Abstract
Manganese(II)-oxidizing bacteria play an integral role in the cycling of Mn as well as other metals and organics. Prior work with Mn(II)-oxidizing bacteria suggested that Mn(II) oxidation involves a multicopper oxidase, but whether this enzyme directly catalyzes Mn(II) oxidation is unknown. For a clearer understanding of Mn(II) oxidation, we have undertaken biochemical studies in the model marine α-proteobacterium, Erythrobacter sp. strain SD21. The optimum pH for Mn(II)-oxidizing activity was 8.0 with a specific activity of 2.5 nmol × min−1 × mg−1 and a Km = 204 µM. The activity was soluble suggesting a cytoplasmic or periplasmic protein. Mn(III) was an intermediate in the oxidation of Mn(II) and likely the primary product of enzymatic oxidation. The activity was stimulated by pyrroloquinoline quinone (PQQ), NAD+, and calcium but not by copper. In addition, PQQ rescued Pseudomonas putida MnB1 non Mn(II)-oxidizing mutants with insertions in the anthranilate synthase gene. The substrate and product of anthranilate synthase are intermediates in various quinone biosyntheses. Partially purified Mn(II) oxidase was enriched in quinones and had a UV/VIS absorption spectrum similar to a known quinone requiring enzyme but not to multicopper oxidases. These studies suggest that quinones may play an integral role in bacterial Mn(II) oxidation.
Keywords: manganese oxidation, PQQ, erythrobacter, multicopper oxidase
INTRODUCTION
It is important to understand the fundamental mechanism of bacterial manganese(II) oxidation because it plays a role in many biogeochemical cycles (Tebo et al. 2004). The amorphous biogenic Mn(IV) oxides produced by Mn(II)-oxidizing bacteria are characterized by their strong reactivity, oxidizing metals and organic molecules such as chromium(III)(Murray and Tebo 2007) and phenolic compounds. In addition to the strong oxidative nature of Mn(IV) oxides, the structure of the oxides leads to the adsorption and sequestration of heavy metals (e.g., Cu, Co, Cd, Zn, Ni, and Pb) resulting in potential applications of Mn(IV) oxides in bioremediation (Tebo et al. 2004).
The physiological basis of microbial Mn(II) oxidation remains enigmatic. Many hypotheses have been put forth on the utility of Mn oxide formation for Mn(II)-oxidizing bacteria. Potential roles range from environmental protection to energy utilization. There may be a common role for Mn(II) oxidation, or the role of Mn(II) oxidation may be as diverse as the bacteria that catalyze this reaction. The three most studied Mn(II)-oxidizing microorganisms represent this diversity(Tebo et al. 2005). Vegetative cells of the γ – proteobacteria Pseudomonas putida MnB1 and GB1 oxidize Mn(II) during stationary phase, the gram positive Bacillus sp. SG-1 oxidizes Mn(II) as “dormant” spores, and the sheathless Leptothrix discophora SS1 secretes Mn(II)-oxidizing activity into the culture medium. Despite these physiological and phylogenetic differences, a multicopper oxidase (MCO) gene was shown to be involved in Mn(II) oxidation in each of these strains (van Waasbergen et al. 1996; Corstjens and De Vrind 1997; Brouwers et al. 1999). Recently, a fourth MCO has been shown to be required for Mn(II) oxidation in the α – proteobacterium Pedomicrobium sp. ACM 3067 (Ridge et al. 2007). In all four of these model organisms these MCOs are hypothesized to encode the catalytic Mn(II) oxidase. Yet, the inability to purify the native Mn(II) oxidase (Adams and Ghiorse 1987; Okazaki et al. 1997) or to express an active Mn(II) oxidase in a heterologous host has largely prevented the biochemical characterization of this interesting enzymatic activity. The first published attempt to purify this protein was in 1987 (Adams and Ghiorse 1987), and 20 years later we are still struggling with this problem. The low native expression levels and large losses in activity with protein separation has hindered our ability to understand and investigate this activity. The roadblocks to purifying and expressing an active Mn(II) oxidase have also prevented researchers from making a conclusive link between the MCO genes and the enzymatic activity.
MCOs are characterized by their spectrally distinct Cu atoms as well as by sequence homology(Solomon et al. 1996). The Cu atoms are integral in catalyzing successive one electron transfers from the substrate to reduce molecular oxygen to water. Most MCOs oxidize organic compounds, especially phenolic compounds, however MCO’s have also been shown to oxidize Fe(II) in yeast (Fet3) (Askwith et al. 1994), bacteria (Huston et al. 2002), and humans (ceruloplasmin) (Lindley et al. 1997). In addition, fungal MCOs (laccases) (Hofer and Schlosser 1999; Schlosser and Hofer 2002) have been described that can oxidize Mn(II) to Mn(III). Some MCOs such as ceruloplasmin show a high degree of substrate specificity and others such as laccase possess a more relaxed substrate specificity oxidizing many different substrates including metals and phenolic compounds (Stoj and Kosman 2003) (Solano et al. 2001) (Kim et al. 2001). Fe(II) oxidation and fungal Mn(II) oxidation by MCOs serve as models for bacterially mediated Mn(II) oxidation. But it is also useful to consider additional mechanisms of Mn(II) and Fe(II) oxidation that could lead to insight into microbial Mn(II) oxidation as well as provide an additional experimental context. For instance, Mn(II) oxidation by fungal Mn peroxidases employ peroxide and a Mn(III) chelator for the oxidation of Mn(II), and Fe(II) can be oxidized directly by cytochromes (Cobley and Haddock 1975), type I copper enzymes (Cox and Boxer 1978), iron-sulfur proteins (Fukumori et al. 1988), and by the iron storage protein ferritin (Harrison and Arosio 1996).
Studies on Mn(II) oxidation in α-proteobacteria have been limited, yet the effect of copper on Mn(II) oxidation in whole cells (Larsen et al. 1999), the non Mn(II) oxidizing phenotype of a MCO mutant (Ridge et al. 2007), and the effect of copper chelators on Mn(II) oxidation in acrylamide gels (Francis et al. 2001) suggest copper may play an important role in Mn(II) oxidation in this phylogenetic group. The marine α-proteobacterium Erythrobacter sp. strain SD21 (Francis et al. 2001) is a Mn(II) oxidizer and an excellent model for Mn(II) oxidation studies. The in vitro Mn(II)-oxidizing activity of this strain is relatively robust and stable (Francis et al. 2001), and therefore well-suited for biochemical characterization and purification. This organism is a close phylogenetical relative of aerobic anoxygentic phototrophs. Aerobic anoxygenic phototrophs are estimated to comprise 11% of the microbial community in the ocean and have been implicated in as much as 5–10% of the photosynthetic potential in the oceans (Kolber et al. 2001; Goericke 2002). Because of their important role in the oceans, understanding how this group of organisms may oxidize Mn(II) is especially intriguing and relevant.
In this paper we describe the use of traditional biochemical techniques to understand Mn(II) oxidation in the marine Erythrobacter sp. SD21. In vitro studies on Mn(II) oxidation presented here indicate that previously unexpected cofactors are involved in Mn(II) oxidation – specifically, the quinone PQQ (pyroloquinoline quinone). Many of the results of our studies on the Mn(II) oxidase in this microorganism suggest similarities to known MCOs, yet there are striking inconsistencies and new findings which indicate that this Mn(II) oxidase is a quinoprotein and may represent a new class of Mn(II)-oxidizing enzymes.
MATERIAL AND METHODS
Bacterial media, and growth conditions
Erythrobacter sp. SD21 (Francis et al. 2001) was grown in 2.8 L Fernbach flasks containing 1 L of natural seawater based K medium (van Waasbergen et al. 1993) on an orbital shaker. When additional copper was added to the media it was added as 10, 50, or 100 µM CuCl2. For growth experiments, optical density was measured at OD600 approximately every 12 hours.
Preparation and fractionation of cell-free extracts of Erythrobacter sp. SD21
Cells of Erythrobacter sp. SD21 were grown to the beginning of stationary phase at an OD600 of 0.5. Cells were harvested by centrifugation (10,000 × g for 20 min at 4°C), washed in 100 ml 20 mM HEPES pH 8 buffer, recentrifuged, and resuspended in 2–4 ml 20 mM HEPES pH 8 buffer. When harvesting more than 10 liters, the initial centrifugation was replaced by tangential flow filtration with 0.2 micron filter cassettes. DNAse (1000 U) was added to the washed cells which were then broken by 3 passages through a French pressure cell at 130 MPa. The broken cells were centrifuged in a Beckman tabletop ultracentrifuge (200,000 × g for 2 hours at 4°C). The soluble cell-free extract (the supernatant, 5–10 mg × ml−1 protein) was used immediately or frozen in small aliquots at −20°C. When specifically noted, cell-free extract was dialyzed with 50,000 MWCO membranes overnight at 4°C in 20 mM HEPES, pH 8, and 20% glycerol. Membrane fractions were prepared by centrifuging the broken cells at 12,000 × g for 30 min at 4°C. The supernatant was removed and centrifuged again at 200,000× g for 2 h at 4°C. The solid pellet was resuspended in 20 mM HEPES pH 8 buffer to a volume equal to the precentrifugation volume and defined as the membrane fraction.
Preparation of cell-free extracts of Pseudomonas putida MnB1
1L cultures of Pseudomonas putida MnB1 were grown to stationary phase in LEPT media (Boogerd and de Vrind 1987). Cells were harvested by centrifugation (10,000 × g for 20 min at 4°C), washed in 100 ml 20 mM HEPES pH 8 buffer, recentrifuged, and resuspended in 15 ml 20 mM HEPES pH 8 buffer. The culture was sonicated on ice for 30 minutes at 20 kHz, 50% pulse (Okazaki et al. 1997). Cell-free extract was obtained as the supernatant from centrifugation (12,000 × g for 15 min at 4°C). The cell-free extract was used immediately or frozen in small aliquots at −20°C.
Mn(II) oxidase assay
The Mn(II) oxidase assay contained approximately 0.5–1.0 mg/ml protein as soluble cell-free extract in a 20 mM HEPES pH 8 buffer, 2 mM MnSO4, 10 µM pyrroloquinoline quinone (PQQ), 200 µM NAD+, and 15 mM CaCl2 unless otherwise noted. Assays were incubated at room temperature on a shaker. To determine the effects of components added to the assay as shown in Table 1, PQQ and NAD+ were omitted. Calcium was omitted from the control to determine the effect of calcium and magnesium, but was included with both the control and tested compound for all other assays. Oxidized Mn was measured colorimetrically using leukoberbelin blue (LBB) (Okazaki et al. 1997). When LBB is oxidized by Mn(III,IV) it forms a blue product. At discrete time points, 50 µL of the assay solution was removed and added to 250 µL 0.04% LBB in 45 mM acetic acid, the assays were incubated for a minimum of 15 minutes for complete reduction of oxidized Mn, centrifuged to remove precipitated protein, and the A620 determined. Duplicat assays varied less than 10%. KMnO4 was used as a standard. There was no background oxidation of Mn(II) in the absence of cell-free extract. Values reported as % of control are the average of two or more replicates.
Table 1.
The effect of various components on the in vitro Mn(II)-oxidizing activity. Control activity is as described in Materials and Methods. % values are an average of a minimum of 2 replicates.
| Component | Effect on Activity (% of control activity) |
|---|---|
| Cofactors/coenzymes | |
| PQQ (10 µM) | 824 |
| NADH (200 µM) | 199 |
| NAD+ (200 µM) | 185 |
| NAD+ (200 µM) + PQQ (10 µM) | 1829 |
| Cytochrome c (10 µM)* | 49 |
| ATP, Mg2+ (2mM, 2 mM)* | 46 |
| Coenzyme Q10 (10 µM) | 100 |
| p-benzoquinone (10 µM) | 118 |
| 1,4-naphthoquinone (10 µM) | 164 |
| Metals | |
| Calcium (15 mM) | 140 |
| Magnesium (15 mM) | 100 |
| Cu2+ (3,5,10 µM) | 100,100,100 |
| Cu2+ (10 µM)* | 55 |
| Cu+ - histidine (10 µM)* | 100 |
| Zn2+ (10 µM)* | 88 |
| Proteases | |
| Trypsin (100 µg/ml, 3 hours) | 17 |
| Proteinase K (100 µg/ml, 3 hours) | 17 |
| Protease from Streptomyces griseus (100 µg/ml, 3 hours) | 15 |
Dialyzed cell-free extract was used in assay.
To determine particulate oxidized Mn, samples were filtered with a 0.2 µm filter to retain particulate Mn. The filters were then washed 3 times with manganese free 20 mM HEPES pH 8 buffer. Total Mn on the filters was then determined. This value was divided by the volume filtered to determine the concentration of particulate oxidized Mn.
The buffers used for optimum pH determination were 20 mM MES pH 5.2, 5.7, 6, 6.4; 20 mM HEPES pH 6.8, 7, 7.6, 7.9, and 8.4. For kinetic studies, all assays at different Mn(II) concentrations were performed in duplicate with oxidized Mn measured at 6–7 time points, each 25 minutes apart. The oxidation rate was linear during this time. Km and Vmax were determined with the graphing software Kaleidagraph (Synergy Softwared) fitted to the Michaelis-Menten kinetic equation.
For inhibitor studies, fresh stocks of 1mM and 10 mM phenylhydrazine, sodium cyanide, o-phenanthroline, and sodium azide were made and added as aliquots to the assay mixture. Phenylhydrazine is oxidized by biogenic Mn oxides, thus the amount of oxides reduced by a given amount of phenylhydrazine in the Mn(II) oxidase assay was determined. Inhibition would be considered to occur if the activity was inhibited in excess of that which would be due to a reaction between oxides and phenylhydrazine. For instance 10 µM phenylhydrazine would reduce the A620 of the LBB measurement by 0.02.
Mn(III)-pyrophosphate trapping experiments were executed as described in Webb, et al (Webb et al. 2005). Mn(II) oxidation by cell-free extracts was performed as described above except in a 5 ml stirred assay with 0.25 mg cell-free extract. 40 µM sodium pyrophosphate was added as the trapping agent and calcium was omitted from the assay to prevent precipitation. The absorbance spectrum was recorded every 5 minutes with an Ocean Optics fiber-optic dip probe spectrometer. Mn(III) pyrophosphate was measured at 458 nm (ε = 6750 M−1).
Protease treated soluble cell-free extract was prepared by incubating 100 µg/ml trypsin, proteinase K, or protease with the soluble cell-free extract for 30 minutes to 3 hours at room temperature. The treated protein was assayed immediately or stored on ice until assaying.
Other assays
Protein concentration was determined by the method of Bradford (Bradford 1976) with bovine serum albumin (BSA) as the standard using a commercially available dye-binding assay (Bio-Rad, Hercules, Calif.). Malate dehydrogenase (Stams et al. 1984) and ATPase (Barnett 1970) activities were determined in spectrophotometric assays by monitoring the absorbance at 340 nm. Quinone concentration was determined by the spectrphotometic NBT/glycinate redox assay (Fluckiger et al. 1995) with authentic PQQ used as the standard. PQQ was determined by the reconstitution of the soluble form of glucose dehydrogenase apoenzyme (van der Meer et al. 1990).
Partial purification of the Mn(II) oxidase
Mn(II) oxidase was partially purified from soluble cell-free extracts of Erythrobacter sp. SD21 prepared as described above. Approximately 10 L of cells were used to obtain the partially purified fraction. 1 ml fractions were collected in all cases with the chromatography at 4° C unless otherwise noted. 20 mls of soluble cell-free extract were injected, 2 ml per run, onto a 24 mL Superose 6 HR 10/30 gel filtration column equilibrated with 40 mM HEPES pH 8, 0.12 M NaCl, 20% glycerol, and 0.1 mg/ml BSA at 0.15 ml/min. The active fractions were combined, concentrated by ultrafiltration (MWCO = 50,000), and the buffer exchanged to 20 mM TRIS pH 7.5, 20% glycerol, and 1 M (NH4)2SO4. The active fraction was then filtered with a 0.2 µm PVDF filter, injected onto two 1ml HiTrap Phenyl HP columns in series equilibrated with room temperature 16 mM TRIS 20% glycerol, 0.8 M (NH4)2SO4, and 1 mg/ml dextran. Proteins were eluted with a decreasing linear gradient of (NH4)2SO4. Active fractions were pooled, concentrated by ultrafiltration (MWCO = 50,000), and buffer exchanged to 20 mM TRIS pH 8, and 20% glycerol. The sample was filtered with a 0.2 µm PVDF filter and then injected onto an anion exchange column (1ml HiTrapQ) equilibrated with 20 mM TRIS pH 8, 20% glycerol, and 0.1 mg/ml avidin. The Mn(II) oxidizing activity eluted in the unbound fraction. This fraction is referred to as the partially purified Mn(II) oxidase.
Gel electrophoresis
Denaturing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out by standard procedures using 8% acrylamide/bis acrylamide separating and 4% acrylamide/bis acrylamide stacking gels (Garfin 1990). Molecular mass standards were obtained from Bio-Rad. Proteins were visualized by Coomassie blue R-250 staining. Mn(II) oxidation zymograms were obtained as described previously (Francis et al. 2001). Protein was reduced, but not heat denatured for the gels stained for Mn(II) oxidation activity.
Absorption spectrum
The spectrum of the partially purified Mn(II) oxidase was recorded on a Perkin Elmer Lambda Bio 20 UV/VIS spectrometer.
Chemicals and solutions
Chemicals of the highest grade were purchased from Sigma-Aldrich or Bio-Rad. All solutions were prepared with milli-Q water (Millipore) or in 20 mM HEPES pH 8. FPLC columns were obtained from GE Healthcare. BSA for protein purification buffers was the initial fractionation from cold ethanol precipitation (Sigma). Avidin used for protein purification was affinity purified (Sigma). Soluble glucose dehydrogenase apoenzyme was obtained from J.A. Duine.
RESULTS
Mn(II) oxidase activity
Soluble cell-free extracts of Erythrobacter sp. SD21 catalyzed the oxidation of Mn(II) as measured by the colorimetric reaction with LBB. When Mn(II) was omitted from the assay or when cell-free extracts were heat treated by boiling for 5 minutes, there was no detectable Mn(II)-oxidizing activity. The activity showed a linear dependence on protein concentration. Incubating the cell-free extracts with proteases for 0.5 to 3 hours resulted in an 83–85% reduction in activity (Table 1) suggesting that the enzyme is sensitive to proteases and that the oxidation of Mn(II) by this organism is indeed enzymatically catalyzed. The Mn(II)-oxidizing protein was stable at room temperature and on ice, but the activity decreased when assayed following incubations for 30 minutes or longer at 30° C or higher. Maximum activity occurred at pH 8 and in the presence of PQQ, Ca2+, and NAD+. Based on Michaelis Menten kinetic parameters, the maximum rate of Mn(II) oxidation was 2.5 ± 0.12 nmol as MnO2 equivalents × min−1 × (mg cell-free extract protein)−1 with an apparent Km for Mn(II) of 204 ± 43 µM. The maximum in vitro rate of Mn(II) oxidation was approximately 4 times greater than the in vivo rate.
Localization of Mn(II) oxidase activity
Localization of the Mn(II)-oxidizing activity from Erythrobacter sp. SD21 by ultracentrifugation yielded a soluble Mn(II) oxidase with 87% of the activity present in the supernatant (Table 2). The distribution of the activity between the soluble and pellet fractions is consistent with the soluble control enzyme, malate dehydrogenase, and suggests that the Mn(II) oxidase is cytoplasmic, periplasmic, or a very loosely associated peripheral membrane protein. Mn(II)-oxidizing activity can also be detected in the culture supernatant following approximately 1000 fold concentration of the protein in the culture supernatant. This activity comigrates in SDS-PAGE gels stained for Mn(II)-oxidizing activity with the cell associated activity, suggesting the Mn(II)-oxidizing activity is very loosely associated with the cell surface or Mn(II)-oxidizing activity is stable following natural cell lysis that may occur during late exponential phase.
Table 2.
Localization of Mn(II) oxidase in Erythrobacter sp. SD21.
| Fraction | Mn(II) oxidase (µM/min) |
ATPase (µM/min) |
Malate dehydrogenase (µM/min) |
|---|---|---|---|
| Total cell-free extract | 0.0097 (100%) | 8.29 (100%) | 609 (100%) |
| Soluble | 0.0085 (87%) | 0 (0%) | 493 (81%) |
| Pellet | 0.0024 (25%) | 3.79 (46%) | 195 (32%) |
ATPase and malate dehydrogenase activities were used as membrane and soluble markers, respectively.
Mn(III) as an intermediate in Mn(II) oxidation in Erythrobacter sp. SD21
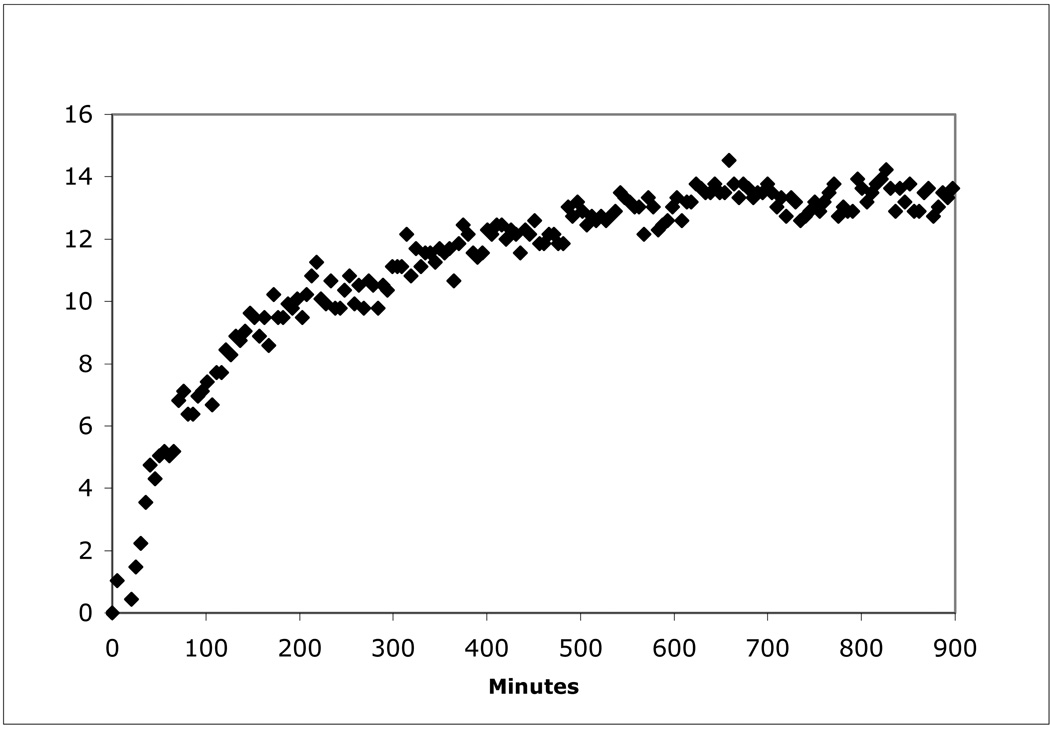
The product of in vitro Mn(II) oxidation is soluble and greater than 95% of oxidized Mn can pass through a 0.2 micron filter, a common technique to detect particulate Mn, suggesting a colloidal Mn(IV) oxide or a soluble Mn(III) species is formed from enzymatic oxidation. The characteristic brown color of Mn(IV) oxides did not develop during the in vitro assay, thus the product is likely to be Mn(III). Mn(III) could be stabilized by chelating with organic acids in the cell-free extract. Using the pyrophosphate trapping protocol (Webb et al. 2005), Mn(III)-pyrophosphate was observed to form in the Mn(II) oxidase assay, further suggesting that Mn(III) is an intermediate in the oxidation of Mn(II) to Mn(IV) oxides. The absorbance of the Mn(III)-pyrophosphate complex did not decrease with time (Figure 1) as was demonstrated with Bacillus sp. SG-1 (Webb et al. 2005) even when the experiment was extended to over 30 hours. In addition, when cells are grown on agar plates, Mn oxides are not confined to the colonies, but are present in a halo around the colony consistent with the production of a diffusible oxidized species such as complexed Mn(III). These experiments and observations suggest that Mn(III) is the product of the enzymatic oxidation and the formation of Mn(IV) oxides by whole cells and in protein gels (Francis et al. 2001) likely occurs by disproportionation of Mn(III).
Figure 1.
Formation of Mn(III) as an intermediate in the oxidation of Mn(II) to Mn(IV) oxides as demonstrated by the pyrophosphate trapping of Mn(III) (Mn(III)-PP). Note that the concentration of Mn(III)-PP does not decrease with time suggesting it is the final product of the enzymatic oxidation of Mn(II). In the absence of Mn(II) there was no change in absorbance. The Mn(III)-PP profile shown is the result of the absorbance in the presence of 40 µM pyrophosphate minus the absorbance in the absence of pyrophosphate. Increasing the concentration of pyrophosphate leads to precipitation and increased noise in the data. The Mn(II) oxidation rate under these conditions was 2 nmol/min/mg. The lower rate can be largely accounted for by the absence of calcium in the assay.
Optimization of the Mn(II) oxidase assay and identification of cofactors
The effect of copper on in vivo and in vitro Mn(II) oxidation was investigated to determine whether copper could stimulate Mn(II) oxidation in Erythrobacter sp. SD21. This stimulation has been shown in other Mn(II)-oxidizing bacteria (van Waasbergen et al. 1996; Larsen et al. 1999; Brouwers et al. 2000a; Zhang et al. 2002). With growing whole cells of Erythrobacter sp. SD21, copper (10–100 µM) addition to the growth media did not affect growth or Mn(II) oxidation. For cultures grown with 50 or 100 µM added Cu(II), the in vitro specific activity of Mn(II) oxidation was determined and found to be the same as in cell extract from cells grown in the absence of copper. Adding Cu(II)Cl2 or Cu(I)-histidine to the in vitro enzyme assay did not affect the rate of Mn(II) oxidation (see Table 1). Both redox states of Cu were tested as the redox state of the Cu may affect loading of the apoenzyme (Musci et al. 1996). Altering the ratio of Cu(II) to Mn(II) (5–1000 nM Cu(II) with 1 mM or 100 µM Mn(II)) also had no effect on in vitro activity. Following buffer exchange by dialysis or ultrafiltration (30,000 MWCO), Mn(II)-oxidizing activity was partially lost. Adding CuCl2 (3 or 5 µM) to the assay did not increase the recovered activity.
We tested the Mn(II)-oxidizing activity of Erythrobacter sp. SD21 to see if this activity was similar to fungal Mn peroxidases and stimulated by H2O2. Peroxide addition (10 µM) had no effect on in vitro Mn(II) oxidation indicating that the bacterial Mn(II) oxidase is not a peroxidase.
When the cell-free extract was separated by anion exchange under “harsh” conditions (in the absence of glycerol and molecular crowding agents), all Mn(II)-oxidizing activity was lost, but the activity could be recovered by combining separated fractions (the unbound fraction with the fraction eluting at 0.4M NaCl with anion exchange). P. putida MnB1 mutants with disruptions in anthranilate synthase had non Mn(II)-oxidizing phenotypes (Caspi et al. 1998) suggesting a quinone may be involved in Mn(II) oxidation (See Effect of PQQ and NAD+ on Mn(II) oxidation in P. putida MnB1), and in fact, PQQ could substitute for the fraction eluting with 0.4 M NaCl and restore activity. Because PQQ was required for activity under these conditions, its potential role in Mn(II) oxidation was investigated. Addition of PQQ to the in vitro Mn(II) oxidation assay increased the activity over 8-fold (see Table 1). PQQ in the absence of cell-free extract from Erythrobacter sp. SD21 did not oxidize Mn(II). When PQQ was added to cell extract of a non Mn(II)-oxidizing strain such as E. coli (G. Dick and B. Tebo, unpublished data) no oxidized Mn was detected. The effect of PQQ concentration in the cell-free extract assay was investigated from 4 to 80 µM, with an optimum effect at 10 µM. Addition of Ca2+ also stimulated activity. This effect may be related to the effect of PQQ (see discussion). We examined other electron acceptors with redox potentials similar to PQQ (E°’ = +90 mV) (Anthony 1996) such as p-benzoquinone (E°’ = +293 mV) and 1,4-naphthoquinone (E°’ = +36 to +64 mV). None of these electron acceptors were able to stimulate the activity to the same extent as PQQ (Table 1).
Interestingly, both NAD+ and NADH stimulated Mn(II) oxidase activity with soluble cell-free extracts. Monitoring A340 for the appearance or disappearance of NADH indicated there was little to no change in the absorbance during the course of the reaction under aerobic or anaerobic conditions. Therefore, oxidation and reduction of NAD+/NADH was either very rapidly cycled during Mn(II) oxidation or it did not occur. Although NAD+ addition was not required for activity in soluble cell-free extracts, the rate of the reaction was dependent on NAD+ in a manner similar to the dependence shown for a substrate with Michealis Menten kinetics (data not shown).
Multicopper oxidase and quinone inhibitors affect Mn oxidation
Bacterial Mn(II) oxidation has been shown to be inhibited by a number of compounds which affect the activity of MCOs, and that inhibition has supported the hypothesis that Mn(II) oxidation is catalyzed by a MCO. The effect of sodium cyanide, o-phenanthroline, and sodium azide on in vitro Mn(II) oxidation in Erythrobacter sp. SD21 was investigated (Table 3). Sodium azide and sodium cyanide are known to inhibit MCOs and quinoproteins (Solomon et al. 1996; Zaitsev et al. 1999), and high concentrations of these inhibitors did inhibit Mn(II) oxidation. Surprisingly, o-phenanthroline, a specific copper chelator and known inhibitor of Mn(II) oxidation in other species, only partially inhibited Mn(II) oxidation at high concentrations—much higher than was required to inhibit Mn(II) oxidation in partially purified preparations of Pseudomonas putida GB-1 (Okazaki et al. 1997). Hydrazines covalently bind to PQQ and are known inhibitors of PQQ utilizing enzymes (van der Meer et al. 1987; Oubrie et al. 1999). Phenylhydrazine inhibited Mn(II) oxidation, and was a very potent inhibitor (10 µM completely inhibited the reaction) when additional PQQ was omitted from the assay. Addition of 10 µM PQQ to the inhibited assay could restore Mn(II) oxidation, suggesting the inhibition by phenylhydrazine is specific for PQQ.
Table 3.
The effect of multicopper oxidase and quinone inhibitors on in vitro Mn(II) oxidation.
| Inhibitor | Rate of Mn(II) oxidation in cell-free extracts of Erythrobacter sp. SD21 (% of activity without inhibition) |
|
|---|---|---|
| Cyanide | 100 µM, 1 mM | 76, 19 |
| o-phenanthroline | 10, 100 µM | 100, 68 |
| Azide | 100 µM, 1 mM | 33, 2 |
| Phenylhydrazine | 20 µM* | 0 |
in the absence of exogenously added 10 µM PQQ. In all other cases, Mn(II) oxidase activity is measured as described in Materials and Methods.
Partial purification of the Mn(II) oxidase from Erythrobacter sp. SD21
Only partial purification of a bacterial Mn(II)-oxidizing enzyme has been achieved to date (Adams and Ghiorse 1987; Okazaki et al. 1997). By using gel filtration, hydrophobic interaction chromatography, and anion exchange chromatography under very “gentle” conditions (such as 20% glycerol and with molecular crowding agents), partial purification was also achieved for the Mn(II) oxidase from Erythrobacter sp. SD21. Complete purification was hindered by unusual responses to chromatographic techniques that are likely a result of interference by the carotenoids and pigments associated with this strain and overall large losses in activity upon protein separation. Mn(II)-oxidizing enzymes are thought to be large complexes (Brouwers et al. 2000b). The activity, as identified from Mn(II)-oxidizing zymograms, migrates in SDS-PAGE gels as a 250 kDa protein (Francis et al. 2001), and an intact complex may be necessary for activity. This is consistent with what was observed during protein purification. The activity could be recovered in greater abundance by using 20% glycerol in all buffers, and molecular crowding agents such as BSA, avidin, and dextran were often required additions to buffers during chromatographic separations to maintain activity. These “gentle” conditions should optimize folding and complex stability (Cheung et al. 2005). Because of the use of protein molecular crowding agents during purification, the specific activity could not be directly determined (Table 4), however, an estimate of the specific activity of the partially purified Mn(II) oxidase can be made based on the protein in the final preparation and the amount of protein added to the buffers for the anion exchange step. This estimate suggests an increase in specific activity to 28 – 56 nmol/min/mg with a 10 – 24 fold purification of activity. The degree of purification can also be assessed by SDS-PAGE, which indicated that although many proteins have been eliminated compared to the soluble cell-free extract, there are still approximately 10 protein bands present in the partially purified Mn(II) oxidase fraction.
Table 4.
A representative partial purification of the Mn(II) oxidase from Erythrobacter sp. SD21.
| Purification step | Activity (nmoles/min) | Activity Recovered (%) |
|---|---|---|
| Soluble cell-free extract | 440 | 100 |
| Gel filtration with BSA | 440 | 100 |
| HIC with dextran | 88 | 20 |
| Anion exchange with avidin | 6.16 | 1.4 |
Characterization of the partially purified Mn(II) oxidase
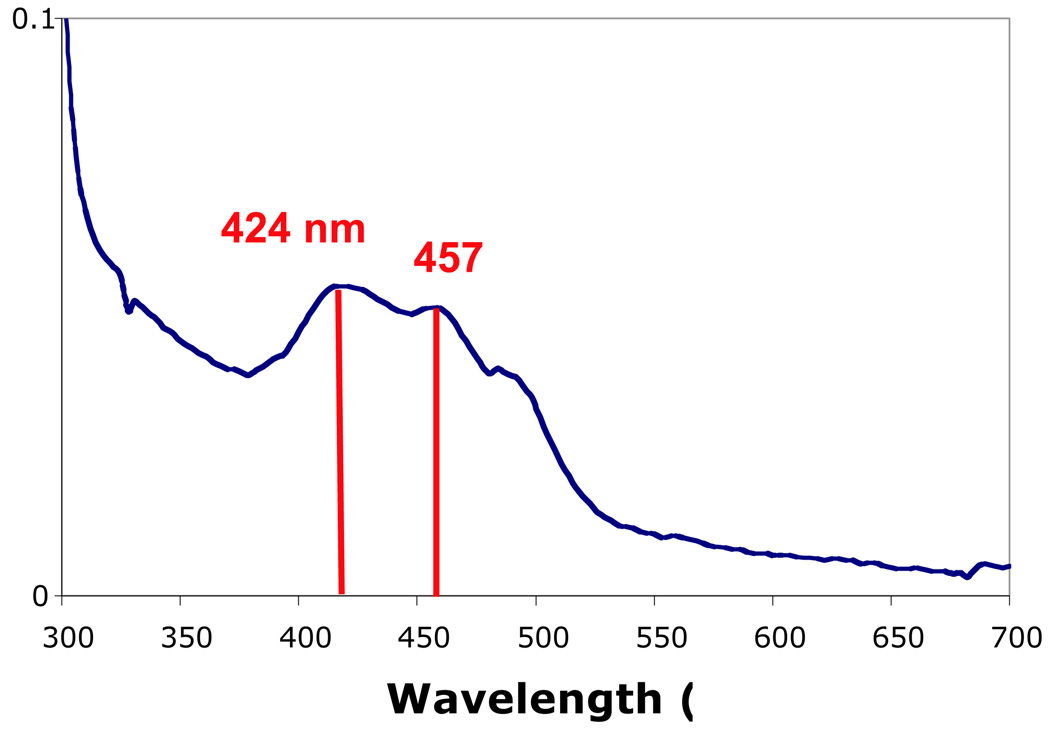
The absorbance spectrum of the partially purified Mn(II) oxidase purified under “gentle” conditions was obtained from 250 to 700 nm. As shown in Figure 2, the spectra show absorbance maxima at 424 nm, 457 nm, and a shoulder around 490 nm. The reduced enzyme (reduced by sulfide or dithionite) was quite similar to the oxidized enzyme (atmospheric O2 or treated with H2O2) and did not show a significant or characteristic shift or change in absorbance peaks. Although the partially purified Mn(II) oxidase is not identical to a PQQ dehydrogenase (Frank et al. 1988), it does show spectral characteristics similar to quinoproteins. The quinone-containing pea diamine oxidase shows absorption maxima at 480 nm for the resting enzyme and at 429, 457, and 332 nm when reduced (Dooley et al. 1987), similar to the partially purified Mn(II) oxidase. MCOs have characteristic absorption spectra with maxima at 330 nm from the Type II copper and 610 nm from the Type I copper (Solomon et al. 1996). Neither peak is observed with the partially purified Mn(II) oxidase. Because the Mn(II) oxidase is enriched and not pure, the spectral characteristics may be caused by other proteins in the mixture. Yet, the spectrum is more consistent with a quinoprotein than with an MCO.
Figure 2.
Absorbance spectra of partially purified Mn(II) oxidase. The striking features are the local absorbance maxima at 424 (421 with the reduced spectra) and 457 nm, similar to quinone containing enzymes. There is no characteristic MCO absorbance at 610 nm. The spectrum does not change shape upon oxidation with peroxide or reduction with sulfide or dithionite. Avidin, which is present in this preparation, does not absorb above 300 nm.
The specific quinone concentration of the partially purified Mn(II) oxidase, as purified under “gentle” conditions, was determined with the NBT/glycinate assay for quinones. The specific quinone concentration in the cell-free extract was 44 pmol × mg−1 protein and in the partially purified fraction 1002 ± 43 pmol × mg−1 protein, a 23 fold enrichment. This is greater than the estimated increase in specific activity, but considering the large losses in activity during purification, some inactive Mn(II) oxidase is likely present in this fraction. Interestingly, despite the requirement for PQQ under normal (“harsh”) protein purification conditions (see above), when extremely “gentle” conditions are employed as used here, additional PQQ is not required for activity, suggesting that under these conditions of high glycerol and the presence of molecular crowding agents, PQQ does not dissociate from the enzyme. Because the NBT/glycinate assay is not specific for PQQ we also reconstituted the activity of soluble glucose dehydrogenase (a known PQQ enzyme) with PQQ extracted from the partially purified fraction. This assay rendered a PQQ concentration of 1130 pmol × mg−1. The absolute concentrations of PQQ are not important since we do not know the concentration of the Mn(II) oxidase, but these experiments do show an enrichment of PQQ with the enriched Mn(II) oxidizing activity.
Effect of PQQ and NAD on Mn(II) oxidation in Pseudomonas putida MnB1
As a comparison to Erythrobacter sp. SD21, we also determined the effect of stimulatory coenzymes on in vitro Mn(II) oxidation in Pseudomonas putida MnB1. The in vitro activity in P. putida MnB1 was quite low with a specific activity of 4.6 pmol × min−1 × (mg cell-free extract)−1. When 10 µM PQQ was added to the assay, the activity increased 7-fold to 33 pmol × min−1 × mg−1. NAD+ increased the activity in the absence of PQQ by 35% but had little effect in the presence of PQQ. This stimulatory effect of PQQ was not seen when added to Bacillus sp. SG-1 (Greg Dick and Bradley Tebo, unpublished data).
Transposon mutants of P. putida MnB1 with disruptions in trpE (Caspi et al. 1998), the α subunit of anthranilate synthase, are unable to oxidize Mn(II) in vivo or in vitro in the absence of PQQ. When 10 µM PQQ is added to the in vitro Mn(II) oxidase assay, the activity is rescued. Both transposon mutants with disruptions in trpE (UT1112 and UT3207) could be rescued in vitro by the addition of 10 µM PQQ. The rescued mutants had a specific activity comparable to wild type activity with added PQQ – 24 picomol/min/mg for UT1112 and 33 picomol/min/mg for UT3207 vs 33 picomol/min/mg for wild type. Anthranilate synthase is involved in the biosynthesis of tryptophan, catalyzing the transformation of chorismate to anthranilate. Chorismate is also upstream in the biosynthesis of quinones as well as tyrosine. One could thus imagine that the pool of quinones, including PQQ may be affected by this mutation. PQQ concentrations in the cell may also be affected by a decrease in tryptophan as it is a positive regulator of tyrosine biosynthesis (Michal 1998) and PQQ is formed from tyrosine.
DISCUSSION
Bacterial Mn(II) oxidation is a phylogenetically widespread trait among the proteobacteria and firmicutes (Tebo et al. 2004) and we are currently on the cusp of understanding the mechanism of this interesting activity in model strains. We may find that the mechanism is conserved amongst these different groups of bacteria suggesting a common ancestral trait, or Mn(II)-oxidizing enzymes may have evolved independently within these strains leading to multiple types of mechanisms. MCOs are suspected to directly catalyze Mn(II) oxidation, but here we report evidence that a quinone is involved in the enzymatic oxidation of Mn(II) in Erythrobacter sp. SD21 and probably P. putida MnB1 as well. The stimulatory effect of PQQ on Mn(II) oxidation (Table 1), its ability to rescue Mn(II)-oxidizing mutants, a partially purified Mn(II) oxidase with an absorbance spectrum similar to a quinoprotein (Figure 2), and the enrichment of PQQ along with Mn(II)-oxidizing activity indicate that a quinone is likely involved in Mn(II) oxidation. PQQ is a noncovalently bound cofactor found in both prokaryotes and eukaryotes, primarily as an electron transferring cofactor for non-NAD+ and non-FAD+ requiring alcohol dehydrogenases. During catalysis, PQQ is reversibly reduced to a hydroquinone (PQQH2) through a semiquinone radical. This class of alcohol dehydrogenases (quinoproteins) contain PQQ as the sole cofactor or in combination with a heme c (quinohemoproteins). PQQ is a unique quinone prosthetic group in that it is not covalently attached nor derived from amino acids of the protein backbone (such as tryptophan tryptophylquinone (TTQ), topaquinone (TPQ), lysine tyrosylquinone (LTQ), and cysteine tryptophylquinone (CTQ)).
The reactions catalyzed by PQQ containing alcohol dehydrogenases and the Mn(II)-oxidizing protein are quite different, therefore, the role of PQQ in Mn(II) oxidation remains a question open for further study. The high redox potential of free Mn(III)/Mn(II) (+1.5V) would not likely be compatible with PQQ (+90 mV) serving as an electron acceptor (Mn(II) + PQQ + H+ → Mn(III) + PQQH). Alternatively, PQQ has been suggested to chelate metal ions such as Mn(II) (Fluckiger et al. 1993) and the role of PQQ could be to chelate Mn(II,III,IV) species at the active site (Mn(II) + Enz-PQQ → Enz-PQQ-Mn(II), Enz-PQQ-Mn(II) + ½ O2 + H+ → Enz-PQQ + Mn(III) + H2O). Mn(II) oxidation could also occur by reacting Mn(II) with superoxide generated from the reduced and semiquinone form of PQQ (Guillen et al. 1997) (Mn(II) + O− + 2H+ → Mn(III) + H2O). The stimulatory role of NAD+ is equally intriguing. It too may serve in electron transfer or as a chelator(Kobayashi et al. 2005). The Erythrobacter sp. SD21 Mn(II) oxidase may have a unique arrangement of cofactors for Mn(II,III) binding and electron transport.
Transmission electron micrographs of Mn(II)-oxidizing microbes have shown the accumulation of Mn oxides extracellularly, thus it has been hypothesized that the Mn(II)-oxidizing protein is on the outermost regions of the cell. This has been demonstrated for Bacillus sp. SG-1 where the Mn(II)-oxidizing protein is located in the exosporium (Francis et al. 2002) and in Leptothrix where the Mn(II)-oxidizing protein is secreted by the sheathless Leptothrix discophora SS1 (Adams and Ghiorse 1987). Localization of the Pseudomonas putida GB1 protein indicated a soluble protein (Okazaki et al. 1997) similar to that identified here with Erythrobacter sp. SD21. These proteins may reside in the periplasm or be loosely associated with the cell surface. A periplasmic or loosely associated cell surface protein would be more consistent with the accumulation of extracellular Mn oxides.
Divalent soft metals such as Mg2+ and Ca2+ are known to be required for or promote the activity of many proteins (Mordasini et al. 2003), and the stimulation of Mn(II) oxidation by Ca2+ (Toyoda and Tebo, unpublished results) may reflect that. The role of Ca2+ may also indicate a more specific role. PQQ requiring enzymes such as methanol dehydrogenase and soluble glucose dehydrogenase (Oubrie et al. 1999) require Ca2+ for binding of PQQ and stabilization of the PQQ semiquinone form (Sato et al. 2001). The effect of Ca2+ on Mn(II) oxidation is consistent with catalysis by a PQQ requiring enzyme.
Is the Mn(II) oxidase in Erythrobacter sp. SD21 a MCO? Unfortunately, the data presented here do not provide a definitive answer, but it certainly raises questions about the widespread role of MCOs in direct oxidation of Mn(II). The absorbance spectra of the partially purified activity does not show characteristics consistent with an MCO, and copper does not affect Mn(II) oxidation by Erythrobacter sp. SD21 in vivo or in vitro as has been shown in other Mn(II)-oxidizing microorganisms (van Waasbergen et al. 1996; Larsen et al. 1999; Brouwers et al. 2000a; Zhang et al. 2002). In addition, in contrast to what has been observed with other Mn(II)-oxidizing protein preparations (Webb et al. 2004), Mn(III) instead of Mn(IV) seems to be the product of Mn(II) oxidation; thus, a different catalytic mechanism may be employed. The Km (204 µM) measured with cell-free extract is similar to that found for Bacillus sp. SG-1 exosporium in the presence of pyrophosphate (196 µM) (Webb et al. 2005), but much higher than that determined for other Mn(II)-oxidizing proteins from vegetative cells: 7 µM with Leptothrix discophora SS-1 (Adams and Ghiorse 1987), 10 µM with Pseudomonas putida GB-1 (Okazaki et al. 1997), 26 µM with Pedomicrobium sp. ACM 3067 (Larsen et al. 1999). The results of inhibition with azide, cyanide, and o-phenanthroline are relatively consistent with what has previously been reported, but do not conclusively suggest the involvement of an MCO because these compounds may also interact with Mn. Although inhibition by these compounds is consistent with an MCO, these compounds affect the activity of other types of metalloenzymes (Hille 1994; Supuran et al. 2004), and azide and cyanide also react directly with PQQ (Frank et al. 1988; Duine 1991). Previous results with Erythrobacter sp. SD21 showed that the MCO organic substrate, ABTS, is transformed by a protein that comigrates with the Mn(II)-oxidizing proteins in SDS-PAGE (Francis et al. 2001) providing evidence that an MCO is involved. The draft sequence of the Erythrobacter SD21 genome has revealed several MCOs, putative PQQ biosynthesis genes, and PQQ requiring enzymes thereby not eliminating the involvement of a MCO or a PQQ requiring enzyme. It is worth noting that of the bacterial MCOs reported in the literature that have been tested for Mn(II) oxidation, none have been able to catalyze Mn(II) oxidation. Since the Mn(II) oxidase is suspected to be a large complex, both an MCO and a quinine requiring enzyme may be required for activity. If specific interprotein electron transfer between an MCO and a quinoprotein is required for Mn(II) oxidation, it may help to explain the difficulties associated with purifying and heterologously expressing an active Mn(II) oxidase. Linking the many MCO genes that have been identified to be involved in Mn(II) oxidation with the biochemical activity continues to await further purification of the activity or mass spectrometry identification.
ACKNOWLEDGEMENTS
We thank Cassandra Gaston, Patrizia Pretto, James McCarthy, and Greg Dick for laboratory assistance and helpful discussions. We thank Hans Duine for generously supplying soluble glucose dehydrogenase apoenzyme. We thank the Gordon and Betty Moore Foundation and The Venter Institute for Genome Sequencing of Erythrobacter sp. SD21. This research was funded by grants from the National Science Foundation (CHE-0089208 and MCB-0630355) and a Superfund Basic Research Program Grant to UCSD (NIEHS ES10337).
REFERENCES
- Adams LF, Ghiorse WC. Characterization of extracellular Mn2+-oxidizing activity and isolation of an Mn2+-oxidizing protein from Leptothrix discophora SS-1. J. Bacteriol. 1987;169:1279–1285. doi: 10.1128/jb.169.3.1279-1285.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony C. Quinoprotein-catalysed reactions. Biochem J. 1996;320(Pt 3):697–711. doi: 10.1042/bj3200697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askwith C, et al. The FET3 gene of S. cerevisiae encodes a multicopper oxidase required for ferrous iron uptake. Cell. 1994;76:403–410. doi: 10.1016/0092-8674(94)90346-8. [DOI] [PubMed] [Google Scholar]
- Barnett RE. Effect of monovalent cations on the ouabain inhibition of the sodium and potassium ion activated adenosine triphosphatase. Biochemistry. 1970;9:4644–4648. doi: 10.1021/bi00826a004. [DOI] [PubMed] [Google Scholar]
- Boogerd FC, de Vrind JP. Manganese oxidation by Leptothrix discophora. J Bacteriol. 1987;169:489–494. doi: 10.1128/jb.169.2.489-494.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brouwers G, de Vrind JPM, Corstjens PLAM, Cornelis P, Baysse C, de Vrind-de Jong EW. cumA, a gene encoding a multicopper oxidase, is involved in Mn2+ oxidation in Pseudomonas putida GB-1. Appl. Environ. Microbiol. 1999;65:1762–1768. doi: 10.1128/aem.65.4.1762-1768.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwers GJ, Corstjens PLAM, de Vrind JPM, Verkamman A, de Kuyper M, de Vrind-de Jong EW. Stimulation of Mn2+ oxidation in Leptothrix discophora SS-1 by Cu2+ and sequence analysis of the region flanking the gene encoding putative multicopper oxidase MofA. Geomicrobiology Journal. 2000a;17:25–33. [Google Scholar]
- Brouwers GJ, Vijgenboom E, Corstjens PLAM, De Vrind JPM, de Vrind-de Jong EW. Bacterial Mn2+ oxidizing systems and multicopper oxidases: An overview of mechanisms and functions. Geomicrobiology Journal. 2000b;17:1–24. [Google Scholar]
- Caspi R, Tebo BM, Haygood MG. c-type cytochromes and manganese oxidation in Pseudomonas putida MnB1. Appl Environ Microbiol. 1998;64:3549–3555. doi: 10.1128/aem.64.10.3549-3555.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung M, Klimov D, Thirumalai D. Molecular crowding enhances native state stability and refolding rates of globular proteins. Proc Natl Acad Sci. 2005;102:4753–4758. doi: 10.1073/pnas.0409630102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobley JG, Haddock BA. The respiratory chain of Thiobacillus ferrooxidans: the reduction of cytochromes by Fe2+ and the preliminary characterization of rusticyanin a novel "blue" copper protein. FEBS Lett. 1975;60:29–33. doi: 10.1016/0014-5793(75)80411-x. [DOI] [PubMed] [Google Scholar]
- Corstjens PL, de Vrind JPM, Westbroek P, de Vrind-de Jong EW. Enzymatic iron oxidation by Leptothrix discophora: identification of an iron-oxidizing protein. Appl Environ Microbiol. 1992;58:450–454. doi: 10.1128/aem.58.2.450-454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corstjens PLAM, De Vrind JPM. Identification and molecular analysis of the Leptothrix discophora SS-1mofA gene, a gene putatively encoding a manganese-oxidizing protein with copper domains. Geomicrobiol. J. 1997;14:91–108. [Google Scholar]
- Cox JC, Boxer DH. The purification and some properties of rusticyanin, a blue copper protein involved in iron(II) oxidation from Thiobacillus ferro-oxidans. Biochem J. 1978;174:497–502. doi: 10.1042/bj1740497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley DM, McGuirl MA, Peisach J, McCracken J. The generation of an organic free radical in substrate-reduced pig kidney diamine oxidase-cyanide. FEBS Lett. 1987;214:274–278. doi: 10.1016/0014-5793(87)80069-8. [DOI] [PubMed] [Google Scholar]
- Duine JA. Quinoproteins: enzymes containing the quinonoid cofactor pyrroloquinoline quinone, topaquinone or tryptophan-tryptophan quinone. Eur J Biochem. 1991;200:271–284. doi: 10.1111/j.1432-1033.1991.tb16183.x. [DOI] [PubMed] [Google Scholar]
- Fluckiger R, Paz M, Mah J, Bishop A, Gallop PM. Characterization of the glycine-dependent redox-cycling activity in animal fluids and tissues using specific inhibitors and activators: evidence for presence of PQQ. Biochem Biophys Res Commun. 1993;196:61–68. doi: 10.1006/bbrc.1993.2216. [DOI] [PubMed] [Google Scholar]
- Fluckiger R, Paz MA, Gallop PM. Redox-cycling detection of dialyzable pyrroloquinoline quinone and quinoproteins. Methods Enzymol. 1995;258:140–149. doi: 10.1016/0076-6879(95)58043-3. [DOI] [PubMed] [Google Scholar]
- Francis CA, Casciotti KL, Tebo BM. Localization of Mn(II)-oxidizing activity and the putative multicopper oxidase, MnxG, to the exosporium of the marine Bacillus sp. strain SG-1. Arch. Microbiol. 2002;178:450–456. doi: 10.1007/s00203-002-0472-9. [DOI] [PubMed] [Google Scholar]
- Francis CA, Co E, Tebo BM. Enzymatic manganese(II) oxidation by a marine α–proteobacterium. Appl. Environ. Microbiol. 2001;67:4024–4029. doi: 10.1128/AEM.67.9.4024-4029.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank J, Jr, Dijkstra M, Duine JA, Balny C. Kinetic and spectral studies on the redox forms of methanol dehydrogenase from Hyphomicrobium X. Eur J Biochem. 1988;174:331–338. doi: 10.1111/j.1432-1033.1988.tb14102.x. [DOI] [PubMed] [Google Scholar]
- Fukumori Y, Yano T, Sato A, Yamanaka T. Fe(II)-oxidizing enzyme purified from Thiobacillus ferrooxidans. FEMS Microbiology Letters. 1988;50:169–172. [Google Scholar]
- Garfin DE. One-Dimensional Gel-Electrophoresis. Methods in Enzymology. 1990;182:425–441. doi: 10.1016/0076-6879(90)82035-z. [DOI] [PubMed] [Google Scholar]
- Goericke R. Bacteriochlorophyll a in the ocean: Is anoxygenic bacterial photosynthesis important? Limnology and Oceanography. 2002;47:290–295. [Google Scholar]
- Guillen F, Martinez MJ, Munoz C, Martinez AT. Quinone redox cycling in the ligninolytic fungus Pleurotus eryngii leading to extracellular production of superoxide anion radical. Arch Biochem Biophys. 1997;339:190–199. doi: 10.1006/abbi.1996.9834. [DOI] [PubMed] [Google Scholar]
- Harrison PM, Arosio P. The ferritins: molecular properties, iron storage function and cellular regulation. Biochim Biophys Acta. 1996;1275:161–203. doi: 10.1016/0005-2728(96)00022-9. [DOI] [PubMed] [Google Scholar]
- Hille R. The reaction mechanism of oxomolybdenum enzymes. Biochim Biophys Acta. 1994;1184:143–169. doi: 10.1016/0005-2728(94)90220-8. [DOI] [PubMed] [Google Scholar]
- Hofer C, Schlosser D. Novel enzymatic oxidation of Mn2+ to Mn3+ catalyzed by a fungal laccase. FEBS Lett. 1999;451:186–190. doi: 10.1016/s0014-5793(99)00566-9. [DOI] [PubMed] [Google Scholar]
- Huston WM, Jennings MP, McEwan AG. The multicopper oxidase of Pseudomonas aeruginosa is a ferroxidase with a central role in iron acquisition. Mol. Microbiol. 2002;45:1741–1750. doi: 10.1046/j.1365-2958.2002.03132.x. [DOI] [PubMed] [Google Scholar]
- Kim C, Lorenz WW, Hoopes JT, Dean JFD. Oxidation of phenolate siderophores by the multicopper oxidase encoded by the Escherichia coli yacK gene. J. Bacteriol. 2001;183:4866–4875. doi: 10.1128/JB.183.16.4866-4875.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A, et al. Transition metal complexes coordinated by an NAD(P)H model compound and their enhanced hydride-donating abilities in the presence of a base. Chemistry. 2005;11:4219–4226. doi: 10.1002/chem.200401211. [DOI] [PubMed] [Google Scholar]
- Kolber ZS, et al. Contribution of aerobic photoheterotrophic bacteria to the carbon cycle in the ocean. Science. 2001;292:2492–2495. doi: 10.1126/science.1059707. [DOI] [PubMed] [Google Scholar]
- Larsen EI, Sly LI, McEwan AG. Manganese(II) adsorption and oxidation by whole cells and a membrane fraction of Pedomicrobium sp. ACM 3067. Arch Microbiol. 1999;171:257–264. [Google Scholar]
- Lindley PF, et al. An X-ray structural study of human ceruloplasmin in relation to ferroxidase activity. Journal of Biological Inorganic Chemistry. 1997;2:454–463. [Google Scholar]
- Matsushita K, Yamashita T, Aoki N, Toyama H, Adachi O. Electron transfer from quinohemoprotein alcohol dehydrogenase to blue copper protein azurin in the alcohol oxidase respiratory chain of Pseudomonas putida HK5. Biochemistry. 1999;38:6111–6118. doi: 10.1021/bi990121f. [DOI] [PubMed] [Google Scholar]
- Michal G, editor. Biochemical Pathways. third edition. Germany: Boehringer Mannheim; 1998. [Google Scholar]
- Mordasini T, Curioni A, Andreoni W. Why do divalent metal ions either promote or inhibit enzymatic reactions? The case of BamHI restriction endonuclease from combined quantum-classical simulations. J Biol Chem. 2003;278:4381–4384. doi: 10.1074/jbc.C200664200. [DOI] [PubMed] [Google Scholar]
- Murray KJ, Tebo BM. Cr(III) is indirectly oxidized by the Mn(II)-oxidizing bacterium Bacillus sp. strain SG-1. Environ Sci Technol. 2007;41:528–533. doi: 10.1021/es0615167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musci G, Di Marco S, Bellenchi GC, Calabrese L. Reconstitution of ceruloplasmin by the Cu(I)-glutathione complex. Evidence for a role of Mg2+ and ATP. J Biol Chem. 1996;271:1972–1978. doi: 10.1074/jbc.271.4.1972. [DOI] [PubMed] [Google Scholar]
- Okazaki M, et al. Partial purification and characterization of manganese-oxidizing factors of Pseudomonas fluorescens GB-1. Appl Environ Microbiol. 1997;63:4793–4799. doi: 10.1128/aem.63.12.4793-4799.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oubrie A, Rozeboom HJ, Dijkstra BW. Active-site structure of the soluble quinoprotein glucose dehydrogenase complexed with methylhydrazine: a covalent cofactor-inhibitor complex. Proc Natl Acad Sci U S A. 1999;96:11787–11791. doi: 10.1073/pnas.96.21.11787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridge JP, Lin M, Larsen EI, Fegan M, McEwan AG, Sly LI. A multicopper oxidase is essential for manganese oxidation and laccase-like activity in Pedomicrobium sp. ACM 3067. Environ Microbiol. 2007;9:944–953. doi: 10.1111/j.1462-2920.2006.01216.x. [DOI] [PubMed] [Google Scholar]
- Sato A, Takagi K, Kano K, Kato N, Duine JA, Ikeda T. Ca2+ stabilizes the semiquinone radical of pyrroloquinoline quinone. Biochem J. 2001;357:893–898. doi: 10.1042/0264-6021:3570893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosser D, Hofer C. Laccase-catalyzed oxidation of Mn2+ in the presence of natural Mn3+ chelators as a novel source of extracellular H2O2 production and its impact on manganese peroxidase. Appl. Environ. Microbiol. 2002;68:3514–3521. doi: 10.1128/AEM.68.7.3514-3521.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano F, Lucas-Elio P, Lopez-Serrano D, Fernandez E, Sanchez-Amat A. Dimethoxyphenol oxidase activity of different microbial blue multicopper proteins. Fems Microbiology Letters. 2001;204:175–181. doi: 10.1111/j.1574-6968.2001.tb10882.x. [DOI] [PubMed] [Google Scholar]
- Solomon EI, Sundaram UM, Machonkin TE. Multicopper oxidases and oxygenases. Chem. Rev. 1996;96:2563–2605. doi: 10.1021/cr950046o. [DOI] [PubMed] [Google Scholar]
- Stams AJM, Kremer DR, Nicolay K, Weenk GH, Hansen TA. Pathway of propionate formation in Desulfobulbus propionicus. Arch Microbiol. 1984;139:167–173. [Google Scholar]
- Stoj C, Kosman DJ. Cuprous oxidase activity of yeast Fet3p and human ceruloplasmin: implication for function. FEBS Lett. 2003;554:422–426. doi: 10.1016/s0014-5793(03)01218-3. [DOI] [PubMed] [Google Scholar]
- Supuran CT, Vullo D, Manole G, Casini A, Scozzafava A. Designing of Novel Carbonic Anhydrase Inhibitors and Activators. Curr Med Chem Cardiovasc Hematol Agents. 2004;2:51–70. [PubMed] [Google Scholar]
- Tebo BM, et al. Biogenic manganese oxides: properties and mechanisms of formation. Annu. Rev. Earth Planet. Sci. 2004;32:287–328. [Google Scholar]
- Tebo BM, Johnson HA, McCarthy JK, Templeton AS. Geomicrobiology of manganese(II) oxidation. Trends Microbiol. 2005;13:421–428. doi: 10.1016/j.tim.2005.07.009. [DOI] [PubMed] [Google Scholar]
- van der Meer RA, Groen BW, van Kleef MA, Frank J, Jongejan JA, Duine JA. Isolation, preparation, and assay of pyrroloquinoline quinone. Methods Enzymol. 1990;188:260–283. doi: 10.1016/0076-6879(90)88043-a. [DOI] [PubMed] [Google Scholar]
- van der Meer RA, Jongejan JA, Duine JA. Phenylhydrazine as probe for cofactor identification in amine oxidoreductases. FEBS Lett. 1987;221:299–304. doi: 10.1016/0014-5793(87)80944-4. [DOI] [PubMed] [Google Scholar]
- van Waasbergen LG, Hildebrand M, Tebo BM. Identification and characterization of a gene cluster involved in manganese oxidation by spores of the marine Bacillus sp. strain SG-1. J. Bacteriol. 1996;178:3517–3530. doi: 10.1128/jb.178.12.3517-3530.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Waasbergen LG, Hoch JA, Tebo BM. Genetic analysis of the marine manganese-oxidizing Bacillus sp. strain SG-1: protoplast transformation, Tn917 mutagenesis, and identification of chromosomal loci involved in manganese oxidation. J Bacteriol. 1993;175:7594–7603. doi: 10.1128/jb.175.23.7594-7603.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb S, Bargar J, Dick GJ, Johnson HA, McCarthy JK, Tebo BM. Insights into the mechanism of enzymatic manganese(II) oxidation by diverse bacterial species. Abst. Papers Amer. Chem. Soc. 2004 [Google Scholar]
- Webb SM, Dick GJ, Bargar JR, Tebo BM. Evidence for the presence of Mn(III) intermediates in the bacterial oxidation of Mn(II) Proc Natl Acad Sci. 2005;102:5558–5563. doi: 10.1073/pnas.0409119102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitsev VN, Zaitseva I, Papiz M, Lindley PF. An X-ray crystallographic study of the binding sites of the azide inhibitor and organic substrates to ceruloplasmin, a multi copper oxidase in the plasma. Journal of Biological Inorganic Chemistry. 1999;4:579–587. doi: 10.1007/s007750050380. [DOI] [PubMed] [Google Scholar]
- Zhang JH, Lion LW, Nelson YM, Shuler ML, Ghiorse WC. Kinetics of Mn(II) oxidation by Leptothrix discophora SS1. Geochimica Et Cosmochimica Acta. 2002;66:773–781. [Google Scholar]