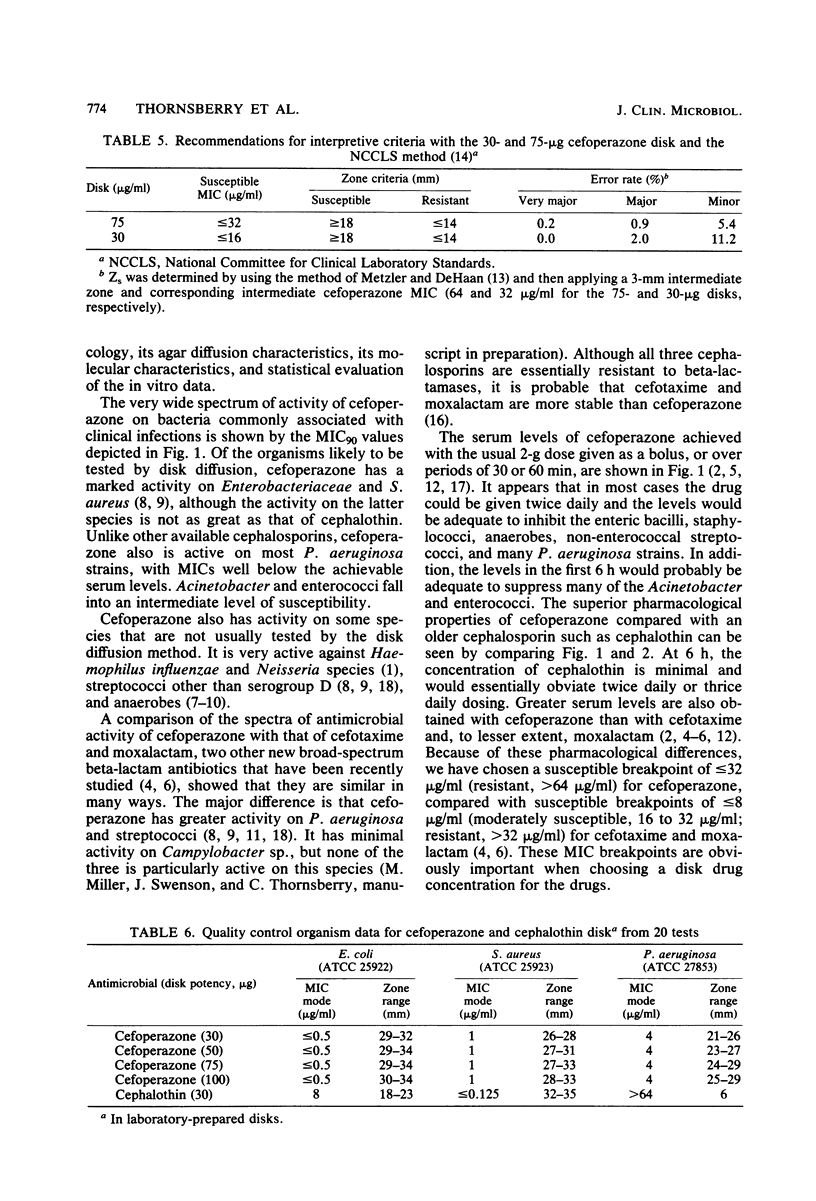
Abstract
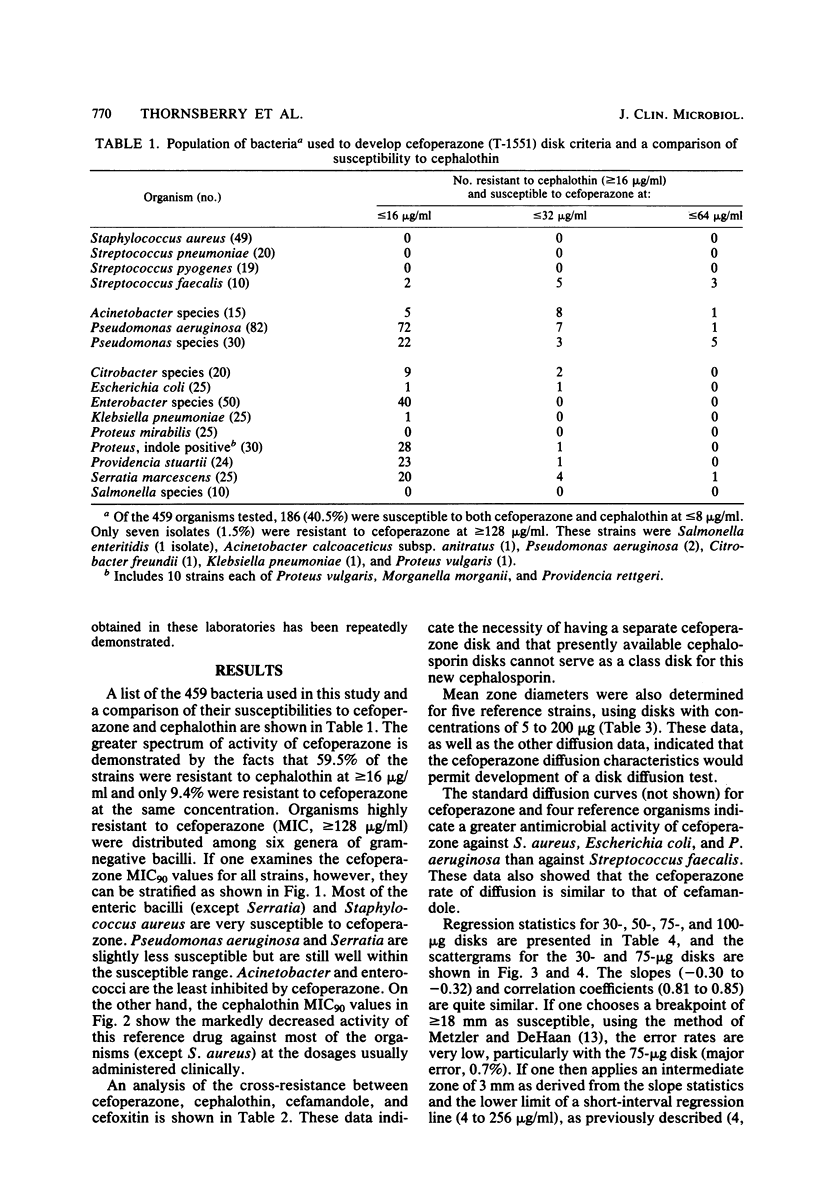
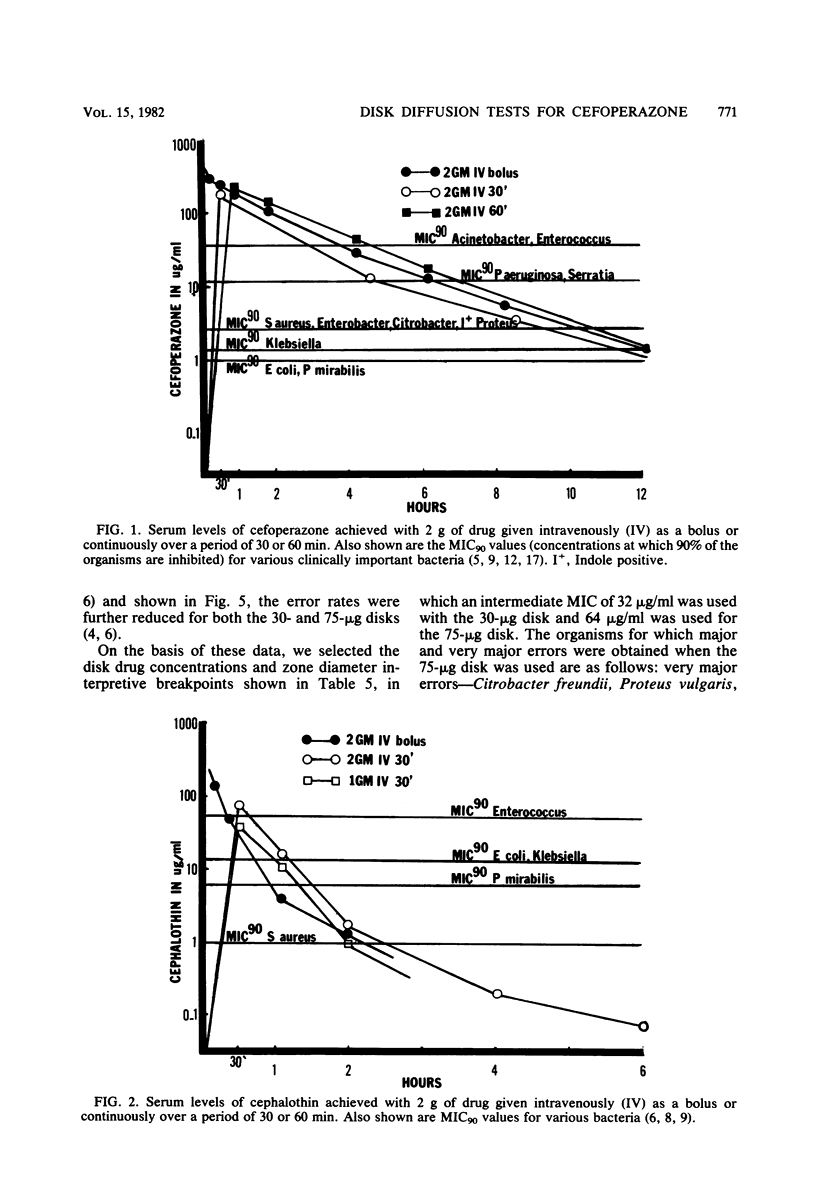
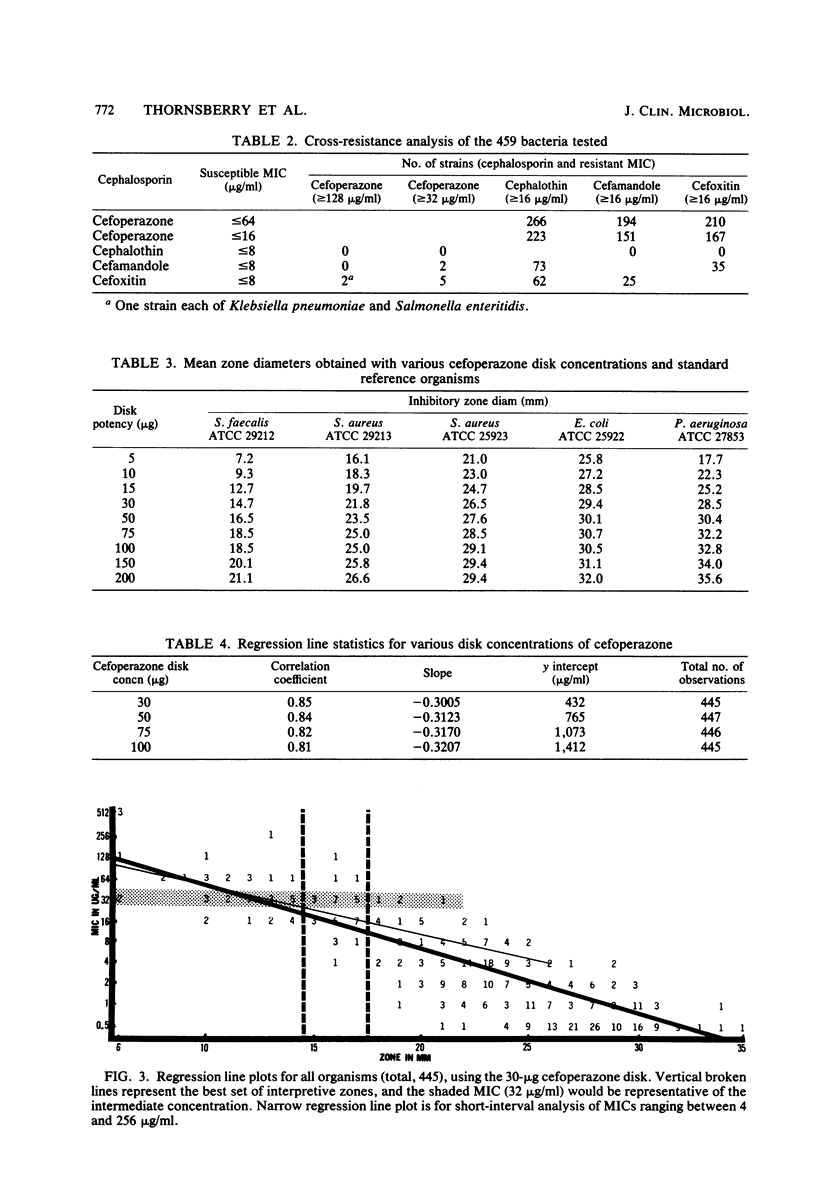
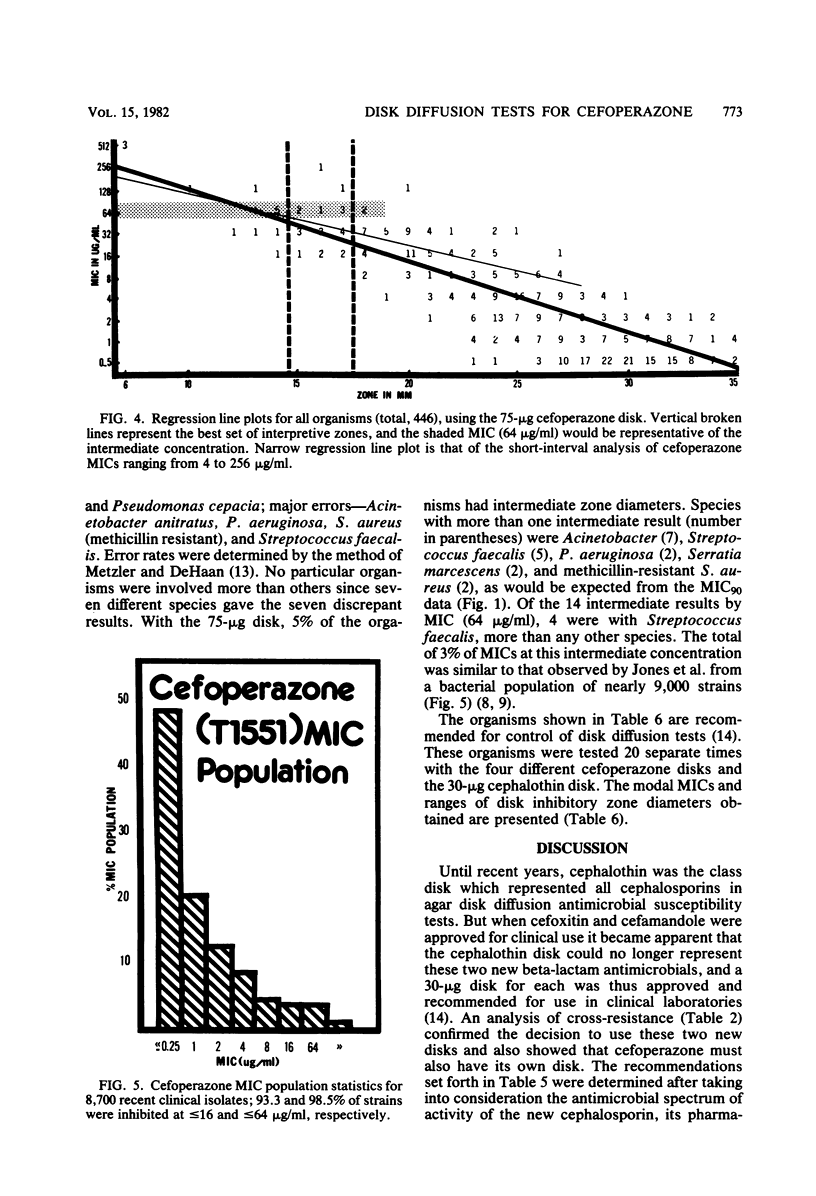
Cefoperazone is a new cephalosporin with a very wide spectrum of activity, including activity against Pseudomonas aeruginosa. It has less activity on enterococci and Acinetobacter. Of the 459 selected bacterial strains tested in this study, only 1.5% (7 strains and 6 genera) had minimum inhibitory concentrations of greater than or equal to 128 micrograms/ml. For a minimum inhibitory concentration breakpoint of less than or equal to 32 micrograms/ml (susceptible), we recommend that the disk diffusion test be done with a 75-micrograms disk and breakpoints of greater than or equal to 18 mm for susceptible, 15 to 17 mm for intermediate, and less than or equal to 14 mm for resistant. Diffusion tests using these criteria yielded only 1.1% very major or major errors.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker C. N., Thornsberry C., Jones R. N. In vitro antimicrobial activity of cefoperazone, cefotaxime, moxalactam (LY127935), azlocillin, mezlocillin, and other beta-lactam antibiotics against Neisseria gonorrhoeae and Haemophilus influenzae, including beta-lactamase-producing strains. Antimicrob Agents Chemother. 1980 Apr;17(4):757–761. doi: 10.1128/aac.17.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balant L., Dayer P., Rudhardt M., Allaz A. F., Fabre J. Cefoperazone: pharmacokinetics in humans with normal and impaired renal function and pharmacokinetics in rats. Clin Ther. 1980;3(Spec Issue):50–59. [PubMed] [Google Scholar]
- Barry A. L., Thornsberry C., Jones R. N., Gerlach E. H. Tentative interpretive standards for disk susceptibility tests with moxalactam (LY127935). Antimicrob Agents Chemother. 1980 Nov;18(5):716–721. doi: 10.1128/aac.18.5.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig W. A. Single-dose pharmacokinetics of cefoperazone following intravenous administration. Clin Ther. 1980;3(Spec Issue):46–49. [PubMed] [Google Scholar]
- Fuchs P. C., Barry A. L., Thornsberry C., Jones R. N., Gavan T. L., Gerlach E. H., Sommers H. M. Cefotaxime: in vitro activity and tentative interpretive standards for disk susceptibility testing. Antimicrob Agents Chemother. 1980 Jul;18(1):88–93. doi: 10.1128/aac.18.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus N. V., Tally F. P., Barza M., Gorbach S. L. Susceptibility of anaerobic bacteria to cefoperazone and other beta-lactam antibiotics. Clin Ther. 1980;3(Spec Issue):34–38. [PubMed] [Google Scholar]
- Jones R. N., Fuchs P. C., Barry A. L., Gavan T. L., Gerlach E. H., Sommers H. M. Antimicrobial activity and spectrum of cefoperazone against recent clinical isolates. Clin Ther. 1980;3(Spec Issue):14–23. [PubMed] [Google Scholar]
- Jones R. N., Fuchs P. C., Barry A. L., Gavan T. L., Sommers H. M., Gerlach E. H. Cefoperazone (T-1551), a new semisynthetic cephalosporin: comparison with cephalothin and gentamicin. Antimicrob Agents Chemother. 1980 Apr;17(4):743–749. doi: 10.1128/aac.17.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye D., Kobasa W., Kaye K. Susceptibilities of anaerobic bacteria to cefoperazone and other antibiotics. Antimicrob Agents Chemother. 1980 Jun;17(6):957–960. doi: 10.1128/aac.17.6.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lode H., Kemmerich B., Koeppe P., Belmega D., Jendroschek H. Comparative pharmacokinetics of cefoperazone and cefotaxime. Clin Ther. 1980;3(Spec Issue):80–88. [PubMed] [Google Scholar]
- Metzler C. M., DeHaan R. M. Susceptibility tests of anaerobic bacteria: statistical and clinical considerations. J Infect Dis. 1974 Dec;130(6):588–594. doi: 10.1093/infdis/130.6.588. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Fu K. P., Aswapokee N., Aswapokee P., Kung K. Comparative activity and beta-lactamase stability of cefoperazone, a piperazine cephalosporin. Antimicrob Agents Chemother. 1979 Aug;16(2):150–157. doi: 10.1128/aac.16.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K. Cefoperazone: absorption, excretion, distribution, and metabolism. Clin Ther. 1980;3(Spec Issue):60–79. [PubMed] [Google Scholar]
- Thornsberry C., Baker C. N., Barry A. L., Jones R. N. Cefoperazone: evaluation of the in vitro activity and an analysis of the disk diffusion test. Clin Ther. 1980;3(Spec Issue):39–45. [PubMed] [Google Scholar]



