Abstract
Otolith-only neurons were recorded extracellularly in the vestibular nuclei before and after cynomolgus monkeys were held on-side for up to 3 hrs. The aim was to determine whether the polarization vectors of these neurons reorient towards the spatial vertical as do canalotolith convergent neurons.1 Otolith input was characterized by tilting the animal 30° from the upright position while positioning the head in different directions in yaw. This determined the response vector orientation (RVO), i.e., the projection of the otolith polarization vector onto the head horizontal plane. Changes in the RVO of otolith-only neurons ranged from 2°–16°, which was on average considerably less than the changes previously noted in canal-otolith convergent vestibulo-only (VO) and vestibular plus saccade (VPS) neurons that ranged up to 109°. Some of the otolith-only neurons had marked sensitivity changes. These findings suggest that otolith-only neurons tend to maintain a head-fixed orientation during prolonged head tilts relative to gravity; some of these neurons even strengthen that orientation sensitivity. In contrast, canal-convergent VO and VPS neurons optimize their response vector orientation to gravity when the head is oriented for prolonged periods.
Keywords: Response vector orientation (RVO), Monkey, Central Vestibular Neurons, Polarization Vector
INTRODUCTION
The polarization vectors of central vestibulo-only (VO) and vestibular-plus-saccade (VPS) neurons that receive convergent semicircular canal and otolith inputs change their orientation towards the gravitational axis after prolonged head tilts. 1 There are multiple otolith afferent inputs to these neurons, and we have suggested that changes in the weights of the convergent otolith afferents 2 could be the source of adaptation of the polarization vectors. Whether such adaptive changes are specific for particular classes of neurons or are a general property of all central otolith-recipient cells is not known. One class of cells, that code head position relative to gravity, receives only-otolith afferent input (otolith-only cells) 3. In this study, we determined whether the polarization vectors of these otolith-only neurons would be changed after prolonged side-down head tilts.
METHODS
The firing rates of five otolith-only neurons were recorded extracellularly in the vestibular nuclei of two cynomolgus monkeys (Macaca fascicularis). The experimental protocol conformed to the Guide for the Care and Use of Laboratory Animals and was approved by the Institutional Animal Care and Use Committee. Polarization vectors of the static otolith input for each otolith-only neuron was characterized by the direction of the response vector orientation (RVO), which is a projection of the otolith polarization vector onto the head horizontal plane.1 The RVO was determined by tilting animals 30° about a spatial horizontal axis from the upright in different head orientations in yaw over at least in 15° increments (Fig. 1, Fig. 2). The head was held in the tilted positions for 30 s or more in each yaw orientation. Only the last 20 s of unit activity recorded after each head tilt was used for analysis to eliminate transient responses. Unit firing rates were converted into sensitivities (imp*s−1/g) 4 and plotted as a function of the direction of the equivalent acceleration of gravity (ag) in head coordinates (Fig. 1 and Fig.2 C, D). Data were fitted with a cosine to determine the RVO - the direction of maximal (spatial) sensitivity of the neuron in head coordinates. This technique is described in greater detail elsewhere.1 The RVO’s were determined before and after each hr with the head reoriented in a side down position for up to three hrs (Fig. 1 and Fig.2). RVO’s are presented as if the monkeys’ head were always held in the left side down (LSD) position (Fig. 3B). Care was taken to ensure that the same unit was recorded over the entire testing procedure. 5
Figure 1. Neuronal response of an otolith-only neuron (Unit #1) to head tilts from the upright from different head orientations in yaw.
A, Direction of the acceleration of gravity (ag, arrows) in head coordinates in 15° increments about a yaw axis from 180° (left) to 360° (right). B, 30° head tilts about the horizontal axis, shown in A by the horizontal line. C, D, Neuronal firing rate (FR) for each head tilt, obtained before (C) and after 2 hrs (D) of head reorientation in a side down position. The horizontal dashed lines represent the average firing rate of this neuron in the upright position.
Figure 2. Neuronal response of the otolith only neuron (Unit #5) to head tilts from the upright for different head orientations in yaw.
Scheme as in Fig. 1.
Figure 3.
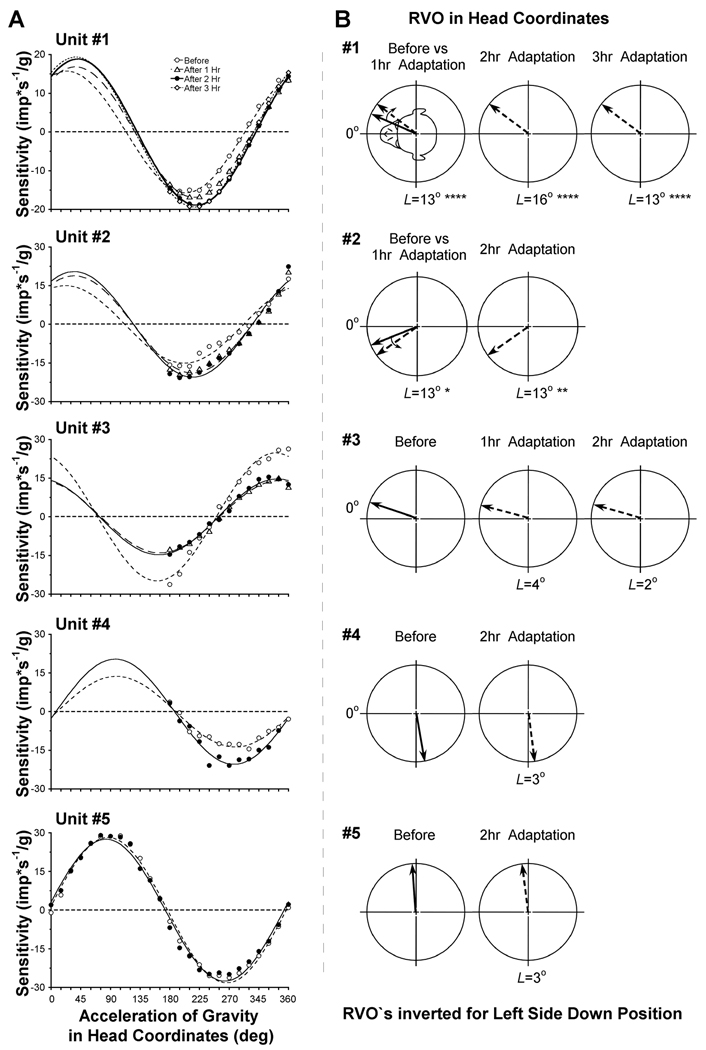
A, Sensitivity of the otolith-only neurons plotted as a function of orientation of the acceleration of gravity (ag) in head coordinates. The sinusoidal fits (lines) to the data measured before (open circles), after 1 hr (open triangles), after 2 hrs (filled circles) and after 3 hrs (open diamonds) of head orientation in side down position. B, RVO of each neuron before (solid arrows) and after 1, 2 and 3 hrs (dotted arrows) of adaptation. The RVO`s are presented as if the head was always positioned left side down (LSD), as shown in the insert for Unit #1.
RESULTS
Figure 1 and Figure 2 demonstrate the firing rates of two otolith-only neurons recorded before (C) and after 2 hr of head reorientation in a side-down position (D). The average firing rate of Unit #1 in the upright head position was initially 47.16±13.42 imp/s and increased to 76.19±28.07 imp/s after being side down for 2 hr (Fig. 1). Before and after adaptation, the unit was maximally inhibited when the head was oriented so that the acceleration of gravity (ag) was ≈195–225° in yaw (A), and the unit was maximally excited when ag was oriented 180° from this position (15–45°). The direction of maximal sensitivity of this neuron was 24±1° before adaptation (Fig. 3A, open circles). This corresponded to a RVO of 336° (or −24° after inverting the value for LSD reorientation, Fig. 3B). The RVO shifted toward the interaural axis by 13° after 1hr of adaptation relative to pre-adaptive measure (p<0.001; Fig. 3A, open triangles). Shifts in the RVO of this neuron were approximately the same after 2 hr (16°; p<0.001; filled circles) and 3 hr of side down adaptation (13°, p<0.001, open diamonds). Thus, the most significant changes in RVO for this neuron were in 1st hr of adaptation (p=0.0004), and further adaptation over the next two hours had only a minor additional effect. The magnitude of the spatial sensitivity gradually increased in this neuron up to about 18% during the experiment (15.7±0.3 imp*s−1/g before adaptation, 16.8±0.4 imp*s−1/g after 1hr, 18.9±0.3 imp*s−1/g after 2hr, and 19.4±0.3 imp*s−1/g after 3hr, p<0.001; Fig. 3A).
Changes were similar in Unit #2 (Fig. 3). The RVO before adaptation was 22±3° and shifted by 13° after 1 hr to 35±3° (p<0.046; Fig. 3A, B, open triangles). After 2 hr of adaptation, the change in the RVO was maintained (35±3° , p=0.0102; filled circles). The spatial sensitivity gradually increased up to 30% during head reorientation in the side-down position from 15.0±0.9 imp*s−1/g before adaptation to 18.9±0.9 and 20.5±1.0 imp*s−1/g after 1st and 2nd hrs of adaptation, respectively.
The RVO of neuron #3 was similar before and after 1 and 2 hr of adaptation. The RVO was 341±2° before adaptation (Fig. 3B, solid arrow), 345±2° after the 1st hr (p>0.05), and 343±2° after the 2nd hr (p>0.05) (Fig. 3B, dotted arrows). The magnitude of the spatial sensitivity, however, decreased significantly by 44% after 1st hr of adaptation (24.9±0.8 imp*s−1/g before adaptation, 14.0±0.4 imp*s−1/g after 1hr, p=0.0002), remaining at this level after the 2nd hr (17.7±0.4, p=0.0004; Fig. 3A).
The RVO of the neuron #4 was 100±2° before adaptation (Fig. 3B, solid arrow) and did not change after 2 hrs of adaptation (103±2°, p>0.05). The magnitude of the spatial sensitivity was 13.7±0.6 imp*s−1/g before adaptation, and significantly increased by 49% up to 20.4±0.8 imp*s−1/g after 2 hrs of adaptation (p=0.0051) (Fig. 3A, filled symbols).
Unit #5, (Figure 2), had comparable firing rates in the upright head position before (40.9±10.3 imp/s) and after 2hrs of adaptation (33.1±11.3 imp/s, p>0.05). The maximal sensitivity and RVO of this neuron were 28.2±0.5 imp*s−1/g and 86° (274° after inverting the value for LSD; Fig. 3A, B), respectively. Maximal sensitivity and RVO were 28.3±0.8 imp*s−1/g and 276±1° after 1st hr (p=0.128, not shown in Fig. 3) and remained at this level (27.5±0.6 imp*s−1/g and 277±1°, p=0.177) after 2nd hr (Fig. 3B, Unit #5).
DISCUSSION
The central otolith-only neurons in this study had different adaptive orientation properties than convergent canal-otolith VO and VPS neurons. The orientations of otolith-only neurons shifted considerably less than canal-otolith convergent neurons, but the sensitivity of some otolith neurons could change appreciably. Only two (#1, #2) of the five neurons that were recorded had significant orientation changes (up to 16°), but these changes were considerably less than those observed in canal-convergent neurons (up to 109°). The three other otolith-only neurons (#3–5) maintained their orientation relative to the head-up position for up to three hrs. We have previously shown that changes in orientation vectors of VO and VPS canal-otolith convergent neurons depend on the angular distance of the original vectors from gravity when the head is placed in the side down position. We postulate that the range of spatial adaptation of the VO and VPS neurons is dependent on the angular disparity of their multiple otolith afferent inputs. If our hypothesis is correct, the small changes in orientation in the otolith-only cells could be due to more limited spatial tuning of the otolith afferent convergent input than to canal-otolith convergent neurons.
Four neurons (#1 – #4) had changes in their sensitivity. This could be an adaptation of these neurons so that their orientation was strengthened as a representation of head orientation relative to gravity. In contrast, there was little or no change in the sensitivity of VO and VPS neurons after reorientation in side down positions.1
If the class of otolith-only neurons do not adapt their RVO’s to prolonged head tilts relative to gravity, as observed in this sample, it would signify that there are differences in function of otolith-only and canal-otolith convergent VO and VPS neurons. The VO and VPS neurons respond to angular head velocity and are closely related to velocity storage, which has spatial orientation properties.6 Otolith-only neurons have no angular head velocity sensitivity and no direct relation to velocity storage. Thus, by adapting their RVO’s to prolonged altered spatial orientation, the VO and VPS neurons could maintain information about angular motion relative to gravity. Otolith-only neurons, in contrast, are likely to provide the central vestibular system with a stable sense of head tilt with regard to gravity, even during prolonged head reorientation.
Acknowledgments
We thank D. Ogorodnikov, S. Tarasenko for technical assistance. This work was supported by NIH grants: DC04996, EY04148, EY11812 and Core Center Grants DC05284 and EY01867.
References
- 1.Eron JN, et al. Adaptation of orientation vectors of otolith-related central vestibular neurons to gravity. J Neurophysiol. 2008 doi: 10.1152/jn.90289.2008. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uchino Y. Otolith and semicircular canal inputs to single vestibular neurons in cats. Biol Sci Space. 2001;15:375–381. doi: 10.2187/bss.15.375. [DOI] [PubMed] [Google Scholar]
- 3.Adrian ED. Discharges from vestibular receptors in the cat. J Physiol. 1943;101:389–407. doi: 10.1113/jphysiol.1943.sp003995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schor RH, Miller AD, Tomko DL. Responses to head tilt in cat central vestibular neurons. I. Direction of maximum sensitivity. J Neurophysiol. 1984;51:136–146. doi: 10.1152/jn.1984.51.1.136. [DOI] [PubMed] [Google Scholar]
- 5.Eron JN, et al. Recording from the same cell in the vestibular nuclei over prolonged periods. J Gravit Physiol. 2007;14:69–70. [PubMed] [Google Scholar]
- 6.Reisine H, Raphan T. Neural basis for eye velocity generation in the vestibular nuclei of alert monkeys during off-vertical axis rotation. Exp Brain Res. 1992;92:209–226. doi: 10.1007/BF00227966. [DOI] [PubMed] [Google Scholar]