Abstract
Esophageal papillomatosis is a very rare condition that is believed to have a benign clinical course. Recent reports underscore the potential development of a malignancy in association with squamous papillomatosis of the esophagus. A case of esophageal papillomatosis complicated by the development of esophageal invasive squamous cell carcinoma diagnosed after esophagectomy, despite multiple nondiagnostic endoscopic biopsies, is described. The patient also developed squamous cell carcinoma in the oral cavity and pyloric channel. The finding of extensive esophageal papillomatosis and unremitting dysphagia symptoms should prompt investigations into an underlying associated malignancy.
Keywords: Endoscopy, Papilloma, Squamous cell carcinoma
Abstract
La papillomatose œsophagienne est une maladie très rare dont l’évolution clinique serait généralement bénigne. Des rapports récents rappellent toutefois le risque de néoplasie en lien avec le papillome épidermoïde de l’œsophage. On décrit ici un cas de papillomatose œsophagienne compliquée d’un carcinome épidermoïde invasif de l’œsophage diagnostiqué après une œsophagectomie et malgré de multiples biopsies endoscopiques non diagnostiques. Le patient a en outre développé un cancer épidermoïde de la cavité buccale et du canal pylorique. La présence de papillomatose œsophagienne étendue et de symptômes de dysphagie rebelle doivent signaler la nécessité d’investiguer rapidement une possible néoplasie sous-jacente.
CASE PRESENTATION
A 70-year-old Caucasian man was referred for evaluation of extensive esophageal nodularity in January 2000. His medical history was significant for hypothyroidism, hyperlipidemia and longstanding diabetes mellitus with microvascular and macrovascular complications including retinopathy, neuropathy, peripheral arterial disease and diffuse three-vessel coronary arterial disease, for which he had been on medical therapy. The patient admitted to social drinking and cessation of smoking almost 25 years previously. An initial endoscopic evaluation for intermittent dysphagia and epigastric abdominal pain at a different centre in October 1999 revealed extensive frond-like esophageal nodularities extending from 22 cm to 39 cm from his incisors. Pathological examination of all the initial sets of esophageal lesion biopsies showed papillary hyperplasia of the squamous epithelium consistent with squamous papillomatosis. The squamous epithelium was thickened and had a ‘finger-like’ architecture over a core of lamina propria. A second endoscopy was performed for further biopsies based on the clinical suspicion of malignancy. However, histology again showed benign squamous cell papillomas with mild reactive atypia. An abdominal and chest computed tomography (CT) scan did not reveal an esophageal lesion, mediastinal lympadenopathy, or other findings suggestive of malignancy or metastasis.
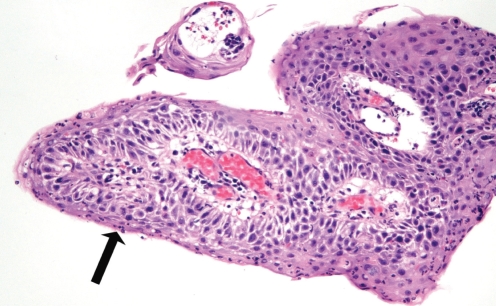
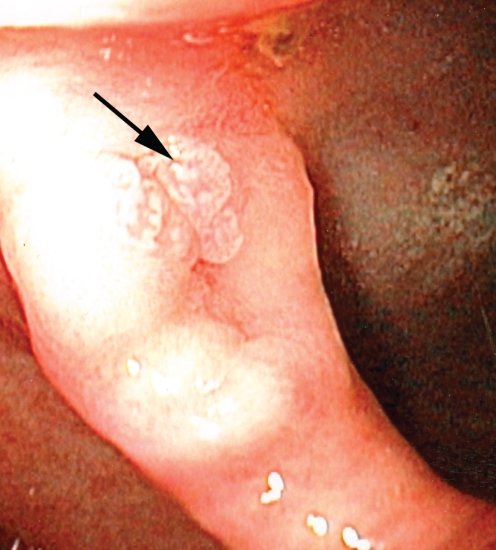
At his first endoscopy at St Michael’s Hospital, Toronto, Ontario, in January 2000, he was positive for papillomatosis of the esophagus, extending from 22 cm to 39 cm. Vital staining using Lugol’s iodine identified many areas of nonstaining. A histopathological examination of biopsies (Figure 1) taken at multiple levels and a review of the previous biopsies revealed intraepithelial inflammation (including eosinophils), and reactive changes including basal layer hyperplasia and keratosis. Focal, mild nuclear atypia was noted. While the possibility of dysplasia was considered, the degree of inflammation lowered the level of suspicion of malignancy and these were diagnosed as reactive changes. Surveillance endoscopy was performed six months later and again revealed extensive verrucous-appearing flat lesions in the mid to distal esophagus. Histological examination following endoscopic mucosal resection again showed squamous epithelial hyperplasia with acute and chronic inflammation.
Figure 1.
Photomicrograph of early biopsy of the esophageal lesions. Note the papillary architecture. Acute inflammation is present within the epithelium (arrow), associated with nuclear changes considered at the time to be reactive in nature (hematoxylin and eosin stain, original magnification ×100)
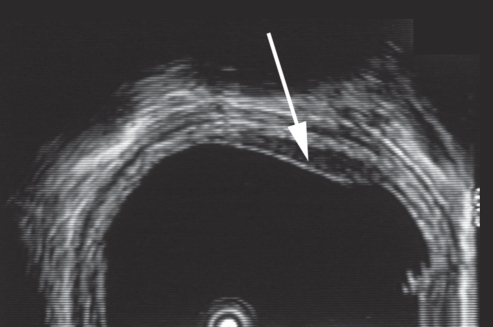
Because of marked esophageal involvement and increasing clinical suspicion of an underlying malignancy, the patient underwent endoscopic ultrasound in October 2000 (Figure 2). Changes in the esophagus were limited to the mucosa without penetration into the muscularis mucosae. Repeat endoscopic mucosal resection findings were consistent with the previous histological diagnosis. Plans to repeat surveillance endoscopy were interrupted in January 2001 due to comorbid medical problems. His longstanding history of diabetes was complicated by the development of an ischemic nonhealing heel ulcer requiring peripheral vascular bypass surgery.
Figure 2.
Endosonographic image of a hypoechoic mucosal thickening (arrow) with intact submucosa
The patient was briefly lost to follow-up. Repeat endoscopy with biopsies was performed in June 2002. The squamous papillary lesion with marked inflammation was once again present; however, there were irregularities at the base of the squamous epithelium suggestive of stromal invasion. Thickening of the distal esophagus was seen on a second CT scan of the chest and abdomen, from 5 cm above the gastroesophageal junction extending 4 cm proximally. Neither an extrinsic mass or mediastinal lymph nodes were seen. One month later, the patient was admitted to hospital because of a food bolus impaction requiring endoscopy. A stricture was identified in the midesophagus and subsequent dilation was performed.
His repeat endoscopic assessment at our centre revealed verrucous esophagus extending from 22 cm to 39 cm. A resistant stricture was noted at 36 cm and required dilation. Biopsy specimens contained similar changes, although in some fragments, the nuclear atypia was increased. There was increased suspicion of a dysplastic lesion and the possibility of a verrucous carcinoma was raised. Possible koilocytes were identified. Due to the possibility of human papilloma virus (HPV) infection, in situ hybridization studies for HPV were performed but were negative for types 6, 11, 16, 18, 31, 33 and 35.
At this point, there was mounting clinical and pathological suspicion of an underlying malignancy, possibly a verrucous carcinoma. The patient decided to proceed with esophagectomy. However, because of his significant comorbid cardiovascular disease as a result of longstanding poorly controlled diabetes, he required coronary artery bypass graft surgery before esophagectomy. His coronary revascularization was uncomplicated. He subsequently underwent a laparoscopically assisted total esophagectomy and gastric pull-up procedure in March 2003, without complications.
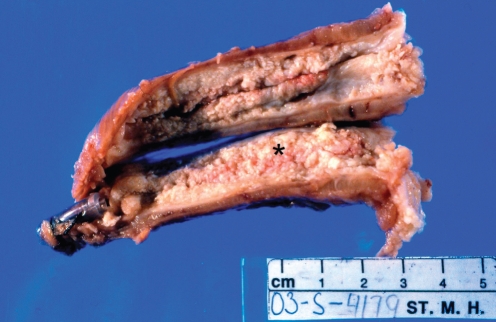
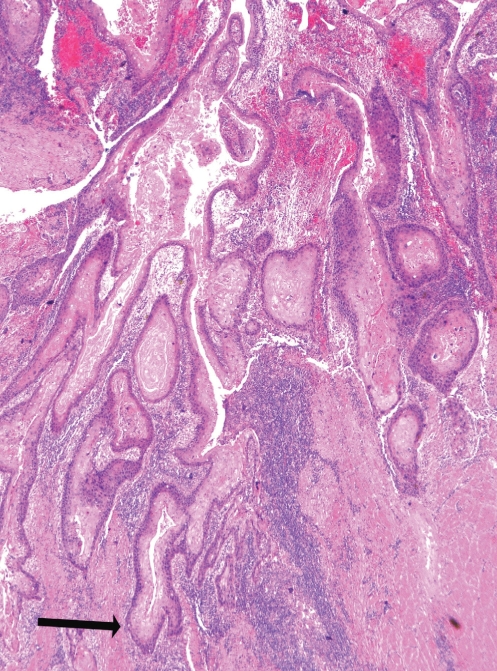
On examination of the esophagectomy specimen, a well-differentiated squamous cell carcinoma (SCC) was identified, with extensive, diffuse papillary and exophytic growth involving most of the esophagus (Figure 3). At the point of greatest depth of invasion, the tumour extended into the muscularis propria at the mid esophagus (Figure 4), correlating with the site of the stricture viewed on endoscopy. There was no lymphatic or perineural invasion and margins were clear of malignancy. On immunohistochemistry, cells in the basal layer stained positively for p53 and the cellular proliferation marker Ki 67. A human epidermal growth factor receptor-2 assay was also performed and was negative. Fourteen of 14 excised nodes were negative for malignancy.
Figure 3.
Photograph of esophagectomy specimen. Note the loss of the normal smooth, white squamous epithelium, replaced by fleshy pink nodular tissue (asterisk)
Figure 4.
Photomicrograph of the invasive squamous cell carcinoma. Nests of well-differentiated malignant squamous cells are invading the muscularis propria (arrow) (hematoxylin and eosin stain, original magnification ×25)
Initial follow-up biopsies of the gastric pull-up procedure six months after surgery contained small amounts of granulation tissue. Eight months after esophagectomy, an ulceration on the right mandibular gum line was noted and biopsied; histology results revealed an invasive SCC. A mandibular and right neck dissection were performed; the tumour was diagnosed as a moderately differentiated SCC with metastases to one neck and one facial lymph node. Dysplastic squamous epithelium was present in the tongue mucosa. The patient also underwent adjuvant radiotherapy.
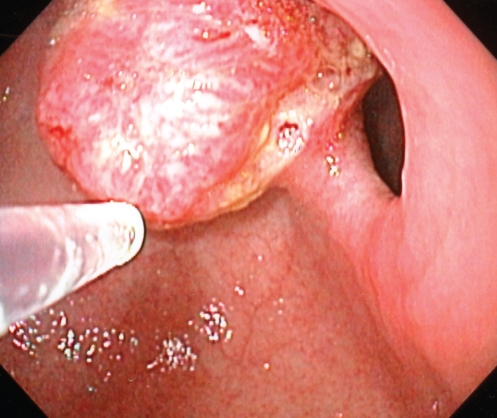
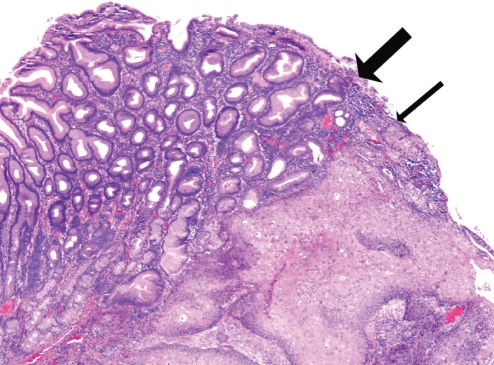
Follow-up endoscopy one year after esophagectomy revealed a small pyloric channel mucosal irregularity (Figure 5). Biopsies contained squamous epithelium with a papillomatous architecture and low-grade dysplasia. Fragments of gastric antral epithelium were also present; an interface between the squamous and gastric mucosa was not identified. As a result, the pathologist considered the possibility that the squamous material was a contaminant originating in the upper esophagus. Because of the relatively benign appearance of this lesion and the slow development of the tumour in the original esophageal lesions, the area was not ablated endoscopically. A follow-up endoscopy one year later revealed a 1.5 cm firm pyloric channel mass (Figure 6). Biopsies of this mass (Figure 7) demonstrated no connection between the squamous and gastric mucosa; however, in these biopsies, the squamous epithelium contained significant nuclear atypia and invasion of the lamina propria was evident, consistent with a well-differentiated invasive SCC. Endoscopic mucosal resection of this area showed a sharp division between the gastric mucosa and the invasive SCC. An abdominal CT scan showed no evidence of obvious local invasion or metastatic disease. On laparotomy, the tumour was found to be invading the left lobe of the liver. Given the present comorbidities, only a gastrojejunostomy was performed. The patient declined any further therapy at the time.
Figure 5.
One-year postesophagectomy endoscopic image of the pyloric channel mucosal irregularity
Figure 6.
Endoscopic image of the pyloric channel mass
Figure 7.
Photomicrograph of the pyloric lesion. Note the sharp division between gastric antral type mucosa (thick arrow) and the invasive squamous epithelium (thin arrow) (hematoxylin and eosin stain, original magnification ×40)
DISCUSSION
Squamous papillomas of the esophagus are epithelial tumours considered to be benign. They are rare, occurring in 0.01% of individuals at autopsy (1) and 0.07% of individuals in an endoscopy series (2). Most lesions are found incidentally, and are small and solitary in nature, usually located in the distal esophagus (1). The pathogenesis of esophageal papillomas is thought to be the result of chronic gastroesophageal reflux resulting in chemical irritation or infection with HPV, or a combination of the two (3). The lesions appear as small, circumferential, pearly, wart-like exophytic growths. Histological examination demonstrates the characteristic finger-like projections of hyperplastic squamous epithelium covering a connective tissue core.
Development of extensive esophageal squamous papillomas, otherwise known as squamous papillomatosis of the esophagus, is even less common. Review of the English literature identifies only eight cases of papillomatosis of the esophagus (3–10). The first reported case involved a patient who received treatment with laser evaporation and alpha-interferon, followed by bleomycin and etoposide phosphate (4). Unfortunately, the patient died after spread of the disease to the bronchi. The second case involved a patient who presented with upper gastrointestinal bleeding due to a duodenal ulcer, and was incidentally found to have a cluster of squamous papillomas in the mid esophagus (3). Ulcer healing was complete in this patient, and no further sequelae resulted. In situ hybridization in this case was positive for HPV types 6 and 11. Sandvik et al (5) reported papillomatosis in the upper two-thirds of the esophagus in a patient presenting with dysphagia and retrosternal pain. Although cells suggestive of koilocytes characteristically seen in HPV-infected squamous epithelium were identified in this patient, in situ hybridization against HPV types 6, 11, 16, 18, 31, 33 and 35 were negative. However, currently available testing for HPV tests only for the most common viral subtypes and a negative test does not rule out HPV infection by a less common subtype.
Early reports of squamous papillomas did not identify a risk or progression to malignancy. However, recent reports underscore the potential development of malignancy in association with squamous papillomas of the esophagus (6–10). One of these reports (6) described a 75-year-old woman with microcytic anemia found to have a polypoid mass at the gastroesophageal junction. Biopsies were consistent with a squamous papilloma and subsequent endoscopic removal was performed. Although it was a squamous papilloma, there was a focus of well-differentiated adenocarcinoma within the lesion. The authors concluded that the malignancy in this case have could have been attributed to a gastric cardia adenocarcinoma, particularly given the patient’s previous Billroth II gastrectomy for peptic ulcer disease. This report was the first to identify the malignant potential in papillomas previously considered benign. In a letter to the editor, Waluga et al (7) described a poorly differentiated SCC arising from the cervical portion of the esophagus in a patient with extensive papillomatosis. Narayani et al (8) reported the development of high-grade dysplasia in a verrucous-appearing esophagus, with earlier biopsies showing changes only consistent with squamous papillomatosis. Reynoso et al (9) presented a case of squamous carcinoma in situ within esophageal papillomatosis, diagnosed after esophagectomy. Biopsies obtained endoscopically did not demonstrate carcinoma. A recent case of esophageal papillomatosis associated with SCC (10) was palliated with photodynamic therapy and a self-expanding metal stent. These reports highlight the difficulty in identifying the malignant areas within these lesions on routine endoscopy and biopsy.
Our patient also demonstrated unusual lesions in follow-up. The SCC of the oral mucosa could have been a second primary squamous lesion, which may be due to a field defect in the squamous mucosa of the oropharyngeal tract. Considering the dysplasia noted in the tongue mucosa, this is considered the most probable origin. It could also represent a metastatic lesion, although this is an unusual site. One previous report (11) of a basaloid SCC of the esophagus metastatic to the gingiva is identified in the literature.
The gastric lesion was of more interest. Endoscopically, this was reported as a superficial lesion of the mucosa initially considered to be an area of intestinalization, which eventually proved to be an invasive SCC. Gastric malignancies occurring synchronously with esophageal SCC have been reported (12,13). However, in these reports, the gastric lesions were adenocarcinomas. Approximately 80 cases of primary SCC of the stomach have been reported; however, on extensive sectioning of the resected specimens, a glandular component is often found, indicating that these are often adenosquamous carcinomas (14–19). These SCCs of the stomach have been reported to occur following corrosive acid burns (18), cyclophosphamide therapy (19) and as a complication of syphilis (17).
While the present case may represent a second primary SCC, possibly due to a field defect involving both squamous and gastric mucosa, it is more probable that it was a secondary lesion from the esophageal primary lesion. Although esophageal SCCs most commonly metastasize to regional lymph nodes, they have been reported to metastasize to a variety of sites including skin (20), umbilicus (21) and the stomach. However, gastric metastases are generally present in lymph nodes or as intramural tumours (22–27), in which they appear to be submucosal masses. One report (28) notes three patients with intraepithelial spread of an esophageal SCC to the gastric mucosa. However, the histopathological criteria noted in this paper describes intraepithelial spread along the gastric mucosa of SCC, suggesting a continuity with the original lesion as opposed to the lesion in our patient, which was distant from the original site of the tumour. Hematological metastasis of esophageal SCCs to the gastric mucosa, identified by biopsy and autopsy sampling, has been reported (29). Metastasis of an esophageal malignancy to an ulcer in the fundus has been reported (30); in this case, it was suggested that the increased cell proliferation at the edge of the ulcer would serve as a good base for implantation of the malignant cells. SCC of the esophagus has also been reported to implant at the insertion site of a gastrostomy tube (31). From these previous reports, it is evident that malignant squamous cells have the ability to directly implant into tissues and grow into detectable metastases. In our patient, there were no reports of gastric ulcers and no gastric biopsies were taken before surgery. It is possible that stress erosions or ulcerations occurred before tumour resection – this allowed a viable fragment of SCC to implant in the distal stomach (ie, a ‘drop metastasis’). However, a metastasis spread by the hematological route must be considered to be a more likely source.
The present case report supports a growing body of literature indicating that squamous papillomas and papillomatosis of the esophagus are not always benign lesions. The finding of extensive papillomatosis and unremitting symptoms should prompt investigations into an underlying malignancy. The present case also emphasizes the difficulties that may occur in diagnosing a malignancy because of the bland nature of the cells and the presence of inflammation in the biopsy specimens that suggested reactive changes. The presence of inflammation is well-recognized by pathologists to be a confounding factor in the diagnosis of dysplastic lesions, although inflammation is more commonly a problem in colonic biopsies of inflammatory bowel disease. Inflammation causes reactive changes within the cells, which can be very similar to that of dysplasia. Treatments to reduce inflammation (if possible) followed by rebiopsy are often recommended. Extensive lesions with symptoms such as the one we have described may require surgical resection for concurrent diagnosis and treatment.
The patient also demonstrated an unusual pattern of tumour recurrence. The oral lesion was favoured to be a second primary lesion; however, the lesion in the pyloric channel was considered to be a metastatic lesion. While this may have occurred through hematogenous spread, it is possible that this was an implanted or drop metastasis. Clinicians should be aware of the malignant potential of these lesions; biopsy of areas of mucosal irregularity in the stomach is recommended.
REFERENCES
- 1.Orlowska J, Jarosz D, Gugulski A, Pachlewski J, Butruk E. Squamous cell papillomas of the esophagus: Report of 20 cases and literature review. Am J Gastroenterol. 1994;89:434–7. [PubMed] [Google Scholar]
- 2.Mosca S, Manes G, Monaco R, Bellomo PF, Bottino V, Balzano A. Squamous papilloma of the esophagus: Long-term follow up. J Gastroenterol Hepatol. 2001;16:857–61. doi: 10.1046/j.1440-1746.2001.02531.x. [DOI] [PubMed] [Google Scholar]
- 3.Politoske EJ. Squamous papilloma of the esophagus associated with the human papilloma virus. Gastroenterology. 1992;102:668–73. doi: 10.1016/0016-5085(92)90118-i. [DOI] [PubMed] [Google Scholar]
- 4.Hording M, Hording U, Daugaard S, Norrild B, Faber V. Human papilloma virus type 11 in a fatal case of esophageal and bronchial papillomatosis. Scand J Infect Dis. 1989;21:229–31. doi: 10.3109/00365548909039974. [DOI] [PubMed] [Google Scholar]
- 5.Sandvik AK, Aase S, Kveberg KH, Dalen A, Folvik M, Naess O. Papillomatosis of the esophagus. J Clin Gastroenterol. 1996;22:35–7. doi: 10.1097/00004836-199601000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Reed PA, Limauro DL, Brodmerkel GJ, Jr, Agrawal RM. Esophageal squamous papilloma associated with adenocarcinoma. Gastrointest Endosc. 1995;41:249–251. doi: 10.1016/s0016-5107(95)70349-7. [DOI] [PubMed] [Google Scholar]
- 7.Waluga M, Hartleb M, Sliwinski ZK, Romanczyk T, Wodolazski A. Esophageal squamous-cell papillomatosis complicated by carcinoma. Am J Gastroenterol. 2000;95:1592–3. doi: 10.1111/j.1572-0241.2000.02106.x. [DOI] [PubMed] [Google Scholar]
- 8.Narayani RI, Young GS. Recurrent proximal esophageal stricture associated with dysplasia in squamous cell papillomatosis. Gastrointest Endosc. 2002;56:591–4. doi: 10.1067/mge.2002.128110. [DOI] [PubMed] [Google Scholar]
- 9.Reynoso J, Davis RE, Daniels WW, Awad ZT, Gatalica Z, Filipi CJ. Esophageal papillomatosis complicated by squamous cell carcinoma in situ. Dis Esophagus. 2004;17:345–7. doi: 10.1111/j.1442-2050.2004.00438.x. [DOI] [PubMed] [Google Scholar]
- 10.Wolfsen HC, Hemminger LL, Geiger XJ, Krishna M, Woodward TA. Photodynamic therapy and endoscopic metal stent placement for esophageal papillomatosis associated with squamous cell carcinoma. Dis Esophagus. 2004;17:187–90. doi: 10.1111/j.1442-2050.2004.00400.x. [DOI] [PubMed] [Google Scholar]
- 11.Ide F, Shimoyama T, Haga H, Horie N. Basaloid squamous cell carcinoma of the esophagus metastatic to the gingiva: A case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:584–7. doi: 10.1016/s1079-2104(97)90124-4. [DOI] [PubMed] [Google Scholar]
- 12.Takemura M, Osugi H, Lee S, et al. A case of synchronous esophageal and gastric cancer successfully treated by combination TS-1/CDDP therapy with irradiation. Gan To Kagaku Ryoho. 2004;31:251–254. [PubMed] [Google Scholar]
- 13.Wakabayashi K, Hayashi S, Masuda H, et al. Remarkable response of simultaneous advanced esophageal and gastric cancer to combined chemotherapy with weekday-on/weekend-off TS-1 plus biweekly cisplatin. Gan To Kagaku Ryoho. 2003;30:1337–42. [PubMed] [Google Scholar]
- 14.Boswell JT, Helwig EB. Squamous cell carcinoma and adenoacanthoma of the stomach. A clinicopathologic study. Cancer. 1965;18:181–92. doi: 10.1002/1097-0142(196502)18:2<181::aid-cncr2820180209>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 15.Bonnheim DC, Sarac OK, Fett W. Primary squamous cell carcinoma of the stomach. Am J Gastroenterol. 1985;80:91–4. [PubMed] [Google Scholar]
- 16.Mori M, Iwashita A, Enjoji M. Squamous cell carcinoma of the stomach: Report of three cases. Am J Gastroenterol. 1986;81:339–42. [PubMed] [Google Scholar]
- 17.Vaughan WP, Straus FH, Paloyan D. Squamous carcinoma of the stomach after luetic linitis plastica. Gastroenterology. 1977;72:945–8. [PubMed] [Google Scholar]
- 18.Eaton H, Tennekoon GE. Squamous carcinoma of the stomach following corrosive acid burns. Br J Surg. 1972;59:382–7. doi: 10.1002/bjs.1800590514. [DOI] [PubMed] [Google Scholar]
- 19.McLoughlin GA, Cave-Bigley DJ, Tagore V, Kirkham N. Cyclophosphamide and pure squamous-cell carcinoma of the stomach. Br Med J. 1980;280:524–5. doi: 10.1136/bmj.280.6213.524-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schoenlaub P, Sarraux A, Grosshans E, Heid E, Cribier B. Survival after cutaneous metastasis: A study of 200 cases. Ann Dermatol Venereol. 2001;128:1310–15. [PubMed] [Google Scholar]
- 21.Dutta U, Kumar M, Sharma SC, Nagi B. Umbilical metastasis with squamous cell carcinoma of esophagus. Indian J Gastroenterol. 2004;23:156–7. [PubMed] [Google Scholar]
- 22.Saito T, Iizuka T, Kato H, Watanabe H. Esophageal carcinoma metastatic to the stomach. A clinicopathologic study of 35 cases. Cancer. 1985;56:2235–41. doi: 10.1002/1097-0142(19851101)56:9<2235::aid-cncr2820560917>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 23.Davis M, Gogel H, McIntire C, Newell JD. Esophageal carcinoma multiplex with gastric metastasis. Gastrointest Radiol. 1989;14:6–8. doi: 10.1007/BF01889145. [DOI] [PubMed] [Google Scholar]
- 24.Kato H, Tachimori Y, Watanabe H, et al. Intramural metastasis of thoracic esophageal carcinoma. Int J Cancer. 1992;50:49–52. doi: 10.1002/ijc.2910500111. [DOI] [PubMed] [Google Scholar]
- 25.Koide N, Yazawa K, Koike S, Adachi W, Amano J, Ishii K. Two cases of gastric involvement of esophageal cancer: Intramural metastasis and intramural lymph node metastasis. Hepatogastroenterology. 1998;45:1619–23. [PubMed] [Google Scholar]
- 26.Kishino T, Yamaguchi Y, Yamagishi T, et al. Submucosal tumor (SMT)-like esophageal squamous cell carcinoma with gastric metastasis. Hepatogastroenterology. 2000;47:1581–4. [PubMed] [Google Scholar]
- 27.Ebihara Y, Hosokawa M, Kondo S, Katoh H. Thirteen cases with intramural metastasis to the stomach in 1259 patients with oesophageal squamous cell carcinoma. Eur J Cardiothorac Surg. 2004;26:1223–5. doi: 10.1016/j.ejcts.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 28.Kuwano H, Baba K, Ikebe M, et al. Gastric involvement of oesophageal squamous cell carcinoma. Br J Surg. 1992;79:328–30. doi: 10.1002/bjs.1800790415. [DOI] [PubMed] [Google Scholar]
- 29.Green LK. Hematogenous metastases to the stomach. A review of 67 cases. Cancer. 1990;65:1596–1600. doi: 10.1002/1097-0142(19900401)65:7<1596::aid-cncr2820650724>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 30.Altorjay A, Gonda G, Ereifej S, Szanto I, Farsang Z, Kiss J. Metastasis of an esophageal carcinoma to a giant gastric ulcer. Hepatogastroenterology. 1999;46:981–2. [PubMed] [Google Scholar]
- 31.Deinzer M, Menges M, Walter K, et al. Implantation metastasis at the exit site after percutaneous endoscopic gastrostomy in esophageal carcinoma. Z Gastroenterol. 1999;37:789–93. [PubMed] [Google Scholar]