Abstract
Glycogen synthase kinase 3 (GSK3) is a critical mediator of many intracellular signaling systems. The activity of GSK3 is regulated by several kinases, with inactivation occurring via phosphorylation of the inhibitory serine-21 (α-isoform) and serine-9 (β-isoform) residues. Here, we investigated whether acute cocaine administration regulates GSK3 activity and if inhibition of GSK3 by valproate or the selective GSK3 inhibitor SB 216763 would attenuate cocaine-induced behaviors in mice. Mice injected with cocaine (20 mg/kg, i.p) showed a reduction in the phosphorylation of GSK3β in the caudate putamen, reflecting an increase in the activity of the kinase. To assess the role of GSK3 in cocaine-induced hyperactivity, mice were pretreated with valproate (50–300 mg/kg, i.p.), SB216763 (0.25–7.5 mg/kg, i.p.), or the appropriate vehicle prior to saline or cocaine (20 mg/kg, i.p.). Valproate or SB 216763 produced significant dose-dependent reductions in cocaine-induced ambulatory and stereotypic activity. Repeated administration of cocaine can result in an augmentation of the locomotor-stimulatory effects of the drug, a phenomenon referred to as sensitization. Mice pretreated with SB 216763 (2.5 mg/kg, i.p.) prior to daily cocaine (20 mg/kg, i.p.) for 5 days showed a significant attenuation of the development of cocaine-induced behavioral sensitization following a cocaine challenge on day 13. These results indicate that cocaine activated GSK3β in the caudate putamen and that pharmacological inhibition of GSK3 reduced both the acute behavioral responses to cocaine and the long-term neuroadaptations produced by repeated cocaine, therefore suggesting a role for GSK3 in the behavioral and neurochemical manifestations associated with cocaine exposure.
Keywords: Cocaine, glycogen synthase kinase-3, locomotion, sensitization, caudate putamen
Introduction
Cocaine is a highly abused psychostimulant with repeated use potentially culminating in drug addiction. Elucidating the molecular mechanisms underlying acute/occasional drug use and repeated drug taking is essential for understanding addiction. As such, the aim of the present study was to investigate the role of the intracellular signaling protein, glycogen synthase kinase 3 (GSK3) on behaviors associated with acute and repeated cocaine administration.
Cocaine is a monoamine transporter inhibitor therefore blocking the reuptake of dopamine, serotonin and norepinephrine into presynaptic neurons resulting in enhanced synaptic levels of these neurotransmitters (Heikkila et al., 1975). Dopaminergic cell bodies originate in the ventral tegmental area and the substantia nigra and project to the nucleus accumbens and caudate putamen, respectively. Dopamine has a functional role in reward processes, both to natural reinforcers and addictive drugs (Koob, 1992). In addition, the importance of dopaminergic transmission in the locomotor-stimulating-effects of cocaine is well established (Kelly and Iversen, 1976; Kalivas et al., 1988), with repeated cocaine administration eliciting a sensitized or increased response to the locomotor-stimulating properties of the drug (Post and Rose, 1976; Robinson and Berrridge, 1993).
It is well established that both acute and repeated cocaine administration alter dopaminergic neurotransmission (for review, see Nestler, 2004). Thus, we chose to investigate GSK3 which has recently gained attention as a kinase that may be critical in both the behavioral and neurochemical underpinnings of dopaminergic signaling (Beaulieu et al., 2004). There is widespread expression of GSK3 in the adult brain, suggesting a fundamental role for this kinase in neuronal signaling pathways (Leroy and Brion, 1999) and its activity is regulated by a number of kinases such as Akt (protein kinase B), with inactivation of GSK3 occurring via phosphorylation at the serine-21 (α-isoform) and serine-9 (β-isoform) residues (Grimes and Jope, 2001). Interestingly, therapeutics used in the treatment of mood disorders and schizophrenia such as lithium, valproate and haloperidol affect GSK3. A therapeutically-relevant dosing regimen of lithium over 4 weeks increases the phosphorylation of the inhibitory serine-9-residue of GSK3β in mouse brain (De Sarno et al., 2002). Likewise, administration of the D2 receptor antagonist and antipsychotic agent haloperidol increases the phosphorylation of serine-9 GSK3β in the rodent brain (Emamian et al., 2004). Valproate also inhibits GSK3β via phosphorylation of the serine-9 residue in neuroblastoma SH-SY5Y cells (De Sarno et al., 2002) and protects against hypoxia-induced serine-9 dephosphorylation of GSK3β in the cortex, hippocampus, and striatum of mice (Roh et al., 2005). Moreover, valproate or specific inhibitors of GSK3 attenuate the increased horizontal activity associated with enhanced extracellular dopamine in dopamine transporter knockout mice (Beaulieu et al. 2004).
Based on previous studies indicating the importance of GSK3 in the regulation of dopamine-dependent behaviors, we investigated the role of this kinase in cocaine-induced activity and locomotor sensitization. In addition, we investigated the ability of cocaine to regulate GSK3 activity in the caudate putamen, a brain region implicated in mediating the cocaine-induced hyperactivity and sensitization (Baker et al., 1996; Kelz et al., 1999).
Materials and Methods
Animals
Male CD-1 mice (8 weeks old) were obtained from Charles River Laboratories (Wilmington, MA). Mice were housed five per plastic cage (28 × 18 × 14 cm) without additional enrichment objects in a temperature- and relative humidity-controlled room with a 12-hr light/dark cycle (lights on at 7:00 a.m.). Animals were housed for seven days prior to behavioral testing and were handled and weighed daily. All animals had access to standard laboratory chow and tap water ad libitum. All animal testing was conducted in accordance with the National Institutes of Health guidelines for the Care and Use of Laboratory Animals and with an approved protocol from Temple University Institutional Animal Care and Use Committee.
Drugs
Cocaine hydrochloride, generously supplied by the National Institute of Drug Abuse, and valproate (Sigma; St. Louis, MO) were dissolved in sterile saline (0.9% NaCl). SB 216763 (Tocris; Ellisville, MO) was dissolved in propylene glycol and brought up to volume in distilled water (70:30). Sterile saline (0.9% NaCl) or 70% propylene glycol were used for control injections.
Immunoblotting
Adult male CD-1 mice were euthanized 30 minutes following acute cocaine (20 mg.kg, i.p.) administration, and the caudate putamen was removed by gross dissection. Tissues were sonicated in boiling 1% SDS, boiled for 5 minutes, aliquotted and stored at −80°C until assayed. Protein concentrations were determined using the Lowry assay (Lowry et. al, 1951). Protein extracts (20 µg) were subjected to SDS-polyacrylamide gel electrophoresis (7.5% Tris-HCl BioRad Ready-gels, Hercules, CA) and transferred for 95 minutes to nitrocellulose membranes. Membranes were subsequently blocked for 1 hour in blocking solution consisting of 5% nonfat dry milk and Tween-TBS and then incubated overnight at 4°C in the following antibodies, phospho-GSK3α-β (1:2000, Cell Signaling, Beverly, MA), total GSK3α-β (1:10000; Santa Cruz, Santa Cruz, CA) and anti-tubulin antibody (1:20000; Sigma, St. Louis, MO). All blots were incubated in the anti-tubulin antibody to correct for differences in protein loading. Following overnight incubation in primary antibodies, membranes were washed in Tween-TBS and incubated in either anti-mouse or anti-rabbit secondary antibody conjugated to horseradish peroxidase (Vector Laboratories, Burlingame, CA) for 1 hour at room temperature. Immunoreactivity was visualized by chemiluminescence following incubation in Supersignal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL) with bands being quantified using the FujiFilm Intelligent Dark Box II, IR LAS-100 Pro V3.1, and Image Gauge V4.22 equipment and software packages. Protein data are expressed as a ratio of phosphorylated protein to total protein.
Behavioral Testing/Drug Administration
All animals were placed in locomotor activity monitors for 30 minutes prior to drug administration and testing. Following the 30 minute habituation period, mice were pretreated with saline or valproate (50–300 mg/kg, i.p.) (Beaulieu et al., 2004) followed by an injection of saline or cocaine (20 mg/kg, i.p.) 45 minutes later. Separate sets of mice were pretreated with vehicle or SB 216763 (0.25–7.5 mg/kg, i.p.) (Beaulieu et al., 2004) followed by an injection of saline or cocaine (20 mg/kg, i.p.) 5 minutes later. Activity was measured for 60 minutes following the second injection using the Digiscan DMicro (Accuscan, Inc., Columbus, OH) system. The activity monitors consist of transparent plastic boxes (45 × 20 × 20 cm) set inside metal frames that are equipped with 16 infrared light emitters and detectors. The number of photocell beam breaks are recorded by a computer interface. Ambulation was recorded as consecutive beams breaks resulting from horizontal movement, while stereotypy was recorded by repetitive beam breaks.
Behavioral Sensitization
Mice were pretreated with vehicle or SB 216763 (2.5 mg/kg, i.p.) followed five minutes later by a second injection of saline or cocaine (20 mg/kg, i.p.). This was repeated once a day for five days. After treatment day 5, animals were left drug-free for 7 days. On day 13, all animals were challenged with cocaine (20 mg/kg, i.p.) in the absence of SB 216763 and activity was recorded for 60 minutes.
Data Analysis
Behavioral data were analyzed using two-way ANOVA with pre-treatment and treatment factors followed by a Bonferroni test for multiple comparisons (GraphPad Prism 4, La Jolla, CA). Immunoblotting data were analyzed using a two-tailed Student t test. EC50 values were determined using nonlinear regression as the mean effect vs. dose (Tallarida, 2000).
Results
Phosphorylated GSK3β is reduced in the caudate putamen following acute administration of cocaine
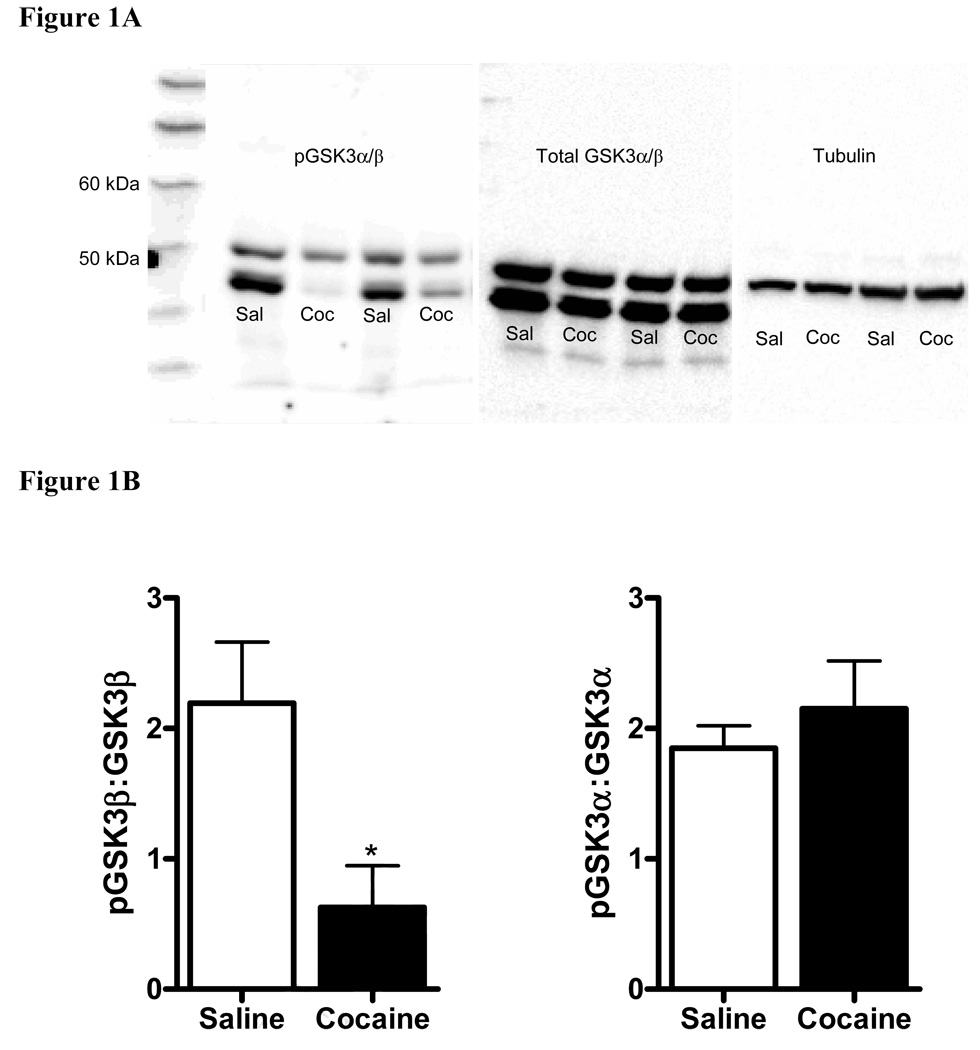
Adult male CD-1 mice were administered cocaine (20 mg/kg, i‥p) or saline and euthanized 30 minutes post-injection. Protein extracts from the caudate putamen were analyzed for levels of phospho-GSK3 and total GSK3 by immunoblot. Figure 1A shows representative immunoblots of tissue extracts from the caudate putamen probed with antibodies recognizing phospho-GSK3α (51 kDa) and phospho-GSK3β (48 kDa), total GSK3α/β and tubulin. As shown in Figure 1B, animals administered cocaine had significantly less phosphorylated GSK3β in the caudate putamen as compared to saline controls (t9=2.641, p=0.0269) indicating that acute cocaine enhanced the activity of GSK3β. In contrast, levels of phosphorylated GSK3α were unchanged in the caudate putamen following acute administration of cocaine (t14=0.7526, p>0.05). Levels of total GSK3:tubulin were unchanged in the caudate putamen following acute administration of cocaine (data not shown).
Figure 1. Phosphorylation of GSK3β but not GSK3α is reduced in the caudate putamen following acute administration of cocaine.
Adult male CD-1 mice were injected with saline or cocaine (20 mg/kg, i.p.) and the caudate putamen obtained 30 minutes later. Representative bands for phospho-GSK3α (51 kDa), GSK3β (48 kDa) and total GSK3α/β are shown from the same immunoblots (1A). Results indicate that acute administration of cocaine reduced the phosphorylation of GSK3β as compared to that in saline-injected controls (*p<0.05) (1B). Levels of phosphorylated GSK3α were unchanged in the caudate putamen following cocaine (1B). Data points represent the mean ± SEM, (n=5–8/group). Data were analyzed by a two-tailed Student t-test.
Acute cocaine-induced activity was attenuated by valproate
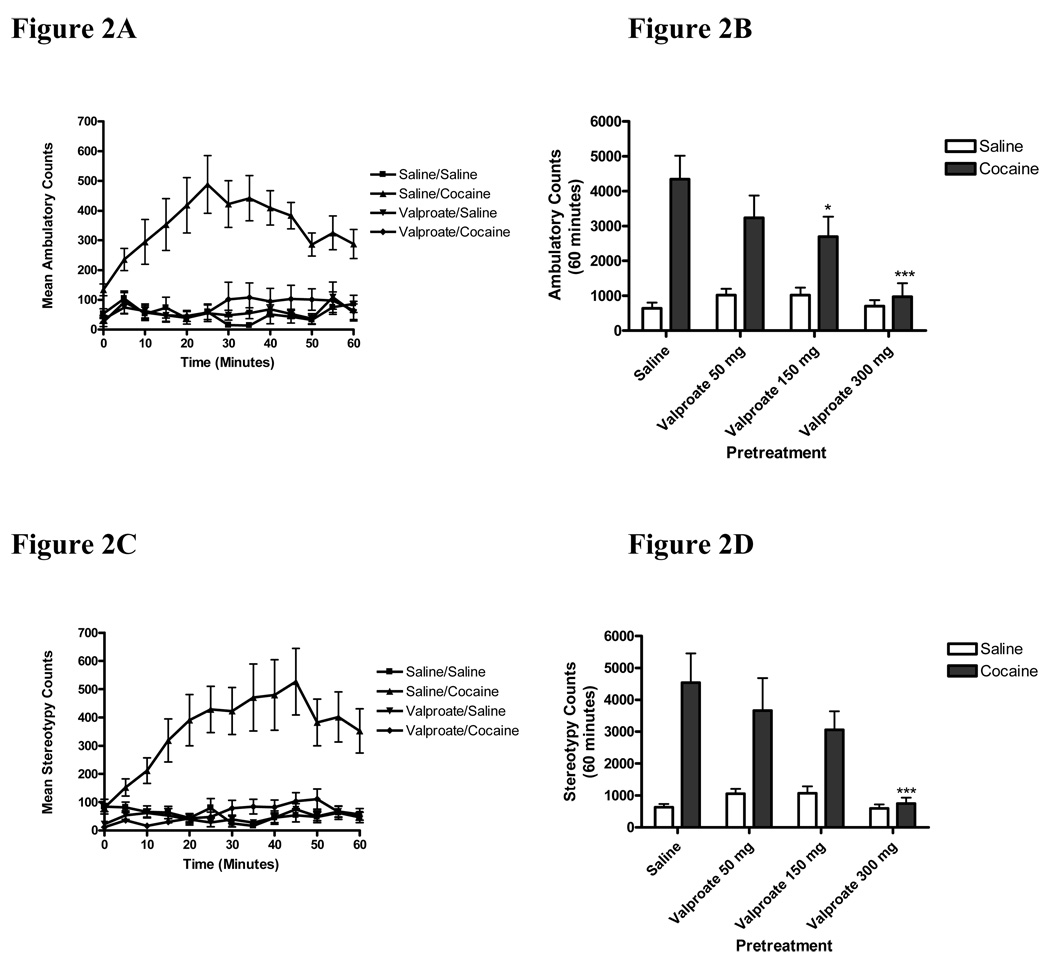
Cocaine-induced ambulatory and stereotypic activity was measured following pretreatment with valproate (50–300 mg/kg, i.p.). Administration of valproate (300 mg/kg) prior to cocaine attenuated cocaine-induced ambulatory activity to baseline values (Figure 2A). The data displayed in Figure 2B represent cumulative ambulatory counts over 60 minutes for animals in each experimental group. Two-way ANOVA of the ambulatory data showed significant interaction, pretreatment and treatment effects (Interaction:F(3,54)=4.348, p=0.0081; Pre-treatment:F(3,54)=4.350, p=0.0081; Treatment:F(1,54)=34.24, p<0.0001). Bonferroni post-hoc analysis revealed that pretreatment with valproate at doses of 150 and 300 mg/kg significantly attenuated cocaine-induced ambulation (*p<0.05; ***p<0.001 sal/coc vs. valproate/coc) (Fig. 2B). Valproate, 50 mg/kg, did not significantly attenuate cocaine-induced ambulation (p>0.05). Nonlinear regression analysis of the mean effect versus dose yielded an EC50 value of 186 mg/kg ± 17mg/kg for valproate in attenuating cocaine-induced ambulation (Table 1).
Figure 2. Valproate attenuated the acute behavioral stimulating effects of cocaine.
Adult male CD-1 mice were pretreated with saline or valproate (50–300 mg/kg, i.p.) 45 minutes prior to an injection of cocaine (20 mg/kg, i.p.) or saline and ambulatory (2A–B) and stereotypic activity (2C–D) were recorded for 60 minutes. The time course of changes in ambulatory (2A) and stereotypy (2C) are shown for mice injected with valproate (300 mg/kg) or saline prior to cocaine (20 mg/kg) or saline. Cummulative hour data are shown in 2B and 2D. Pretreatment of mice with valproate (150 or 300 mg/kg, i.p.) significantly attenuated cocaine-induced ambulatory activity (2B) while pretreatment with valproate (300 mg/kg, i.p.) significantly attenuated cocaine-induced stereotypy (2D). Valproate when administered with saline yielded no significant effect on ambulatory (2B) or stereotypic (2D) activity at any dose. Data were analyzed by a two-way ANOVA and Bonferroni post-hoc analysis (*p<0.05; ***p<0.001; vs. sal/coc). Data points represent mean ± SEM of cumulative ambulatory or stereotypy counts over 60 minutes (n=7–11/group).
Table 1.
EC50 values (mg/kg) for valproate and SB 216763 on cocaine-induced ambulatory and stereotypic activity.
| Pretreatment/Treatment | Ambulatory (mg/kg) | Stereotypy (mg/kg) |
|---|---|---|
| Valproate/Cocaine | 186 ± 17 | 186 ± 15 |
| SB 216763/Cocaine | 1.21 ± 0.176 | 1.28 ± 0.210 |
Administration of valproate (300 mg/kg) prior to cocaine reduced cocaine-induced stereotypic activity to baseline values (Figure 2C). The data displayed in Figure 2D represent cumulative stereotypy counts over 60 minutes. Two-way ANOVA of the stereotypy data showed significant interaction, pretreatment and treatment effects (Interaction:F(3,54)=3.170, p=0.0315; Pre-treatment:F(3,54)=3.845, p=0.0144; Treatment:F(1,54)=24.44, p<0.0001). Bonferroni post-hoc analysis revealed that pretreatment with valproate, 300 mg/kg, significantly attenuated cocaine-induced stereotypy (***p<0.001; sal/coc vs. valproate/coc; Fig 2D). Acute cocaine-induced stereotypic activity was not significantly changed following pretreatment of valproate, 50 or 150 mg/kg, (p>0.05). Nonlinear regression analysis of the mean effect versus dose yielded an EC50 value of 186 mg/kg ± 15mg/kg for valproate in attenuating cocaine-induced stereotypy (Table 1). Valproate alone had no effect on baseline ambulation (Figure 2B) or stereotypy (Figure 2D) (p>0.05 valproate/sal vs. veh/sal).
Inhibition of GSK3 attenuated acute cocaine-induced activity
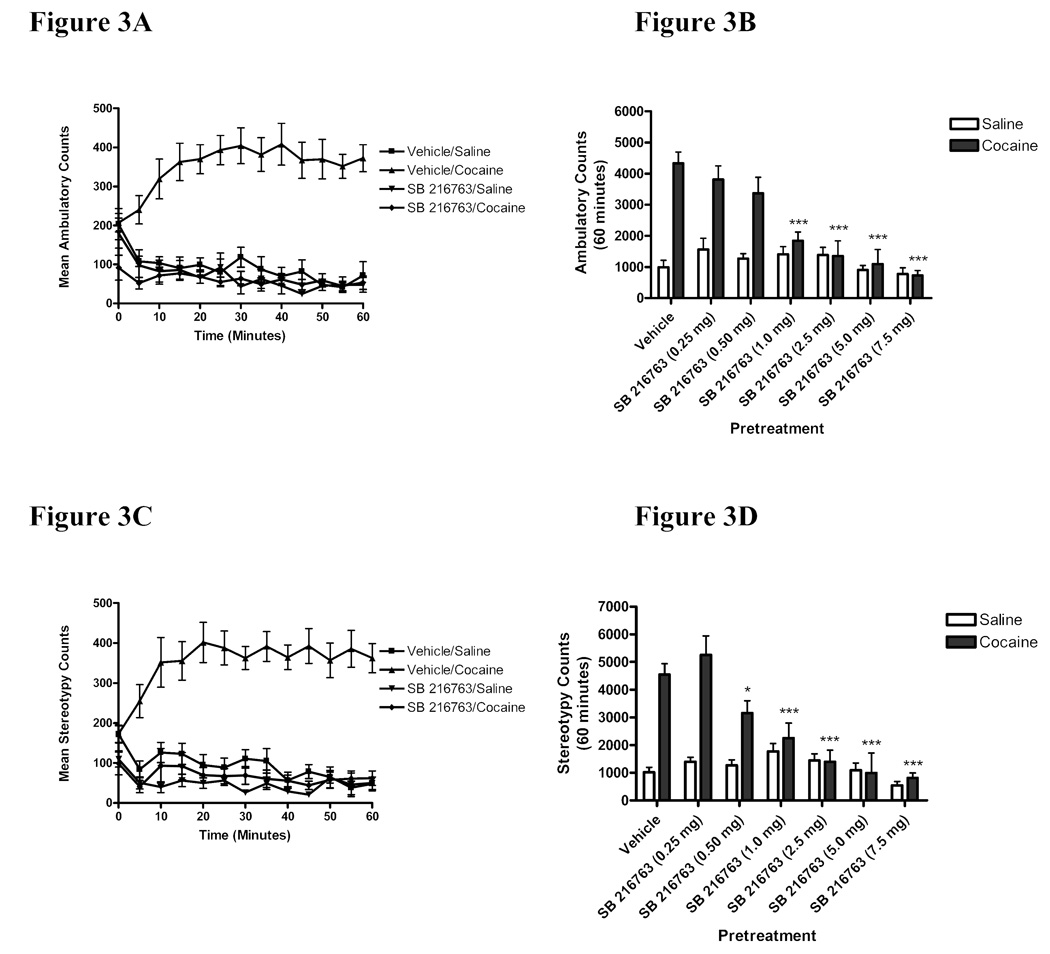
Acute cocaine-induced ambulatory and stereotypic activities were measured following pretreatment with the GSK3 inhibitor SB 216763 (0.25–7.5 mg/kg, i.p.). Pretreatment with SB 216763 (7.5 mg/kg) prior to cocaine blocked cocaine-induced ambulatory activity (Figure 3A). Figure 3B represents the cumulative ambulatory counts over 60 minutes for animals in each experimental group. Two-way ANOVA indicated significant interaction, pretreatment, and treatment effects of SB 216763 on cocaine-induced ambulatory activity (Interaction:F(6,104)=7.621, p<0.0001; Pretreatment:F(6,104)=10.31, p<0.0001; Treatment:F(1,104)=39.78, p<0.0001). Bonferroni post-hoc analyses indicated that pretreatment with SB 216763 at doses of 1.0–7.5 mg/kg significantly attenuated cocaine-induced ambulatory activity (***p<0.001; veh/coc vs. SB 216763/coc; Fig. 3B). Nonlinear regression analysis of the mean effect versus dose yielded an EC50 value of 1.21 mg/kg ± 0.176mg/kg for SB 216763 in attenuating cocaine-induced ambulation (Table 1). Acute cocaine-induced ambulatory activity was not significantly changed following pretreatment with the lower doses of SB 216763 (0.25–0.5 mg/kg) (p>0.05). In addition, SB 216763 when administered with saline had no effect on ambulatory activity (p>0.05; SB/sal vs. veh/sal; Fig 3B).
Figure 3. Inhibition of GSK3 attenuated the acute behavioral stimulating effects of cocaine.
Adult male CD-1 mice were pretreated with vehicle or the selective GSK3 inhibitor SB 216763 (0.25–7.5 mg/kg) 5 minutes prior to injection of cocaine (20 mg/kg, i.p.) or saline and ambulatory (3A–B) and stereotypic activity (3C–D) were recorded for 60 minutes. The time course of changes in ambulatory (3A) and stereotypy (3C) are shown for mice injected with SB 216763 (7.5 mg/kg) or saline prior to cocaine (20 mg/kg) or saline. Cummulative hour data are shown in 3B and 3D. Mice pretreated with SB 216763 (1.0–7.5 mg/kg) exhibited a significant attenuation of cocaine-induced ambulation (3B). Likewise, pretreatment with SB 216763 (0.5–7.5 mg/kg) significantly attenuated stereotypic activity (3D). SB 216763 alone had no effect on either ambulatory (3B) or stereotypic (3D) activity. Data were analyzed by a two-way ANOVA and Bonferroni post-hoc analysis (*p<0.05, ***p<0.001 vs. veh/coc). Data points represent the means ± SEM of cumulative ambulatory or stereotypy counts over 60 minutes (n=6–17/group).
Administration of SB 216763 (7.5 mg/kg) prior to cocaine attenuated cocaine-induced stereotypic activity to baseline values (Figure 3C). Two-way ANOVA analysis of the cumulative stereotypy counts over 60 minutes (Figure 3D) indicated significant interaction, pretreatment and treatment effects of SB 216763 (Interaction:F(6,103)=8.980, p<0.0001; Pretreatment:F(6,103)=11.18, p<0.0001; Treatment:F(1,103)=43.48, p<0.0001). Bonferroni post-hoc analysis revealed that pretreatment with SB 216763 at doses of 0.5–7.5 mg/kg significantly attenuated cocaine-induced stereotypic activity (*p<0.05; ***p<0.001; veh/coc vs. SB 216763/coc; Fig 3D). Nonlinear regression analysis of the mean effect versus dose yielded an EC50 value of 1.28 mg/kg ± 0.210mg/kg for SB 216763 in attenuating cocaine-induced stereotypy (Table 1). Acute cocaine-induced stereotypic activity was not significantly changed following pretreatment with SB 216763 (0.25 mg/kg) (p>0.05). Further, SB 216763 when administered with saline had no effect on stereotypic activity at any dose tested (p>0.05; SB/sal vs. veh/sal; Fig 3D).
Inhibition of GSK3 prevented the development of cocaine-induced locomotor sensitization in mice
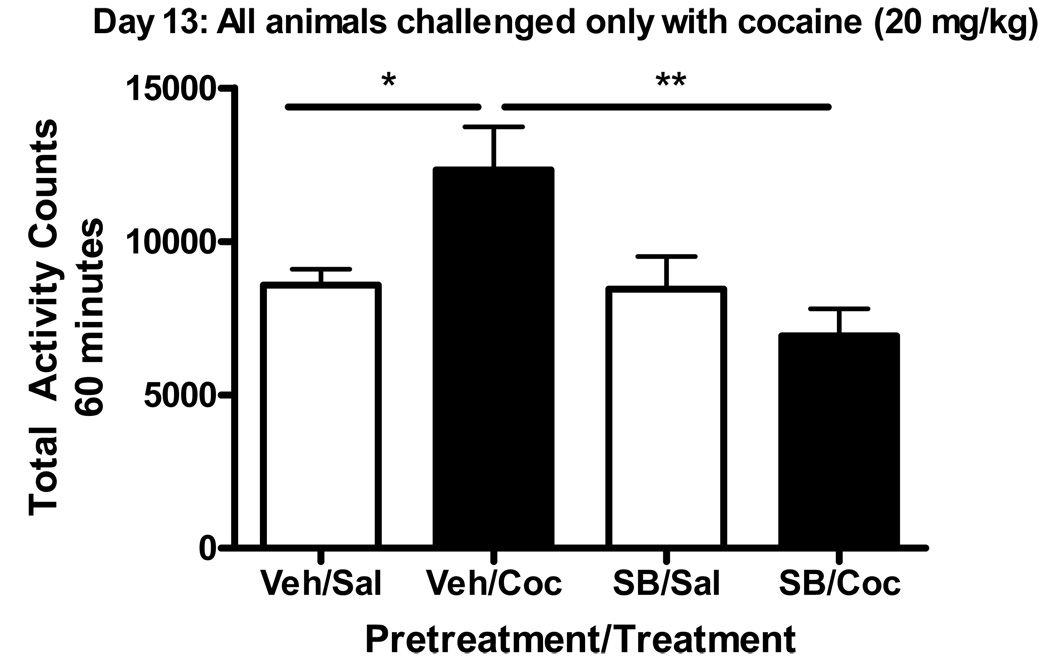
Adult male CD-1 mice were pretreated with vehicle or SB 216763 (2.5 mg/kg) prior to daily administration of cocaine (20 mg/kg) for 5 days. All animals were drug-free for 7 days and subsequently challenged with cocaine (20 mg/kg) on day 13 in the absence of SB 216763, and activity was monitored for 60 minutes (Figure 4). Two-way ANOVA analysis indicated significant interaction and pretreatment effects (Interaction:F(1,25)=6.320, p=0.0187; Pretreatment:F(1,25)=6.978, p=0.0140; Treatment:F(1,25)=1.119, p=0.3002). Bonferroni post hoc analysis indicated that animals administered daily cocaine had higher activity counts following a cocaine challenge as compared to those given daily saline, indicating the development of sensitization (*p<0.05; veh/sal vs. veh/coc). In addition, animals injected with SB 216763 plus cocaine for 5 days had significantly lower activity after the cocaine challenge than those pretreated with vehicle followed by cocaine (**p<0.01; veh/coc vs. SB/coc), indicating that SB 216763 blocked the development of behavioral sensitization.
Figure 4. Inhibition of GSK3 prevented the development of cocaine-induced locomotor sensitization.
Activity counts/60 min are shown for mice injected with cocaine (20 mg/kg) on day 13 of the study. On days 1–5, mice received either SB 216763 (2.5 mg/kg) or vehicle plus cocaine (20 mg/kg). Mice administered cocaine (20 mg/kg) on days 1–5 exhibited a sensitized locomotor response to a cocaine challenge on day 13 as compared to saline-injected controls given a cocaine challenge (veh/sal vs. veh/coc, *p<0.05). Pretreatment with SB 216763 (2.5 mg/kg, i.p.) on days 1–5 attenuated cocaine-induced sensitization following a cocaine challenge (20 mg/kg, i.p.) on day 13 (veh/coc vs. SB/coc, **p<0.01). Data points represent mean ± SEM (n=6–8/group). Data were analyzed using a two-way ANOVA and Bonferroni post-hoc analysis.
Discussion
Our data support the hypothesis that GSK3 is critical for cocaine-induced behaviors. Data presented herein demonstrate that cocaine activates GSK3β in the caudate putamen, as evidenced by a reduction in phosphorylated GSK3β 30 minutes following cocaine administration. We also show that both valproate, which inhibits GSK3, and the selective inhibitor of GSK3 SB 216763 dose-dependently attenuated acute cocaine-induced hyperactivity. Further, GSK3 activity is necessary for the development of the sensitized locomotor response associated with repeated cocaine administration as selective inhibition of GSK3 prevented the development of cocaine-induced behavioral sensitization.
In the present study, pretreatment with valproate dose-dependently attenuated acute cocaine-induced ambulatory and stereotypic activity. Valproate is an anticonvulsant and mood stabilization agent used to treat a number of disease states including epilepsy and mania (Jeavons and Clark, 1974; Bowden et al., 1994). Valproate acts by enhancing the inhibitory actions of GABA via inhibition of GABA degradation, decreasing GABA turnover and increasing GABA synthesis (Owens and Nemeroff, 2003; Johannessen and Johannessen, 2003). Valproate is used clinically in combination with atypical and typical antipsychotics for the treatment of bipolar disorder and schizophrenia (Basan et al., 2004). Valproate is also a direct inhibitor of histone deacetylase (Gottlicher et al., 2001; Phiel et al., 2001) and has neuroprotective effects by suppressing apoptosis (Kanai et al., 2004). Valproate can also regulate the function of GSK3, as valproate has been shown to inhibit GSK3 activity in human neuroblastoma SHSY5Y cells (Chen et al., 1999). Valproate can alter behaviors induced by increases in extracellular dopamine, such as those seen in mice lacking the dopamine transporter. Dopamine transporter knockout mice have 5-fold higher levels of striatal synaptic dopamine (Giros et al., 1996). Administration of valproate to these mice attenuates their heightened ambulatory and stereotypic activity (Beaulieu et al., 2004). Although valproate can block dopamine-dependent behaviors, valproate alone does not significantly change extracellular dopamine levels in the nucleus accumbens or caudate putamen (Biggs et al., 1992). Previous studies have show that valproate can attenuate the development but not the expression of methamphetamine- and cocaine-induced behavioral sensitization in mice (Li et al., 2005), as well as both the development and expression of methylphenidate sensitization (Yang et al., 2000a, b). While our studies demonstrating that valproate can attenuate acute cocaine-induced locomotion agree with previous studies with other psychostimulants (Li et al., 2005), it is difficult to ascertain whether the actions of valproate on such activity are the result of alterations in GABAergic functionality or perhaps inhibition of GSK3. As such, we chose to further evaluate the role of GSK3 on cocaine-induced behaviors using the specific GSK3 inhibitor SB 216763.
SB 216763 is a malemide derivative and inhibits GSK3 in an ATP-competitive manner with a Ki of 9 nM (Coghlan et al., 2000). Here, we show that pretreatment with SB 216763 dose-dependently attenuated cocaine-induced ambulatory and stereotypic activity. These results parallel previous studies highlighting the importance of the beta isoform of GSK3 in behaviors associated with psychostimulant administration or altered dopaminergic transmission. For example, SB 216763 dose-dependently attenuates the hyper-locomotion seen in dopamine transporter knockout mice (Beaulieu et al., 2004). Further, GSK3β heterozygote null mice display a decreased locomotor response to acute amphetamine as compared to wild-type controls (Beaulieu et al., 2004), whereas transgenic mice expressing a constitutively active mutated form of GSK3β exhibit an increase in locomotor activity in response to a novel environment as compared to wild-type controls (Prickaerts et al., 2006), thus giving further credence to the importance of GSK3β in dopamine-stimulated hyperactivity. These studies along with our data suggest that GSK3 is a mediator of the acute hyper-locomotor responses induced by psychostimulant administration, as selective inhibition of the kinase attenuates both acute amphetamine (Beaulieu et al., 2004) and cocaine-induced locomotion in mice (current study).
Although a number of studies indicate the importance of GSK3 in normal and acute psychostimulant-induced behaviors, molecular models encompassing how GSK3 regulates psychostimulant-induced behaviors have yet to be fully established. Previous studies suggest that GSK3 may be associated with a number of intracellular signaling cascades and its regulation is dependent upon both temporal and spatial perturbations of specific neurotransmitters and receptor subtypes in the brain. Our data indicate that GSK3β is activated by the indirect dopaminergic agonist cocaine. Here, we show that acute administration of cocaine reduced the phosphorylation of GSK3β at the regulatory serine-9 residue in the caudate putamen 30 minutes post-injection. In addition, our data suggest that only the β-isoform of GSK3 is regulated by acute cocaine as phosphorylated levels of the α-isoform were unchanged in the caudate putamen following cocaine. These data are consistent with previous studies focusing on the relationship between GSK3 and dopamine as acute administration of amphetamine attenuates phosphorylation of serine-9 GSK3β in the mouse striatum (Beaulieu et al., 2004). In addition, striatal preparations from dopamine transporter knockout mice show a reduction in the inhibitory serine-9 phosphorylation of GSK3β (Beaulieu et al., 2004), suggesting that the kinase is regulated by extracellular dopamine. Further, analysis of the striatum of dopamine transporter knockout mice shows an increase in the phosphorylation of Akt (Beaulieu et al., 2004), an upstream kinase capable of regulating GSK3β phosphorylation. Intracerebroventricular injection of the cAMP analog 8-Br-cAMP to mice lacking the dopamine transporter increases the phosphorylation of DARPP-32 at Thr-34 without changing Akt or GSK3β phosphorylation (Beaulieu et al., 2004), suggesting that the relationship between dopamine and GSK3β may be independent of cAMP.
In addition to identifying a role for GSK3 in acute cocaine-induced behaviors, we also investigated the importance of GSK3 in the development of cocaine-induced behavioral sensitization. Identification of new molecular targets to prevent sensitized responses to drugs of abuse is salient in that the etiology of sensitization may model certain aspects of addictive behavior such as drug craving and potential relapse (Robinson and Berrridge, 1993). Here, we show that when daily cocaine administration is preceded by the GSK3 inhibitor SB 216763, the development of cocaine-induced locomotor sensitization is prevented. This is the first study indicating a role for GSK3 in the behavioral manifestations of repeated cocaine administration. The mechanism by which GSK3 prevents the development of cocaine-induced sensitization is currently unknown; however there are a number of neural and anatomical substrates underlying the development of cocaine-induced sensitization, one of which involves dopamine. For example, administration of the dopamine D1 receptor antagonist SCH 23390 prior to daily cocaine prevents the development of sensitization (McCreary and Marsden, 1993), and dopamine D1 receptor knockout mice do not show locomotor sensitization to cocaine as compared to wildtype controls (Karlsson et al., 2008). The development of cocaine sensitization can also be blocked by the dopamine D2 receptor antagonist haloperidol (Karler et al., 1994). These studies indicate the importance of dopamine receptors in the induction of cocaine sensitization. As administration of the D2 receptor antagonists haloperidol and raclopride inhibit GSK3 (Emamian et al., 2004; Beaulieu et al., 2004), it may be that D2 receptor antagonists interfere with cocaine sensitization by a mechanism involving GSK3 inhibition.
Another mechanism by which inhibition of GSK3 may prevent the development of cocaine sensitization is by interfering with glutamatergic transmission. Animals sensitized to cocaine show an increase in extracellular glutamate in the core of the nucleus accumbens as compared to non-sensitized and saline pretreated animals (Pierce et al., 1996), and pretreatment with the NMDA receptor antagonist MK-801 prevents the development of cocaine sensitization in mice (Karler et al., 1989), therefore highlighting the importance of glutamate in cocaine-induced behavioral sensitization. Of note, stimulation of the NMDA receptor causes an activation of GSK3 via protein phosphatase-1 in the adult mouse brain (Szatmari et al., 2005), whereas inhibition of GSK3 reduces the whole cell current of the NMDA receptor in cortical pyramidal neurons and can cause NMDA receptor internalization (Chen et al., 2007). In addition, the NMDA receptor antagonist memantine can increase the inhibitory phosphorylation of the serine-9 residue of GSK3β in the cerebral cortex, striatum, and hippocampus of mice (De Sarno et al., 2006). This suggests that the cellular and behavioral manifestations associated with the development of cocaine sensitization may be due to an interaction between the NMDA receptor and GSK3β. Future studies to identify the specific intracellular signaling cascade involved in GSK3-induced regulation of cocaine sensitization and plasticity are warranted.
In summary, the results of our study indicate that cocaine activates GSK3β and that activation of GSK3 is critical to the acute locomotor-stimulating effects of cocaine, as well as to the development of cocaine sensitization. Investigations determining the role of GSK3 in other facets of addiction-related behaviors such as drug-induced reward and reinstatement are currently underway in our laboratory. The importance of GSK3 in the behavioral and neurochemical adaptations associated with other drugs of abuse may yield important insights as to the role of GSK3 in addiction.
Acknowledgements
This work was supported by NIH R01 DA09580 (EMU), T32 DA07237 (EMU) and P30 DA13429 (Adler/EMU).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baker DA, Khroyan TV, O'Dell LE, Fuchs RA, Neisewander JL. Differential effects of intra-accumbens sulpiride on cocaine-induced locomotion and conditioned place preference. The Journal of Pharmacology and Experimental Therapeutics. 1996;279:392–401. [PubMed] [Google Scholar]
- Basan A, Kissling W, Leucht S. Valproate as an adjunct to antipsychotics for schizophrenia: A systematic review of randomized trials. Schizophrenia Research. 2004;70:33–37. doi: 10.1016/j.schres.2004.01.016. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Yao WD, Kockeritz L, Woodgett JR, Gainetdinov RR, Caron MG. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:5099–5104. doi: 10.1073/pnas.0307921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs CS, Pearce BR, Fowler LJ, Whitton PS. Regional effects of sodium valproate on extracellular concentrations of 5-hydroxytryptamine, dopamine, and their metabolites in the rat brain: An in vivo microdialysis study. Journal of Neurochemistry. 1992;59:1702–1708. doi: 10.1111/j.1471-4159.1992.tb11001.x. [DOI] [PubMed] [Google Scholar]
- Bowden CL, Brugger AM, Swann AC, Calabrese JR, Janicak PG, Petty F, Dilsaver SC, Davis JM, Rush AJ, Small JG. Efficacy of divalproex vs lithium and placebo in the treatment of mania. the depakote mania study group. JAMA : The Journal of the American Medical Association. 1994;271:918–924. [PubMed] [Google Scholar]
- Chen G, Huang LD, Jiang YM, Manji HK. The mood-stabilizing agent valproate inhibits the activity of glycogen synthase kinase-3. Journal of Neurochemistry. 1999;72:1327–1330. doi: 10.1046/j.1471-4159.2000.0721327.x. [DOI] [PubMed] [Google Scholar]
- Chen P, Gu Z, Liu W, Yan Z. Glycogen synthase kinase 3 regulates N-methyl-D-aspartate receptor channel trafficking and function in cortical neurons. Molecular Pharmacology. 2007;72:40–51. doi: 10.1124/mol.107.034942. [DOI] [PubMed] [Google Scholar]
- Coghlan MP, Culbert AA, Cross DA, et al. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chemistry & Biology. 2000;7:793–803. doi: 10.1016/s1074-5521(00)00025-9. [DOI] [PubMed] [Google Scholar]
- De Sarno P, Bijur GN, Zmijewska AA, Li X, Jope RS. In vivo regulation of GSK3 phosphorylation by cholinergic and NMDA receptors. Neurobiology of Aging. 2006;27:413–422. doi: 10.1016/j.neurobiolaging.2005.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sarno P, Li X, Jope RS. Regulation of akt and glycogen synthase kinase-3 beta phosphorylation by sodium valproate and lithium. Neuropharmacology. 2002;43:1158–1164. doi: 10.1016/s0028-3908(02)00215-0. [DOI] [PubMed] [Google Scholar]
- Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, Gogos JA. Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nature Genetics. 2004;36:131–137. doi: 10.1038/ng1296. [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Gottlicher M, Minucci S, Zhu P, et al. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. The EMBO Journal. 2001;20:6969–6978. doi: 10.1093/emboj/20.24.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Progress in Neurobiology. 2001;65:391–426. doi: 10.1016/s0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- Heikkila RE, Orlansky H, Cohen G. Studies on the distinction between uptake inhibition and release of (3H)dopamine in rat brain tissue slices. Biochemical Pharmacology. 1975;24:847–852. doi: 10.1016/0006-2952(75)90152-5. [DOI] [PubMed] [Google Scholar]
- Jeavons PM, Clark JE. Sodium valproate in treatment of epilepsy. British Medical Journal. 1974;2:584–586. doi: 10.1136/bmj.2.5919.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannessen CU, Johannessen SI. Valproate: Past, present, and future. CNS Drug Reviews. 2003;9:199–216. doi: 10.1111/j.1527-3458.2003.tb00249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P, DuMars LA, Skinner C. Behavioral and neurochemical effects of acute and daily cocaine administration in rats. The Journal of Pharmacology and Experimental Therapeutics. 1988;245:485–492. [PubMed] [Google Scholar]
- Kanai H, Sawa A, Chen RW, Leeds P, Chuang DM. Valproic acid inhibits histone deacetylase activity and suppresses excitotoxicity-induced GAPDH nuclear accumulation and apoptotic death in neurons. The Pharmacogenomics Journal. 2004;4:336–344. doi: 10.1038/sj.tpj.6500269. [DOI] [PubMed] [Google Scholar]
- Karler R, Calder LD, Bedingfield JB. Cocaine behavioral sensitization and the excitatory amino acids. Psychopharmacology. 1994;115:305–310. doi: 10.1007/BF02245070. [DOI] [PubMed] [Google Scholar]
- Karler R, Calder LD, Chaudhry IA, Turkanis SA. Blockade of "reverse tolerance" to cocaine and amphetamine by MK-801. Life Sciences. 1989;45:599–606. doi: 10.1016/0024-3205(89)90045-3. [DOI] [PubMed] [Google Scholar]
- Karlsson RM, Hefner KR, Sibley DR, Holmes A. Comparison of dopamine D1 and D5 receptor knockout mice for cocaine locomotor sensitization. Psychopharmacology. 2008;200:117–127. doi: 10.1007/s00213-008-1165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly PH, Iversen SD. Selective 6OHDA-induced destruction of mesolimbic dopamine neurons: Abolition of psychostimulant-induced locomotor activity in rats. European Journal of Pharmacology. 1976;40:45–56. doi: 10.1016/0014-2999(76)90352-6. [DOI] [PubMed] [Google Scholar]
- Kelz MB, Chen J, Carlezon WA, Jr, et al. Expression of the transcription factor deltaFosB in the brain controls sensitivity to cocaine. Nature. 1999;401:272–276. doi: 10.1038/45790. [DOI] [PubMed] [Google Scholar]
- Koob GF. Drugs of abuse: Anatomy, pharmacology and function of reward pathways. Trends in Pharmacological Sciences. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- Leroy K, Brion JP. Developmental expression and localization of glycogen synthase kinase-3beta in rat brain. Journal of Chemical Neuroanatomy. 1999;16:279–293. doi: 10.1016/s0891-0618(99)00012-5. [DOI] [PubMed] [Google Scholar]
- Li JX, Han R, Deng YP, Chen SQ, Liang JH. Different effects of valproate on methamphetamine- and cocaine-induced behavioral sensitization in mice. Behavioural Brain Research. 2005;161:125–132. doi: 10.1016/j.bbr.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. The Journal of Biological Chemistry. 1951;193:265–275. [PubMed] [Google Scholar]
- McCreary AC, Marsden CA. Cocaine-induced behaviour: Dopamine D1 receptor antagonism by SCH 23390 prevents expression of conditioned sensitization following repeated administration of cocaine. Neuropharmacology. 1993;32:387–391. doi: 10.1016/0028-3908(93)90161-u. [DOI] [PubMed] [Google Scholar]
- Mueser KT, McGurk SR. Schizophrenia. Lancet. 2004;363:2063–2072. doi: 10.1016/S0140-6736(04)16458-1. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular mechanisms of drug addiction. Neuropharmacology. 2004;47 Suppl 1:24–32. doi: 10.1016/j.neuropharm.2004.06.031. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Nemeroff CB. Pharmacology of valproate. Psychopharmacology Bulletin. 2003;37 Suppl 2:17–24. [PubMed] [Google Scholar]
- Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, Klein PS. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. The Journal of Biological Chemistry. 2001;276:36734–36741. doi: 10.1074/jbc.M101287200. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. The Journal of Neuroscience. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post RM, Rose H. Increasing effects of repetitive cocaine administration in the rat. Nature. 1976;260:731–732. doi: 10.1038/260731a0. [DOI] [PubMed] [Google Scholar]
- Prickaerts J, Moechars D, Cryns K, Lenaerts I, van Craenendonck H, Goris I, Daneels G, Bouwknecht JA, Steckler T. Transgenic mice overexpressing glycogen synthase kinase 3beta: A putative model of hyperactivity and mania. The Journal of Neuroscience. 2006;26:9022–9029. doi: 10.1523/JNEUROSCI.5216-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Research.Brain Research Reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Roh MS, Eom TY, Zmijewska AA, De Sarno P, Roth KA, Jope RS. Hypoxia activates glycogen synthase kinase-3 in mouse brain in vivo: Protection by mood stabilizers and imipramine. Biological Psychiatry. 2005;57:278–286. doi: 10.1016/j.biopsych.2004.10.039. [DOI] [PubMed] [Google Scholar]
- Szatmari E, Habas A, Yang P, Zheng JJ, Hagg T, Hetman M. A positive feedback loop between glycogen synthase kinase 3beta and protein phosphatase 1 after stimulation of NR2B NMDA receptors in forebrain neurons. The Journal of Biological Chemistry. 2005;280:37526–37535. doi: 10.1074/jbc.M502699200. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ. Drug Synergism and Dose-Effect Data Analysis. Boca Raton: CRC/Chapman and Hall; 2000. [Google Scholar]
- Yang P, Beasley A, Eckermann K, Swann A, Dafny N. Valproate prevents the induction of sensitization to methylphenidate (ritalin) in rats. Brain Research. 2000;887:276–284. doi: 10.1016/s0006-8993(00)02996-6. [DOI] [PubMed] [Google Scholar]
- Yang P, Beasley A, Swann A, Dafny N. Valproate modulates the expression of methylphenidate (ritalin) sensitization. Brain Research. 2000;874:216–220. doi: 10.1016/s0006-8993(00)02500-2. [DOI] [PubMed] [Google Scholar]