receptor agonist [66]. However, 2-propylthio-β,γ-dichloromethylene-ATP (AR-C67085, 36, an antagonist of the P2Y12 receptor) is the most potent reported agonist of the P2Y11 receptor (EC50 = 8.9 μM) [57, 67].
The effects of replacement of the ATP ribose moiety by a (N)- or a (S)-methanocarba-constrained carbocyclic ring were studied at the human P2Y4 receptor [36]. The potency of (N)-methanocarba-ATP 21 (EC50 = 34.5 μM) is equivalent to unmodified ATP (EC50 = 17 μM). In contrast, (S)-methanocarba-ATP 22 produces no effect at the P2Y11 receptor at a concentration of 100 μM. It is possible that not only (N)-methanocarba analogues but also the (N)-conformation of the ribose ring of ATP analogues are preferred at the P2Y11 receptor. In addition, these data demonstrate that the oxygen atom of the ATP ribose ring is not essential for agonist potency.
The preferences of green fluorescent protein (GFP)-tagged P2Y11 receptor stably expressed in 1321N1 astrocytoma cells for ATP-α-S and ATP-α-S diastereoisomers were the focus of recent studies [66]. Rp-ATP-α-S (EC50 = 0.27 μM) and Sp-ATP-α-B (EC50 = 0.34 μM) isomers are more potent than their respective Sp (EC50 = 1.71 μM) and Rp (EC50 = 2.38 μM) isomers. Moreover, these compounds are more potent than ATP (EC50 = 2.83 μM). In addition, both Rp- and Sp-2-MeS-ATP-α-S (EC50 = 0.64 and 2.64 μM) are more potent at the P2Y11 receptor than is 2-MeS-ATP (EC50 = 13.8 μM) [66]. Interestingly, for the P2Y1 receptor, these ligands display the opposite stereoselectivity, but for the P2Y2 and P2Y4 receptors, Rp-UTP-α-S (EC50 P2Y2 = 5.4 μM, EC50 P2Y4 = 27 μM) is more potent than the Sp-UTP-α-S- analogue (EC50 P2Y2 = 14 μM, EC50 P2Y4 = 81 μM) [16].
Various nonnucleotide derivatives have been shown to inhibit P2Y11 receptors (Fig. 4). Suramin 48a, a drug used to treat trypanosomiasis and onchocerciasis, weakly antagonizes various P2Y and P2X receptors, and its SAR is known in detail [52]. Suramin also inhibits G protein signaling intracellularly. P2Y11 receptor antagonists derived from suramin exhibit nanomolar potency [68]. NF157 48b has a pKi value of 7.35 at the P2Y11 receptor and displays the following selectivity ratios (x-fold) over P2Y1 (>650), P2Y2 (>650), P2X2 (1), P2X2 (3), P2X3 (8), P2X4 (>22), and P2X7 (>67) receptors.
ATP derivatives as P2Y2 receptor agonists
The P2Y2 receptor is activated by both uracil and adenine 5′-triphosphates (Fig. 1). The SAR of UTP derivatives at the P2Y2 receptor are treated separately below.
In contrast to UTP 3, which can activate only P2Y2 and P2Y4 receptors, ATP 1 is an agonist for three P2Y receptor subtypes P2Y2 (EC50 = 0.085 μM), P2Y1 (EC50 = 1.5 μM), and P2Y11 (EC50 = 17.3 μM) [36]. For this reason the design of ATP analogues with P2Y2 receptor selectivity is a difficult problem.
BzATP and ATP-γ-S are weaker than ATP, and ATP-α-S is inactive at the mouse P2Y2 receptor [69, 70]. Substitution of the α,β-oxygen atom of ATP by a methylene group (α,β-MeATP) results in a ligand that is virtually ineffective at the P2Y2 receptor [71]. In addition, replacement of the β,γ oxygen atom in the 5′-triphosphate chain of ATP by a β,γ-methylene or β,γ-imido group leads to ligands with no effect at the P2Y2 receptor at concentration of 1 mM [69, 70]. Although the P2Y1 receptor can be potently activated by 2-MeS-ATP 11, this ligand is inactive at the P2Y2 subtype. However, 2-Cl-ATP is a weak agonist of the P2Y2 receptor (EC50 = 2.30 μM). The potency of 8-Br-ATP is reported to be EC50 = 23.0 μM [72].
Studies of the preferred conformation of the ribose ring indicate that at the P2Y2 receptor (N)-methanocarba-ATP (21, EC50 = 0.091 μM) and (N)-methanocarba-UTP (62, EC50 = 0.0159 μM) are equipotent to ATP and UTP, respectively. In contrast, (S)-methanocarba-ATP (22, EC50 = 3.7 μM) is significantly weaker than ATP.
Uracil nucleotide-responsive P2Y receptors
SAR of P2Y2 and P2Y4 receptors for 5′-triphosphates
The most prominent tissues of distribution of these UTP-responsive receptors are P2Y2 (vascular cells, epithelial cells) and P2Y4 (gastrointestinal cells, placenta, cochlear cells). These two receptors display the highest identity in the sequences of their TM domains (66.8%) of all P2Y receptor subtypes.
Phosphate modifications and dinucleotides The P2Y2 receptor is activated nearly equipotently by UTP 3 (EC50 = 0.049 μM) and ATP 3 (EC50 = 0.085 μM) (Fig. 1). However, it is not activated by the 5′-diphosphates UDP 4 and ADP 2. An early publication showed UDP to be active, but this was later attributed to the presence of UTP in commercial preparations of UDP [73]. Uridine γ-thiodiphosphate (UTP-γ-S) 9 is a selective agonist for P2Y2 (EC50 = 0.24 μM) and P2Y4 (EC50 = 1.6 μM) receptors [43].
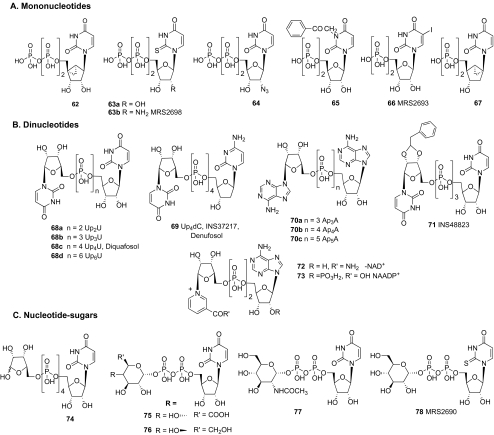
The P2Y2 receptor is activated by dinucleotide molecules [72] as well as uracil or adenine triphosphates. The dinucleotides Up4U (INS365, Diquafosol) 68c and Up4dC (INS37217, Denufosol) 69 (Fig. 5) have the optimal phosphate chain length for activation of the P2Y2 receptor (EC50 = 0.1 and 0.22 μM) and tend to be more resistant to degradation by nucleotidases than nucleoside triphosphates [59, 60]. Up4-[5′]-ribose 74 activates the P2Y2 receptor with an EC50 of 1.88 μM, i.e., ninefold less potent than Up4U 68c. Up4-[5′]-ribose 74 still activates the P2Y2 receptor and serves as a truncated form of Up4U 68c for purposes of modeling receptor docking [74]. On the other hand, Up2U 68a, containing only two phosphate groups, is nearly inactive as an agonist, and Up6U 68d is 98-fold weaker than Up4U at the P2Y2 receptor.
The model of receptor-docked Up4U is consistent with a tetraphosphate chain having the optimal length for dinucleotide binding [74]. The phosphate side chain of diuridine diphosphates apparently is too short to allow uridineII to reach its binding pocket. However, in the case of Up6U, its phosphate chains are too large to be fully accommodated inside the receptor. Also, as discussed by Brunschweiger and Müller [4], among various linear dinucleotides, only dinucleoside tetraphosphates have the same number of negatively charged oxygen atoms as UTP.
Ribose and uracil modifications Both 2′-deoxy-2′-amino-UTP and 2-thio-UTP 63a preserve the agonist potency of UTP at the P2Y2 receptor (Fig. 5a). Combination of these two modifications yields 2′-amino-2-thio-UTP, compound 63b, synergizes to enhance both potency (8 nM EC50) and selectivity (300-fold P2Y2-selective versus P2Y4). 2′-Amine acetylation reduces potency, and trifluoroacetylation produces intermediate potency.
Modification at position 5, such as 5-bromo-UTP (EC50 = 0.75 μM) and 5-iodo-UTP (EC50 = 0.83 μM), suggests that introducing a small hydrophobic group might be beneficial at the P2Y2 receptor. However, 5-methyl UTP (EC50 = 0.48 μM) does not enhance potency, and the 5-amino (EC50 = 5.6 μM) and 5-azido (EC50 = 1.8 μM) analogues are less potent at the P2Y2.
2′-Deoxy-UTP (EC50 = 1.08 μM) is 22-fold less potent than UTP (EC50 = 0.049 μM); replacement of the 2′-hydroxyl group by a 2′-methoxy group (EC50 = 14.3 μM) reduces the potency further (290-fold weaker than UTP). Thus, the hydroxyl group at the 2′-position appears to be important for agonist activities as a donor of H-bonding to the P2Y2 receptor.
AR-C126313 52 and its higher-molecular-weight analogue AR-C118925 53 are selective antagonists of the P2Y2 receptor [75] (Fig. 4). Recently, flavonoid derivatives, e.g., 54, [76], and derivatives of Reactive Blue 2 46a, such as 1-amino-4-(2-methoxyphenyl)-2-sulfoanthraquinone (PSB-716) 46b [77], have been explored as P2Y2 receptor antagonists. Flavonoid antagonists reduce the amplitude of the P2Y2 receptor response to UTP but not the EC50 value, indicating allosteric antagonism [76].
New ligand tools are needed to distinguish P2Y2 and P2Y4 receptor subtypes pharmacologically and to extend the characterization of the few existing tools to a wider range of species. Selective antagonists of the P2Y4 receptor have not yet been identified. Suramin 48a is more potent as a competitive antagonist at the human P2Y2 receptor than at the P2Y4 receptor [4, 5]. The order of potency of agonists is useful in this regard [16]. 2′-Azido-2′-deoxyUTP 64 is fivefold more potent in activation of phospholipase C (PLC0 at the human P2Y4 receptor than at the P2Y2 receptor (EC50 = 0.54 μM vs. 0.78 μM). 4-Thio-UTP (EC50 = 0.023 μM) is 15-fold more potent than 2-thio-UTP (EC50 = 0.35 μM) in activating the human P2Y4 receptor.
SAR of P2Y6 receptor for 5′-diphosphates
The major tissue distribution of these UDP-responsive receptors includes vascular smooth muscle cells, microglial cells, and neutrophils. This receptor exhibits significant homology with the TM domains of other P2Y1-like receptors, such as P2Y1 (43.0%), P2Y2 (47.7%), P2Y4 (48.2%), and P2Y11 (31.6%).
UDP 4 is a selective agonist at the P2Y6 receptor. UDP-β-S 10 (EC50 = 47 nM) is more potent than UDP (EC50 = 300 nM) in activation of the P2Y6 receptor and is more stable toward ectonucleotidases. 5-Br-UDP (EC50 = 800 nM) and the dinucleotide triphosphate (71, INS48823) are potent and/or stable agonists of the P2Y6 receptor (EC50 = 125 nM) [72, 78] (Figs. 1 and 5). The SAR and molecular modeling of the uracil nucleotide-activated P2Y6 receptor were investigated recently [79, 80]. Novel UDP analogues were synthesized and assayed for activity at the human P2Y6 receptor.
The P2Y6 receptor is generally selective for 5′-diphosphate derivatives. Thus, uridine 5′-monophosphate, 2′-deoxyuridine 3′,5′-bisphosphate, a cyclic 3′,5′-diphosphate analogue, and a uridine 3′-diphosphate derivative are inactive as either agonists or antagonists.
Within a series of uracil dinucleoside 5′,5′-polyphosphates, the triphosphates (68b and 71) are the most potent and selective (EC50 = 0.2 and 0.125 μM). The simple Up2U 68a displays low potency at the P2Y6 receptor.
Modification of ribose hydroxyl groups of UDP has not yielded more potent P2Y6 receptor agonists. Substitution with a 2′-azido or 2′-amino group reduces potency at the P2Y6 receptor. Removal of either the 2′- or 3′-hydroxyl group of UDP yields a >100-fold decrease in potency. The (S) conformation of the ribose moiety is preferred for ligand recognition by the P2Y6 receptor [79]. A UDP derivative locked in the (N) conformation by an (N)-methanocarba ring is completely inactive, and the 2′-deoxy-(S)-methanocarba derivative 67 is moderately potent in activating the P2Y6 receptor (Fig. 5A). This is a rare example of the use of molecular modeling to predict the conformational preference in a putative GPCR binding site for rational ligand design. The computational prediction led to the subsequent synthesis of a novel analogue containing a rigid carbocyclic ribose substitute that is more potent than the corresponding 9-(2′-deoxyriboside) as a P2Y6 agonist.
The uracil ring was modified at the 2-, 3-, 4-, and 5-positions. Modification at the 3-position with N-CH3 markedly decreases potency, but halogenation of the 5-position in the iodo analogue 66 (Fig. 5A) yields a molecule that is equal in potency (EC50 = 0.15 µM) to UDP and is similar in potency to a 5-bromo analogue previously reported to be roughly equipotent to UDP [80]. A 5-iodo modification maintains potency at the P2Y6 receptor, and 5-halo substitution of the uracil ring may therefore provide a basis for achieving selectivity at this subtype. In a series of uracil-modified compounds, 2-thio-UDP (EC50 = 0.06 μM) and 4-thio-UDP (EC50 = 0.08 μM) are five- to six-fold less potent than UDP. 4-Thioether derivatives and 4-carboxyalkylthio derivatives exhibit lower potency than the parent compound 4-thio-UDP. 3-Phenacyl-UDP 65 (EC50 = 70 nM) is a potent and selective P2Y6 receptor agonist [81].
Only one class of antagonists of the P2Y6 receptor has been reported. The diisothiocyanate derivative MRS2578 55 (EC50 = 0.037 μM) is a potent, insurmountable antagonist of P2Y6 nucleotide receptors [82] (Fig. 4). Related compounds 56 and 57 have varied selectivity for P2Y receptor subtypes. These isothiocyanate derivatives likely bind covalently to P2Y receptors; they are hydrophobic and have limited stability in aqueous solution.
SAR of P2Y14 receptor for UDP-sugars
The most atypical P2Y receptor is the P2Y14 receptor, which is distributed in the immune system, including in dendritic cells and the central nervous system. The identity of amino acid residues of TM domains of the P2Y14 receptor with P2Y12 and P2Y13 subtypes is 57.0% and 52.8%, respectively. However, in contrast to other P2Y receptors, the P2Y14 receptor is not activated by uridine 5′-di- or triphosphates or by adenine nucleotides [83]. The P2Y14 receptor is the only subtype to respond principally to UDP-glucose 5 (EC50 = 0.35 μM) and other UDP-sugars, such as UDP-glucuronic acid 75 (EC50 = 102 nM), and the endogenous nucleotides UDP-galactose 76 (EC50 = 0.67 μM) and UDP-N-acetylglucosamine 77 (EC50 = 4.38 μM) (Fig. 5C). The SAR of analogues of UDP-glucose at the P2Y14 receptor was recently systematically explored [84]. Novel analogues modified on the nucleobase, ribose moiety, and glucose moieties were synthesized and characterized biologically at the recombinant human P2Y14 receptor.
The P2Y14 receptor appears to be the most restrictive of the P2Y family with respect to modification of the nucleobase, ribose, and phosphate moieties. Most of the novel analogues of 5 modified on the uracil or ribose moieties are inactive. All compounds modified at the 5-position of uracil moiety (i.e., with azido, amino, and iodo) or at the 2′, 3′ hydroxy groups of ribose ring are inactive. Analogues with other nucleosides, such as adenosine, guanosine, cytidine, thymidine, deoxyuridine and deoxycytidine, are inactive. The dinucleotides Up2U 68a and Up4U 68c are inactive at the P2Y14 receptor.
Among the analogues of 5, a 2-thiouracil analogue, MRS2690 78, is tenfold more potent (EC50 = 49 nM). A 4-thiouracil (EC50 = 0.29 μM) analogue is equipotent to 5, but a 4-methyl-4-thiouracil moiety is not tolerated. The 2- and 4-thio modifications preserve potency at all uracil nucleotide-responsive subtypes but most effectively at the P2Y2 and P2Y4 receptors.
The high potency of analogues 75 (UDP-glucuronic acid) and 76 (UDP-galactose) suggests a flexibility of structural modification at the glucose C4 and C6 positions. The 12-fold-lower potency of 77 (UDP-N-acetylglucosamine) in comparison with 5 suggests a limited tolerance for steric bulk at the glucose C2 position. Modification of the glucose moiety of 5 to yield, for example, UDP-fructose, UDP-mannose, and UDP-inositol, preserved agonist potency at the P2Y14 receptor, as predicted in a modeling study [85].
Conclusion
The medicinal chemistry of several of the P2Y receptors has recently acquired detailed qualitative and quantitative SARs. Selective agonists (for P2Y1, P2Y2, and P2Y6 receptors) and selective antagonists (for P2Y1, P2Y2, P2Y6, P2Y11, P2Y12, and P2Y13 receptors) have been identified. At the P2Y1 and P2Y12 receptors, 5′-diphosphate agonist derivatives have been transformed into potent and selective antagonists by alteration of the phosphate moieties and nucleobase and substitution pattern and introduction of carbocyclic substitution of the ribose moiety. Empirical and computational methods have identified conformations of the ribose moiety that enhance affinity at the P2Y1 and P2Y6 receptors have been identified through—and these conformations have been chemically locked to provide novel—selective ligands.
Screening of chemically diverse compound libraries has begun to yield new lead compounds for the development of P2Y receptor antagonists, e.g., directly acting P2Y12 receptor antagonists with antithrombotic activity. Challenges remaining include identification of selective agonists for the P2Y4, P2Y11, and P2Y13 receptors and of selective antagonists for the P2Y4 and P2Y14 receptors. The discovery of new, selective P2Y receptor ligands and optimization of existing structural leads hold promise for the identification of new clinical agents that exploit nucleotide receptor signalling.
Acknowledgment
Support from the NIDDK Intramural Research Program is acknowledged.
References
- 1.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Fumagalli M, King BF, Gachet C, Jacobson KA, Weisman GA (2006) International Union of Pharmacology LVIII Update on the P2Y G protein-coupled nucleotide receptors: From molecular mechanisms and pathophysiology to therapy. Pharmacol Rev 58:281–341 [DOI] [PMC free article] [PubMed]
- 2.Costanzi S, Mamedova L, Gao ZG, Jacobson KA (2004) Architecture of P2Y nucleotide receptors: Structural comparison based on sequence analysis, mutagenesis, and homology modeling. J Med Chem 47:5393–5404 [DOI] [PMC free article] [PubMed]
- 3.Ivanov AA, Costanzi S, Jacobson KA (2006) Defining the nucleotide binding sites of P2Y receptors using rhodopsin-based homology modeling. J Comput Aided Mol Des 20:417–426 [DOI] [PubMed]
- 4.Brunschweiger A, Müller CE (2006) P2 receptors activated by uracil nucleotides–an update. Curr Med Chem 13:289–312 [DOI] [PubMed]
- 5.von Kügelgen I (2006) Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther 110:415–432 [DOI] [PubMed]
- 6.Houston D, Jacobson KA, Costanzi S, Harden TK (2008) Development of selective high affinity antagonists, agonists, and radioligands for the P2Y1 receptor. Comb Chem High Throughput Screen (in press) [DOI] [PMC free article] [PubMed]
- 7.Jacobson KA, Jarvis MF, Williams M (2002) Perspective: Purine and pyrimidine (P2) receptors as drug targets. J Med Chem 45:4057–4093 [DOI] [PMC free article] [PubMed]
- 8.Boeynaems J-M, Wilkin F, Marteau F, Duhant X, Savi P, Gonzalez NS, Robaye B, Communi D (2003) P2Y Receptors: New subtypes, new functions. Drug Devel Res 59:30–35 [DOI]
- 9.Jankowski V, Tölle M, Vanholder R, Schönfelder G, van der Giet M, Henning L, Schlüter H, Paul M, Zidek W, Jankowski J (2005) Uridine adenosine tetraphosphate: a novel endothelium- derived vasoconstrictive factor. Nat Med 11:223–227 [DOI] [PubMed]
- 10.Fricks I, Maddiletti S, Carter R, Lazarowski ER, Nicholas RA, Jacobson KA, Harden TK (2008) UDP is a competitive antagonist at the human P2Y14 receptor and a full agonist at the rat P2Y14 receptor. J Pharm Exp Therap 325:588–594 [DOI] [PMC free article] [PubMed]
- 11.Colgan SP, Eltzschig HK, Eckle T, Thompson LF (2006) Physiological roles for ecto-5′-nucleotidase (CD73). Purinergic Signal 2:315–360 [DOI] [PMC free article] [PubMed]
- 12.Lazarowski ER, Boucher RC, Harden TK (2000) Constitutive release of ATP and evidence for major contribution of ecto-nucleotide pyrophosphatase and nucleoside diphosphokinase to extracellular nucleotide concentrations. J Biol Chem 275:31061–31068 [DOI] [PubMed]
- 13.Gerevich Z, Zadori Z, Müller C, Wirkner K, Schröder W, Rubini P, Illes P (2007) Metabotropic P2Y receptors inhibit P2X3 receptor channels via G protein-dependent facilitation of their desensitization. Br J Pharmacol 151:226–236 [DOI] [PMC free article] [PubMed]
- 14.Communi D, Suarez Gonzalez N, Detheux M, Brezillon S, Lannoy V, Parmentier M, Boeynaems JM (2001) Identification of a Novel Human ADP Receptor Coupled to Gi. J Biol Chem 276:41479–41485 [DOI] [PubMed]
- 15.Fischer B, Chulkin A, Boyer JL, Harden TK, Gendron FP, Beaudoin AR, Chapal J, Hillaire-Buys D, Petit P (1999) 2-Thioether 5′-O-(1-thiotriphosphate)adenosine derivatives as new insulin secretagogues acting through P2Y-receptors. J Med Chem 42:3636–3646 [DOI] [PubMed]
- 16.Jacobson KA, Costanzi S, Ivanov AA, Tchilibon S, Besada P, Gao ZG, Maddileti S, Harden TK (2006) Structure activity and molecular modeling analyses of ribose- and base-modified uridine 52-triphosphate analogues at the human P2Y2 and P2Y4 receptors. Biochem Pharmacol 71:540–549 [DOI] [PMC free article] [PubMed]
- 17.Müller C (2002) P2-Pyrimidinergic receptors and their ligands. Curr Pharm Des 8:2353–2369 [DOI] [PubMed]
- 18.Houston D, Ohno M, Nicholas RA, Jacobson KA, Harden TK (2006) [32P]2-iodo-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate ([32P]MRS2500), a novel radioligand for quantification of native P2Y1 receptors. Brit J Pharmacol 147:459–467 [DOI] [PMC free article] [PubMed]
- 19.Scarborough RM, Laibelman AM, Clizbe LA, Fretto LJ, Conley PB, Reynolds E, Sedlock M, Jantzen H-M (2001) Novel tricyclic benzothiazolo[2,3-c]thiadiazine antagonists of the platelet ADP receptor (P2Y12). Bioorg Med Chem Lett 11:1805–1808 [DOI] [PubMed]
- 20.Nahum V, Zundorf G, Levesque SA, Beaudoin AR, Reiser G, Fischer B (2002) Adenosine 5′-O-(1-boranotriphosphate) derivatives as novel P2Y1 receptor agonists. J Med Chem 45:5384–5396 [DOI] [PubMed]
- 21.van Rhee AM, Fischer B, van Galen PJM, Jacobson KA (1995) Modelling the P2Y purinoceptor using rhodopsin as template. Drug Des Discov 13:133–154 [PMC free article] [PubMed]
- 22.Springthorpe B, Bailey A, Barton P, Birkinshaw TN, Bonnert RV, Brown RC, Chapman D, Dixon J, Guile SD, Humphries RG, Hunt SF, Ince F, Ingall AH, Kirk IP, Leeson PD, Leff P, Lewis RJ, Martin BP, McGinnity DF, Mortimore MP, Paine SW, Pairaudeau G, Patel A, Rigby AJ, Riley RJ, Teobald BJ, Tomlinson W, Webborn PJ, Willis PA (2007) From ATP to AZD6140: the discovery of an orally active reversible P2Y12 receptor antagonist for the prevention of thrombosis. Bioorg Med Chem Lett 17:6013–6018 [DOI] [PubMed]
- 23.Halbfinger E, Major DT, Ritzmann M, Ubl J, Reiser G, Boyer JL, Harden KT, Fischer B (1999) Molecular recognition of modified adenine nucleotides by the P2Y1-receptor1. A synthetic, biochemical, and NMR approach. J Med Chem 42:5325–5337 [DOI] [PubMed]
- 24.Fischer B, Boyer JL, Hoyle CH, Ziganshin AU, Brizzolara AL, Knight GE, Zimmet J, Burnstock G, Harden TK, Jacobson KA (1993) Identification of potent, selective P2Y-purinoceptor agonists: structure-activity relationships for 2-thioether derivatives of adenosine 5′-triphosphate. J Med Chem 36:3937–3946 [DOI] [PMC free article] [PubMed]
- 25.Brown SG, King BF, Kim YC, Burnstock G, Jacobson KA (2000) Activity of novel adenine nucleotide derivatives as agonists and antagonists at recombinant rat P2X receptors. Drug Dev Res 49:253–259 [DOI] [PMC free article] [PubMed]
- 26.Marteau F, Le Poul E, Communi D, Labouret C, Savi P, Boeynaems JM, Gonzalez NS (2003) Pharmacological characterization of the human P2Y13 receptor. Mol Pharmacol 64:104–112 [DOI] [PubMed]
- 27.Cristalli G, Mills DC (1993) Identification of a receptor for ADP on blood platelets by photoaffinity labelling. Biochem J 291:875–881 [DOI] [PMC free article] [PubMed]
- 28.Cristalli G, Podda GM, Costanzi S, Lambertucci C, Lecchi A, Vittori S, Volpini R, Zighetti ML, Cattaneo M (2005) Effects of 5′-Phosphate Derivatives of 2-Hexynyl Adenosine and 2-Phenylethynyl Adenosine on Responses of Human Platelets Mediated by P2Y Receptors. J Med Chem 48:2763–2766 [DOI] [PubMed]
- 29.Boyer JL, Siddiqi S, Fischer B, Romera-Avila T, Jacobson KA, Harden TK (1996) Identification of potent P2Y-purinoceptor agonists that are derivatives of adenosine 5′-monophosphate. Brit J Pharmacol 118:1959–1964 [DOI] [PMC free article] [PubMed]
- 30.Hollopeter G, Jantzen HM, Vincent D, Li G, England L, Ramakrishnan V, Yang RB, Nurden P, Nurden A, Julius D, Conley PB (2001) Identification of the platelet ADP receptor targeted by anti-thrombotic drugs. Nature 409:202–207 [DOI] [PubMed]
- 31.Färber K, Markworth S, Pannasch U, Nolte C, Prinz V, Kronenberg G, Gertz K, Endres M, Bechmann I, Enjyoji K, Robson SC, Kettenmann H (2008) The ectonucleotidase cd39/ENTPDase1 modulates purinergic-mediated microglial migration. Glia 56:331–341 [DOI] [PubMed]
- 32.Burnstock G, Fischer B, Maillard M, Ziganshin A, Ralevic V, Knight G, Brizzolara A, von Isakovios A, Boyer JL, Harden TK, Jacobson KA (1994) Structure activity relationships for derivatives of adenosine-5′-triphosphate as agonists at P2 purinoceptors: heterogeneity within P2X- and P2Y-subtypes. Drug Dev Res 31:206–219 [DOI] [PMC free article] [PubMed]
- 33.Sharon E, Lévesque SA, Munkonda MN, Sévigny J, Ecke D, Reiser G, Fischer B (2006) Fluorescent N2,N3-epsilon-adenine nucleoside and nucleotide probes: synthesis, spectroscopic properties, and biochemical evaluation. Chembiochem 7:1361–1374 [DOI] [PMC free article] [PubMed]
- 34.Nandanan E Camaioni E, Jang SY, Kim Y-C, Cristalli G, Herdewijn P, Secrist JA, Tiwari KN, Mohanram A, Harden TK, Boyer JL, Jacobson KA (1999) Structure activity relationships of bisphosphate nucleotide derivatives as P2Y1 receptor antagonists and partial agonists. J Med Chem 42:1625–1638 [DOI] [PMC free article] [PubMed]
- 35.Nandanan E, Jang S-Y, Moro S, Kim HO, Siddiqui MA, Russ P, Marquez VE, Busson R, Herdewijn P, Harden TK, Boyer JL, Jacobson KA (2000) Synthesis, biological activity, and molecular modeling of ribose-modified deoxyadenosine bisphosphate analogues as P2Y1 receptor ligands. J Med Chem 43:829–842 [DOI] [PMC free article] [PubMed]
- 36.Kim HS, Ravi RG, Marquez VE, Maddileti S, Wihlborg A-K, Erlinge D, Malmsjö M, Boyer JL, Harden TK, Jacobson KA (2002) Methanocarba modification of uracil and adenine nucleotides: High potency of northern ring conformation at P2Y1, P2Y2, or P2Y4 and P2Y11, but not P2Y6 receptors. J Med Chem 45:208–218 [DOI] [PMC free article] [PubMed]
- 37.Ravi RG, Kim HS, Servos J, Zimmermann H, Lee K, Maddileti S, Boyer JL, Harden TK, Jacobson KA (2002) Adenine nucleotide analogues locked in a northern methanocarba conformation: Enhanced stability and potency as P2Y1 receptor agonists. J Med Chem 45:2090–2100 [DOI] [PMC free article] [PubMed]
- 38.Chhatriwala M, Ravi RG, Patel RI, Boyer JL, Jacobson KA, Harden TK (2004) Induction of novel agonist selectivity for the ADP-activated P2Y1 receptor versus the ADP-activated P2Y12 and P2Y13 receptors by conformational constraint of an ADP analogue. J Pharmacol Exp Therfs 311:1038–1043 [DOI] [PMC free article] [PubMed]
- 39.Boyer JL, Romero-Avila T, Schachter JB, Harden TK (1996) Identification of competitive antagonists of the P2Y1-receptor. Mol Pharmacol 50:1323–1329 [PubMed]
- 40.Raboisson P, Baurand A, Cazenave JP, Gachet C, Schultz D, Spiess B, Bourguignon JJ (2002) A general approach toward the synthesis of C-nucleoside pyrazolo[1,5-a]-1,3,5-triazines and their 3′,5′-bisphosphate C-nucleotide analogues as the first reported in vivo stable P2Y1-receptor antagonists. J Org Chem 67:8063–8071 [DOI] [PubMed]
- 41.Costanzi S, Tikhonova IG, Ohno M, Roh EJ, Joshi BV, Colson A-O, Houston D, Maddileti S, Harden TK, Jacobson KA (2007) P2Y1 antagonists: combining receptor-based modeling and QSAR for a quantitative prediction of the biological activity based on consensus scoring. J Med Chem 50:3229–3241 [DOI] [PubMed]
- 42.Xu B, Stephens A, Kirschenheuter G, Greslin AF, Cheng X, Sennelo J, Cattaneo M, Zighetti ML, Chen A, Kim SA, Kim HS, Bischofberger N, Cook G, Jacobson KA (2002) Acyclic analogues of adenosine bisphosphates as P2Y receptor antagonists: Phosphate substitution leads to multiple pathways of inhibition of platelet aggregation. J Med Chem 45:5694–5709 [DOI] [PMC free article] [PubMed]
- 43.Cattaneo M, Lecchi A, Joshi BV, Ohno M, Besada P, Tchilibon S, Lombardi R, Bischofberger N, Harden TK, Jacobson KA (2004) Antiaggregatory activity in human platelets of potent antagonists of the P2Y1 receptor. Biochem Pharmacol 68:1995–2002 [DOI] [PMC free article] [PubMed]
- 44.Ingall AH, Dixon J, Bailey A, Coombs ME, McInally JI, Hunt SF, Kindon ND, Theobald BJ, Willis PA, Humphries R, Leff P, Clegg JA, Smith JA, Tomlinson W (1999) Antagonists of the platelet P2T receptor: A novel approach to antithrombotic therapy. J Med Chem 42:213–220 [DOI] [PubMed]
- 45.van Giezen JJ, Humphries RG (2005) Preclinical and Clinical Studies with Selective Reversible Direct P2Y12 Antagonists. Semin Thromb Hemost 31:195–204 [DOI] [PubMed]
- 46.Douglass J, Patel RI, Yerxa B, Shaver SR, Watson PS, Bednarski K, Plourde R, Redick C, Brubaker K, Jones AC, Boyer JL (2008) Lipophilic modifications to dinucleoside polyphosphates and nucleotides that confer antagonist properties at the P2Y12 platelet receptor. J Med Chem 51:1007–1025 [DOI] [PubMed]
- 47.Savi P, Pereillo JM, Uzabiaga MF, Combalbert J, Picard C, Maffrand JP, Pascal M, Herbert JM (2002) Identification and biological activity of the active metabolite of Clopidogrel. Thromb Haemost 84:891–896 [PubMed]
- 48.Jakubowski JA, Matsushima N, Asai F, Naganuma H, Brandt JT, Hirota T, Freestone S, Winters KJ (2007) A multiple dose study of prasugrel (CS-747), a novel thienopyridine P2Y12 inhibitor, compared with clopidogrel in healthy humans. Br J Clin Pharmacol 63:421–430 [DOI] [PMC free article] [PubMed]
- 49.Fretz H, Houille O, Hilpert K, Peter O, Breu V, Giller T, Valdenaire O, Riederer M (2005) Novel pyrazolidine-3,5-dione derivatives are P2Y12 receptor antagonists and inhibit ADP-triggered blood platelet aggregation. 229th National Meeting of the American Chemical Soc, San Diego, CA, Abstract MEDI 80
- 50.Wang YX, Vincelette J, da Cunha V, Martin-McNulty B, Mallari C, Fitch RM, Alexander S, Islam I, Buckman BO, Yuan S, Post JM, Subramanyam B, Vergona R, Sullivan ME, Dole WP, Morser J, Bryant J (2007) A novel P2Y12 adenosine diphosphate receptor antagonist that inhibits platelet aggregation and thrombus formation in rat and dog models. Thromb Haemost 97:847–855 [PubMed]
- 51.Herpin TF, Morton G, Rehfuss RP, Lawrence RM, Poss MA, Roberge JY, Gungor T. Aminobenzazoles as P2Y1 receptors inhibitors. WO2005070920. Date of publication is August 4, 2005
- 52.Lambrecht G, Braun K, Damer M, Ganso M, Hildebrandt C, Ullmann H, Kassack MU, Nickel P (2002) Structure-activity relationships of suramin and pyridoxal-5’-phosphate derivatives as P2 receptor antagonists. Curr Pharm Des 8:2371–2399 [DOI] [PubMed]
- 53.Kim YC, Lee J-S, Sak K, Marteau F, Mamedova L, Boeynaems J-M, Jacobson KA (2005) Synthesis of pyridoxal phosphate derivatives with antagonist activity at the P2Y13 receptor. Biochem Pharmacol 70:266–274 [DOI] [PMC free article] [PubMed]
- 54.Communi D, Govaerts C, Parmentier M et al (1997) Cloning of a human purinergic P2Y receptor coupled to phospholipase C and adenylyl cyclase. J Biol Chem 272:31969–31973 [DOI] [PubMed]
- 55.Conigrave AD, Fernando KC, Gu B, Tasevski V, Zhang W, Luttrell BM, Wiley JS (2001) P2Y11 receptor expression by human lymphocytes: evidence for two cAMP-linked purinoceptors. Eur J Pharmacol 426:157–163 [DOI] [PubMed]
- 56.Nguyen TD, Meichle S, Kim US, Wong T, Moody MW (2001) P2Y11, a purinergic receptor acting via cAMP, mediates secretion by pancreatic duct epithelial cells. Am J Physiol Gastrointest Liver Physiol 280:G795–G804 [DOI] [PubMed]
- 57.Communi D, Robaye B, Boeynaems J-M (1999) Pharmacological characterization of the human P2Y11 receptor. Br J Pharmacol 128:1199–1206 [DOI] [PMC free article] [PubMed]
- 58.White PJ, Webb TE, Boarder MR (2003) Characterization of a Ca2+ response to both UTP and ATP at human P2Y11 receptors: evidence for agonist-specific signaling. Mol Pharmacol 63:1356–1363 [DOI] [PubMed]
- 59.Shaver SR, Rideout JL, Pendergast W, Douglas JG, Brown EG, Boyer JL, Patel RI, Redick CC, Jones AC, Picher M, Yerxa BR (2005) Structure–activity relationships of dinucleotides: Potent and selective agonists of P2Y receptors. Purinerg Signal 1:183–191 [DOI] [PMC free article] [PubMed]
- 60.Yerxa BR, Sabater JR, Davis CW, Stutts MJ, Lang-Furr M, Picher M, Jones AC, Cowlen M, Dougherty R, Boyer J, Abraham WM, Boucher RC (2002) Pharmacology of INS37217 [P1-(Uridine 5′)-P4- (2′-deoxycytidine 5′)tetraphosphate, Tetrasodium Salt], a Next-Generation P2Y2 Receptor Agonist for the Treatment of Cystic Fibrosis. J Pharmacol Exp Ther 302:871–880 [DOI] [PubMed]
- 61.Patel K, Barnes A, Camacho J, Paterson C, Boughtflower R, Cousens D, Marshall F (2001) Activity of diadenosine polyphophates at P2Y receptors stably expressed in 1321N1 cells. Eur J Pharmacol 430:203–210 [DOI] [PubMed]
- 62.Moreschi I, Bruzzone S, Nicholas RA, Fruscione F, Sturla L, Benvenuto F, Usai C, Meis S, Kassack MU, Zocchi E, De Flora A (2006) Extracellular NAD+ is an agonist of the human P2Y11 purinergic receptor in human granulocytes. J Biol Chem 281:31419–31429 [DOI] [PubMed]
- 63.Moreschi I, Bruzzone S, Bodrato N, Usai C, Guida L, Nicholas RA, Kassack MU, Zocchi E, De Flora A (2008) NAADP+ is an agonist of the human P2Y11 purinergic receptor. Cell Calcium 43:344–355 doi:10.1016/j.ceca.2007.06.006 [DOI] [PubMed]
- 64.van der Weyden L, Adams DJ, Luttrell BM, Conigrave AD, Morris MB (2000) Pharmacological characterization of the P2Y11 receptor in stable transfected haematological cell lines. Mol Cell Biochem 213:75–81 [DOI] [PubMed]
- 65.van der Weyden L, Rakyan V, Luttrell BM, Morris MB, Conigrave AD (2000) Extracellular ATP couples to cAMP generation and granulocytic differentiation in human NB4 promyelocytic leukaemia cells. Immunol Cell Biol 78:467–473 [DOI] [PubMed]
- 66.Ecke D, Tulapurkar ME, Nahum V, Fischer B, Reiser G (2006) Opposite diastereoselective activation of P2Y1 and P2Y11 nucleotide receptors by adenosine 52-O-(α-boranotriphosphate) analogues. Br J Pharmacol 149:416–423 [DOI] [PMC free article] [PubMed]
- 67.Balogh J, Wihlborg AK, Isackson H, Joshi BV, Jacobson KA, Arner A, Erlinge D (2005) Phospholipase C and cAMP-dependent positive inotropic effect of ATP in mouse cardiomyocytes via P2Y11-like receptors. J Mol Cell Cardiol 39:223–230 [DOI] [PMC free article] [PubMed]
- 68.Ullmann H, Meis S, Hongwiset D, Marzian C, Wiese M, Nickel P, Communi D, Boeynaems JM, Wolf C, Hausmann R, Schmalzing G, Kassack MU (2005) Synthesis and structure-activity relationships of suramin-derived P2Y11 receptor antagonists with nanomolar potency. J Med Chem 48:7040–7048 [DOI] [PubMed]
- 69.Erb L, Lustig KD, Sullivan DM, Turner JT, Weisman GA (1993) Functional expression and photoaffinity labeling of a cloned P2U Purinergic receptor. Proc Natl Acad Sci 90:10449–10453 [DOI] [PMC free article] [PubMed]
- 70.Erb L, Garrad R, Wang Y, Quinn T, Turner JT, Weisman GA (1995) Site-directed mutagenesis of P2U purinoreceptors. J Biol Chem 270:4185–4188 [DOI] [PubMed]
- 71.Parr CE, Sullivan DM, Paradiso AM, Lazarowski ER, Burch LH, Olsen JC, Erb L, Weisman GA, Boucher RC, Turner JT (1995) Cloning and expression of a human P2U nucleotide receptor, a target for cystic fibrosis pharmacotherapy. Proc Natl Acad Sci 91:3275–3279 [DOI] [PMC free article] [PubMed]
- 72.Lazarowski ER, Watt WC, Stutts MJ, Boucher RC, Harden TK (1995) Pharmacological selectivity of the cloned human P2U-purinoreceptor: potent activation by diadenosine tetraphosphate. Brit J Pharm 116:1619–1627 [DOI] [PMC free article] [PubMed]
- 73.Nicholas RA, Watt WC, Lazarowski ER, Li Q, Harden TK (1996) Uridine nucleotide selectivity of three phospholipase C-activating P2 receptors: identification of a UDP-selective, a UTP-selective, and an ATP- and UTP-specific receptor. Mol Pharmacol 50:224–229 [PubMed]
- 74.Ivanov AA, Ko H, Cosyn L, Maddileti S, Besada P, Fricks I, Costanzi S, Harden TK, Van Calenbergh S, Jacobson KA (2007) Molecular modeling of the human P2Y2 receptor and design of a selective agonist, 2′-amino-2′-deoxy-2-thio-UTP. J Med Chem 50:1166–1176 [DOI] [PMC free article] [PubMed]
- 75.Kindon N, Meghani P, Thom S (1998) World Pat. 98/54180
- 76.Kaulich M, Streicher F, Mayer R, Müller I, Müller CE (2003) Flavonoids - novel lead compounds for the development of P2Y2 receptor antagonists. Drug Devel Res 59:72–81 [DOI]
- 77.Weyler S, Baqi Y, Hillmann P, Kaulich M, Hunder AM, Müller IA, Müller CE (2008) Combinatorial synthesis of anilinoanthraquinone derivatives and evaluation as non-nucleotide-derived P2Y2 receptor antagonists. Bioorg Med Chem Lett 18:223–227 [DOI] [PubMed]
- 78.Korcok J, Raimundo LN, Du X, Sims SM, Dixon SJ (2005) P2Y6 nucleotide receptors activate NF-κB and increase survival of osteoclasts. J Biol Chem 280:16909–16915 [DOI] [PubMed]
- 79.Costanzi S, Joshi BV, Maddileti S, Mamedova L, Gonzalez-Moa M, Marquez VE, Harden TK, Jacobson KA (2005) Human P2Y6 receptor: Molecular modeling leads to the rational design of a novel agonist based on a unique conformational preference. J Med Chem 48:8108–8111 [DOI] [PMC free article] [PubMed]
- 80.Besada P, Shin DH, Costanzi S, Ko HJ, Mathé C, Gagneron J, Gosselin G, Maddileti S, Harden TK, Jacobson KA (2006) Structure activity relationship of uridine 5′-diphosphate analogues at the human P2Y6 receptor. J Med Chem 49:5532–5543 [DOI] [PMC free article] [PubMed]
- 81.El-Tayeb A, Qi A, Müller CE (2006) Synthesis and structure-activity relationships of uracil nucleotide derivatives and analogues as agonists at human P2Y2, P2Y4, and P2Y6 receptors. J Med Chem 49:7076–7087 [DOI] [PubMed]
- 82.Mamedova L, Joshi BV, Gao ZG, von Kügelgen I, Jacobson KA (2004) Diisothiocyanate derivatives as potent, insurmountable antagonists of P2Y6 nucleotide receptors. Biochem Pharmacol 67:1763–1770 [DOI] [PMC free article] [PubMed]
- 83.Abbracchio MP, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Miras-Portugal MT, King BF, Gachet C, Jacobson KA, Weisman GA, Burnstock G (2003) Characterization of the UDP-glucose receptor (re-named here the P2Y14 receptor) adds diversity to the P2Y receptor family. Trends Pharmacol Sci 24:52–55 [DOI] [PMC free article] [PubMed]
- 84.Ko H, Fricks I, Ivanov AA, Harden TK, Jacobson KA (2007) Structure activity relationship of uridine 5′-diphosphoglucose (UDP-glucose) analogues as agonists of the human P2Y14 receptor. J Med Chem 50:2030–2039 [DOI] [PMC free article] [PubMed]
- 85.Ivanov AA, Fricks I, Harden TK, Jacobson KA (2007) Molecular dynamics simulation of the P2Y14 receptor Ligand docking and identification of a putative binding site of the distal hexose moiety. Bioorg Med Chem Lett 17:761–766 [DOI] [PMC free article] [PubMed]
- 86.Pfefferkorn JA, Choi C, Winters T, Kennedy R, Chi L, Perrin LA, Lu G, Ping YW, McClanahan T, Schroeder R, Leininger MT, Geyer A, Schefzick S, Atherton J (2008) P2Y1 receptor antagonists as novel antithrombotic agents. Bioorg Med Chem Lett 18:3338–3343 [DOI] [PubMed]
- 87.Ye H, Chen C, Zhang H-C, Haertlein BJ, Parry TJ, Damiano BP, Maryanoff BE (2008) Carba-nucleosides as Potent Antagonists of the Adenosine 5′-Diphosphate (ADP) Purinergic Receptor (P2Y12) on Human Platelets. ChemMedChem 3(5):732–736 [DOI] [PubMed]
- 88.Lieu HD, Conley PB, Andre P, Leese PT, Romanko K, Phillips DR, Jurek M, Meloni A, Hutchaleelaha A, Gretler DD (2007) Initial intravenous experience with PRT060128 (PRT128), an orally available, direct-acting, and reversible P2Y12 inhibitor. J Thromb Haemost 5(Suppl 1) (Abstract)
- 89.Tantry US, Bliden KP, Gurbel PA (2007) AZD6140. Expert Opin Investig Drugs 16:225–229 [DOI] [PubMed]