Abstract
Many studies of drug self-administration in primates have shown that faster infusions of a drug are more reinforcing than slower infusions. Similar effects have not been shown in rats. We assessed the influence of delivery rate by allowing rats to choose between the same dose of intravenous cocaine delivered over two different infusion speeds. Rats were trained in chambers containing two nose-poke response devices. In Experiment 1, responses in one nose poke delivered 0.3 mg/kg/inj of cocaine over 10 sec, and responses in the other delivered the same dose over 100 sec. In Experiment 2, the same procedure was used, but with 1.0 mg/kg/inj dose delivered over 1.7 versus 100 sec. During acquisition, most rats preferred the faster infusion. When the delivery rates associated with the nose pokes were reversed, rats trained with 0.3 mg/kg/inj failed to switch nose-poke preference, but half the rats trained with 1.0 mg/kg/inj did switch. In experiment 3, the choice was between 1 mg/kg cocaine delivered over 1.7 sec and no reinforcement. Here, rats quickly learned to respond in the nose-poke associated with cocaine and quickly switched their choice during reversal. In Experiment 4, two groups of rats were allowed to choose between food delivered with a delay of 1 versus 5 sec or 1 versus 10 sec, respectively. Rats preferred the shorter delay during initial training. In reversal, some rats in the 1 vs 5 sec group failed to reverse, while all the rats in the 1 vs 10 sec group reversed. These results show that faster infusions of cocaine are clearly more reinforcing during acquisition, but delivery rate may not be as important to the maintenance of self-administration once it has been established. The results with food suggest that these findings represent general principles of behavior and are not unique to drug self-administration.
Keywords: Cocaine, self-administration, infusion duration, choice, rat
It is widly accepted that the onset rate of a drug’s effects influences its abuse liability. Evidence for this “rate hypothesis” (Gorelick, 1998) in humans comes from the observation that drugs that have a quick onset (i.e., enter the brain more rapidly) are more likely to be abused than drugs with slower onsets (Gossop et al., 1992; Hatsukami et al., 1996). In addition, injection drug users report that they typically inject an abused drug over a very short period of time (3–10 sec; Zernig et al., 2003). In the human laboratory, rate of infusion has been investigated for both cocaine and opioids. Both Abreu et al. (2001) and Nelson et al. (2006) reported stronger subjective effects for cocaine when it was administered faster. In the Abreu et al. (2001) study, rate of infusion was studied by administering the same dose of cocaine over periods of 2, 15 or 60 sec. Subjective report measures were then taken. In general, subjective reports of “high” and “liking” were higher for the shorter infusion durations (faster onset). Marsch et al. (2001) reported similar effects for morphine administration in humans.
In non-human primates, the effect of infusion duration has been studied on the self-administration of cocaine by a number of investigators (Balster and Schuster, 1973; Kato et al., 1987; Kimmel et al., 2007; Panlilio et al., 1998; Woolverton and Wang, 2004). The study by Panlilio et al. is illustrative of the general effect. Rhesus monkeys were trained to respond on a key for i.v. cocaine on a fixed-ratio (FR) schedule with a 5-min timeout. While the dose was held constant, the infusion duration was varied from 10 to 240 sec. For all the monkeys studied, as the duration of the infusion increased, rates of self-administration decreased. Winger et al. (2002) studied the effect of drug onset rate for NMDA antagonists by studying 3 drugs with different inherent rates of onset. While both ketamine and phencyclidne have at least some immediate behavioral effects, dizocilpine has few immediate effects in rhesus monkeys. In self-administration studies, both ketamine and phencyclidine were stronger reinforcers than was dizocilpine. In addition to drug self-administration, infusion duration also influences other behavioral effects of abused drugs. For example, rapid delivery of cocaine is more likely to produce locomotor sensitization (Samaha and Robinson, 2005).
While both human and non-human primate laboratory studies clearly support the rate hypothesis of self-administered drugs, results from rodents have not been as clear. In a study by Crombag et al. (2008), the rate of drug delivery was varied during acquisition (5 vs 100 sec) or during maintenance (5, 25, 50 or 100 sec) for rats responding on a FR 1 schedule of cocaine or amphetamine self-administration. They found that the duration of the injection had no effect on either the acquisition or maintenance of self-administration for either drug. Liu et al. (2005) also studied the effects of injection duration on cocaine self-administration. Animals were initially trained on a progressive ratio (PR) schedule and then switched to an FR schedule. Different groups of rats were trained with different infusion durations (5, 25 or 50 sec). Liu et al. found a small, but significant, effect of injection speed on the FR schedule, with the shorter duration infusions maintaining slightly fewer infusions per hour. Given that, on the descending limb of the dose-effect function, larger doses of cocaine typically maintain lower response rates in rats responding on an FR schedule with a short timeout, these results may suggest that the shorter duration infusions were functioning similarly to larger doses of cocaine.
The reasons for the discrepancy in the effects of infusion duration on rat versus primate drug self-administration are not clear. It may be that differences in the effectiveness of fast vs. slow injections would become more apparent if rats were allowed to directly compare the same drug reinforcer given at different speeds. Choice procedures have been used successfully in drug self-administration studies to evaluate behavioral and pharmacological factors involved in drug self-administration (Hart et al., 2000; Negus, 2003). Therefore, in the current study, rats were trained in a 2-response choice procedure, where two responses were reinforced with the same dose of cocaine. However, for one response cocaine was given rapidly and for the other response cocaine was administered more slowly. After acquisition the responses associated with the fast and slow injections were reversed. To determine if the observed effects were unique to cocaine, rats were also trained on a choice procedure for food using different delays of reinforcement.
METHOD
Subjects
The subjects (n = 49) were experimentally naïve, male Sprague-Dawley rats, weighing approximately 350–380 g at the beginning of the experiment. They were housed in a room with a 12:12 hr reverse light-dark cycle (lights on at 2200 h) and at an average ambient temperature of 23° C for the duration of the experiment. The animals had free access to water. All rats were housed individually and food was restricted to maintain a weight of approximately 350 g. The guidelines of the Institutional Animal Care and Use Committee at the National Institute on Drug Abuse/Intramural Research Program and the Guide for the Care and Use of Laboratory Animals were followed at all times.
For intravenous (i.v.) drug administration, jugular-vein catheters were implanted according to the procedure described in detail by Panlilio et al. (1996). Briefly, approximately 3 cm of Silastic tubing (Dow Corning, 0.44 mm i.d., 0.9 mm o.d.) was inserted into the right jugular vein and connected to vinyl tubing (Dural Plastics, 0.5 mm i.d., 1.0 mm o.d.) that exited the back at the midscapular region, and was plugged with an obturator. Immediately following catheter implantation, a 20-mm nylon screw was cemented to the skull to serve as a head mount. A metal spring, through which tubing connecting the fluid swivel on the top of the cage to the catheter was fed, was attached to this head mount. Catheters were flushed before and after each training session with 0.1 ml of a saline solution containing 1.25 units/ml heparin and 0.08 mg/ml gentamicin.
Apparatus
Ten training chambers (ENV-008CT, Med Associates, St. Albans VT) were enclosed individually in sound-attenuation chambers equipped with fans for ventilation and background noise (ENV-018M, Med Associates). Each chamber had a grid floor and two nose-poke response holes (ENV-114BM, Med Associates), one on each side of a food trough into which 45-mg food pellets (BioServ, Frenchtown, NJ) could be delivered. The nose-poke holes could be illuminated from inside the hole by a dim yellow light. A houselight (ENV-215M, Med Associates) was situated above and on the wall behind the nose-poke holes. Cocaine was delivered through Tygon tubing inside a metal spring, suspended through the ceiling from a single-channel fluid swivel (Instech, Plymouth Meeting, PA). For Experiment 1, this tubing was attached via a Y-connector to two 10-ml syringes controlled by two motor-driven syringe pumps outside the sound attenuation chamber. The pumps (PHM-100, Med Associates) ran at different speeds, but delivered that same concentration and volume (0.2 ml/inj) of cocaine solution. For Experiment 2, the volume was increased to 0.3 ml/inj to reduce the potential for variability in injection amounts. The slow pump was replaced by a variable speed pump (PHM-107, Med Associates) for Experiment 2. A 20 ml syringe was used for the slow pump and a 30 ml syringe was used for the fast pump. For Experiment 3 only the fast pump configuration of Experiment 2 was used. Experimental events were controlled by a MED-PC computer system (Med Associates).
Procedure
Experiment 1
Following surgery and at least 7 days of recovery, the catheters were connected, and the rats (n = 14) were placed in the training chambers every weekday. Sessions began with the illumination of the houselight and the two nose-poke holes. A nose-poke response in either hole was immediately reinforced with an injection of 0.3 mg/kg cocaine. Cocaine was delivered via the fast pump (10 sec infusion) for responses in one hole and via the slow pump (100 sec infusion) for responses in the other hole. Which hole (right or left) was associated with which pump was counterbalanced across rats. There was a 110 sec timeout (including infusion time), during which the houselight and hole lights were turned off, following each response. Sessions continued for 2 hours or until 25 injections had been received for responding in one hole. Animals were trained until responding stabilized (less than ±20% variation in rate with no consistent upward or downward trend) for 3 consecutive days. Once stable acquisition was evident, the holes that were associated with the fast and slow pumps were reversed. All other details of the procedure remained the same.
Experiment 2
Experiment 2 was identical to Experiment 1 with the following exceptions. The fast pump was operated for 1.7 sec instead of 10 sec. There was no maximum on the numbers of injections per session. Session terminated only after 2 hours had elapsed. The dose of cocaine was 1.0 mg/kg/inj for all rats (n = 17).
Experiment 3
Experiment 3 was identical to Experiment 2 except that responding was reinforced by 1.0 mg/kg cocaine given over 1.7 sec for one nose-poke, while responding for the other nose-poke was not reinforced (no injection was given). The response for the non-reinforced choice started the timeout identically to the reinforced choice. All other details of the experiment remained the same. Rats (n = 8) were trained for at least 10 days or until stability prior to reversal.
Experiment 4
In Experiment 4, nose-poke responses were reinforced by a single 45 mg food pellet rather than cocaine. To facilitate training, food was restricted to maintain the rats at 85% of their initial free-feeding weight. There was a delay between the nose-poke and the delivery of food. There was no change in stimuli with the response, but the houselight and nose-poke light were turned off following the delivery of the food pellet. The response started a 110-sec timeout that terminated with the houselight and nose-poke lights being turned back on. Responses made during the timeout were recorded, but had no programmed consequence. Five rats were initially trained for 4 days with delays of 1 sec or 5 sec associated with the different holes (counterbalanced across rats). A second group of 5 rats was trained for 4 days with delays of 1 sec or 10 sec. Following the initial 4 days of training, the nose-pokes associated with each delay were reversed and rats continued training for an additional 10 days.
Drugs
Cocaine hydrochloride (NIDA, Baltimore, MD) was dissolved in sterile saline. The concentration of the infusion was adjusted according to the weight of the rat to give either 0.3 or 1.0 mg/kg/injection (as the salt) during self-administration sessions.
Data Analysis
The primary dependent variable was the number of non-timeout responses for each nose-poke hole. Statistical analysis was performed on responses per session using SAS software (SAS Institute, Cary NC) with proc mixed. The factors were reinforcement (fast vs. slow pump speed in Experiments 1 and 2, cocaine vs. no cocaine in Experiment 3, and long vs short delay in Experiment 4) and day of training (up to 15 days for analysis of acquisition, and the last three days pre-reversal and the last three days post-reversal to assess the effects of reversal). For significant main effects or interactions, paired comparisons were performed using the Tukey-Kramer procedure, maintaining a significance level of 0.05 within each set of comparisons.
RESULTS
Experiment 1
Of the 14 rats trained in Experiment 1, 3 were lost due to catheter failure prior to 10 days of training. Of the remaining 11 rats, 6 showed evidence of acquisition of nose-poke responding within 15 days of the beginning of the experiment. One other rat showed evidence of acquisition after 15 days. Of these 7 rats, 6 acquired responding to the fast nose-poke hole. The eighth rat acquired initially to the slow nose-poke hole, but eventually switched responding to the fast nose-poke hole. The other 4 rats showed minimal or no sign of acquisition. The results of these 4 rats were not included in the following analysis.
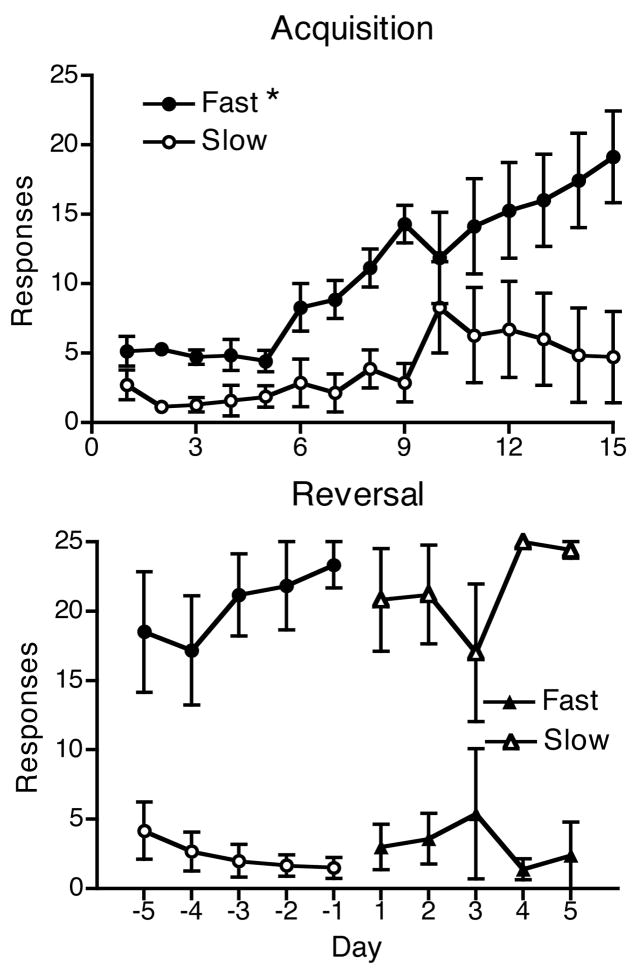
The top panel of Fig. 1 shows the average responding in each nose-poke hole for the first 15 days of training for those rats that showed at least minimal evidence of acquisition (n = 7). By day 6 there was already some separation between the nose-poke responses associated with the fast and slow pump and by the end of the 15 days there was a clear separation between the responses, with a clear preference for the faster injection. Analysis-of-variance revealed a significant Day (F14,84 = 2.28, p < 0.05) and Pump Speed (F1,6 = 7.97, p < 0.01) effect, but no Day × Pump Speed interaction.
Fig. 1.
Reinforced responses for rats trained to self-administer 0.3 mg/kg/inj cocaine (Experiment 1). One response was associated with a 10-sec infusion (Fast) and the other with a 100-sec (Slow) infusion. The top panel shows the first 15 days of acquisition for 8 rats. The difference between the two responses (Fast vs Slow) was significant (p < 0.01). The bottom panel shows the reinforced responses for 5 rats during the final 5 days of acquisition and the first 5 days following the reversal of the reinforcers associated with each response. Circles represent acquisition while triangles represent reversal. Closed symbols are the fast pump responses while open symbols are the slow pump responses. While the differences between the Fast and Slow responses were significantly different for each phase (p’s < 0.01), there was no evidence of reversal. *Difference in main effect of infusion duration, p < 0.05.
Once acquisition occurred, training continued for at least 3 days. The nose-poke holes associated with the fast and slow pumps were then reversed. The bottom panel of Fig. 1 shows the average results for the 5 rats that maintained patent catheters for at least 5 days following the reversal. Note that data for days 11–15 in the upper panel of Figure 1 are not identical to the data for the last 5 days of the acquisition phase in the lower panel because some animals were trained for more than 15 days to reach 3 days of stable behavior and because data for animals that did not complete reversal training are not included in the lower panel. During reversal (5–12 days total), all 5 rats that completed the phase continued to respond almost exclusively in the hole where self-administration was originally acquired. Analysis of the last 3 days of acquisition (pre) and the last 3 days of reversal (post) revealed a significant Day × Pump Speed interaction (F5,20 = 45.14, p < 0.0001). Paired comparisons revealed that responding in the hole associated with the fast pump and the slow pump differed on each day, with more responding to the fast pump nose-poke in the pre-phase and more responding to the slow pump nose-poke after reversal.
Experiment 2
Of the 17 rats trained for Experiment 2, 2 were lost due to catheter failure before 10 days of training. Of the remaining 15 rats, all showed evidence of acquisition, with 14 of the rats acquiring to the nose-poke associated with the faster injection. The top panel of Fig. 2 shows the average results for the 15 rats that acquired cocaine self-administration. On the first training day, rats responded approximately equally in the nose-poke holes associated with the fast and slow pump. However, clear separation between the fast and slow pump was evident early in training. By the end of 10 days of training, rats were taking an average of 20 cocaine injections via the fast pump. Any responding for the slow pump was due almost exclusively to a single rat that acquired to the slow pump and continued to respond in that nose-poke throughout training. Analysis of the acquisition phase revealed a significant effect of Days (F9,126 = 2.79, P < 0.01), Pump Speed (F1,14 = 136.5, P < 0.001) and Days × Pump interaction (F9,126 = 2.44, P < 0.05). Paired comparisons showed that responding for the nose-poke associated with the fast pump was different from that associated with the slow pump for every day beyond day 3.
Fig. 2.
Reinforced responses for rats trained to self-administer 1.0 mg/kg/inj cocaine (Experiment 2). One response was associated with a 1.7-sec infusion (Fast) and the other with a 100-sec (Slow) infusion. The top panel shows the first 10 days of acquisition for 14 rats. The difference between the two responses (Fast vs Slow) was significant for every day beyond day 3 (p’s < 0.05). The bottom panel shows the reinforced responses for 10 rats during the final 5 days of acquisition and the first 10 days following the reversal of the reinforcers associated with each response. Circles represent acquisition while triangles represent reversal. Closed symbols are the fast pump responses while open symbols are the slow pump responses. While the differences between the Fast and Slow responses were significantly different for the acquisition phase (p’s < 0.01), they were not significantly different in the reversal phase. *Differences between durations for a given day, p < .05.
The bottom panel of Fig. 2 shows the results for the nose-poke hole reversal phase of Experiment 2. Average results are shown for the 12 animals that continued training for at least 8 days following reversal. The average responding during the 5 days prior to reversal and the 10 days following reversal are shown. Note that data for days 6–10 in the upper panel of Figure 2 are not identical to the data for the last 5 days of the acquisition phase in the lower panel because some animals were trained for more than 10 days to reach 3 days of stable behavior and because data for animals that did not complete reversal training are not included in the lower panel. On the first day of reversal there was a small increase in responding in the nose-poke associated with the slow pump (i.e. previously associated with the fast pump). Responding for the slow pump then dropped over the next 3 days. Unlike Experiment 1, responding in the hole associated with the fast pump (previously the slow pump) increased on the first day of reversal and then increased to around 20/day by day 2 of reversal. Over the course of reversal training 6 of the 12 rats showed some evidence of reversal. For all of these rats, evidence of reversal was seen before reversal day 5, with one rat showing evidence of reversal on day 1, 3 on day 2 and one on day 4 of the reversal phase. For the 5 rats that did not reverse, one rat acquired to the slow infusion nose-poke and therefore in reversal was already responding to the fast infusion nose-poke. Analysis of the last 3 acquisition days and the last 3 reversal days (including days beyond day 5) showed a significant effect of Pump Speed (F1,11 = 31.29, p < 0.01), but no main or interaction effect for Days. Planned comparisons (Tukey) of nose-poke responding over days show only a significant effect on the last 2 days of acquisition, where responding for the nose-poke associated with the fast pump was higher. The responses did not differ in reversal.
Experiment 3
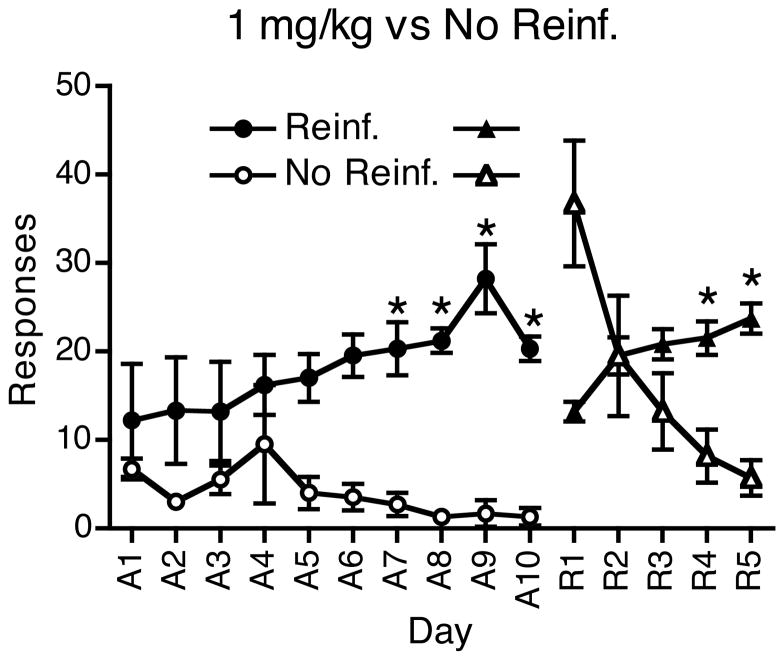
To define a boundary condition and confirm that most rats are indeed capable of switching preference with drug self-administration, in Experiment 3 rats were allowed to choose between a nose-poke that delivered cocaine and a one that did not. Of the 8 rats that began training, on rat died and one rat lost its catheter prior to completing training. All the remaining 6 rats met the acquisition criterion by 10 days of training. For these sessions, there was a significant interaction of Pump (i.e., cocaine vs no injection) and Day (F9,45 = 2.28, p < 0.05). Paired comparisons indicated that responding in the cocaine hole was significantly higher than in the no-injection hole on days 7–10. All 6 rats completed 5 days of reversal training. Following reversal there was an increase in responding for the non-reinforced choice (the previously reinforced choice), but responding for the non-reinforced choice quickly decreased over days (Fig. 3). Responding for the reinforced choice (the previously non-reinforced choice) was evident on day 1 of reversal, and by day 2 of reversal, 3 of the 5 rats were responding more for the reinforced choice. By day 3 of reversal 5 rats were responding more for the reinforced choice. Statistical analysis of the last 3 days of acquisition (day A8–A10 in Figure 3) and the last 3 days of reversal (days R3–R5 in Figure 3) showed a significant interaction of Pump (i.e., cocaine vs no injection) and Day (F5,25 = 3.54, p < 0.05). Paired comparisons indicated that responding in the cocaine hole was significantly higher (p’s < 0.05) than in the no-injection hole on each of the last three days of acquisition, and on the last 2 days of reversal (R4 and R5).
Fig. 3.
Reinforced responses for rats trained to respond for 1 mg/kg cocaine (Experiment 3). Results are shown for the acquisition (A1–A10) phase and reversal phase (R1–R5) for 6 rats trained with one response associated with 1 mg/kg cocaine given over 1.7 sec and the other associated with no reinforcement. Circles represent acquisition while triangles represent reversal. Closed symbols are the responses reinforced with 1 mg/kg cocaine, while open symbols are non-reinforced responses. *Differences between responses for a given day, p < .05.
Experiment 4
The top panel of Fig. 4 shows the results of the 1 vs 5 sec delay food-reinforcement group in Experiment 4. The rats (n = 5) rapidly learned to respond for food (days A1–A4), with responding occurring almost exclusively in the hole associated with the 1 sec delay by day 4 of acquisition. When the holes associated with the 1 and 5 sec delay were reversed (days R1–R10), rats initially continued to respond almost exclusively in the hole previously associated with the 1 sec delay (now associated with the 5 sec delay). One rat switched to the hole associated with the 1 sec delay on day 4 of reversal, a second rat on day 9 and a third on day 10. Two rats continued to respond almost exclusively in the hole now associated with the 5 sec delay throughout the 10 days of the reversal phase. Analysis revealed a significant Day × Delay interaction (F13,52 = 37.77, p < 0.001). Paired comparisons showed that rates of responding in the hole associated with the 1-sec and 5-sec delays differed on acquisition days A3 and A4 and on reversal days R1–R3. No other days were significantly different.
Fig. 4.
Reinforced responses for rats trained to respond for food pellets (Experiment 4). The top panel shows the acquisition (A1–A4) phase and reversal phase (R1–R10) for 5 rats trained with one response associated with a 1-sec delay and the other associated with a 5-sec delay. The bottom panel shows the acquisition (A1–A4) phase and reversal phase (R1–R10) for 5 rats trained with one response associated with a 1-sec delay and the other associated with a 10-sec delay. Circles represent acquisition while triangles represent reversal. Closed symbols are the short delay responses while open symbols are the long delay responses. *Differences between delays for a given day, p < .05.
The bottom panel of Fig. 4 shows the results of the 1 vs 10 sec delay group (n = 5). Acquisition to the 1 sec delay response appeared to occur even more rapidly than for the 1 vs 5 sec delay group, with the difference in the responses reaching significance on day 2 of acquisition as opposed to day 3 for the 1 vs 5 sec delay group. Again, by day 4 all the rats responded almost exclusively in the hole associated with the 1 sec delay. On the first day of reversal, the rats responded almost exclusively in the hole that had been previously associated with the 1 sec delay, but by day 2, two rats had begun responding in the hole now associated with the shorter delay. By reversal day 6, all 5 rats had begun responding in the hole associated with the shorter delay, and by day 10 all 5 rats were responding almost exclusively in the hole associated with the shorter delay. Analysis revealed a significant Day × Delay interaction (F13,52 = 39.57, p < 0.001). Paired comparisons showed that rats responding in the hole associated with the 1-sec and 10-sec delays differed on acquisition days A2–A4 and on reversal days R1, R2 and R6–R10.
DISCUSSION
These results clearly show that for rats faster infusions of cocaine are more reinforcing than slower infusions during acquisition of drug self-administration. These findings are consistent with the majority of studies previously performed comparing fast vs. slow infusions in humans (Abreu et al., 2001; Nelson et al., 2006) and non-human primates (Balster and Schuster, 1973; Kato et al., 1987; Kimmel et al., 2007; Panlilio et al., 1998; Woolverton and Wang, 2004). Since the only difference between the infusions delivered for the two nose-pokes was the speed of the infusion (i.e., dose, volume, and concentration were held constant within Experiment 1 and within Experiment 2), there is no question that the choice of nose pokes was determined by infusion speed alone.
In Experiment 1, acquisition was relatively slow with a self-administration dose of 0.3 mg/kg/inj and a 110-sec timeout. However, by the end of 15 days of training there was a clear separation between the fast and slow infusions, with the rats making a clear majority of their responses in the nose-poke hole associated with the fast cocaine infusion. In Experiment 2, acquisition was faster, but again, by the end of 10 days of training rats responded almost exclusively in the nose-poke associated with the faster infusion. It seems most likely that the higher cocaine dose led to faster acquisition in Experiment 2, but we cannot rule out the possibility that the increased speed of the faster injection in Experiment 2 vs. Experiment 1 also enhanced the rate of acquisition. Despite these differences in rate of acquisition between Experiments 1 and 2, the faster infusion was a more effective reinforcer in both experiments.
The most likely reason that infusion speed had such a clear effect in the current study in rats, while previous studies have shown only small effects of infusion duration on cocaine self-administration (Crombag et al., 2008; Liu et al., 2005), is the use of a choice procedure during acquisition. The choice procedure allows rats to sample the two different infusion durations within a short period of time, potentially enhancing discriminability. In addition, having to make these comparisons during acquisition mitigates against the possibility that habit formation, in which behavior becomes less sensitive to its immediate consequences with extended training (Dickinson, 1985; Dickinson et al., 1995), would overshadow the effect of infusion duration. In the Crombag et al. (2008) study, while infusion duration was studied in acquisition, a moderate dose was used for initial acquisition (0.5 mg/kg/inj) with the dose increasing to 0.7 mg/kg/inj by the end of the acquisition phase. In the present study, differential responding to the various infusion durations developed faster with a higher dose of cocaine, suggesting that the basic procedure used by Crombag et al. might also reveal effects of infusion duration if a higher dose were used.
Crombag et al. (2008) also studied the effects of infusion duration during maintenance of cocaine self-administration, but again found no difference in responding across infusion durations. This finding contrasts with the Liu et al. (2005) study that also investigated the effects of infusion duration on FR responding for cocaine self-administration during maintenance. In the Liu et al. (2005) study, infusion duration did have a small, but significant effect on cocaine self-administration. Rats responding for a 1.5 mg/kg/inj dose of cocaine given over 5 secs took about 8 injections/hour. At a 50-sec infusion duration, the rats took around 10 injections/hour. As lower doses of cocaine typically support more FR responding when investigating the descending limb of the dose-effect function, these results suggest that the slower infusion was functionally equivalent to a lower cocaine dose. One possible reason for this difference between the two studies is that Liu et al. (2005) trained animals to stability, while Crombag et al. (2008) used single day substitutions for each infusion duration. In the present study, differential responding for fast and slow infusions developed over a number of days, and even preference for a fast infusion over no infusion took a few days to develop (Experiment 3).
The results of the present study indicate that infusion duration may have a stronger influence during acquisition than maintenance of drug self-administration. In Experiment 1, when the nose-poke holes associated with the fast and slow infusions were reversed, none of the rats switched responding to the other hole during the first 5 days of reversal training. Even rats whose catheter patency was maintained for a longer period following reversal failed to switch their responses to the other hole. This was despite the fact that a clear preference was observed for the faster infusion during the acquisition phase. In Experiment 2, using the larger dose of cocaine and a shorter short-duration infusion, more evidence of reversal was seen. Some rats eventually switched to the nose-poke associated with the faster infusion. But, even in Experiment 2, some rats continued to respond in the hole associated with the slow infusion for periods as long as those rats in Experiment 1. These results suggest that once a response is learned, changing the duration of the infusion within the parameters used here does not greatly affect its ability to support self-administration behavior in rats. A likely explanation for the lack of reversal is the development of habit formation (Dickinson, 1985; Dickinson et al., 1995), where responding becomes insensitive to changes in reinforcement.
The failure of behavior to reverse when the outcome is switched is not a general effect of drug self-administration as shown by the results of Experiment 3. When the choice is between a reinforced nose-poke and a non-reinforced nose-poke, rats quickly change their preference to the reinforced nose-poke. In fact, the change in preference was quicker than that seen with food reinforcement when the choices were between responses reinforced with the 1- versus 10-sec delay. Clearly, rats are capable of switching preference when responses are reinforced by drug self-administration.
The pattern of effects observed with cocaine self-administration for infusion duration do not appear to be unique to drug self-administration. In studies performed with a food reinforcer using delay of reinforcement, which is somewhat analogous to infusion duration, parallel effects were observed. All the rats preferred the shorter delay during acquisition, a finding that agrees with a number of previous studies (e.g., Chung and Herrnstein, 1967; Gentry and Marr, 1980). When the differences between the delays were larger, potentially making those differences more discriminable, a quicker separation between the two responses was observed. The higher dose and shorter infusion duration for the fast reinforcer used in Experiment 2 may also have allowed the differences in infusion duration to be more discriminable, speeding the response separation. When the nose-pokes associated with the two delays were reversed, some rats continued to respond in the nose-poke associated with the longer delay, an effect that was clearer when the delays were closer (1 vs 5) and thus maybe less discriminable to the rat. Similarly, for the cocaine self-administration experiments, less evidence of reversal was seen in Experiment 1 where the differences in infusion duration may have been less discriminable. It is likely that two different delays could have been chosen for the food study where preference would have been seen in acquisition for the short delay, but a reversal would not have been observed in the majority of rats. While changing the delay of reinforcement for food does not directly mimic the change in infusion duration, the results of the food study clearly show that the probability of reversal can be influenced by changes that would affect the discriminability of the choices. These changes in discriminabilty did not influence the initial choice in acquisition for either drug or food, but did influence the probability of reversal for both drug and food. Thus, the findings with cocaine self-administration do not appear to be a unique property of drug self-administration.
A number of factors, both pharmacokinetic and pharmacodynamic, could contribute to faster drug administration being more reinforcing to animals and humans. In addition to reaching the peak drug level faster, a higher level can be reached with a faster administration of the drug (see Panlilio et al., 1998). These two factors together or alone may increase the reinforcing effectiveness of a drug. To assess the potential differences between fast and slow infusions, we modeled whole-body cocaine levels under the parameters of the present study assuming an 18.1 min half-life for cocaine (Barbieri et al., 1992; for details of the whole-body drug level modeling procedure, see Panlilio et al., 2003). We found that differences in peak concentration would be minimal as long as the duration of the longest drug infusion is substantially shorter than the half-life of the drug. Under the parameters used in the Experiments 1 and 2, peak whole-body cocaine levels produced by an acute injection at the slowest and fastest pump speeds would differ by only about 3 percent. In addition, changing the speed of infusion does not greatly affect the duration of the drug’s actions in the body when infusion durations are substantially less than the half-life of the drug (Panlilio et al., 1998). Therefore, it seems most likely that the rate of rise in whole-body drug levels, rather than the peak level or duration of effect, would be the primary factor responsible for the differences in behavior observed here.
Differences in brain levels of cocaine for the two different infusion durations are potentially even more relevant than whole-body levels. When we modeled brain levels of acute cocaine infusions (see Nicola and Deadwyler, 2000; Pan et al., 1991), we again found very little difference in the peak brain level of cocaine between the two infusion durations. Like with whole-body drug levels, the rate of increase in the brain is much more striking than the difference in the peak level, further supporting the conclusion that the difference in onset rate is the most likely factor in the behavioral differences observed.
The rate of delivery can also affect a number of indices of brain function. For example, Samaha et al. (2004) reported that a faster infusion of cocaine induces greater increases in the immediate early genes c-fos and arc in rat brain than do slower infusions. Faster infusions also led to increased inhibition of the re-uptake of dopamine in the nucleus accumbens core (Samaha et al., 2004). Woolverton and Wang (2004) reported a decrease in the rate of dopamine transporter binding with slower cocaine infusions. However, the maximum occupancy at the end of the infusion was no different across infusion durations from 10 to 600 sec. This result suggests that drug self-administration may be sensitive to the different levels of occupancy that would occur immediately after infusion. Porrino et al. (1993) reported that doses of cocaine given i.p. and i.v. that led to similar increases in locomotor activity had different effects on brain glucose metabolism in rats. Following i.p. infusions only those areas of the brain directly related to locomotor activity showed increases in glucose metabolisms. Following i.v. administration, the increase in glucose metabolism included more brain regions and included areas thought to be important to drug self-administration. Brown and Kiyatkin (2005) studied the effects of different infusion durations on rat brain temperature in the nucleus accumbens and ventral tegmental area. Changes in temperature in these areas may reflect changes in brain metabolism (Kiyatkin, 2004) and therefore may be a marker for the ongoing activity state of these brain regions. They found that shorter infusion durations led to greater increases in brain temperature in the nucleus accumbens and ventral tegmental area. Given all these findings it is probably not surprising that rats prefer to self-administer cocaine for faster infusion rates.
In conclusion, faster infusions of cocaine are clearly more effective reinforcers during acquisition of cocaine self-administration in rats. These results support evidence that human drug abusers are more likely to abuse drugs that have more rapid onsets (Gossop et al., 1992; Hatsukami et al., 1996) and that abusers typically self-administer i.v. drugs with a short injection duration (Zernig et al., 2003). Once drug self-administration is acquired, however, delivery rate may not play as important a role in the maintenance of drug self-administration. The weaker effect of delivery rate during maintenance might be due to habit formation (Dickinson, 1985; Dickinson et al., 1995), in which the response comes under the control of external stimuli rather than the immediate reinforcing effects of the drug. It seems unlikely, however, that delivery rate plays no role in the maintenance of drug self-administration, as some rats in the current experiment did switch their responding to the response associated with the faster infusion duration during reversal. There are probably infusion duration parameters and cocaine doses where infusion duration would have an even stronger effect during maintenance. Finally, these findings regarding the effects of infusion duration on choice behavior do not appear to be unique to drug self-administration. Parallel behavioral effects can be observed with food as the reinforcer by manipulating the delay of reinforcement.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, NIDA. The authors would like to thank Kevin Thornton, Peter Riley and Reeva Morton for their assistance in conducting the experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abreu ME, Bigelow GE, Fleisher L, Walsh SL. Effect of intravenous injection speed on responses to cocaine and hydomorphone in humans. Psychopharmacology. 2001;154:76–84. doi: 10.1007/s002130000624. [DOI] [PubMed] [Google Scholar]
- Balster RL, Schuster CR. Fixed-interval schedule of cocaine reinforcement: Effect of dose and infusion duration. J Exp Anal Behav. 1973;20:119–129. doi: 10.1901/jeab.1973.20-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri EJ, Ferko AP, DiGregorio GJ, Ruch EK. The presence of cocaine and benzoylecgonine in rat cerebrospinal fluid after intravenous administration of cocaine. Life Sci. 1992;51:1739–1746. doi: 10.1016/0024-3205(92)90303-7. [DOI] [PubMed] [Google Scholar]
- Brown PL, Kiyatkin EA. Brain temperature change and movement activation induced by intravenous cocaine delivered at various injection speeds in rats. Psychopharmacology. 2005;181:299–308. doi: 10.1007/s00213-005-2244-0. [DOI] [PubMed] [Google Scholar]
- Chung S-H, Herrnstein RJ. Choice and delay of reinforcement. J Exp Anal Behav. 1967;10:67–64. doi: 10.1901/jeab.1967.10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Ferrario CR, Robinson TE. The rate of intravenous cocaine or amphetamine delivery does not influence drug-taking and drug-seeking behavior in rats. Pharmacol Biochem Behav. 2008;90:797–804. doi: 10.1016/j.pbb.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson A. Actions and habits: the development of behavioural autonomy. Phil Trans R Soc Lond B. 1985;308:67–78. [Google Scholar]
- Dickinson A, Balleine B, Watt A, Gonzalez F, Boakes RA. Motivational control after extended instrumental training. Animal Leaning Behav. 1995;23:197–206. [Google Scholar]
- Gentry GD, Marr MJ. Choice and reinforcement delay. J Exp Anal Behav. 1980;33:27–37. doi: 10.1901/jeab.1980.33-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick DA. The rate hypothesis and agonist substitution approaches to drug abuse treatment. Adv Pharmacol. 1998;42:995–997. doi: 10.1016/s1054-3589(08)60914-x. [DOI] [PubMed] [Google Scholar]
- Gossop M, Griffiths P, Powis B, Strang J. Severity of dependence and route of administration of heroin, cocaine and amphetamines. Brit J Addiction. 1992;87:1527–1536. doi: 10.1111/j.1360-0443.1992.tb02660.x. [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Foltin RW, Fischman MW. Alternative reinforcers differentially modify cocaine self-administration by humans. Behav Pharmacol. 2000;11:87–91. doi: 10.1097/00008877-200002000-00010. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Fischman MW. Crack cocaine and cocaine hydrochloride. Are the differences myth or reality? JAMA. 1996;276:1580–1588. [PubMed] [Google Scholar]
- Kato S, Wakasa Y, Yanagita T. Relationships between minimum reinforcing doses and injection speed in cocaine and pentobarbital self-administration in crab-eating monkeys. Pharmacol Biochem Behav. 1987;28:407–410. [PubMed] [Google Scholar]
- Kimmel HL, O’Conner JA, Carroll FI, Howell LL. Faster onset and dopamine transporter selectivity predict stimulant and reinforcing effects of cocaine analogs in squirrel monkeys. Pharmacol Biochem Behav. 2007;86:45–54. doi: 10.1016/j.pbb.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA. Brain hyperthermia during physiological and pathological conditions: causes, mechanisms, and functional implications. Curr Neurovas Res. 2004;1:77–90. doi: 10.2174/1567202043480233. [DOI] [PubMed] [Google Scholar]
- Liu Y, Roberts DC, Morgan D. Sensitization of the reinforcing effects of self-administered cocaine in rats: effects of dose and intravenous injection speed. Eur J Neurosci. 2005;22:195–200. doi: 10.1111/j.1460-9568.2005.04195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsch LA, Bickel WK, Gadger GJ, Rathmell JP, Swedberg MDB, Jonzon B, Norsten-Hoog C. Effects of infusion rate of intravenously administered morphine on physiological, psychomotor, and self-reported measures in humans. J Pharmacol Exp Ther. 2001;299:1056–1065. [PubMed] [Google Scholar]
- Negus SS. Rapid assessment of choice between cocaine and food in rhesus monkeys: Effects of environmental manipulations and treatment with d-amphetamine and flupenthixol. Neruopsychopharmacology. 2003;28:919–931. doi: 10.1038/sj.npp.1300096. [DOI] [PubMed] [Google Scholar]
- Nelson RA, Boyd SJ, Ziegelstein RC, Herning R, Cadet JL, Henningfield JE, Schuster CR, Contoreggi C, Gorelick DA. Effect of rate of administration on subjective and physiological effects of intravenous cocaine in humans. Drug Alcohol Depen. 2006;82:19–24. doi: 10.1016/j.drugalcdep.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Deadwyler SA. Firing rate of nucleus accumbens neurons is dopamine-dependent and reflects the timing of cocaine-seeking behavior in rats on a progressive ratio schedule of reinforcement. J Neurosci. 2000;20:5526–5537. doi: 10.1523/JNEUROSCI.20-14-05526.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H-T, Menacherry S, Justice JB., Jr Differences in the pharmacokinetics of cocaine in naive and cocaine-experienced rats. J Neurochem. 1991;56:1299–1306. doi: 10.1111/j.1471-4159.1991.tb11425.x. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Goldberg SR, Gilman JP, Jufer R, Cone EJ, Schindler CW. Effects of delivery rate and non-contingent infusion of cocaine on cocaine self-administration in rhesus monkeys. Psychopharmacology. 1998;137:253–258. doi: 10.1007/s002130050618. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Katz JL, Pickens RW, Schindler CW. Variability of drug self-administration in rats. Psychopharmacology. 2003;167:9–19. doi: 10.1007/s00213-002-1366-x. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Weiss SJ, Schindler CW. Cocaine self-administration increased by compounding discriminative stimuli. Psychopharmacology. 1996;125:202–208. doi: 10.1007/BF02247329. [DOI] [PubMed] [Google Scholar]
- Porrino LJ. Functional consequences of acute cocaine treatment depend on route of administration. Psychopharmacology. 1993;112:343–351. doi: 10.1007/BF02244931. [DOI] [PubMed] [Google Scholar]
- Samaha A-H, Robinson TE. Why does the rapid delivery of drugs to the brain promote addiction? Trends Pharmacol. Sci. 2005;26:82–87. doi: 10.1016/j.tips.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Samaha AN, Mallet N, Ferguson SM, Gonon F, Robinson T. The rate of cocaine administration alters gene regulation and behavioral plasticity: implications for addiction. J Neurosci. 2004;24:6362–6370. doi: 10.1523/JNEUROSCI.1205-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winger G, Hursch SR, Casey KL, Woods JH. Relative reinforcing strength of three N-methyl-d-aspartate antagonists with different onsets of action. J Pharmacol Exp Ther. 2002;301:690–697. doi: 10.1124/jpet.301.2.690. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Wang Z. Relationship between injection duration, transporter occupancy and reinforcing strength of cocaine. Eur J Pharmacol. 2004;486:251–257. doi: 10.1016/j.ejphar.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Zernig G, Giacomuzzi S, Riemer Y, Wakonigg G, Sturm K, Saria A. Intravenous drug injection habits: drug users’ self-reports versus researchers’ perception. Pharmacology. 2003;68:49–56. doi: 10.1159/000068731. [DOI] [PubMed] [Google Scholar]