Abstract
Transgenic mice expressing human HOX11 in B lymphocytes die prematurely from lymphomas that initiate in the spleen and frequently disseminate to distant sites. Preneoplastic hematopoiesis in these mice is unperturbed. We now report that expression of the HOX11 transgene does not affect the ability of dendritic cells (DCs) to process and present foreign peptides and activate antigen-specific T cell responses. We also show that nontransgenic DCs presenting peptides derived from the human HOX11 protein are highly efficient stimulators of autologous T cells, whereas transgenic T cells are nonresponsive to peptides derived from the HOX11 transgene and the murine Meis1 protein. HOX11 transgenic mice thus show normal development of tolerance to immunogenic antigens expressed throughout B cell maturation. DCs pulsed with cell lysates prepared from lymphomas, obtained from HOX11 transgenic mice with terminal lymphoma, activate T cells from nontransgenic and premalignant transgenic mice, whereas T cells isolated from lymphomatous transgenic mice are nonresponsive to autologous tumor cell antigens. These data indicate that HOX11 lymphoma cells express tumor-rejection antigens that are recognized as foreign in healthy transgenic mice and that lymphomagenesis is associated with the induction of anergy to tumor antigen-specific T cells. These findings are highly relevant for the development of immunotherapeutic protocols for the treatment of lymphoma.
Conventional approaches using combination chemotherapy regimens of increasing intensity for the treatment of patients with advanced stages or relapsed low-grade non-Hodgkin's lymphoma do not lead to cure. Aggressive strategies using high-dose therapy with autologous-stem-cell support may prolong remissions, but most patients ultimately relapse, become refractory to treatment, and die from disease progression or complications of treatment (1). Because the overall prognosis for patients with non-Hodgkin's lymphoma is poor, with a median survival of 7–9 years, new approaches to treatment are necessary. A promising new area of research for the treatment of both solid and hematopoietic malignancies, including non-Hodgkin's lymphoma, is dendritic cell (DC)-based immunotherapy (2). This approach is based on the hypothesis that although tumor cells are ineffective at presenting tumor-specific antigens, host DCs are highly capable of triggering potent antitumor responses if appropriately manipulated to present tumor-specific antigens. Some promising protocols for providing or pulsing DCs with antigens to process and present involve loading ex vivo-expanded DCs with tumor-cell-derived or in vitro-transcribed (IVT) RNA, specific tumor-antigen-derived peptides, or crude tumor cell lysates. Theoretically, successfully engineered DCs may be used as cancer vaccines that activate antitumor-specific immune responses in patients with primary or refractory malignant disease.
Mouse transplantable tumor models have established the potential of tumor-peptide-pulsed DCs to stimulate naïve CD4+ and CD8+ T cells and to confer tumor-specific protective immunity (3, 4). Inoculation of mice with relatively small numbers of DCs pulsed with tumor-specific peptide induces potent in vivo cytotoxic T lymphocyte (CTL) responses. Furthermore, immunization of mice with DCs pulsed with tumor cell lysates confers protective immunity against subsequent lethal challenges with live tumor cells. Results of human clinical trials testing DC-based therapies for the treatment of melanoma, lymphoma, and advanced prostate cancer demonstrated clinical responses in a proportion of treated patients, although the therapeutic benefits were less dramatic than would have been predicted from murine tumor transplantation studies (5–7). It therefore appears that although immune responses may be induced in healthy patients by using antigen-presenting DCs, the lack of therapeutic efficacy may be associated with an overall compromised host immune system or an acquired ability of tumor cells to evade detection by the host immune system (8–16).
Although mouse models using transplantable tumor cell lines are useful to test basic concepts and rapidly provide an indication of the efficacy of a therapeutic approach, they do not accurately recapitulate tumor initiation and progression and thus are of somewhat limited practical value. Transgenic mice expressing oncogenes provide an attractive alternative in that tumorigenesis often involves a predictable subset of cells and occurs within the context of the whole animal. Such model systems may ultimately prove to be important tools to study tumor–host interactions during all stages of tumorigenesis and may provide excellent preclinical models to accurately assess the efficacy and safety of therapeutic approaches.
HOX11 is a divergent homeodomain-containing transcription factor that was isolated from the breakpoint of the nonrandom t(10;14)(q24;q11) chromosome translocation (17). This translocation is found in the malignant cells of approximately 5% of patients with T cell acute lymphoblastic leukemias. The translocation places the HOX11 coding sequence under the transcriptional control of T cell receptor α/δ regulatory elements, resulting in ectopic expression of normal HOX11 protein in thymocytes (18–20). To assess the oncogenic potential of HOX11, we targeted its expression in lymphocytes of transgenic mice by placing the human cDNA under the transcriptional control of immunoglobulin heavy chain (IgH) or lymphocyte-specific tyrosine kinase p56lck (LCK) regulatory sequences. Only IgHμ-HOX11 mice expressing low levels of HOX11 were viable (21). These mice become terminally ill during their second year of life with more than 85% developing clonal IgM+IgD+ mature B cell lymphomas. A premalignant phase characterized by splenic lymphoid hyperplasia may be detected by as early as 4 weeks of age. HOX11 transgenic mice provide a clinically relevant model of a human subtype of indolent nonfollicular lymphoma; splenic marginal zone lymphoma (22). Patients with splenic marginal zone lymphoma are generally asymptomatic and show good performance status. At the time of clinical presentation, however, the disease is usually disseminated and prognosis, even with aggressive treatment regimes, is extremely poor (23). Like splenic marginal zone lymphoma, the lymphomas developing in HOX11 mice are low-grade indolent lymphomas that slowly progress leading, ultimately, to premature death (21). The lymphocytes observed in the hyperplastic spleens are small and show maturation to plasma cells. Progression to lymphoma is associated with enlarged marginal zones, early marginal-zone lymphoma, and the appearance of large lymphoma cells.
We now report that premalignant HOX11 transgenic mice have functionally competent DCs and T cells capable of inducing antigen-specific immune responses. Furthermore, tumor-associated antigens expressed by lymphoma cells from HOX11 transgenic mice elicit an immune response in naïve mice. However, transgenic mice with terminal lymphomas are nonresponsive to tumor antigens presented by DCs, suggesting that lymphomagenesis in HOX11 transgenic mice is associated with the induction of anergy to tumor-antigen-specific T cells.
Materials and Methods
Mice.
HOX11 transgenic mice were generated as described (21). HOX11 transgenic lines were established by breeding transgenic mice with either CD-1 or C57BL/6 mice. HOX11 transgenic mice bred into the C57BL/6 genetic background were used in all studies except those outlined in Fig. 1a. Three- to 4-week old male and female C57BL/6 (H-2b), C3H/He (H-2K), and CD-1 mice were purchased from Charles River Breeding Laboratories and were bred and maintained in microisolators under pathogen-free conditions in the Sunnybrook animal facility. All animal manipulations and housing were in accordance with the Sunnybrook Research Institute Animal Care Committee guidelines.
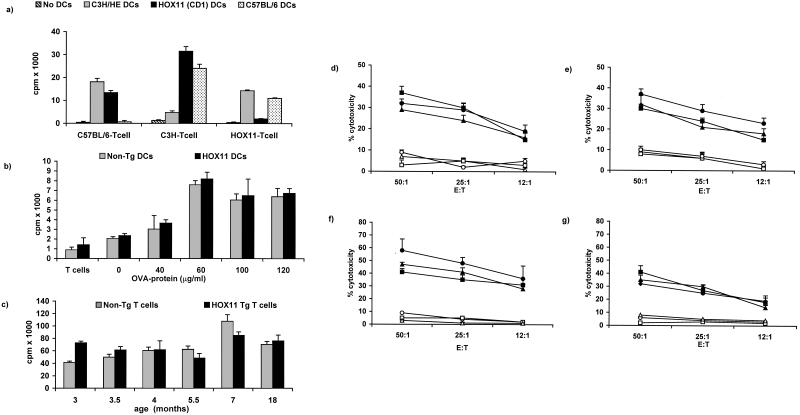
Figure 1.
(a) Activation of T cells by allogeneic DCs. DCs isolated from C3H/HE (H-2K), C57BL/6 (H-2b), or HOX11 transgenic mice inbred for more than 20 generations onto the CD1 genetic background were cocultured with allogeneic T cells. T cell activation was assessed by [3H]thymidine incorporation. Negative controls included the coculture of autologous DCs and T cells. (b) Activation of nontransgenic and HOX11 transgenic T cells by autologous DCs presenting OVA protein. DCs isolated from nontransgenic and transgenic mice, bred onto the C57BL/6 genetic background, were pulsed with OVA protein and cocultured with autologous T cells. Cultures consisting of unpulsed DCs and autologous T cells were established to confirm OVA-specific T cell activation. T cells cultured in the absence of DCs served as a negative control. (c) Phytohemagglutinin-induced proliferation of splenic T cells isolated from age- and sex-matched HOX11 transgenic and nontransgenic mice. (d and e) In vitro CTL assays to compare OVA-specific cytolytic activity of C57BL/6, nontransgenic, and HOX11 transgenic T cells. Bone marrow-derived DCs, isolated from age- and sex-matched mice, were pulsed with OVA protein (60 μg/ml) and used to stimulate autologous T cells. C57BL/6 (●), nontransgenic (■), or HOX11 transgenic (▴) CTLs were assayed for lytic activity against autologous DCs pulsed with OVA protein (d) or E.G7-OVA target cells (e). Nonspecific C57BL/6 (○), nontransgenic (□), and HOX11 transgenic (▵) CTL activity was assessed against unpulsed DC targets (d) or EL4 target cells (e). (f and g) In vivo activation of OVA-specific CTLs in C57BL/6, nontransgenic, and HOX11 transgenic mice. Mice were injected with OVA-pulsed bone marrow-derived DCs and assayed for the in vivo generation of OVA-specific CTLs in C57BL/6 (●), nontransgenic (■), or HOX11 transgenic (▴) mice by using DCs pulsed with OVA protein (f) or E.G7-OVA cells (g) as targets. Nonspecific C57BL/6 (○), nontransgenic (□), and HOX11 transgenic (▵) CTL activity was assessed against unpulsed DC targets (f) or EL4 target cells (g).
Cell Lines.
The EL4 cell line [American Type Culture Collection (ATCC), TIB-39] was established from a T cell lymphoma induced in a C57BL/6 (H-2b) mouse. The E.G7-OVA cell line (ATCC, CRL-2113) was derived by transfection of EL4 cells with a plasmid carrying the chicken ovalbumin (OVA) and neomycin phosphotransferase (G418 resistance) genes. All cell lines were maintained in complete medium (CM) (RPMI 1640 medium, GIBCO/BRL), supplemented with 10% heat-inactivated fetal calf serum, 2 mM L-glutamine, penicillin (0.1 U/ml), and streptomycin (0.1 mg/ml). E.G7-OVA culture medium was adjusted to contain sodium bicarbonate (1.5 g/liter), glucose (4.5 g/liter), 10 mM Hepes, 1 mM sodium pyruvate, 0.05 mM 2-mercaptoethanol, and G418 (GIBCO/BRL; 0.4 mg/ml).
Isolation of DCs from Spleen and Bone Marrow.
Murine femur-derived bone marrow cells, resuspended in Hepes-buffered Hanks' salt solution (GIBCO/BRL) containing 2% fetal calf serum (HF), were treated with 0.165 M NH4Cl-Tris (pH 7.4) to lyse erythrocytes. Bone marrow-derived DCs were expanded in vitro by culturing bone marrow cells at 37°C in 5% CO2/95% air for 6–12 days in CM supplemented with granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4) derived from cells transduced with murine cDNAs encoding GM-CSF (a gift from Nicholas Gough, AMRAD, Victoria, Australia) or IL-4 (24) (a gift from Fritz Melchers, Basel Institute for Immunology, Basel, Switzerland). DCs were isolated by using CD11c microbeads and the MiniMACS magnetic cell sorting system (Miltenyi Biotec, Auburn, CA). Splenic DCs were isolated by collagenase D (Boehringer Mannheim) digestion of spleens followed by centrifugation of splenocyte suspensions in dense BSA (Cohn fraction V; Intergen, Purchase, NY). DCs were isolated from the low-buoyant-density fraction. Flow cytometric analysis indicated the purity of freshly isolated or cultured DCs was 80–90% as revealed by CD11c+ mAb (PharMingen) staining and lack of expression of lineage-specific markers.
Isolation of T Cells from Mouse Spleens.
Single-cell suspensions were treated with 0.165 M NH4Cl-Tris (pH 7.4) for 15 min on ice and washed with HF buffer. T cells were isolated by using nylon wool columns or labeled with CD90 (Thy1.2) microbeads (10 μl of beads per 107 cells) and isolated by using the MiniMACS magnetic cell sorting system. Cells eluted by either purification method were ≈90% CD3+, as determined by flow cytometric analysis.
Pulsing of DCs with IVT RNA or Cell Lysates.
The pGEMHOX11 vector was generated by cloning nucleotides 165–2,058 of the human HOX11 cDNA into the SmaI site of the multiple cloning site of pGEM7Zf (Promega), thereby placing the HOX11 cDNA downstream of the T7 promoter. The 1,550-bp human Pbx1a cDNA (25), subcloned into the SP65 expression vector, was a gift from Michael Cleary (Stanford Univ., Stanford, CA). The 2.3-kb murine Meis1 cDNA, provided by Neal Copeland (National Cancer Institute–Frederick Cancer Research and Development Center, Frederick, MD), was subcloned into the pTarget mammalian expression vector system. Vectors were linearized to minimize plasmid sequences from the IVT mRNA. In vitro transcription of HOX11, Meis1, and Pbx1a was carried out at 37°C for 1 h by using the Promega Riboprobe In Vitro Transcription System according to the manufacturer's instructions. Cell lysates were prepared by freezing cell suspensions in liquid nitrogen followed by thawing cells at room temperature. After five or six freeze–thaw cycles, lysates were centrifuged at 320 × g for 10 min, and supernatants were sterilized with a 0.22-μm (pose size) filter (Millipore). Pulsing of DCs with IVT RNA, cell lysates, or OVA protein was performed as described by Boczkowski et al. (26). Pulsed DCs were washed, irradiated at 2,000 rads, and added (1 × 104 cells per well) to T cells (1 × 105 cells per well) in 96-well plates.
Phytohemagglutinin T Cell Stimulation and Proliferation Assays.
T cells were isolated from spleens of 3.5- to 18-month-old mice and seeded into 96-well plates at 3–5 × 105 cells per well in 200 μl CM containing phytohemagglutinin (Sigma; 5 μg/ml). T cell proliferation was assayed by [3H]thymidine incorporation (27).
Mixed Lymphocyte Reactions (MLRs).
MLRs were established as outlined by Kruisbeek and Shevach (27).
CTL Assay.
CTL activity was measured by a DNA fragmentation assay (28, 29).
Immunization and CTL Induction in Mice.
Approximately 1–5 × 105 pulsed or nonpulsed DCs resuspended in 200 μl of PBS were injected i.p. into C57BL/6, nontransgenic, and HOX11 transgenic mice. Mice were given a booster injection twice at 7-day intervals with pulsed or nonpulsed DCs and then killed. Spleens were removed, red cells were lysed, and T cells were assayed for antigen-specific CTL activity. Autologous or syngeneic, ex vivo-expanded bone marrow-derived DCs pulsed with IVT mRNA, OVA protein, or cell lysates were used to stimulate antigen-specific T cells. Targets used to assess CTL activity included IVT RNA-pulsed DCs, DCs pulsed with lymphoma cell lysates, B220+ lymphoma cells, or EG.7 OVA cells. Negative control target cells included IVT Meis1-RNA-pulsed DCs, unpulsed DCs, nontransformed B cells activated with lipopolysaccharide (LPS) and EL4 cells. Lymphoma cells and nontransformed B cells were isolated from spleens by using B220+ microbeads and the MiniMACS magnetic cell sorting system and cultured in CM supplemented with NIH 3T3 cell supernatants. CTL assays were set up at effector:target (E:T) ratios of 100:1 to 12:1 in 200 μl of CM.
Results
Immunocompetence of HOX11 Transgenic DCs and T cells.
To ensure that expression of the HOX11 transgene in hematopoietic cells did not perturb DC or T cell function, our initial experiments were aimed at assessing the ability of transgenic DCs to process and present antigens and activate T cells and the ability of HOX11 transgenic T cells to respond to mitogenic or foreign antigen stimulation. The absolute numbers of HOX11 transgenic and nontransgenic DCs determined directly after isolation from bone marrow or spleen or after expansion in the presence of GM-CSF and IL-4 were similar (data not presented). Flow cytometric analysis indicated that 80–90% of the isolated cells were DCs as shown by lack of expression of lineage-specific markers and expression of CD11c, a cell surface antigen predominantly but not exclusively expressed on DCs. Staining of bone marrow- or spleen-derived DCs with antibodies specific for major histocompatibility complex (MHC) class I and class II antigens, the activation antigen CD40 and the costimulatory molecules B7–1, B7–2, and heat-stable antigen (HSA) revealed similar expression profiles between HOX11 transgenic and nontransgenic mice (data not presented). Expression profiles of bone marrow-derived DCs were consistent with an immature phenotype, as indicated by low-level expression of MHC class II, CD40, B7–1, and B7–2. In contrast, expression of the above antigens was up-regulated on a high proportion of splenic DCs, indicative of normal differentiation to a mature DC phenotype. Therefore, both bone marrow- and spleen-derived HOX11 transgenic DCs expressed a normal array of cell surface antigens, proliferated normally in response to GM-CSF and IL-4, and differentiated to a mature phenotype. We assessed the ability of HOX11 transgenic DCs to process and present MHC class I and class II-associated peptides and activate T cells in MLR and CTL assays. In vitro MLR assays revealed that DCs isolated from C3H/He, C57BL/6, and HOX11 transgenic mice induced similar levels of allogeneic T cell proliferation relative to unstimulated T cells (Fig. 1a). MLR assays also demonstrate that HOX11 transgenic and nontransgenic DCs presenting peptides derived from the chicken OVA were equally efficient stimulators of autologous T cells (Fig. 1b).
The ability of HOX11 transgenic T cells to respond to mitogenic stimulation and foreign antigens was demonstrated in standard proliferation and CTL assays. T cells derived from spleens of age- and sex-matched HOX11 transgenic or nontransgenic mice ranging in age from 3 to 18 months showed similar proliferative responses after phytohemagglutinin stimulation (Fig. 1c). In addition, in vitro MLR assays revealed similar proliferative responses of C57BL/6 and HOX11 transgenic T cells after stimulation by allogeneic C3H/He DCs (data not shown). To further assess HOX11 transgenic T cell function, antigen-specific cytolytic activity, induced in vitro (Fig. 1 d and e) or in vivo (Fig. 1 f and g) by stimulation with autologous or syngeneic DCs presenting OVA peptides, was compared with antigen-specific CTL activity of C57BL/6 and nontransgenic mice. The data indicated that HOX11 transgenic mice were fully competent in their ability to activate T cells and lyse target cells presenting OVA peptides. Furthermore, HOX11 CTL activity was antigen-specific in that minimal lysis of unpulsed DCs and EL-4 target cells was noted after both in vitro and in vivo stimulation of T cells by DCs presenting OVA peptides. The above studies indicate that expression of the HOX11 transgene does not compromise the ability of HOX11 mice to mount antigen-specific immune responses.
Immunogenicity of HOX11-Derived Peptides.
To determine whether HOX11 transgenic T cells are rendered nonresponsive to the HOX11 protein as a result of expression of the human HOX11 cDNA throughout B cell differentiation, we assessed T cell responses to HOX11-derived peptides presented by DCs in naïve and HOX11 transgenic mice. DCs isolated from C57BL/6, nontransgenic, and HOX11 transgenic mice were pulsed with IVT human HOX11 mRNA and injected into syngeneic recipients. As a negative mRNA control, C57BL/6, nontransgenic, and HOX11 transgenic mice were injected with DCs pulsed with IVT mRNA derived from the cDNA of Meis1, the murine homeodomain-containing transcription factor. Positive controls included the injection of mice with syngeneic DCs pulsed with either OVA protein or IVT mRNA derived from the cDNA of PBX1a, the human homeodomain-containing transcription factor. Mice were boosted twice and then assayed for in vivo generation of antigen-specific CTLs. The results of these studies revealed that C57BL/6 and nontransgenic DCs pulsed with IVT human HOX11 mRNA were able to induce the in vivo activation of anti-HOX11 specific CTLs capable of in vitro lysis of target cells presenting human HOX11-derived peptides (Fig. 2a). In contrast, DCs presenting human HOX11-derived peptides were unable to induce anti-HOX11-specific CTLs in HOX11 transgenic mice (Fig. 2a). All mice showed similar levels of in vivo generation of OVA-specific CTLs after the injection of OVA-pulsed DCs, and the generation of CTLs was antigen specific, as indicated by minimal lysis of EL-4 and unpulsed DC target cells (Fig. 2c). Because C57BL/6 and HOX11 transgenic mice were equally efficient in their generation of anti-human PBX1a-specific CTLs (Fig. 2b), it is unlikely that the absence of anti-HOX11-specific CTLs in HOX11 transgenic mice was caused by a defect in the ability of HOX11 transgenic DCs to process and present peptides derived from IVT mRNA. Meis1-specific CTLs were not expanded in mice injected with murine Meis1 pulsed DCs, indicating that recipient mice were tolerant to the constitutively expressed murine Meis1 protein (Fig. 2a). Thus, these studies demonstrated that although human HOX11-derived peptides presented by DCs were highly immunogenic and elicited an immune response in naïve C57BL/6 and nontransgenic recipients, immunologically competent HOX11 transgenic mice were nonresponsive to the constitutively expressed HOX11 transgene.
Figure 2.
In vivo generation of cytotoxic T cells after injection of DCs presenting HOX11-, PBX1a-, OVA-, and Meis1-derived peptides. (a) DCs isolated from C57BL/6, nontransgenic, and HOX11 transgenic mice were pulsed with IVT HOX11 mRNA and injected into C57BL/6 (●), nontransgenic (■), and transgenic (▴) mice and analyzed for the in vivo generation of anti-HOX11-specific CTLs. Cytolytic activity was assessed with autologous DC targets pulsed with human HOX11 mRNA. Cytolytic activity in C57BL/6 mice (○) after the injection of DCs pulsed with Meis1 IVT mRNA was assessed against autologous DC targets presenting Meis1-derived peptides. (b) Generation of human PBX1-specific CTLs in C57BL/6 (●) and HOX11 transgenic (▴) mice injected with syngeneic DCs pulsed with IVT human PBX1 mRNA. Cytolytic activity was assessed against autologous DC targets presenting human PBX1a-derived peptides. Nonspecific C57BL/6 (○) and HOX11 transgenic (▵) CTL activity was assessed against unpulsed DC targets. (c) The generation of OVA-specific T cells after the injection of OVA-pulsed DCs into C57BL/6 (●), nontransgenic (■), and HOX11 transgenic (●) mice. Cytolytic activity was assessed against autologous DC targets presenting OVA-derived peptides. Nonspecific C57BL/6 (○), nontransgenic (□), and HOX11 transgenic (▵) CTL activity was assessed against EL4 target cells.
Immunogenicity of Lymphoma Cell Lysates in Nontransgenic and Premalignant Transgenic Mice.
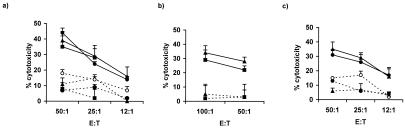
The immunogenicity of HOX11 lymphoma cell lysates was assessed for their ability to induce anti-lymphoma-specific CTL responses in vivo (Fig. 3). Lymphoma cells were obtained from three HOX11 transgenic mice, all of which were shown by histopathological analysis to have splenic lymphoma with dissemination of lymphoma cells to the lungs, kidney, liver, and thymus. Lymphoma cell lysates were generated from two HOX11 transgenic mice with solid tumor masses. The third HOX11 transgenic mouse had terminal lymphoma in which the spleen was comprised almost exclusively of lymphoma cells. Cell lysates from this mouse were generated from purified B220+ cells or total unsorted spleen cells. In vivo studies revealed that DCs presenting lymphoma cell antigens induced strong anti-lymphoma-specific CTL responses in C57BL/6, nontransgenic, and in premalignant HOX11 transgenic mice. Efficient lysis of target DCs pulsed with B220+ lymphoma cells (Fig. 3a), spleen-derived B220+ lymphoma cells (Fig. 3b), and with total spleen/lymphoma cell lysates (Fig. 3c) was detected. Cytolytic activity was lymphoma-antigen-specific in that CTLs generated after injection of DCs pulsed with B220+ lymphoma cell lysates showed minimal lysis of unpulsed DC targets (Fig. 3 a and c) and nontransformed LPS-activated splenic B cells (Fig. 3b). Moreover, minimal cytolytic activity against DCs presenting control B cell antigens was noted after injection of C57BL/6 mice with DCs pulsed with C57BL/6-derived B cell lysates (Fig. 3a). Antigen-specific CTLs were expanded in vivo rather than in vitro, as revealed by the lack of target cell lysis after the injection of mice with PBS (Fig. 3c). These studies demonstrate that lymphomagenesis in HOX11 transgenic mice is associated with the generation of immunogenic tumor-associated antigens capable of inducing an immune response in naïve, nontransgenic mice and in young, premalignant HOX11 transgenic mice.
Figure 3.
Immunogenicity of HOX11 transgenic lymphoma cell lysates. Cell
lysates were generated from a spleen that contained lymphoma cells
almost exclusively. Cell lysates were prepared from either
B220+ sorted spleen cells (a and
b) or unsorted, total spleen cells (c).
DCs isolated from 4- to 6-week-old C57BL/6, nontransgenic, or HOX11
transgenic mice were pulsed with either B220+ lymphoma cell
lysates or total spleen/lymphoma cell lysates and injected into
6-week-old C57BL/6, nontransgenic, or HOX11 transgenic recipients.
Mice were given booster injections twice at 7-day intervals and killed
21 days after the initial injection, and their spleens were used as a
source of T cells. Generation of anti-lymphoma-antigen-specific
CTLs in C57BL/6 ( ), nontransgenic
(
), nontransgenic
( ), or HOX11 transgenic (
), or HOX11 transgenic ( )
mice was assessed against target DCs pulsed with B220+
lymphoma cell lysates (a), B220+ lymphoma
cells isolated from the spleen (b), or target DCs pulsed
with total spleen/lymphoma cell lysates (c). In
vivo generation of CTLs was lymphoma antigen specific as
revealed by minimal lysis of unpulsed C57BL/6
(---●---),
nontransgenic
(---■---), or HOX11
transgenic
(---▴---)
DC targets (a and c) and LPS-activated,
nontransformed B cells (b). The immunogenicity of
nontransformed B cell lysates was assessed after injection of DCs
pulsed with C57BL/6-derived nontransformed B220+ cell
lysates into C57BL/6 mice. Target cells in these assays were DCs
pulsed with C57BL/6 B220+ cell lysates
(---○---)
(a). In vivo rather than in
vitro generation of antigen-specific CTLs was demonstrated by
injection of PBS into C57BL/6
(---○---)
recipients with target cell lysis being assessed against total
spleen/lymphoma cell lysates (c). These studies were
repeated with two additional lymphoma cell lysates with similar
results.
)
mice was assessed against target DCs pulsed with B220+
lymphoma cell lysates (a), B220+ lymphoma
cells isolated from the spleen (b), or target DCs pulsed
with total spleen/lymphoma cell lysates (c). In
vivo generation of CTLs was lymphoma antigen specific as
revealed by minimal lysis of unpulsed C57BL/6
(---●---),
nontransgenic
(---■---), or HOX11
transgenic
(---▴---)
DC targets (a and c) and LPS-activated,
nontransformed B cells (b). The immunogenicity of
nontransformed B cell lysates was assessed after injection of DCs
pulsed with C57BL/6-derived nontransformed B220+ cell
lysates into C57BL/6 mice. Target cells in these assays were DCs
pulsed with C57BL/6 B220+ cell lysates
(---○---)
(a). In vivo rather than in
vitro generation of antigen-specific CTLs was demonstrated by
injection of PBS into C57BL/6
(---○---)
recipients with target cell lysis being assessed against total
spleen/lymphoma cell lysates (c). These studies were
repeated with two additional lymphoma cell lysates with similar
results.
Tolerance to Lymphoma-Associated Antigens by Autologous T Cells in Lymphomatous HOX11 Transgenic Mice.
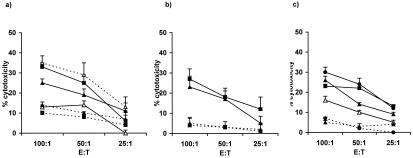
To determine whether lymphomagenesis in HOX11 transgenic mice was associated with the induction of anergy of lymphoma antigen-specific T cells, T cells from HOX11 transgenic mice with terminal lymphoma were assessed for their ability to lyse target cells presenting autologous lymphoma antigens. The immunogenicity of the lymphoma cells was demonstrated in in vitro and in vivo assays by the activation of naïve C57BL/6, nontransgenic, and HOX11 transgenic T cells by autologous DCs presenting HOX11-derived lymphoma antigens (Fig. 4 a–c). T cell lysis was antigen specific in that T cells from C57BL/6, nontransgenic, and premalignant HOX11 mice showed efficient lysis of target DCs pulsed with HOX11 B220+ lymphoma cell lysates (Fig. 4a) or total spleen/lymphoma cell lysates (Fig. 4c), and lysis of B220+ lymphoma cells (Fig. 4b). Minimal lysis of LPS-stimulated C57BL/6 B cells (Fig. 4b) or nonpulsed DC targets (Fig. 4 a and c) was seen. In contrast, T cells from terminally ill HOX11 mice were unable to efficiently lyse target DCs pulsed with HOX11 B220+ lymphoma cell lysates or total spleen lymphoma cell lysates (Fig. 4 a and c). However, terminal HOX11 transgenic mice generated OVA-specific CTL responses against DCs presenting OVA peptides (Fig. 4a) or peptides derived from human PBX1a (data not presented), which were comparable to in vitro OVA responses of T cells isolated from premalignant, young HOX11 transgenic mice (Fig. 1 d and e), thereby minimizing the possibility of a global immunosuppression in these mice. Our data thus indicate that T cells encountering lymphoma-associated antigens were rendered nonresponsive to these antigens during the course of lymphoma progression thereby providing a likely mechanism by which lymphoma cells evade immune detection in HOX11 transgenic mice.
Figure 4.
Induction of lymphoma-antigen-specific T cell tolerance in terminal
HOX11 transgenic mice. DCs and T cells were isolated from nontransgenic
mice ( ), healthy HOX11 transgenic mice
(
), healthy HOX11 transgenic mice
( ), and HOX11 transgenic mice with terminal
lymphoma (––▵––). Age- and sex-matched
C57BL/6 mice (
), and HOX11 transgenic mice with terminal
lymphoma (––▵––). Age- and sex-matched
C57BL/6 mice ( ) were also included. Cell lysates
were prepared from either B220+ sorted lymphoma cells or
unsorted, total spleen/lymphoma cells. T cells were stimulated twice
at 6-day intervals with autologous DCs pulsed with either lymphoma cell
lysates or OVA protein, harvested, and assayed for anti-HOX11
lymphoma-antigen-specific cytolytic activity against DC targets pulsed
with B cell lymphoma lysates (a) or DC targets pulsed
with total spleen/lymphoma lysates (c).
Anti-OVA-specific CTL activity was assessed against DC targets pulsed
with OVA protein
(---▵---)
(a). Generation of nonspecific C57BL/6
(---●---),
nontransgenic
(---■---), or HOX11
transgenic
(---▴---)
CTLs was assessed against unpulsed DC targets (a and
c). These findings were confirmed by using lymphoma cell
lysates from five additional HOX11 mice with terminal lymphoma.
(b) Immunogenicity of lymphoma cell lysates and the
in vivo generation of anti-lymphoma-specific CTLs in
elderly, healthy nontransgenic and transgenic mice. Five 14-month-old
littermates were used; one nontransgenic mouse, three premalignant
transgenic mice, and one HOX11 transgenic mice with terminal lymphoma.
The four healthy littermates were injected with DCs pulsed with
B220+ lymphoma cell lysate originating from the fifth
littermate, they were given booster injections twice and then killed,
and their spleens were used as a source of T cells. In
vivo generation of lymphoma-specific cytolytic activity was
assessed against B220+ lymphoma cell targets and
LPS-activated C57BL/6 B cells.
) were also included. Cell lysates
were prepared from either B220+ sorted lymphoma cells or
unsorted, total spleen/lymphoma cells. T cells were stimulated twice
at 6-day intervals with autologous DCs pulsed with either lymphoma cell
lysates or OVA protein, harvested, and assayed for anti-HOX11
lymphoma-antigen-specific cytolytic activity against DC targets pulsed
with B cell lymphoma lysates (a) or DC targets pulsed
with total spleen/lymphoma lysates (c).
Anti-OVA-specific CTL activity was assessed against DC targets pulsed
with OVA protein
(---▵---)
(a). Generation of nonspecific C57BL/6
(---●---),
nontransgenic
(---■---), or HOX11
transgenic
(---▴---)
CTLs was assessed against unpulsed DC targets (a and
c). These findings were confirmed by using lymphoma cell
lysates from five additional HOX11 mice with terminal lymphoma.
(b) Immunogenicity of lymphoma cell lysates and the
in vivo generation of anti-lymphoma-specific CTLs in
elderly, healthy nontransgenic and transgenic mice. Five 14-month-old
littermates were used; one nontransgenic mouse, three premalignant
transgenic mice, and one HOX11 transgenic mice with terminal lymphoma.
The four healthy littermates were injected with DCs pulsed with
B220+ lymphoma cell lysate originating from the fifth
littermate, they were given booster injections twice and then killed,
and their spleens were used as a source of T cells. In
vivo generation of lymphoma-specific cytolytic activity was
assessed against B220+ lymphoma cell targets and
LPS-activated C57BL/6 B cells.
Discussion
HOX11 transgenic mice provide an attractive model system in which to design and assess immunotherapeutic protocols for the treatment of low-grade non-Hodgkin's lymphoma. Successful immunotherapy for the treatment of cancer requires an intact, functional immune system capable of eliciting an antigen-specific immune response. Because the possibility existed that expression of the HOX11 transgene in hematopoietic cells resulted in perturbations in the ability of HOX11 transgenic mice to mount an immune response, it was essential to thoroughly characterize transgenic DCs and T cells. We previously reported that B and T lymphopoiesis in young HOX11 transgenic mice were unperturbed with phenotypically normal lymphocyte subpopulations in bone marrow, thymus, and the periphery (21). In this report, we demonstrated that HOX11 transgenic DCs express a normal array of DC accessory molecules required for T cell activation and are able to process and present foreign antigens and activate antigen-specific T cells. Studies to assess HOX11 transgenic T cells indicated that transgenic mice ranging in age from 3 to 18 months have normal T cell proliferative responses to mitogenic stimulation and to stimulation by foreign antigens presented by DCs. Therefore, expression of the HOX11 transgene does not compromise the ability of transgenic mice to mount an antigen-specific immune response.
The utility of HOX11 transgenic mice as a model system to assess immunotherapeutic protocols is also dependent on the generation of lymphoma associated antigens against which an immune response can be initiated. We therefore assessed the immunogenicity of peptides derived from the human HOX11 protein and HOX11 lymphoma cell lysates. Our studies showed that human HOX11-derived peptides, when presented on DCs, are immunogenic in naïve C57BL/6 and nontransgenic mice. In contrast, T cell responses specific for anti-HOX11 peptides are not detected after immunization of HOX11 transgenic mice with DCs presenting human HOX11-derived antigens. This finding was predictable given that the HOX11 protein is expressed throughout B cell differentiation in transgenic mice, and thus anti-HOX11 specific T cells would likely be deleted during thymocyte development or rendered nonresponsive. HOX11 is normally expressed during a restricted period of embryogenesis, ectopic expression of human HOX11 in murine lymphocytes contributes to lymphomagenesis, and deregulated expression of HOX11 has been reported in patients with T cell acute lymphoblastic leukemia (17). Thus, our data suggest that the HOX11 protein might be an attractive target against which immune responses could be directed in immunotherapy protocols for human T cell acute lymphoblastic leukemia.
Our studies to assess the immunogenicity of spontaneously arising lymphomas in HOX11 transgenic mice demonstrated that tumor cell lysates are highly immunogenic in naïve and in premalignant transgenic mice. Lymphoma antigens are also immunogenic in elderly (>12 months) syngeneic transgenic mice with splenic lymphoid hyperplasia or lymphoma. Thus, lymphoma progression in HOX11 transgenic mice is likely associated with the acquisition of genomic mutations and deregulated gene expression that result in the generation of lymphoma-associated antigens.
A critical question addressed in this report was whether the exposure of autologous T cells to lymphoma-associated antigens results in the induction of antigen-specific T cell tolerance. We demonstrated that T cells isolated from HOX11 transgenic mice with terminal lymphoma are minimally activated by stimulation with DCs presenting immunogenic lymphoma antigens. Furthermore, there was no evidence to suggest that lymphomagenesis is associated with global immunosuppression in these mice because their T cells show normal levels of proliferation and cytolytic activity after activation by DCs presenting OVA- or PBX1a-derived peptides. Therefore, lymphoid hyperplasia with progression to lymphoma in HOX11 transgenic mice is associated with the tolerization of anti-lymphoma antigen-specific T cells.
A variety of mechanisms may be used by malignant cells to abrogate an efficient immune response against antigenic tumors (refs. 10, 16, 30–32, and references within). A better understanding of mechanisms used by tumor cells to evade immune detection and the development of strategies to restore T cell responsiveness and to increase the efficiency of the host immune system to recognize and eliminate malignant cells will be critical for successful development of antitumor immunotherapy protocols. These studies will require approaches that do not separate the processes of host immune rejection from the in vivo environment of tumor progression. Transgenic mouse models that accurately recapitulate all stages of tumorigenesis will be powerful model systems in which to address these questions.
Acknowledgments
We thank Drs. Michael Cleary and Neil Copeland for providing cDNAs for PBX1a and Meis1 and Drs. Nicholas Gough and Fritz Melchers for providing cytokine-producing cell lines. We gratefully acknowledge Dr. Megan Lim for histopathological analysis of mouse tissues and Dr. Gillian Wu for critical advice provided during preparation of this manuscript. We also thank Josephine Correia and Denise Vanner for the excellent assistance with animal husbandry and the pathology laboratory technologists of SD Laboratories, Sunnybrook & Women's College Health Sciences Centre, for preparation of tissue sections. Research in the investigators laboratories is supported by grants from the Medical Research Council of Canada and the National Cancer Institute of Canada.
Abbreviations
- DC
dendritic cell
- CTL
cytotoxic T lymphocyte
- MLR
mixed lymphocyte reaction
- IVT
in vitro-transcribed
- OVA
chicken ovalbumin protein
- LPS
lipopolysaccharide
- CM
complete medium
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.240221297.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.240221297
References
- 1.Coiffier B, Thieblemont C, Felman P, Salles G, Berger F. Semin Hematol. 1999;36:198–208. [PubMed] [Google Scholar]
- 2.Gilboa E, Nair S K, Lyerly H K. Cancer Immunol, Immunother. 1998;46:82–87. doi: 10.1007/s002620050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fields R C, Shimizu K, Mule J J. Proc Natl Acad Sci USA. 1998;95:9482–9487. doi: 10.1073/pnas.95.16.9482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paglia P, Chiodoni C, Rodolfo M, Colombo M P. J Exp Med. 1996;183:317–322. doi: 10.1084/jem.183.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nestle F O, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 6.Ashley D M, Faiola B, Nair S, Hale L P, Bigner D D, Gilboa E. J Exp Med. 1997;186:1177–1182. doi: 10.1084/jem.186.7.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nair S K, Boczkowski D, Morse M, Cumming R I, Lyerly H K, Gilboa E. Nat Biotechnol. 1998;16:364–369. doi: 10.1038/nbt0498-364. [DOI] [PubMed] [Google Scholar]
- 8.Ishida T, Oyama T, Carbone D P, Gabrilovich D I. J Immunol. 1998;161:4842–4851. [PubMed] [Google Scholar]
- 9.Oyama T, Ran S, Ishida T, Nadaf S, Kerr L, Carbone D P, Gabrilovich D I. J Immunol. 1998;160:1224–1232. [PubMed] [Google Scholar]
- 10.Staveley-O'Carroll K, Sotomayor E, Montgomery J, Borrello I, Hwang L, Fein S, Pardoll D, Levitsky H. Proc Natl Acad Sci, USA. 1998;95:1178–1183. doi: 10.1073/pnas.95.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cardoso A A, Schultze J L, Boussiotis V A, Freeman G J, Seamon M J, Laszlo S, Billet A, Sallan S E, Gribben J G, Nadler L M. Blood. 1996;88:41–48. [PubMed] [Google Scholar]
- 12.Theobald M, Biggs J, Hernandez J, Lustgarten J, Labadie C, Sherman L A. J Exp Med. 1997;185:833–841. doi: 10.1084/jem.185.5.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wick M, Dubey P, Koeppen H, Siegel C T, Fields P E, Chen L, Bluestone J A, Schreiber H. J Exp Med. 1997;186:229–238. doi: 10.1084/jem.186.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flood P M, Liu X, Alexander R, Schreiber H, Haque S. J Immunother. 1998;21:307–316. doi: 10.1097/00002371-199807000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez J, Lee P P, Davis M M, Sherman L A. J Immunol. 2000;164:596–602. doi: 10.4049/jimmunol.164.2.596. [DOI] [PubMed] [Google Scholar]
- 16.Lee P P, Yee C, Savage P A, Fong L, Brockstedt D, Weber J S, Johnson D, Swetter S, Thompson J, Greenberg P D, Roederer M, Davis M M. Nat Med. 1999;5:677–685. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 17.Dubé I D, Raimondi S C, Pi D, Kalousek D K. Blood. 1986;67:1181–1184. [PubMed] [Google Scholar]
- 18.Dubé I D, Kamel-Reid S, Yuan C C, Lu M, Wu X, Corpus G, Raimondi S C, Crist W M, Carroll A J, Minowada J, Baker J B. Blood. 1991;78:2996–3003. [PubMed] [Google Scholar]
- 19.Hatano M, Roberts C W, Minden M, Crist W M, Korsmeyer S J. Science. 1991;253:79–82. doi: 10.1126/science.1676542. [DOI] [PubMed] [Google Scholar]
- 20.Kennedy M A, Gonzalez-Sarmiento R, Kees U R, Lampert F, Dear N, Boehm T, Rabbitts T H. Proc Natl Acad Sci USA. 1991;88:8900–8904. doi: 10.1073/pnas.88.20.8900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hough M, Reis M, Singaraja R, Bryce D, Kamel-Reid S, Dardick I, Breitman R, Dubé I. Proc Natl Acad Sci USA. 1998;95:13853–13858. doi: 10.1073/pnas.95.23.13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris N L, Jaffe E S, Stein H, Banks P M, Chan J K, Cleary M L, Delsol G, De Wolf-Peeters C, Falini B, Gatter K C. Blood. 1994;84:1361–1392. [PubMed] [Google Scholar]
- 23.Berger F, Felman P, Thieblemont C, Pradier T, Baseggio L, Bryon P A, Salles G, Callet-Bauchu E, Coiffier B. Blood. 2000;95:1950–1956. [PubMed] [Google Scholar]
- 24.Karasuyama H, Melchers F. Eur J Immunol. 1988;18:97–104. doi: 10.1002/eji.1830180115. [DOI] [PubMed] [Google Scholar]
- 25.Monica K, Galili N, Nourse J, Saltman D, Cleary M L. Mol Cell Biol. 1991;11:6149–6157. doi: 10.1128/mcb.11.12.6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boczkowski D, Nair S K, Snyder D, Gilboa E. J Exp Med. 1996;184:465–472. doi: 10.1084/jem.184.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kruisbeek A, Shevach E. In: Current Protocols in Immunology. Coligan J E, Kruisbeek A, Margulies D, Shevach E, Strober W, editors. Vol. 1. New York: Wiley; 1997. pp. 3.12.1–3.12.14. [Google Scholar]
- 28.Matzinger P. J Immunol Methods. 1991;145:185–192. doi: 10.1016/0022-1759(91)90325-a. [DOI] [PubMed] [Google Scholar]
- 29.Wunderlich J, Shearer G, Livingstone A. In: Current Protocols in Immunology. Coligan J E, Kruisbeek A, Margulies D, Shevach E, Strober W, editors. Vol. 1. New York: Wiley; 1997. pp. 3.11.1–3.11.20. [Google Scholar]
- 30.Anichine A, Molla A, Mortarini R, Tragni G, Bersani I, Di Nicola M, Gianne A, Pilotti S, Dunbar R, Cerundolo V, Parmiani G. J Exp Med. 1999;190:651–667. doi: 10.1084/jem.190.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ochsenbein A F, Klenerman P, Karrer U, Ludewig B, Pericin M, Hengartner H, Zinkernagel R M. Proc Natl Acad Sci USA. 1999;96:2233–2238. doi: 10.1073/pnas.96.5.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schreiber H. Fundamental Immunology. New York: Raven; 1999. [Google Scholar]