Abstract
During vaginal delivery dual injuries of the pudendal nerve and the external urethral sphincter (EUS), along with other injuries, are correlated with later development of stress urinary incontinence. It is not known how combinations of these injuries affect neuromuscular recovery of the micturition reflex. We investigated the EUS electromyogram (EMG) and the pudendal nerve motor branch potentials (PNMBP) during voiding 4 days, 3 weeks or 6 weeks after injury; including vaginal distension (VD), pudendal nerve crush (PNC), both PNC and VD (PNC+VD), and pudendal nerve transection (PNT); and in controls. Pudendal nerve and urethral specimens were excised and studied histologically. No bursting activity was recorded in the EUS EMG during voiding 4 days after all injuries, as well as 3 weeks after PNC+VD. Bursting activity demonstrated recovery 3 weeks after either VD or PNC and 6 weeks after PNC+VD, but the recovered intraburst frequency remained significantly decreased compared to controls. Bursting results of PNMBP were similar to the EMG, except bursting in PNMBP 4 days after VD and the recovered intraburst frequency was significantly increased compared to controls after PNC and PNC+VD. After PNT, neither the EUS nor the pudendal nerve recovered by 6 weeks after injury. Our findings indicate bursting discharge during voiding recovers more slowly after PNC+VD than after either PNC or VD alone. This was confirmed histologically in the urethra and the pudendal nerve and may explain why pudendal nerve dysfunction has been observed years after vaginal delivery.
Keywords: Injury, Motor unit, Muscle, EMG, Nerve, neuromuscular junction, neurotransmission, repair
Introduction
Vaginal childbirth can cause damage to the pudendal nerve, the levator ani, and pelvic organ fascial supports as well as the urethral and anal sphincters (Dietz and Wilson 2005, Goldberg et al. 2005). The risk factors for injury include operative vaginal delivery, long second stage of delivery, and fetal macrosomia (Nassar et al. 2003, Hankins and Rowe 1996). Therefore, vaginal delivery represents a potent determinant of stress urinary incontinence (SUI) (Goldberg et al. 2005). However, it is much less clear how the combination of these traumas affects the rate of recovery during the micturition reflex.
We have recently developed a dual injury model in the rat to better simulate the combination of traumas of childbirth, including injuries both to the external urinary sphincter (EUS) via vaginal distension (VD) and to its innervation, the pudendal nerve, via a bilateral pudendal nerve crush (PNC) (Jiang et al. 2008). Both single injury models have been previously utilized to demonstrate, among other results, that increased duration of VD results in increased time to recovery (Pan et al. 2007); the pudendal nerve regenerates by 2 weeks later (Damaser et al. 2007); and that the urethra, vagina, and bladder become ischemic after VD (Damaser et al. 2005). However, it is not clear how combinations of these injuries affect neuromuscular recovery of voiding.
In normal rats, voiding is characterized by interrupted bursting of the EUS electromyogram (EMG) (Maggi et al. 1986a, Kruse et al. 1993). The presence of the bursting EUS EMG activity is considered a hallmark for normal and effective micturition reflex recovery in rats after neurological injuries (Kruse et al. 1993). The bursting EUS EMG activity may disappear acutely after injury but shows signs of recovery at later time points (Cheng and de Groat 2004, Chang et al. 2007, Chang and Havton 2008).
In rats, the pudendal nerve is divided into sensory and motor nerve branches in Alcock’s canal, making the rat a useful model for electrophysiological study (McKenna and Nadelhaft 1986) (McKenna and Nadelhaft 1989). We have recorded pudendal nerve motor branch potentials (PNMBP) to obtain a functional measure of pudendal efferent recovery after injury (Jiang et al. 2008). The goal of this study was to determine how different simulated childbirth injuries affect the recovery of electrophysiological bursting discharge both in the EUS EMG and PNMBP during voiding, after single and combined simulated childbirth injuries, and to relate them to histological outcomes of the pudendal nerve and urethra.
Materials and Methods
All procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the Cleveland Clinic. Sixty-seven female virgin Sprague-Dawley rats (200 - 250g) were randomized into five groups: uninjured control, VD, PNC, PNC+VD, and bilateral pudendal nerve transection (PNT). The recordings, including filling cystometrogram (CMG), EUS EMG, and PNMBP, were performed in the VD and PNC groups either 4 days (n = 5) or 3 weeks (n = 5) after injury. In PNC+VD and PNT groups, recordings were made 4 days (n = 5), 3 weeks (n = 6), or 6 weeks (n = 6) after injury. Controls (n = 13) consisted of unmanipulated age-matched animals.
Simulated Childbirth Injuries
During VD, PNC, PNC+VD and PNT procedures, rats were anesthetized with i.p. ketamine (100 mg/kg) and xylazine (10 mg/kg). For VD, the vagina was first accommodated with increasing sizes of bouge à boule urethral dilators (24F, 26F, 28F, 30F, and 32F). A modified 10F Foley balloon catheter was inserted into the vagina and the balloon was inflated with 3 ml water for 4 hours, as previously described (Pan et al. 2007). For PNC, the pudendal nerve was accessed from a postero-lateral gluteal approach in anesthetized animals. The ilium and sacrum were opened slightly, and all branches of the pudendal nerve were isolated and crushed bilaterally with a Castroviejo needle holder twice for 30 seconds as previously described (Damaser et al. 2003). Animals in the PNC+VD group received both of the above procedures with PNC performed first. Rats in the PNT group underwent a procedure similar to that of PNC except that all branches of the pudendal nerve were transected bilaterally instead of being crushed. A segment of nerve (∼2 mm long) was removed from the transection site to prevent neuroregeneration. All injury rats received buprenophrine (0.1 mg/kg s.c.) for postoperative analgesia.
Electrophysiological recordings during voiding
All rats were anaesthetized with urethane (1.2 g/kg) intraperitoneally. The pubic symphysis was exposed through a vertical midline incision and the rectus abdominis muscles were cut to expose the pubic symphysis. The inferior epigastric artery and vein were then ligated and cut bilaterally. The urethra was exposed by opening the pubic symphysis with forceps. Bipolar parallel platinum electrodes (30-gauge needles 2 mm apart) for recording EUS EMG were placed on the outside of the mid-urethra at the location of the EUS. The electrodes were connected to an amplifier (Model P511 AC Amplifier, Astro-Med, Inc., Providence, RI) with 3 Hz - 3 KHz band pass frequencies and electrophysiological recording system (DASH 8X, Astro-Med, Inc.; 10 KHz sampling rate).
For PNMBP recordings, the pubis and ischium were partly removed using forceps to separate and enlarge the right side of the ischiorectal fossa where Alcock’s canal is located. The pudendal nerve motor branch to the EUS was identified among the Alcock’s canal and separated from the sensory and anal sphincter motor branches of the pudendal nerve using dissecting needle under a surgical microscope. The pudendal motor branch to the EUS was guided over an identical set of recording electrodes that were placed in a warm (37°C) paraffin oil bath. As above, the electrodes were connected to an amplifier and the electrophysiological recording system. No PNMBP recordings could be made after PNT.
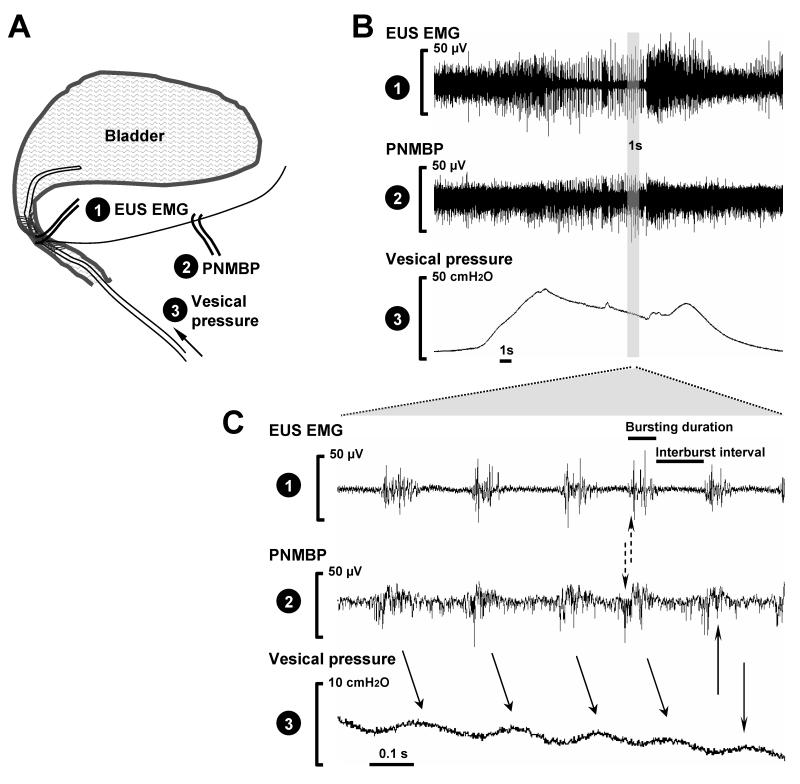
A labeled polyethylene catheter (PE-50) was inserted into the bladder via the urethra, and was connected to both a pressure transducer (model P122; Astro-Med, Inc.) and a syringe pump (model 200; KD Scientific, New Hope, PA). The vesical pressure was referenced to air pressure at the level of the bladder. Vesical pressure, EUS EMG, and PNMBP were recorded simultaneously by the above recording system while the bladder was filled with saline (5 ml/h) (Fig. 1A). Rats were free to void via the urethra around the urethral catheter.
Fig .1.
A. Schematic diagram of the electrophysiological recordings for external urethral sphincter electromyogram (EUS EMG) and pudendal nerve motor branch potential (PNMBP) and vesical pressure during filling cystometrogram on urethane-anesthetized rats. B. Example simultaneous recordings in a control rat showing EUS EMG activity, PNMBP bursting, and bladder pressure during voiding. C. Extended view of a one second sample during bursting. The solid arrows indicate bursting associated with high frequency oscillations. The dashed arrows indicate the time delay between EUS EMG and PNMBP bursting.
Data analysis
Quantitative assessment of PNMBP and EUS EMG was performed by determining the mean rectified amplitude and the mean firing frequency of both signals. Power supply interference (60 Hz and 120 Hz) was filtered out by digital band stop filtering (59 - 61 Hz and 119 - 121 Hz; Myosotic SignaPoint 2007, Myosotic LLC, Woodinville, WA). During voiding, a bursting episode was identified and archived as a one-second recording sample (Fig. 1B, shaded bar) using AstroVIEWX (Astro-Med, Inc.).
In each rat, 3 - 4 one-second samples were analyzed. In each one, frequency of bursting, interburst interval , and burst duration were determined (Fig. 1C). Mean rectified intraburst amplitude and frequency were also determined with a threshold set of 0.2 μV according to the amplifier’s fiducial value before recording. A mean of each outcome variable was calculated for each animal. Group means for each experimental group were calculated from these values at each time point and are presented as mean ± standard error of the mean. One way ANOVA was used to test for significant differences in these variables between groups with Dunnett’s posthoc test and p < 0.05 indicating a significant difference (SAS 9.1; SAS Institute, NY).
Urethral and pudendal nerve histology
Intracardiac perfusion fixation was performed immediately after the electrophysiological recordings as previously described (Kerns et al. 2000). The pudendal nerve was dissected free, osmicated (2% osmium tetroxide), and prepared for embedding with the sectioning face 2-3 mm distal to the nerve crush site. It was post fixed, stained with aqueous 2% osmium tetroxide and 1% uranyl acetate dehydrated in graded alcohol rinses, and embedded in epoxy resin. It was then transversely sectioned (1 μm) on an ultramicrotome and stained with methylene blue-azure II. The EUS branch of the pudendal nerve was identified in the cross-section for qualitative analysis. The urethra and vagina were harvested en bloc and fixed in 10% neutral buffered formalin. The mid-urethra and anterior vagina were embedded in paraffin with the sectioning face located 6 mm distal to the urethral orifice. Full-thickness cross sections (5 μm) were deparaffinized, hydrated with distilled water and stained with Masson’s trichrome for qualitative assessment.
Results
Electrophysiological bursting during voiding in control rats
High frequency oscillations (HFO) in bladder pressure were identified during voiding in urethane-anesthetized uninjured control rats. Both the EUS EMG and the PNMBP demonstrated bursting activity during voiding associated with HFO in bladder pressure (Fig. 1). Each wave of the HFO was associated with the bursting event in both EUS EMG and PNMBP with approximately a 50 ms time delay between the middle of the bursting event and the peak of the pressure wave. Each EUS EMG bursting event was associated with PNMBP bursting with approximately a 10 ms time delay between the two (Fig. 1).
EUS EMG bursting during voiding after injury
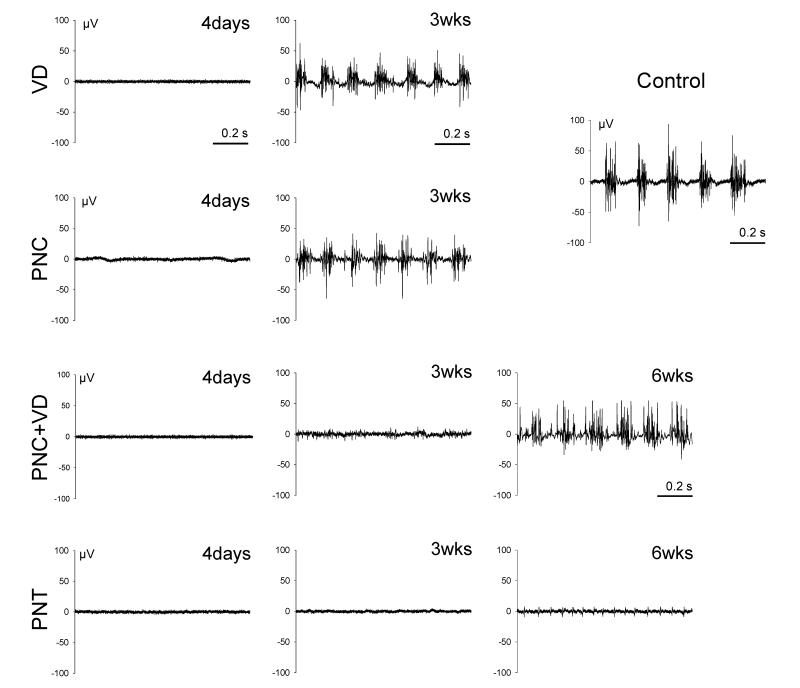
Four days after all injuries, EUS EMG demonstrated no bursting activity during voiding (Fig. 2). Likewise, none of the injury groups demonstrated HFO during voiding 4 days after injury. Three weeks after either VD or PNC, EUS EMG demonstrated recovery of bursting activity during voiding but the intraburst frequency was significantly decreased compared to controls (Table 1). In contrast, the intraburst amplitude was not significantly different compared to controls. The frequency of bursting was significantly increased 3 weeks after VD or PNC compared to controls, but the burst duration and the interburst interval were not significantly different (Table 1).
Fig. 2.
Examples of EUS EMG bursting activity (1 second duration) during voiding in controls and after vaginal distension (VD), pudendal nerve crush (PNC), both PNC and VD (PNC+VD), and pudendal nerve transection (PNT).
Table 1.
EUS EMG bursting parameters (mean ± SEM)
| Frequency of bursting (Hz) |
Interburst interval (ms) |
Burst duration (ms) |
Intraburst amplitude (μV) |
Intraburst frequency (Hz) |
||
|---|---|---|---|---|---|---|
| Control (n=13) | 5.73 ± 0.19 | 99.0 ± 10.6 | 69.9 ± 6.1 | 12.4 ± 1.9 | 531 ± 20 | |
| VD | 4-day (n=5) | 0 ± 0 | -- | -- | -- | -- |
| 3-week (n=5) | 7.25 ± 0.13* | 83.9 ± 11.6 | 59.5 ± 10.2 | 8.0 ± 1.8 | 372 ± 25* | |
| PNC | 4-day (n=5) | 0 ± 0 | -- | -- | -- | -- |
| 3-week (n=5) | 6.89 ± 0.31* | 70.7 ± 5.0 | 70.9 ± 2.5 | 7.0 ± 0.6 | 422 ± 34* | |
| PNC+VD | 4-day (n=5) | 0 ± 0 | -- | -- | -- | -- |
| 3-week (n=6) | 0 ± 0 | -- | -- | -- | -- | |
| 6-week (n=6) | 6.44 ± 0.20* | 58.4 ± 4.3* | 104.0 ± 4.8* | 13.0 ± 2.8 | 306 ± 36* | |
| PNT | 4-day (n=5) | 0 ± 0 | -- | -- | -- | -- |
| 3-week (n=6) | 0 ± 0 | -- | -- | -- | -- | |
| 6-week (n=6) | 0 ± 0 | -- | -- | -- | -- | |
indicates a significant difference compared to controls. -- indicates no bursting activity was recorded in EUS EMG and therefore, no value for the quantitative variables could be obtained. VD, vaginal distension; PNC, pudendal nerve crush; PNC+VD, pudendal nerve crush followed by vaginal distension; PNT, pudendal nerve transection.
Three weeks after PNC+VD, EUS EMG showed no bursting activity during voiding, but demonstrated recovered 6 weeks after injury (Fig. 2). As with both single injuries, the intraburst frequency of firing was significant decreased compared to controls but amplitude of the burst was not significantly different (Table 1). The frequency of bursting and the interburst interval were significantly increased compared to controls, whereas the interburst interval was significantly decreased. After PNT, EUS EMG could not be recorded at any time point (Fig. 2) consistent with complete bilateral denervation of the EUS.
PNMBP bursting during voiding after injury
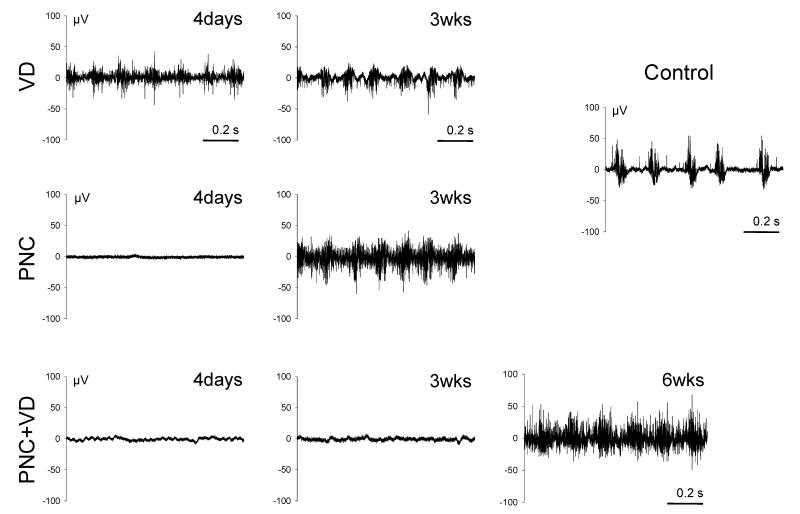
Four days after injury, only rats that underwent VD demonstrated bursting activity during voiding in the PNMBP signal (Fig. 3). Compared to controls (1038 ± 61 Hz), frequency of the potential within each burst (1365 ± 139 Hz, p = 0.0151) was significantly increased but amplitude (Control: 9.0 ± 1.2 μV; VD: 7.0 ± 1.7 μV) was not. Three weeks after either VD or PNC, the intraburst frequency was significantly increased compared to controls (VD: 1630 ± 139 Hz, p < 0.0001; PNC: 1357±126 Hz, p = 0.0175), but as at 4 days after VD, amplitude was not significantly different. Neither frequency (1037 ± 111 Hz) nor amplitude (8.9 ± 1.1 μV) was significantly different 6 weeks after PNC+VD compared to controls indicative of recovery. PNMBP testing was impossible after PNT since the pudendal nerve had been transected bilaterally.
Fig. 3.
Examples of pudendal nerve motor branch potentials (PNMBP) bursting activity (1 second duration) during voiding in controls and after vaginal distension (VD), pudendal nerve crush (PNC), both PNC and VD (PNC+VD), and pudendal nerve transection (PNT).
Histology
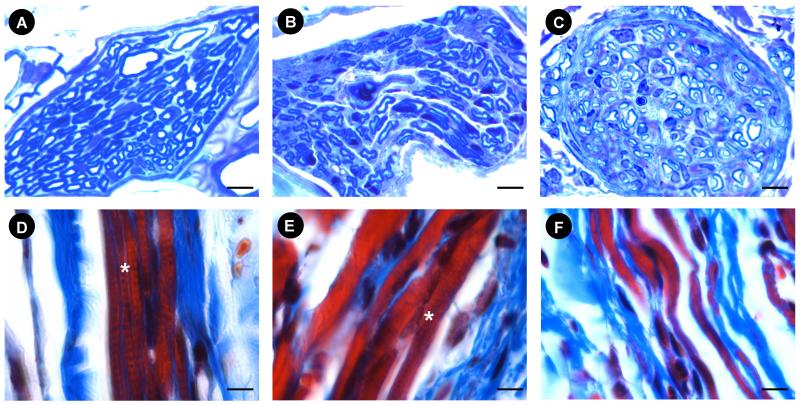
The pudendal nerve motor branch courses in Alcock’s canal and bifurcates into two branches to innervate the EUS and the external anal sphincter (EAS) (Fig. 4). The ventral branch of the PNMB incorporates the fibers which innervate the EUS and contains both myelinated and non-myelinated axons. Six weeks after PNC+VD, there were small myelinated axons with pudendal nerve motor branch distal to the crush site, but they demonstrated distorted myelin figures and Wallerian degeneration, suggesting that neuroregeneration was still in process (Fig. 5). Six weeks after PNT, the evidence of Wallerian degeneration distal to the transection site included distorted axons and myelin figures. The nerve was not as well recovered as 6 weeks after PNC+VD, indicating a more severe injury.
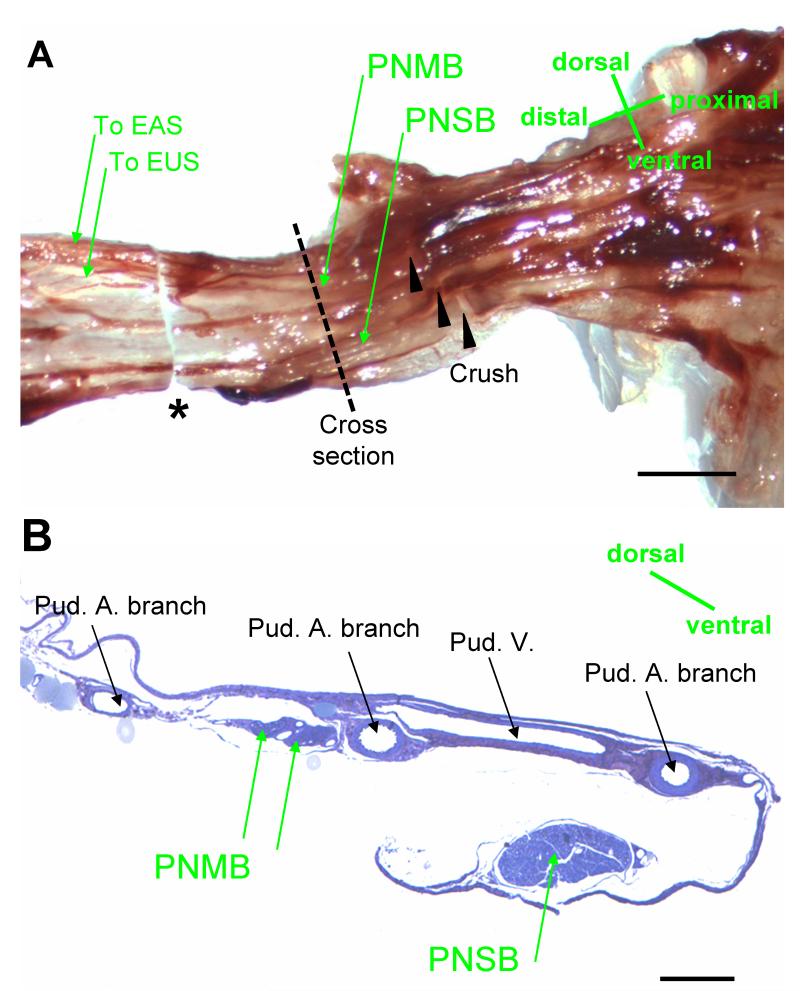
Fig. 4.
Nerves in Alcock’s canal in female rats. A. the pudendal nerve showing the location of nerve crush. The sample for embedding was indicated between the dashed line and * (the distal cut section). EUS: external urinary sphincter; EAS: external anal sphincter; PNMB: pudendal nerve motor branch; PNSB: pudendal nerve sensory branch. Bar = 1 mm. B. Alcock’s canal cross section at the proximal end of the sample shown in A (the dashed line), showing the PNMB, the PNSB, the pudendal artery (Pud. A), and the pudendal vein (Pud. V). Bar = 250 μm.
Fig. 5.
Examples of the pudendal nerve motor branch (PNMB) and external urethral sphincter (EUS) in controls (A, D); 6 weeks after PNC+VD (B, E), and 6 weeks after PNT (C, F). Nerve stain (A, B, C) = toluidine blue; Muscle stain (D, E, F) = Masson’s trichrome. * indicates the cross striation (D, E). Bar = 5 μm.
In controls, the striated muscle of the EUS contains several layers of striated myofibers bound continuously together around the urethral lumen with repeated and regular cross-striations (Fig. 5). Six weeks after PNC+VD, the EUS demonstrated recovery from injury since some of fibers demonstrate striations, although they are not as clear and trim as the striations in EUS of controls; while some of the fibers showed no striations (Fig. 5). Six weeks after PNT, the EUS showed typical signs of neurogenic atrophy. The EUS is thin, contains small muscle bundles, and no striations can be found (Fig. 5).
Discussion
Both the EUS and the pudendal nerve can be injured during vaginal delivery (Rogers and Leeman 2007) although the mechanistic pathway has not been identified. These injuries are associated with development of SUI (Goldberg et al. 2005). In this study, we used several simulated childbirth injuries to determine the effects on EUS and pudendal nerve function during filling CMG. Our recordings indicated that in the bursting signal in the EUS EMG is transferred from the pudendal nerve motor branch with a time delay when it transits the neuromuscular junction during voiding. Bursting activity in EUS EMG and rhythmic contractions and relaxations of the EUS during voiding have been demonstrated previously (Maggi et al. 1986b, Chang et al. 2007) and have been correlated with efficiency of bladder emptying. They serve a critical role in normal voiding in rats (Yoshiyama et al. 2000). Therefore, the presence of a bursting EUS EMG and PNMBP activity can also serve as a sign of recovery of functional voiding after injury.
In this study, both controls and injury groups were studied in the same urethral exposure, including removal of parts of the pubis and ischium. These procedures enable the simultaneous recordings of EUS EMG and PNMBP with a ventrolateral approach. PNMBP demonstrated bursting activity and can be used as a functional measure of nerve injury and extent of functional nerve recovery after injury. EUS EMG records motor unit activity in the muscle. It has been used as a functional measure of muscle injury and extent of reinnervation after injury (Chemali and Tsao 2005). Control rats and model rats were studied in the same urethral exposure condition. The main aim of this study was to determine if the micturition reflex recovery time was different when these models were applied in combination with one another and we were not assessing the effects of each individual injury. Thus, the establishment of sham arms to these models is not critically necessary.
Four days after VD, the bursting activity in EUS EMG disappeared since VD injures the EUS, as has been previously shown histologically (Pan et al. 2007). In contrast, the busting activity remained in PNMBP activity 4 days after VD, although there was a significant increase in the intraburst frequency compared to controls. This demonstrates that VD affects the pudendal nerve motor branch firing rate, likely as a short term response to the muscle injury to the EUS. It is also possible that the increased intraburst frequency could be affected by a reflexive response after VD since the damage of VD involves not only the EUS and the distal pudendal nerve, but also the afferent innervation of the vagina, cervix, pelvic diaphragm, and/or rectum via the pelvic, hypogastric, and/or other somatic nerves (Martinez-Gomez et al. 1992, Pacheco et al. 1989, Komisaruk et al. 1972, Peters et al. 1987).
Four days after the other injuries, including PNC, PNC+VD, and PNT, there was no bursting activity in either the EUS EMG or the PNMBP. Three weeks after the single injuries, VD or PNC, restoration of the bursting pattern during voiding in both EUS EMG and the PNMBP was observed. We have previously shown that 2 weeks represents an early time point of recovery after PNC or VD (Damaser et al. 2007, Pan et al. 2007), consist with the electrophysiological recordings in this study. The intraburst frequency of firing in the EUS EMG after injury was significantly lower than that of control. In contrast, the intraburst frequency of firing in the PNMBP recording was significantly increased compared to controls, suggesting that the firing rate of motor units in the PNMBP increase after injury to either the nerve or the EUS, likely to compensate for the injury because motor units are required and recruited in reflex or voluntary movement (Cope and Clark 1993). Nevertheless, the increased PNMBP intraburst frequency cannot bring about a proportionate increase in that of EUS EMG, but a decreased intraburst frequency. It shows insufficient transmission between PNMBP and EUS EMG activity compared to controls and may indicates the injury and variable repair between pudendal nerve and EUS still remains at the time point of the recordings.
Three weeks after a dual injury of PNC+VD, there was no bursting activity during voiding in both EUS EMG and PNMBP, indicating that neuroregeneration is slowed or delayed with the addition of VD to the PNC, likely because of the simultaneous injury to its target organ, the EUS. This mechanism could involve regulation of neurotrophins, which play a major role in regeneration of injured motoneurons (Yan et al. 1994, Friedman et al. 1995). After muscle injury, some neurotrophins are significantly decreased or even become undetectable, including brain derived neurotrophic factor (BDNF) and neurotrophins-4 (NT-4) to enable neuromuscular junction (NMJ) repair (Sakuma et al. 2001). The same neurotrophic factors, in contrast are upregulated in the target muscle after nerve injury (Funakoshi et al. 1995). Therefore, the same factors both inhibit NMJ reformation, and promote neuroregeneration if the nerve and muscle are injured simultaneously as likely occurs in the dual injury model and in vaginal childbirth. We postulate that the muscle injury delays nerve recovery. In addition, delayed nerve recovery can postpone muscle recovery since the nerve innervates the muscle.
Six weeks after PNC+VD, the recovery of some myelinated axons in the pudendal nerve motor branch distal to the crush site may account for the bursting activity in PNMBP observed during voiding. However, the distorted myelin and Wallerian degeneration indicate that they have not fully recovered. This is supported by functional data that the frequency of bursting, the burst duration, and the interburst interval showed significant differences compared to controls. Some of the EUS fibers did not show striations, indicating that they are denervated and are waiting for reinnervation. Bursting in EUS EMG may recover to control values with greater time after injury. In contrast, PNT is a permanent injury to the nerve. Six weeks after PNT, both the pudendal nerve and the EUS histology show extensive damage without evidence of regeneration or reinnervation. Likewise, bursting during voiding showed no signs of recovery in EUS EMG.
In this study, we demonstrated bursting activity during voiding in both the EUS EMG and PNMBP and recovery at different rates after different simulated child birth injuries. The recovery of bursting activity during voiding occurs more slowly after PNC+VD than after either PNC or VD alone, as was also observed in histology of the urethra and the pudendal nerve. The intraburst frequency was increased in PNMBP and decreased in EUS EMG after injury, suggesting that the activity of motor units does not transmit efficiently between the pudendal nerve and the EUS, since they remain in the process of repair, as demonstrated by histology.
Acknowledgements
This work was supported in part by NIH RO1 HD38679-08, the Cleveland Clinic, and the Rehabilitation Research and Development service of the Department of Veterans Affairs
References
- Chang HY, Cheng CL, Chen JJ, de Groat WC. Serotonergic drugs and spinal cord transections indicate that different spinal circuits are involved in external urethral sphincter activity in rats. Am. J. Physiol Renal Physiol. 2007;292:F1044–F1053. doi: 10.1152/ajprenal.00175.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HY, Havton LA. Re-established micturition reflexes show differential activation patterns after lumbosacral ventral root avulsion injury and repair in rats. Exp Neurol. 2008 doi: 10.1016/j.expneurol.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemali KR, Tsao B. Electrodiagnostic testing of nerves and muscles: when, why, and how to order. Cleve. Clin. J Med. 2005;72:37–48. doi: 10.3949/ccjm.72.1.37. [DOI] [PubMed] [Google Scholar]
- Cheng CL, de Groat WC. The role of capsaicin-sensitive afferent fibers in the lower urinary tract dysfunction induced by chronic spinal cord injury in rats. Exp. Neurol. 2004;187:445–454. doi: 10.1016/j.expneurol.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Cope TC, Clark BD. Motor-unit recruitment in self-reinnervated muscle. J Neurophysiol. 1993;70:1787–1796. doi: 10.1152/jn.1993.70.5.1787. [DOI] [PubMed] [Google Scholar]
- Damaser MS, Broxton-King C, Ferguson C, Kim FJ, Kerns JM. Functional and neuroanatomical effects of vaginal distention and pudendal nerve crush in the female rat. Journal of Urology. 2003;170:1027–1031. doi: 10.1097/01.ju.0000079492.09716.43. [DOI] [PubMed] [Google Scholar]
- Damaser MS, Samplaski MK, Parikh M, Lin DL, Rao S, Kerns JM. Time course of neuroanatomical and functional recovery after bilateral pudendal nerve injury in female rats. Am. J. Physiol Renal Physiol. 2007;293:F1614–F1621. doi: 10.1152/ajprenal.00176.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaser MS, Whitbeck C, Chichester P, Levin RM. Effect of vaginal distension on blood flow and hypoxia of urogenital organs of the female rat. J Appl. Physiol. 2005;98:1884–1890. doi: 10.1152/japplphysiol.01071.2004. [DOI] [PubMed] [Google Scholar]
- Dietz HP, Wilson PD. Childbirth and pelvic floor trauma. Best. Pract. Res. Clin. Obstet. Gynaecol. 2005;19:913–924. doi: 10.1016/j.bpobgyn.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Friedman B, Kleinfeld D, Ip NY, Verge VM, Moulton R, Boland P, Zlotchenko E, Lindsay RM, Liu L. BDNF and NT-4/5 exert neurotrophic influences on injured adult spinal motor neurons. J Neurosci. 1995;15:1044–1056. doi: 10.1523/JNEUROSCI.15-02-01044.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakoshi H, Belluardo E, Arenas E, Yamamoto Y, Casabona A, Persson H, Ibanez C. Muscle-derived neurotrophin-4 as an activity-dependent trophic signal for adult motor neurons. Science. 1995;268:1495–1499. doi: 10.1126/science.7770776. [DOI] [PubMed] [Google Scholar]
- Goldberg RP, Abramov Y, Botros S, Miller JJ, Gandhi S, Nickolov A, Sherman W, Sand PK. Delivery mode is a major environmental determinant of stress urinary incontinence: results of the Evanston-Northwestern Twin Sisters Study. Am. J. Obstet. Gynecol. 2005;193:2149–2153. doi: 10.1016/j.ajog.2005.08.055. [DOI] [PubMed] [Google Scholar]
- Hankins GD, Rowe TF. Operative vaginal delivery--year 2000. Am. J. Obstet. Gynecol. 1996;175:275–282. doi: 10.1016/s0002-9378(96)70135-7. [DOI] [PubMed] [Google Scholar]
- Jiang HH, Pan HQ, Gustilo-Ashby AM, Glaab GB, Zaszczurynski PJ, Damaser MS. Dual simulated childbirth injuries result in slowed recovery of pudendal nerve and urethral function. Neurourol. Urodyn. 2008 doi: 10.1002/nau.20632. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns JM, Damaser MS, Kane JM, Sakamoto K, Benson JT, Shott S, Brubaker L. Effects of pudendal nerve injury in the female rat. Neurourol. Urodyn. 2000;19:53–69. doi: 10.1002/(sici)1520-6777(2000)19:1<53::aid-nau7>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Komisaruk BR, Adler NT, Hutchison J. Genital sensory field: enlargement by estrogen treatment in female rats. Science. 1972;178:1295–1298. doi: 10.1126/science.178.4067.1295. [DOI] [PubMed] [Google Scholar]
- Kruse MN, Belton AL, de Groat WC. Changes in bladder and external urethral sphincter function after spinal cord injury in the rat. Am. J. Physiol. 1993;264:R1157–R1163. doi: 10.1152/ajpregu.1993.264.6.R1157. [DOI] [PubMed] [Google Scholar]
- Maggi CA, Giuliani S, Santicioli P, Meli A. Analysis of factors involved in determining urinary bladder voiding cycle in urethan-anesthetized rats. Am. J. Physiol. 1986a;251:R250–R257. doi: 10.1152/ajpregu.1986.251.2.R250. [DOI] [PubMed] [Google Scholar]
- Maggi CA, Santicioli P, Meli A. The nonstop transvesical cystometrogram in urethane-anesthetized rats: a simple procedure for quantitative studies on the various phases of urinary bladder voiding cycle. J. Pharmacol. Methods. 1986b;15:157–167. doi: 10.1016/0160-5402(86)90064-1. [DOI] [PubMed] [Google Scholar]
- Martinez-Gomez M, Chirino R, Beyer C, Komisaruk BR, Pacheco P. Visceral and postural reflexes evoked by genital stimulation in urethane-anesthetized female rats. Brain Res. 1992;575:279–284. doi: 10.1016/0006-8993(92)90091-m. [DOI] [PubMed] [Google Scholar]
- McKenna KE, Nadelhaft I. The organization of the pudendal nerve in the male and female rat. J. Comp Neurol. 1986;248:532–549. doi: 10.1002/cne.902480406. [DOI] [PubMed] [Google Scholar]
- McKenna KE, Nadelhaft I. The pudendo-pudendal reflex in male and female rats. J. Auton. Nerv. Syst. 1989;27:67–77. doi: 10.1016/0165-1838(89)90130-6. [DOI] [PubMed] [Google Scholar]
- Nassar AH, Usta IM, Khalil AM, Melhem ZI, Nakad TI, bu Musa AA. Fetal macrosomia (> or =4500 g): perinatal outcome of 231 cases according to the mode of delivery. J. Perinatol. 2003;23:136–141. doi: 10.1038/sj.jp.7210877. [DOI] [PubMed] [Google Scholar]
- Pacheco P, Martinez-Gomez M, Whipple B, Beyer C, Komisaruk BR. Somato-motor components of the pelvic and pudendal nerves of the female rat. Brain Res. 1989;490:85–94. doi: 10.1016/0006-8993(89)90433-2. [DOI] [PubMed] [Google Scholar]
- Pan HQ, Kerns JM, Lin DL, Liu S, Esparza N, Damaser MS. Increased duration of simulated childbirth injuries results in increased time to recovery. Am. J. Physiol Regul. Integr. Comp Physiol. 2007;292:R1738–R1744. doi: 10.1152/ajpregu.00784.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters LC, Kristal MB, Komisaruk BR. Sensory innervation of the external and internal genitalia of the female rat. Brain Res. 1987;408:199–204. doi: 10.1016/0006-8993(87)90372-6. [DOI] [PubMed] [Google Scholar]
- Rogers RG, Leeman LL. Postpartum genitourinary changes. Urol. Clin. North Am. 2007;34:13–21. doi: 10.1016/j.ucl.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Sakuma K, Watanabe K, Sano M, Uramoto I, Nakano H, Li Y-J, Kaneda S, Sorimachi Y, Yoshimoto K, Yasuhara M, Totsuka T. A possible role for BDNF, NT-4, and TrkB in the spinal cord and muscle of rat subjected to mechanical overload, bupivacaine injection, and axotomy. Brain Research. 2001;907:1–19. doi: 10.1016/s0006-8993(01)02288-0. [DOI] [PubMed] [Google Scholar]
- Yan Q, Matheson C, Lopez OT, Miller JA. The biological responses of axotomized adult motoneurons to brain-derived neurotrophic factor. Journal of Neuroscience. 1994;14:5281–5291. doi: 10.1523/JNEUROSCI.14-09-05281.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama M, deGroat WC, Fraser MO. Influences of external urethral sphincter relaxation induced by alpha-bungarotoxin, a neuromuscular junction blocking agent, on voiding dysfunction in the rat with spinal cord injury. Urology. 2000;55:956–960. doi: 10.1016/s0090-4295(00)00474-x. [DOI] [PubMed] [Google Scholar]