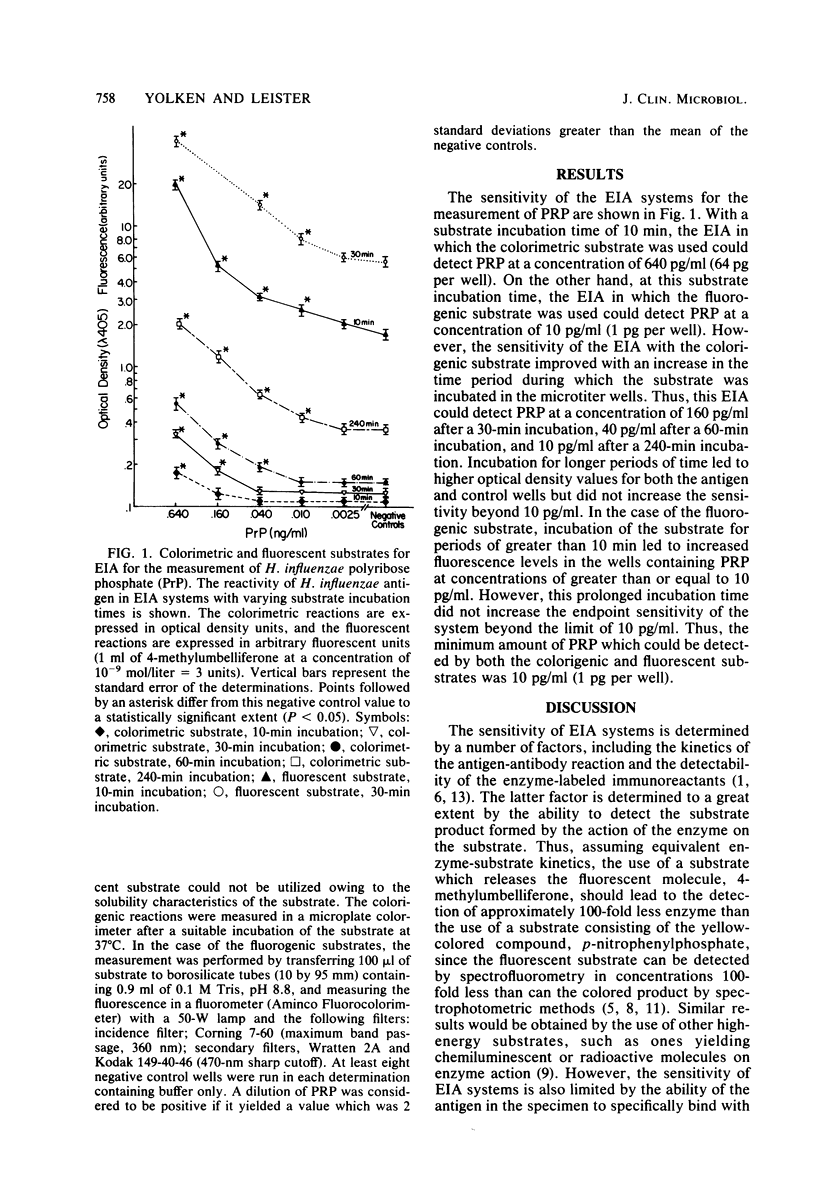
Abstract
A variety of substrates can be employed in enzyme immunoassays (EIAs) for the measurement of enzyme-labeled immunoreactants. We compared the sensitivities of a fluorescent and a colorigenic substrate in an EIA system for the measurement of Haemophilus influenza purified polyribose phosphate. After a 10-min substrate incubation, the EIA in which the fluorescent substrate was used could detect 10 pg of polyribose phosphate per ml, whereas the EIA in which the colorigenic substrate was used required the addition of 640 pg of polyribose phosphate per ml to generate a positive reading. However, the use of longer substrate incubation periods led to an increase in sensitivity of the colorigenic EIA. After an incubation period of 240 min, the sensitivity was equal to that of the EIA in which the fluorescent substrate was used. These results suggest that the ultimate limit of sensitivity of EIA systems is determined by the nature of the antigen-antibody reactions. However, the use of high-energy substrates in EIA systems can allow for the attainment of maximal sensitivity after short enzyme-substrate incubation periods.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ekins R. More sensitive immunoassays. Nature. 1980 Mar 6;284(5751):14–15. doi: 10.1038/284014a0. [DOI] [PubMed] [Google Scholar]
- Forghani B., Dennis J., Schmidt N. J. Visual reading of enzyme immunofluorescence assays for human cytomegalovirus antibodies. J Clin Microbiol. 1980 Nov;12(5):704–708. doi: 10.1128/jcm.12.5.704-708.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel M. E., Gerhard W. The rapid determination of binding constants for antiviral antibodies by a radioimmunoassay. An analysis of the interaction between hybridoma proteins and influenza virus. Mol Immunol. 1979 Feb;16(2):101–106. doi: 10.1016/0161-5890(79)90051-8. [DOI] [PubMed] [Google Scholar]
- Harris C. C., Yolken R. H., Krokan H., Hsu I. C. Ultrasensitive enzymatic radioimmunoassay: application to detection of cholera toxin and rotavirus. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5336–5339. doi: 10.1073/pnas.76.10.5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi E., Iwasa S., Kondo K., Hori M. Chemiluminescence-linked immunoassay for detection of mumps virus antibodies. J Clin Microbiol. 1980 Aug;12(2):140–143. doi: 10.1128/jcm.12.2.140-143.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepple J., Moxon E. R., Yolken R. H. Indirect enzyme-linked immunosorbent assay for the quantitation of the type-specific antigen of Haemophilus influenzae b: a preliminary report. J Pediatr. 1980 Aug;97(2):233–237. doi: 10.1016/s0022-3476(80)80480-x. [DOI] [PubMed] [Google Scholar]
- Puget K., Michelson A. M., Avrameas S. Light emission techniques for the microestimation of femtogram levels of peroxidase. Application to peroxidase (and other enzymes)-coupled antibody-cell antigen interactions. Anal Biochem. 1977 May 1;79(1-2):447–456. doi: 10.1016/0003-2697(77)90420-1. [DOI] [PubMed] [Google Scholar]
- Segal E., Berg R. A., Pizzo P. A., Bennett J. E. Detection of Candida antigen in sera of patients with candidiasis by an enzyme-linked immunosorbent assay-inhibition technique. J Clin Microbiol. 1979 Jul;10(1):116–118. doi: 10.1128/jcm.10.1.116-118.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev A., Greenberg A. H., McAlpine P. J. Detection of attograms of antigen by a high-sensitivity enzyme-linked immunoabsorbent assay (HS-ELISA) using a fluorogenic substrate. J Immunol Methods. 1980;38(1-2):125–139. doi: 10.1016/0022-1759(80)90337-3. [DOI] [PubMed] [Google Scholar]
- Wisdom G. B. Enzyme-immunoassay. Clin Chem. 1976 Aug;22(8):1243–1255. [PubMed] [Google Scholar]
- Yolken R. H. Enzyme-linked immunosorbent assay (ELISA): a practical tool for rapid diagnosis of viruses and other infectious agents. Yale J Biol Med. 1980 Jan-Feb;53(1):85–92. [PMC free article] [PubMed] [Google Scholar]
- Yolken R. H., Kim H. W., Clem T., Wyatt R. G., Kalica A. R., Chanock R. M., Kapikian A. Z. Enzyme-linked immunosorbent assay (ELISA) for detection of human reovirus-like agent of infantile gastroenteritis. Lancet. 1977 Aug 6;2(8032):263–267. doi: 10.1016/s0140-6736(77)90951-5. [DOI] [PubMed] [Google Scholar]
- Yolken R. H., Stopa P. J. Enzyme-linked fluorescence assay: Ultrasensitive solid-phase assay for detection of human rotavirus. J Clin Microbiol. 1979 Sep;10(3):317–321. doi: 10.1128/jcm.10.3.317-321.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]



