Abstract
Salmonella typhimurium is a facultative pathogen capable of entering and replicating in both professional and non-professional antigen presenting cells. Control of infection requires MHC class II restricted CD4 T-helper cell responses. Here we show that Salmonella infection induced polyubiquitination of HLA-DR, a post-translational modification that led to removal of mature, peptide loaded, αβ dimers from the cell surface. Immature αβIi complexes were unaffected. Surface expression of all class II isotypes, HLA-DP, -DQ, and -DR, was reduced in infected cells, but other cell-surface molecules that traffic through class II peptide loading compartments were unaffected. A Salmonella strain carrying a mutation in ssaV did not induce ubiquitination of class II, implicating Salmonella T3SS-2 effector proteins in the process. T3SS-2 effectors, with established or proposed roles in ubiquitination, were not required for class II down-regulation, suggesting that an additional T3SS-2 effector is involved in regulating MHC class II ubiquitination. Although recognized as a viral immune evasion strategy, here, we demonstrate that bacteria can control surface MHC expression through ubiquitination.
Salmonellae are Gram-negative bacteria that cause in a range of animal species. In humans, infection with the serovar Salmonella typhi results in approximately 21 million cases of typhoid fever annually and 200,000 deaths (1). Infection occurs by the oral route and requires virulence factors transported into the host cell by 2 Salmonella-encoded type III secretion systems (T3SS). T3SS-1 is encoded within Salmonella pathogenicity island-1 (SPI-1) and is required for the initial invasion of host cells, while T3SS-2, which is encoded within SPI-2, is required for survival and replication of bacteria within Salmonella-containing vacuoles (SCVs).
Infecting bacteria are generally cleared from the circulation but can survive and replicate in cells of the reticuloendothelial system. Clearance requires both CD4+ and CD8+ T cell-mediated responses, but CD4+ responses are of most importance. In mice lacking CD4+ T cells, early phases of infection are controlled but ultimately bacterial numbers increase, resulting in death (2). MHC genes have a role in controlling infection and particular haplotypes in humans are associated with either resistance or susceptibility to typhoid fever (3). Bone marrow-derived dendritic cells (DCs) infected with Salmonella show reduced ability to present antigen to T cells (4), providing further evidence that Salmonella interferes with MHC class II antigen presentation.
MHC class II molecules play an essential role in adaptive immune responses by presenting peptide antigens to CD4 restricted T cells (5). Class II α and β chains assemble in the endoplasmic reticulum (ER), together with invariant chain (Ii), to form nonomeric complexes (αβIi)3 (6). These complexes exit the ER and follow the secretory pathway to the trans-Golgi network (TGN). Dileucine based sorting signals, present in Ii, are responsible for targeting into endosomal-lysosomal compartments, either directly from the TGN, or indirectly via the cell surface (7–9). Experiments where clathrin or AP-2 are ablated suggest that a significant proportion of (αβIi)3 traffics via the plasma membrane (10). Once in the endosomal-lysosomal system, Ii is sequentially degraded leaving a short peptide fragment, CLIP, in the peptide-binding groove (11). HLA-DM and -DO, two class II related molecules, regulate the exchange of CLIP for antigenic peptide (12, 13). The complex nature of the class II antigen-processing pathways provides extensive opportunities for pathogen interference (14).
Post-translational regulation of surface class II expression is a key feature of the activation of DCs, the most potent professional antigen presenting cells (APCs) known. Upon sensing invading pathogens, DCs redirect their intracellular pool of MHC class II from late endosomal antigen processing compartments to the cell surface (15, 16). Synchronized relocation of the co-stimulatory molecule B7.2 ensures that the mature DCs exhibit full co-stimulatory capacity (17). Redistribution is controlled through regulated ubiquitination of class II (15, 16).
Salmonella interferes with MHC antigen presentation by specifically reducing cell surface HLA-DR expression (18). We set out to determine the molecular mechanism underlying this precise targeting of MHC class II molecules, which are crucial to CD4 T-cell responses.
Results
Infection by Salmonella Results in Loss of Surface Class II Expression, but Does Not Affect Other Surface Receptors.
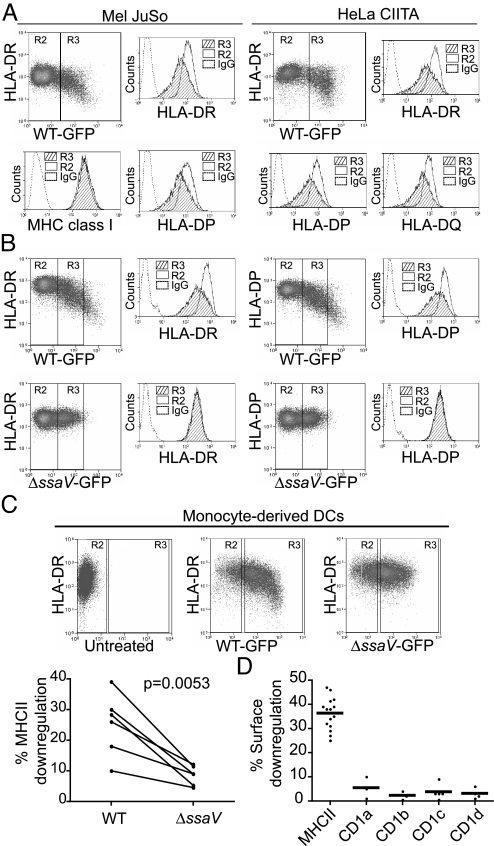
To examine the specificity of Salmonella induced DR down-regulation, we determined if all class II isotypes were influenced. Infection of Mel JuSo and HeLa-CIITA cells resulted in loss of HLA-DP, -DQ, and -DR surface expression (Fig. 1A). Salmonella specifically targeted MHC class II, as MHC class I levels remained unchanged. The Salmonella protein SsaV is an essential component of the SPI-2 secretion apparatus. Infection by ssaV mutant Salmonella did not result in loss of surface class II expression (Fig. 1B). We confirmed these observations in primary monocyte derived DCs (Fig. 1C). Exposure to Salmonella increased surface class II expression above that seen in the immature DCs, consistent with activation by LPS (Gate R2). Only in cells infected by WT Salmonella was this decreased (Gate R3). Surface DR expression was reduced upon infection with WT Salmonella, but not the ssaV-deficient strain in DCs obtained from 6 independent donors (Fig. 1C). The specificity for class II was further confirmed in cells expressing CD1a-d. Upon infection, no alteration in CD1a-d surface expression was observed (Fig. 1D). As CD1a-d traffic extensively through class II containing endocytic compartments, a general disturbance in endocytic trafficking due to Salmonella infection appeared an unlikely explanation for the infection-induced loss of surface class II. We conclude that Salmonella infection reduced surface class II expression in primary human DCs and cells lines, by a mechanism that required the function of the SPI-2 T3SS.
Fig. 1.
Salmonella infection results in selective loss of surface MHC class II expression. (A) Mel JuSo and HeLa-CIITA cells were infected with Salmonella-GFP and HLA-DP, -DQ, and -DR expression was examined in GFP positive (infected, R3) and GFP negative (uninfected, R2) cells. Panels show representative FACS dot plots of HLA-DR against GFP expression, in infected Mel JuSo and HeLa-CIITA cells. Histograms show surface DR, DP, DQ, and MHC class I expression in infected (hatched) and uninfected (clear) cells. Isotype control is represented by a dashed line. Data are representative of more than 3 independent experiments. (B) Mel JuSo were infected with WT and ssaV Salmonella-GFP strains and DP and DR examined in infected and uninfected cells. Panels show representative FACS dot plots of HLA-DR and HLA-DP expression in cells infected with similar numbers of bacteria (R3). Histograms show surface DR and DP expression in infected (hatched) and uninfected (clear) cells. Isotype control is represented by the dashed line. Data are representative of more than 3 independent experiments. (C) Immature DCs were left uninfected or infected with WT or ssaV strains for 30 min and the entire population of cells processed for FACS analysis 16–20 h post infection. DR is only reduced by infection with WT Salmonella (upper panels). Comparison of DR down-regulation in DCs derived from 6 independent donors infected with WT or ssaV strains is shown in the lower panel. (D). Mel JuSo cells, stably expressing either CD1a, CD1b, CD1c, or CD1d, were infected with WT Salmonella. Surface expression of each CD1 isotype, together with HLA-DR, was measured by FACS. Dots show the percentage surface down-regulation of both HLA-DR and CD1. Error bars, means.
MHC Class II Is Removed from the Surface of Salmonella-Infected Cells.
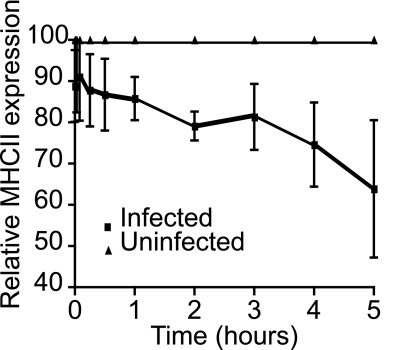
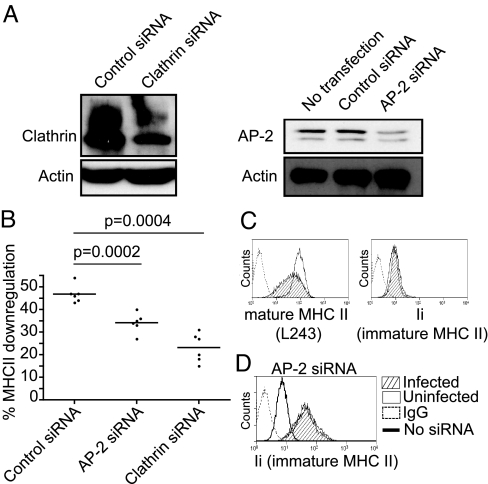
To determine if class II was removed from the surface of infected cells, cell surface DR molecules were labeled with L243 and allowed to internalise for various times. L243 remaining at the surface was then quantified by FACS. Cells infected with Salmonella showed enhanced surface disappearance of DR compared to uninfected cells (Fig. 2). This implied that Salmonella influenced the internalisation and or recycling of mature class II from the cell surface. We used siRNA interference to determine if this process was AP-2 or clathrin dependent. Upon infection we observed less down-regulation of class II in AP2- and clathrin-depleted cells (Fig. 3B). This implied that Salmonella-induced loss of mature peptide loaded MHC class II from the cell surface was both clathrin- and AP2-dependent.
Fig. 2.
Salmonella infection induces loss of MHC class II from the cell surface. Mel JuSo cells were infected with Salmonella and, 15 h post-infection, surface HLA-DR was labeled with L243. At various times post-labeling MHC class II remaining at the cell surface was detected with PE-conjugated rabbit anti-mouse antibody and measured by FACS. The figure shows mean fluorescence levels of HLA-DR remaining at the surface in infected cells, plotted as a % of the level in uninfected cells, which was set at 100%. Means are derived form 4 independent experiments.
Fig. 3.
Salmonella induced internalisation of mature DRαβ, but not immature αβIi complexes, is clathrin- and AP2-dependent. (A) Mel JuSo cells were transfected with clathrin, AP-2, or control siRNA oligonucleotides as described in Materials and Methods. Western blot analysis of cell lysates prepared after day 5 of treatment shows the level of clathrin and AP-2 depletion. (B) Analysis of Salmonella induced surface HLA-DR down-regulation in cells depleted for AP-2 or clathrin. Cells depleted for AP-2 or clathrin were infected with Salmonella at day 5 of depletion and surface DR was assessed 16–20 h post-infection as in Fig. 2. Dots represent percentage down regulation of surface DR. Error bars, means. P values, P = 0.0002 and P = 0.0004. (C) Histograms show levels of mature (L243) and immature (Ii) surface class II in infected (hatched) and uninfected (open) Mel JuSo cells. L243 recognizes only mature DRαβ dimers and Ii is used to monitor immature αβIi surface expression. (D) Levels of immature surface αβIi complexes in Mel JuSo cells were increased by partial depletion of AP-2 by siRNA treatment. No loss of αβIi from the cell surface was evident upon infection by Salmonella.
L243 recognizes only mature DRαβ dimers (19). To determine if immature class II was also targeted we used Ii to follow αβ/Ii complexes. This is possible as all surface Ii is present in αβ/Ii complexes (20). No reduction in Ii was observed in infected cells (Fig. 3C). To confirm this in cells expressing higher levels of αβ/Ii, we used partial depletion of the adaptor protein AP-2 to increase expression of αβ/Ii complexes at the cell surface (10, 21). We titrated the level of AP-2 RNAi oligonucleotides such that the level of αβ/Ii was increased, but mature class II was still down-regulated upon infection. Partial depletion of the adaptor protein AP-2 increased αβ/Ii surface expression by more than an order of magnitude and this was not altered by infection (Fig. 3D). Therefore Salmonella specifically targeted mature and not immature class II αβ/Ii complexes.
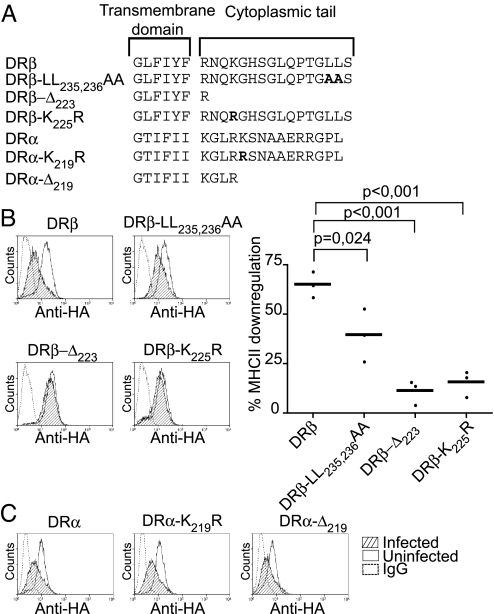
The Cytoplasmic Tail of DRβ Is Required for Salmonella-Induced Down-Regulation.
Class II molecules contain a number of motifs in their cytoplasmic tails that regulate surface expression and endocytosis. Using WT and mutated DRα and β chains, we investigated which features of MHC class II were important for Salmonella-induced down-regulation. HA-tagged DRα and DRβ constructs were both detected at the plasma membrane of stably transfected Mel JuSo cells and both were down-regulated upon infection with Salmonella (Fig. 4). Substitution of Lys219, a known target for ubiquitination of DRα (22), or deletion of the entire DRα cytoplasmic tail, had no influence upon down-regulation. Inactivation of the DRβ encoded di-leucine sorting signal (DRB-LL235,236AA) partially prevented Salmonella-induced class II suppression (Fig. 4B). More strikingly, DRβ constructs lacking their cytoplasmic tail (DRB-Δ233), or with a single lysine to arginine mutation (DRB-K225R), were largely resistant to the Salmonella-induced effect (Fig. 4B). This suggested that Lys225, a known substrate for ubiquitination by MARCH I and MARCH VIII E3 ligases (23, 24), was targeted for ubiquitination by Salmonella.
Fig. 4.
Salmonella-induced class II down-regulation requires residues in the cytoplasmic tail of the DRβ chain. (A) Amino acid sequence of the carboxyl terminal region of the HA-DRα and HA-DRβ constructs used to generate stable Mel JuSo transfectants. Substituted residues are shown in bold. (B) Left panels show representative FACS histogram profiles of surface HA expression in infected and uninfected HA-DRβ transfectants and the percentage down-regulation is shown on the far right. Dots show results from independent experiments. P values, P = 0.024, P < 0.001. (C) FACS profiles show surface HA expression of tagged DRα constructs in infected and uninfected Mel JuSo cells. Histograms show levels of surface HA-DR expression in infected (hatched) and uninfected (open) Mel JuSo cells.
Salmonella Infection Induces T3SS-Dependent Ubiquitination of HLA-DR.
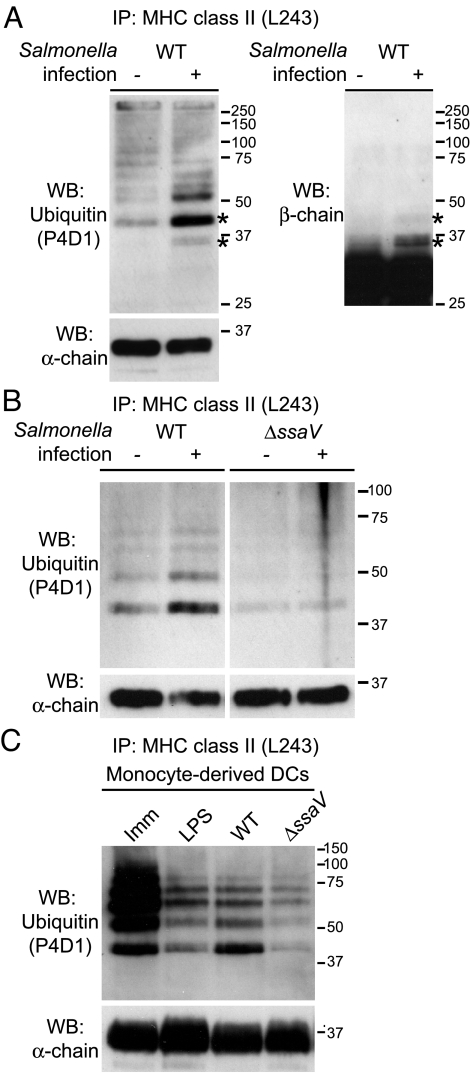
Regulated ubiquitination of HLA-DRβ has recently been shown to control surface MHC class II expression in maturing DCs (15, 16, 23, 25). We examined if Salmonella influenced levels of HLA-DR ubiquitination. Mel JuSo cells, exposed to GFP-expressing Salmonella, were sorted into infected and uninfected populations. HLA-DR was immunoprecipitated from cell lysates and associated ubiquitin detected by western blot with the anti-ubiquitin antibody P4D1. Elevated levels of ubiquitin were associated with HLA-DR immunoprecipitated from infected compared to uninfected cells (Fig. 5A). By densitometry, this was calculated as a 3- to 4-fold increase. The profile of bands was similar in both cell populations and was consistent with the addition of up to 6 ubiquitin molecules. There was no reduction in the total level of HLA-DR in infected cells, as detected by probing with TAL1B5, a DRα specific reagent. This suggested that ubiquitination influenced the intracellular distribution of class II but not its degradation. This is in agreement with our previous observations, demonstrating similar levels of MHC class II in infected compared to uninfected cells (18). Constitutive ubiquitination of DR is observed in cells before exposure to Salmonella and the profile of ubiquitinated products is similar to that induced by expression of transfected MARCH I (Fig. S1).
Fig. 5.
Salmonella infection results in enhanced ubiquitination of HLA-DR. (A and B) Mel JuSo cells were infected with WT or ssaV mutant Salmonella and FACS sorted into infected and uninfected cell populations. Immunoprecipitated HLA-DR was subject to western blot analysis with anti-ubiquitin (P4D1), anti-DRα (TAL1B5), and anti-DRβ LC2.1 antibodies. (A) Left panel shows HLA-DR immunoprecipitates probed with P4D1. Bottom panel shows similar loading of HLA-DR by detection with TAL1B5. Right panel shows that several bands corresponding to ubiquitinated HLA-DR are also detected with the anti-DRβ chain antibody, LC2.1 (*). (B) HLA-DR was immunoprecipitated from Mel JuSo cells that were infected or uninfected with WT (left) or ssaV mutant Salmonella (right). Immunoprecipitates were subject to western blot analysis with the anti-ubiquitin antibody P4D1. Total DRα is monitored with TAL1B5 (bottom panel). (C) Immature monocyte-derived DCs were left untreated (Imm), treated with Salmonella-LPS (100 ng/mL Sigma) for 16–20 h (LPS), or infected with WT (WT) or mutant Salmonella (ΔssaV) for 16–20 h. Salmonella infection rates were >70% as measured by GFP FACS analysis. HLA-DR was immunoprecipated with L243 from 1 × 106 cells and after western blot transfer probed with P4D1 (upper panel). Levels of DRα were monitored with TAL1B5 as a loading control (lower panel).
As both DRα and DRβ are subject to ubiquitination by MARCH E3 ligases (22), we investigated if Salmonella-induced ubiquitination was associated with one or both chains. In immunoprecipitates from infected cells, several weak bands, migrating above the position of unmodified DRβ, were detected with the DRβ specific reagent, LC2.1 (Fig. 5A, *). The predominant modification corresponded to the addition of a single ubiquitin molecule. These bands co-migrated with ones previously detected using the anti-ubiquitin reagent P4D1 (Fig. 5A, *). As expected, only a minor proportion of the total DRβ was modified by ubiquitination at steady state. The anti-ubiquitin reagent clearly detected a ladder of polyubiquitinated products, and the predominant signal corresponded to the addition of 2 ubiquitin molecules (Fig. 5A, *). This apparent contradiction is most likely explained by the fact that recognition by P4D1 is sensitive to both polyubiquitination and the nature of the particular linkages involved. We were unable to detect any DRα associated bands migrating above the position of the unmodified protein in Salmonella-infected cells. This is consistent with the mutational analysis of the DRβ cytoplasmic tail. RT-PCR analysis showed that the enhanced ubiquitination of DR was not due to up-regulation of MARCH I or MARCH VIII gene expression (SI Materials and Methods and Fig. S1 B and C).
We also investigated how Salmonella influenced ubiquitination of class II in professional APCs. HLA-DR isolated from immature monocyte-derived DCs was heavily ubiquitinated (Fig. 5C). Upon stimulation with Salmonella LPS, ubiquitination was severely reduced, as expected (Fig. 5C). Exposure to Salmonella resulted in reduced ubiquitination, but importantly there was more ubiquitination of DR in cells infected by WT Salmonella than the ssaV mutant. LPS has profound effects on DC maturation, by comparing cells infected by mutant versus WT Salmonella we are able to separate LPS induced effects from those associated with SPI-2 effectors. Therefore in primary professional APCs Salmonella induced the ubiquitination of HLA-DR and this required a functional SPI-2 T3SS (Fig. 5 B and C).
Surface Expression of B7.2 and CD8-DR Reporter Molecules Are Not Influenced by Salmonella Infection.
DCs regulate class II surface expression through maturation-dependent ubiquitination of DRβ, a process controlled by MARCH-I down-regulation (25). As MARCH E3 ligases also regulate surface expression of B7.2 (17), we examined whether Salmonella additionally regulated B7.2 surface expression. Mel JuSo cells stably expressing B7.1 or B7.2 were transfected with MARCH I-GFP or MARCH VIII-GFP and levels of class II and B7 determined. Transfection with MARCH I or MARCH VIII reduced class II (73–87%) and B7.2 (65–75%) and did not influence B7.1 (0–8%) expression. Infection by Salmonella led to class II down-regulation (39%), but levels of B7.1 (4%) and B7.2 (2%) were unaltered (Fig. S2A).
CD8 reporter constructs comprising the extracellular domain of CD8α and the transmembrane and cytoplasmic tail of DRβ (CD8-DRβ) or DRα (CD8-DRα) are ubiquitinated by both MARCH I and MARCH VIII (22). We investigated if Salmonella influenced ubiquitination of these reporter molecules. Mel JuSo cells stably expressing CD8-DRβ were transfected with the E3 ligase MARCH VIII (c-MIR), and surface levels of CD8 and class II monitored by FACS. CD8-DRβ was subject to regulation by MARCH VIII and this required DRβ Lys225 (Fig. S2B). Upon infection DR expression was reduced, but there was no change in the expression of CD8-DRβ.
Discussion
We previously reported that Salmonella infection led to a post-translational reduction in cell surface MHC class II expression (18). This was not due to alteration in class II biosynthesis, maturation or degradation in infected cells. Steady-state levels of total class II and mature SDS-stable peptide loaded class II were also unaltered in infected cells. By demonstrating elevated polyubiquitination of MHC class II in Salmonella-infected DCs, the current data establish a mechanism for this down-regulation. In DCs, regulated ubiquitination of MHC class II is the central mechanism controlling maturation-dependent surface class II expression (15, 16). Our observations provide a mechanism by which Salmonella can influence the initiation of the adaptive immune response. By enhancing ubiquitination of MHC class II, Salmonella may reduce the ability of cells to present antigen to CD4 restricted T cells, the most important T-cell subset required for resolution of infection (2). This is consistent with recent studies showing that antigen presentation by DCs is inhibited upon infection with Salmonella (4).
ssaV mutant Salmonella failed to induce ubiquitination of class II, implicating the SPI-2 translocation system, and presumably SPI-2 effector proteins, in the process. A number of different explanations could account for these observations:
The simplest explanation is that Salmonella encodes a SPI-2 effector protein that directly ubiquitinates MHC class II. Salmonella encodes a number of effectors with proposed roles in ubiquitination including SopA (26), sseL (27), SlrP, Ssph1, and Ssph2 (28). Infection studies using Salmonella strains deficient in these effectors, together with an Ssph1/Ssph2 double mutant, failed to identify potential candidates. As few effector proteins are encoded within SPI-2, we also examined the entire Salmonella genome for proteins related to mammalian components of the ubiquitination system, but found no obvious candidates. This is not entirely surprising given the evolutionary distance between mammalian and bacterial genomes, and does not preclude their existence. For example SopA has no obvious sequence similarity with mammalian proteins, but is a HECT-type E3 ligase (26).
A second possibility is that the bacterial effectors act by enhancing the activity of a host cell E3 ligase. Although this is an attractive mechanism it is unlikely to involve a simple up-regulation of MARCH I or MARCH VIII activity. Were this the case then co-regulated receptors such as B7.2 and the CD8-DR reporter molecules would also be influenced.
A third explanation is that Salmonella orchestrates a mechanism that influences MHC class II ubiquitination indirectly. Cycles of ubiquitination and deubiquitination are proposed to regulate class II endocytosis at the plasma membrane and sorting at the MVB (16). A simple mechanism could involve a bacterial effector that specifically reduced deubiquitination of class II. Although bacterial deubiquitinases, such as YopJ/P (29), SseL (27), and ChlaDub1 (30), have been identified, factors that regulate deubiquitination activity remain to be described.
More elaborate possibilities involve re-localisation of class II, or ubiquitin enzymes, to facilitate their interaction. MARCH I and VIII co-localize with class II in Lamp 1-positive compartments (22). Salmonella interacts extensively with the endocytic pathway, causing redistribution and aggregation of late endocytic, Lamp 1-positive compartments. It is possible that differences in ubiquitination could result from this redistribution.
Salmonella targets mature and not immature class II complexes and infection induces enhanced ubiquitination and removal of mature class II from the cell surface, consistent with ubiquitination occurring at the plasma membrane or in the early endocytic pathway. This is compatible with the normal maturation-dependent down-regulation of class II molecules in DCs, which involves ubiquitination at the same cellular locations and targets mature and not immature complexes (16, 25). We observed that Salmonella-induced down-regulation was both clathrin- and AP-2-dependent. However, internalization of mature αβ dimers is reported to be clathrin, AP-2 and dynamin-independent (31). One possibility to explain this difference is that Salmonella diverts class II from a clathrin-independent to a clathrin-dependent pathway. Alternatively, AP-2 and clathrin could be utilised by Salmonella for other processes and the effect of their depletion on class II could be indirect.
Any explanation of our findings must fit with the intriguing observation that CD8-DRβ is efficiently regulated by both MARCH I and MARCH VIII but is not influenced by Salmonella, even though the molecule contains both the ubiquitination and dileucine signals. Information in addition to that contained in the transmembrane and cytoplasmic tail of DRβ must therefore be required. Potentially, this could be supplied through association with DRα. A role for both α and β chain cytoplasmic tails in targeting class II is not without precedent. Pinet et al. described an antigen presentation pathway involving internalisation of cell surface class II (32). This was distinct from the conventional pathway and required signals jointly contributed by the cytoplasmic tails of DRα and DRβ. HLA-DRα is subject to polyubiquitination, a modification that could provide a signal (22). Against this, CD8-DRα is not itself regulated by Salmonella and DRβ/DRA-Δ219 complexes are still subject to down-regulation. It will be important to determine which extracellular, transmembrane, and cytoplasmic tail regions of DRα and DRβ are required.
Together these data identify 2 endocytic signals in the cytoplasmic tail of DRβ that influence Salmonella-induced class II regulation. Ubiquitination of Lys225, is the major, essential determinant, consistent with the presence of this conserved motif in HLA-DP and HLA-DQ β chains, which were also down-regulated by Salmonella. This may work in concert with, or independently from, the dileucine motif, a signal known to be involved in endocytosis of mature class II (33). As ubiquitination functions as a signal for endocytosis, ubiquitination of Lys225 by Salmonella provides a viable explanation for the reduction in surface class II expression seen upon infection.
It is clear that bacteria possess the ability to sensitize cellular proteins for ubiquitination (34), and modulate the innate immune response to infection (35). Here we provide a mechanism by which the pathogen can influence the initiation of adaptive immune responses.
Materials and Methods
Cell Culture and Antibodies.
Mel JuSo and HeLa-CIITA cells were maintained in DMEM, 10% FCS. Transfections were performed using Effectene (Qiagen), according to manufacturer's instructions. Stable transfectants were selected in the presence of the appropriate antibiotic (1 mg/mL geneticin, 100 μg/mL Zeocin) and positive cells sorted using a MoFlo flow cytometer (Cytomation). DCs were derived from peripheral blood mononuclear cells (PBMCs) isolated from Buffy Coats (British National Transfusion Service) using Lymphoprep (Axis-Shield). Autologous serum was collected, filtered, and heat-inactivated. Monocytes were isolated using a Negative Isolation Kit (Dynal) and differentiated into DCs by culturing for 7 to 9 days in RPMI, 1% autologous serum, 50 ng/mL GM-CSF, and 50 ng/mL IL-4 (Peprotech). DCs purity was 85% to 98% (CD14-, CD11c+, DC-Sign+). All antibodies used in this study are listed in Table S1.
Plasmid Constructs.
B7.1, B7.2, CD1a, CD1b, CD1c, and CD1d constructs were generated by RT-PCR of mRNA isolated from purified human monocyte and macrophages and cloned into pCDNA3.1/Zeo. The HA–tagged HA-DRB, HA-DRA, CD8-DRB, and CD8-DRB-K225R constructs have been described (22). All PCR was performed with KOD HiFi DNA Polymerase according to manufacturer's instructions (Calbiochem), and constructs were subject to DNA sequence analysis to verify authenticity. Human MARCH VIII (cMIR) was a gift from Professor Satoshi Ishido.
Bacterial Strains, Infection, and FACS.
Plasmid pFVP25.1, carrying GFP, was introduced into WT 12023 (ATCC) and its isogenic ssaV mutant (18). Bacteria were grown in LB broth at 37 °C, supplemented with ampicillin or kanamycin (50 μg/mL). Mel JuSo and HeLa-CIITA cells were infected with log-phase bacteria for 20 min at a MOI of 50:1. DCs were infected with stationary-phase Salmonella in RPMI containing 10% autologous serum for 30 min at a MOI approximately 50:1. Cells were washed and cultured in fresh medium containing gentamicin (50 μg/mL) for 1 h. The antibiotic concentration was then reduced to 5 μg/mL until the cells were processed 16–20 h post infection. Immature DCs were processed using exactly the same protocol except for the addition of Salmonella. Maturation of DCs was routinely monitored by CD80 and MHC class II expression (Fig. 1C). In all cases similar levels of activation were observed in cells exposed to WT compared to ssaV-mutant Salmonella.
For FACS analysis cells were detached using Cell Dissociation Buffer (Sigma) and incubated for 30 min on ice, with appropriate antibodies, in FACS buffer (5% FCS, 2 mM EDTA in PBS). After washing, cells were incubated with RPE anti-mouse (Dako), fixed in 2% formaldehyde and analyzed using a FACScan™ Flow Cytometer and Summit software (BD Biosciences). FACS analysis was performed on cells gated for similar levels of GFP expression. In all cases % down-regulation was calculated as mean fluorescence of infected cells (Gate R3)/mean fluorescence of uninfected cells (Gate R2) × 100. Graphic representation and statistical analysis were performed using Graphpad Prism Software. Comparison of distributions was performed using a paired test with 95% confidence intervals with P ≤ 0.05 considered significant. Infected and uninfected cell populations were isolated by FACS sorting GFP+ve and GFP−ve cells, 16–20 h postinfection, using a MoFlo cell sorter.
Immunoprecipitation and Western Blot.
Cells were harvested using Cell Dissociation Buffer (Sigma) and lysed in lysis buffer [PBS, 1% Nonidet P-40, 50 mM Tris (pH 7.5), 5 mM EDTA, 150 mM NaCl, protease inhibitors mixture (Roche Diagnostics), and 5 mM iodoacetamide] for 30 min at 4 °C. Immunoprecipitations were performed with Sepharose-coupled L243 as described (22). Lysates were extensively washed and incubated for 5 min at 95 °C in SDS/PAGE loading buffer before standard SDS/PAGE and transfer to Hybond™ ECL™ membrane (Amersham). Each track corresponds to lysate from approximately 1 × 106 cells. Membranes were blocked in PBS, 5% skim milk, and 0.1% Tween-20 (Sigma) overnight at 4 °C before probing. Detection was performed using ECL Plus™ Western Blotting Detection Reagents (Amersham).
Internalization Assay.
Mel JuSo cells were infected with Salmonella-GFP. Fifteen h postinfection cells were washed and resuspended in cold DMEM. Surface HLA-DR was labeled with L243 for 30 min at 4 °C. Cells were washed in ice cold medium, resuspended in culture medium and returned to 37 °C. At various time points aliquots were removed, resuspended in ice cold FACS buffer containing phycoerythrin-conjugated rabbit anti-mouse Ig. After 30 min incubation cells were washed to remove free secondary antibody, fixed in 2% formaldehyde, washed and analyzed by FACS to quantify HLA-DR-L243 complexes remaining at the cell surface. GFP-negative cells were considered uninfected.
siRNA Regulation.
Mel JuSo cells were seeded into 6-well plates. On the following day (day 1) cells were transfected with the oligonucleotides using Oligofectamine (Invitrogen), according to manufacturer's instructions (Invitrogen). On day 2 or 3, cells were reseeded into 9-cm dishes 8 h before a second transfection with the same oligonucleotide. Cells were harvested and analyzed on day 5. The siRNA target sequences for AP-2, clathrin heavy chain, and the control siRNA were AAGUGGAUGCCUUUCGGGUCA, AAUCCAAUUCGAAGACCAAUU, and AAACTTGTCGACGAGAAGCAA, respectively.
Supplementary Material
Acknowledgments.
We thank Dr. M. Espeli and Dr. R. Apps for helpful discussion, Prof. S. Ishido (Yokohama, Japan) for providing the c-MIR/MARCH VIII, Prof. J. Kaufmann (Cambridge, U.K.) for providing LC2.1, Prof. P. Lehner for MARCH I-GFP, and N. Miller for FACS sorting. This work was supported by the Wellcome Trust and the MRC. N.L. was supported by a Marie-Curie Research Training Network, MICROBAN contract number: MRTN-CT-2003-504227 with part funding from the National Institute for Health Research Cambridge Biomedical research Centre. S.M. was supported by the Fondation pour la Recherche Médicale (Equipe FRM) and by the ANR (Programme M.I.E. Salmo-sensor).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906735106/DCSupplemental.
References
- 1.Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ. 2004;82:346–353. [PMC free article] [PubMed] [Google Scholar]
- 2.Hess J, Ladel C, Miko D, Kaufmann SH. Salmonella typhimurium aroA- infection in gene-targeted immunodeficient mice: major role of CD4+ TCR-alpha beta cells and IFN-gamma in bacterial clearance independent of intracellular location. J Immunol. 1996;156:3321–3326. [PubMed] [Google Scholar]
- 3.Dunstan SJ, et al. Genes of the class II and class III major histocompatibility complex are associated with typhoid fever in Vietnam. J Infect Dis. 2001;183:261–268. doi: 10.1086/317940. [DOI] [PubMed] [Google Scholar]
- 4.Cheminay C, Mohlenbrink A, Hensel M. Intracellular Salmonella inhibit antigen presentation by dendritic cells. J Immunol. 2005;174:2892–2899. doi: 10.4049/jimmunol.174.5.2892. [DOI] [PubMed] [Google Scholar]
- 5.Bryant PW, Lennon-Dumenil AM, Fiebiger E, Lagaudriere-Gesbert C, Ploegh HL. Proteolysis and antigen presentation by MHC class II molecules. Adv Immunol. 2002;80:71–114. doi: 10.1016/S0065-2776(02)80013-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cresswell P. Assembly, transport, and function of MHC class II molecules. Annu Rev Immunol. 1994;12:259–293. doi: 10.1146/annurev.iy.12.040194.001355. [DOI] [PubMed] [Google Scholar]
- 7.Bakke O, Dobberstein B. MHC class II-associated invariant chain contains a sorting signal for endosomal compartments. Cell. 1990;63:707–716. doi: 10.1016/0092-8674(90)90137-4. [DOI] [PubMed] [Google Scholar]
- 8.Watts C. The exogenous pathway for antigen presentation on major histocompatibility complex class II and CD1 molecules. Nat Immunol. 2004;5:685–692. doi: 10.1038/ni1088. [DOI] [PubMed] [Google Scholar]
- 9.Hiltbold EM, Roche PA. Trafficking of MHC class II molecules in the late secretory pathway. Curr Opin Immunol. 2002;14:30–35. doi: 10.1016/s0952-7915(01)00295-3. [DOI] [PubMed] [Google Scholar]
- 10.McCormick PJ, Martina JA, Bonifacino JS. Involvement of clathrin and AP-2 in the trafficking of MHC class II molecules to antigen-processing compartments. Proc Natl Acad Sci USA. 2005;102:7910–7915. doi: 10.1073/pnas.0502206102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cresswell P. Chemistry and functional role of the invariant chain. Curr Opin Immunol. 1992;4:87–92. doi: 10.1016/0952-7915(92)90131-w. [DOI] [PubMed] [Google Scholar]
- 12.Denzin LK, Cresswell P. HLA-DM induces CLIP dissociation from MHC class II alpha beta dimers and facilitates peptide loading. Cell. 1995;82:155–165. doi: 10.1016/0092-8674(95)90061-6. [DOI] [PubMed] [Google Scholar]
- 13.Denzin LK, Sant'Angelo DB, Hammond C, Surman MJ, Cresswell P. Negative regulation by HLA-DO of MHC class II-restricted antigen processing. Science. 1997;278:106–109. doi: 10.1126/science.278.5335.106. [DOI] [PubMed] [Google Scholar]
- 14.Brodsky FM, Lem L, Solache A, Bennett EM. Human pathogen subversion of antigen presentation. Immunol Rev. 1999;168:199–215. doi: 10.1111/j.1600-065x.1999.tb01294.x. [DOI] [PubMed] [Google Scholar]
- 15.Shin JS, Ebersold M, Pypaert M, Delamarre L, Hartley A, Mellman I. Surface expression of MHC class II in dendritic cells is controlled by regulated ubiquitination. Nature. 2006;444:115–118. doi: 10.1038/nature05261. [DOI] [PubMed] [Google Scholar]
- 16.van Niel G, et al. Dendritic cells regulate exposure of MHC class II at their plasma membrane by oligoubiquitination. Immunity. 2006;25:885–894. doi: 10.1016/j.immuni.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Goto E, et al. c-MIR, a human E3 ubiquitin ligase, is a functional homolog of herpesvirus proteins MIR1 and MIR2 and has similar activity. J Biol Chem. 2003;278:14657–14668. doi: 10.1074/jbc.M211285200. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell EK, Mastroeni P, Kelly AP, Trowsdale J. Inhibition of cell surface MHC class II expression by Salmonella. Eur J Immunol. 2004;34:2559–2567. doi: 10.1002/eji.200425314. [DOI] [PubMed] [Google Scholar]
- 19.Roche PA, Cresswell P. Proteolysis of the class II-associated invariant chain generates a peptide binding site in intracellular HLA-DR molecules. Proc Natl Acad Sci USA. 1991;88:3150–3154. doi: 10.1073/pnas.88.8.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stumptner-Cuvelette P, et al. HIV-1 Nef impairs MHC class II antigen presentation and surface expression. Proc Natl Acad Sci USA. 2001;98:12144–12149. doi: 10.1073/pnas.221256498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dugast M, Toussaint H, Dousset C, Benaroch P. AP2 clathrin adaptor complex, but not AP1, controls the access of the major histocompatibility complex (MHC) class II to endosomes. J Biol Chem. 2005;280:19656–19664. doi: 10.1074/jbc.M501357200. [DOI] [PubMed] [Google Scholar]
- 22.Lapaque N, Jahnke M, Trowsdale J, Kelly AP. The HLA-DRalpha chain is modified by poly-ubiquitination. J Biol Chem. 2009;284:7007–7016. doi: 10.1074/jbc.M805736200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohmura-Hoshino M, et al. Inhibition of MHC class II expression and immune responses by c-MIR. J Immunol. 2006;177:341–354. doi: 10.4049/jimmunol.177.1.341. [DOI] [PubMed] [Google Scholar]
- 24.Matsuki Y, et al. Novel regulation of MHC class II function in B cells. EMBO J. 2007;26:846–854. doi: 10.1038/sj.emboj.7601556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Gassart A, et al. MHC class II stabilization at the surface of human dendritic cells is the result of maturation-dependent MARCH I down-regulation. Proc Natl Acad Sci USA. 2008;105:3491–3496. doi: 10.1073/pnas.0708874105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Higashide WM, McCormick BA, Chen J, Zhou D. The inflammation-associated Salmonella SopA is a HECT-like E3 ubiquitin ligase. Mol Microbiol. 2006;62:786–793. doi: 10.1111/j.1365-2958.2006.05407.x. [DOI] [PubMed] [Google Scholar]
- 27.Rytkonen A, et al. SseL, a Salmonella deubiquitinase required for macrophage killing and virulence. Proc Natl Acad Sci USA. 2007;104:3502–7. doi: 10.1073/pnas.0610095104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quezada CM, Hicks SW, Galan JE, Stebbins CE. A family of Salmonella virulence factors functions as a distinct class of autoregulated E3 ubiquitin ligases. Proc Natl Acad Sci USA. 2009;106:4864–4869. doi: 10.1073/pnas.0811058106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou H, et al. Yersinia virulence factor YopJ acts as a deubiquitinase to inhibit NF-kappa B activation. J Exp Med. 2005;202:1327–1332. doi: 10.1084/jem.20051194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Misaghi S, Balsara ZR, Catic A, Spooner E, Ploegh HL, Starnbach MN. Chlamydia trachomatis-derived deubiquitinating enzymes in mammalian cells during infection. Mol Microbiol. 2006;61:142–150. doi: 10.1111/j.1365-2958.2006.05199.x. [DOI] [PubMed] [Google Scholar]
- 31.Walseng E, Bakke O, Roche PA. Major histocompatibility complex class II-peptide complexes internalize using a clathrin- and dynamin-independent endocytosis pathway. J Biol Chem. 2008;283:14717–14727. doi: 10.1074/jbc.M801070200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinet V, Vergelli M, Martin R, Bakke O, Long EO. Antigen presentation mediated by recycling of surface HLA-DR molecules. Nature. 1995;375:603–606. doi: 10.1038/375603a0. [DOI] [PubMed] [Google Scholar]
- 33.Zhong G, Romagnoli P, Germain RN. Related leucine-based cytoplasmic targeting signals in invariant chain and major histocompatibility complex class II molecules control endocytic presentation of distinct determinants in a single protein. J Exp Med. 1997;185:429–438. doi: 10.1084/jem.185.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boyer L, Lemichez E. Targeting of host-cell ubiquitin and ubiquitin-like pathways by bacterial factors. Nat Rev Microbiol. 2004;2:779–788. doi: 10.1038/nrmicro1005. [DOI] [PubMed] [Google Scholar]
- 35.Neish AS, et al. Prokaryotic regulation of epithelial responses by inhibition of IkappaB-alpha ubiquitination. Science. 2000;289:1560–1563. doi: 10.1126/science.289.5484.1560. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.