Abstract
Rationale and Objectives
The aims of this study were to determine the feasibility of diffusion-weighted magnetic resonance imaging (DWI) in the detection of bowel inflammation and to investigate the changes in apparent diffusion coefficient (ADC) values in the inflamed bowel in patients with Crohn's disease.
Materials and Methods
Eleven patients who underwent magnetic resonance enterography (including DWI) for Crohn's disease and colonoscopy or surgery within 4 weeks of examination were recruited. Two radiologists reviewed diffusion-weighted images and ADC maps to evaluate for inflammation in each bowel segment (terminal ileum, cecum, ascending colon, transverse colon, descending colon, and rectosigmoid colon) and measured the ADC values of each bowel segment. Endoscopic and pathologic results were correlated with DWI findings.
Results
Fifty-three segments (19 with inflammation, 34 normal) were included. DWI detected inflammation in 18 of 19 segments (94.7%) and showed normal results in 28 of 34 segments (82.4%). On diffusion-weighted images, bowel segments with inflammation revealed higher signal compared to normal segments. Artifact levels were none or minimal in 10 of 11 patients (90.9%) and moderate in one patient. On quantitative analysis, ADC values of inflamed and normal bowel were measured as 0.47 – 2.60 × 10−3 and 1.39 – 4.03 × 10−3 mm2/s, respectively (P < .05).
Conclusion
DWI with parallel imaging is a feasible technique for the detection of inflammation in patients with Crohn's disease. ADC values are decreased in inflamed bowel segments, indicating restricted diffusion.
Keywords: Diffusion-weighted MRI, Crohn's disease, bowel
Magnetic resonance (MR) imaging has been increasingly used for the diagnosis and follow-up of patients with inflammatory bowel disease. Its lack of ionizing radiation, excellent soft-tissue contrast resolution, and potential to perform real-time and functional imaging are the important advantages of MR imaging that make it well suited for imaging the gastrointestinal tract (1). Currently, evaluation of the bowel wall by MR imaging is based on its signal on T2-weighted images, thickness, and the degree of contrast enhancement (1). Although the results of MR imaging using these criteria are promising, several clinically important issues, such as the accurate estimation of the extent of disease, reliable differentiation between chronic and active inflammation, and monitoring response to treatment, remain as diagnostic challenges.
A new possibility to expand the capability of MR imaging is to apply new MR applications that can give additional information about the structural organization of tissues on bowel imaging. Diffusion-weighted imaging (DWI) reflects the changes in the water mobility caused by interactions with cell membranes, macromolecules, and alterations of the tissue environment. DWI has been widely used for intracranial diseases but has only recently been applied to the abdomen. Initial results suggest that it can be useful for the evaluation of various hepatic, renal, and pancreatic diseases (2), (3) and (4). The available published descriptions of DWI for the evaluation of the bowel are focused on the detection of colorectal cancer (5), (6) and (7). To our knowledge, the use of DWI for the detection of bowel wall inflammation and its associated features has not been previously described.
Especially after the emergence of nephrogenic systemic fibrosis as a rare but serious complication of gadolinium-based contrast agents, there is less willingness to use intravenous contrast agents in MR imaging, and DWI is becoming important as an alternative method to obtain information that could otherwise be obtained from extracellular contrast enhancement. The purposes of our study were to determine the possibility of a role for DWI in the detection of bowel inflammation and investigate the changes in apparent diffusion coefficient (ADC) values of the inflamed bowel in patients with Crohn's disease (CD).
Materials and methods
A retrospective search of the institutional computer database was performed for all patients who had undergone MR enterography for the indication of suspected or known CD and who also underwent subsequent colonoscopy or bowel resection within 4 weeks of MR enterography between July 2007 and February 2008. Institutional review board approval was obtained, and informed consent was waived for this retrospective study, which was compliant with the Health Insurance Portability and Accountability Act.
A total of 32 MR enterographic examinations were performed for suspected or known CD during the specified time. Eleven patients (seven women, four men; mean age, 36.8 years; range, 21–74 years) who also underwent subsequent colonoscopy or bowel resection within 4 weeks of MR enterography were included in the study to undergo either endoscopy or pathology as the gold standard. Two patients had partial colectomy (one right hemicolectomy and one cecectomy) and ileal resection prior to MR enterography.
MR Imaging Protocol
The MR imaging examinations were performed with a 1.5-T GE Signa unit (GE Healthcare, Milwaukee, WI). Patients fasted for 6 hours before the MR imaging examinations. VoLumen 1350 mL (E-Z-EM Inc, Lake Success, NY) was administered orally to every patient over 45 minutes before the study. Glucagon 1 mg (Glucagen; Bedford Laboratories, Bedford, OH) was administered intramuscularly when the patient was placed in the scanner, immediately before starting the examination.
After acquiring a standard three-plane scout image, the following sequences were obtained through the abdomen and pelvis using a four-channel, phased-array body coil: (1) axial and coronal fast imaging employing steady-state acquisition with and without fat suppression (repetition time [TR], 3.4 ms; echo time [TE], 1.4 ms; matrix, 224 × 224; flip angle, 45°; slice thickness, 7 mm; gap, 0 mm); (2) axial and coronal T2-weighted single-shot fast spin echo with and without fat suppression (TR, infinite; TE, 90 ms; matrix, 256 × 256; slice thickness, 6 mm; gap, 0 mm); (3) pre- and postcontrast T1-weighted liver acquisition with volume acceleration, with additional dynamic postcontrast images (TR, 3.5–3.9 ms; TE, 1.6–1.9 ms; matrix, 192 × 256; flip angle, 10°; interpolated slice thickness, 2.2 mm); and (4) axial and/or coronal diffusion-weighted images (b values, 0 and 600 s/mm2; TR, 8000 ms; TE, 75 ms; matrix, 128 × 128–224; slice thickness, 7 mm; gap, 0 mm; number of signals acquired, 4). The upper abdomen and pelvis were scanned separately. The field of view ranged between 32 and 40 cm, and an ASSET factor of 2 was used in all sequences. Acquisition time for the DWI sequences covering the abdomen and pelvis ranged from 5 to 8 minutes.
Image Analysis
The bowel was divided into six segments: terminal ileum, cecum, ascending colon, transverse colon, descending colon, and rectosigmoid colon. In patients who had previous ileocolectomy and ileocecal anastomosis, the small-bowel loop segments (up to 10 cm) anastomosed to the colon (neoterminal ileum) was regarded as the “terminal ileum.” The perianal region and small-bowel loops other than terminal ileum were not specifically assessed in this study.
Qualitative Analysis
DWI of the bowel (b values, 0 and 600 s/mm2) was retrospectively evaluated by two radiologists (with a combined 12 years of body MR experience) who were blinded to the clinical and endoscopic examination and surgical results. Pixelwise ADC maps were generated using a commercially available software workstation system (Advanced Workstation; GE Medical Systems, Milwaukee, WI).
Each segment was graded for the presence of inflammation on a four-point confidence scale on the basis of wall thickening and wall signal on DWI and the ADC map as follows: 0 = definitely absent (imperceptible wall, both in signal and in thickness), 1 = probably absent (normal thickness, signal intensity and thickness are similar to the surrounding bowel segments), 2 = probably present (normal wall thickness, but signal intensity is increased on DWI and decreased on ADC map), and 3 = definitely present (thick bowel wall, and signal intensity is increased on DWI and decreased on ADC map). The bowel wall was considered to be thickened when it was >3 mm. Grading scores of 0 and 1 were regarded as indicating normal bowel wall, and scores of 2 and 3 were regarded as indicating bowel wall inflammation on DWI.
DWI of the bowel was also evaluated for the presence of artifacts limiting evaluation of the bowel segments on a four-point scale as follows: 0 = no artifacts, 1 = minimal artifacts (no significant impact on evaluation), 2 = moderate artifacts (significant impact on evaluation), and 3 = severe artifacts (nondiagnostic).
Quantitative Analysis
ADCs were calculated for each bowel segment. ADC measurements were performed for each segment by two different radiologists, blinded to the clinical, endoscopic, and surgical results, on a workstation with commercially available diffusion analysis software (Advantage Windows version 4.2.3; GE Healthcare). For the ADC measurements, the images were magnified, and oval regions of interest were placed on the largest possible area covering the bowel wall. The measurements were made from the area of brightest signal in the bowel wall. Region-of-interest areas varied between 12 and 30 mm2. The mean of the two ADC values was accepted as the ADC value of the segment.
Endoscopic and Surgical Findings
The reports of endoscopic examinations, surgical procedures, and pathologic results were reviewed by a third radiologist who was not involved in the image analysis. Visualization of inflamed mucosa on endoscopy or evidence of bowel inflammation in the biopsy or resected surgical specimen was accepted as proof of inflammation and noted for each segment. Qualitative and quantitative DWI findings were compared with this clinical gold standard.
Statistical Analysis
Sensitivity and specificity were calculated for qualitative inflammation score, with pathologic findings of inflammation as the gold standard. Because there was a potential intrapatient correlation in ADC values among several segments of the same patient, a mixed-effects linear regression model was used to examine whether ADC values were different between normal and inflamed bowel walls. If the intrapatient correlation was zero, the mixed-effects linear model was reduced to a Student's t test. Bland-Altman 95% limits of agreement were used to assess the interobserver agreement in ADC values. Paired t tests were used to examine whether there was a difference between the measurements of two radiologists. A receiver-operating characteristic curve was constructed for ADC values, and the area under the curve was a measure of the overall ability of discriminating inflamed and normal bowels. Statistical analysis was performed using Stata version 9.0 (StataCorp LP, College Station, TX).
Results
Four patients underwent ileocolectomy following MR enterography, and seven patients underwent colonoscopy within the specified period. Colonoscopy was incomplete in two patients (up to the sigmoid colon in one patient and up to the descending colon in the other patient), and the terminal ileum could not be visualized in one patient. A total of 53 segments could be evaluated by endoscopy (n = 31) or surgery (n = 22). On the basis of these gold-standard evaluations, 34 bowel segments were normal, and 19 segments demonstrated inflammation. Confirmation of disease activity by endoscopy or surgery, inflammation grading score on a per segment basis, and artifact level are summarized in Table 1.
Table 1.
Disease Activity by Endoscopy or Surgery, Inflammation Grading Score on a per Segment Basis, and Artifact Level
| Patient | Terminal Ileum |
Cecum | Ascending Colon |
Transverse Colon |
Descending Colon |
Rectosigmoid Colon |
|---|---|---|---|---|---|---|
| 1 | N/A | N/A | N/A | N/A | Inf (3) | Inf (1) |
| 2 | N/A | N/A | N/A | N/A | N/A | N (1) |
| 3 | N (1) | Inf (2) | Inf (2) | N (2) | Inf (3) | Inf (2) |
| 4 | N (1) | Inf (3) | Inf (3) | Inf (2) | Inf (3) | Inf (2) |
| 5 | N (1) | N (1) | N (1) | Inf (3) | Inf (3) | Inf (2) |
| 6 | Inf (2) | X | X | N (2) | N (2) | N (2) |
| 7 | N | N | N | N | N | N |
| 8 | Inf | N | N | N | N | N |
| 9 | N | N | N | N | N | N |
| 10 | Inf | X | N | N | N | N |
| 11 | N/A | Inf | Inf | N | N | N |
Inf, inflamed segment; N, normal segment; N/A, segment could not be evaluated by colonoscopy; X, segment was previously excised.
Numbers in parenthesis are artifact level grading scores: 0 = no artifacts, 1 = minimal artifacts (no significant impact on evaluation), 2 = moderate artifacts (significant impact on evaluation), and 3 = severe artifacts (nondiagnostic).
Qualitative Evaluation
Of the 34 normal bowel segments, DWI detected 28 (grading score 0 or 1), yielding a specificity of 82.4% (Fig 1). Six normal bowel segments (one ascending, two transverse, one descending, and two rectosigmoid) in four patients were characterized as inflamed by DWI (grading score 2) without surgical, endoscopic, or pathologic confirmation and were therefore classified as “false-positive” results. Of the 19 inflamed segments, DWI detected 18 (grading score 2 or 3), yielding a sensitivity of 94.7% (Figure 2 and Figure 3). Inflammation could not be detected (grading score 1) in one rectosigmoid segment (patient 1). Artifacts were either minimal or not present in 10 of 11 patients (90.9%) and were moderate in one patient. The artifacts that were encountered were distortion and ghosting.
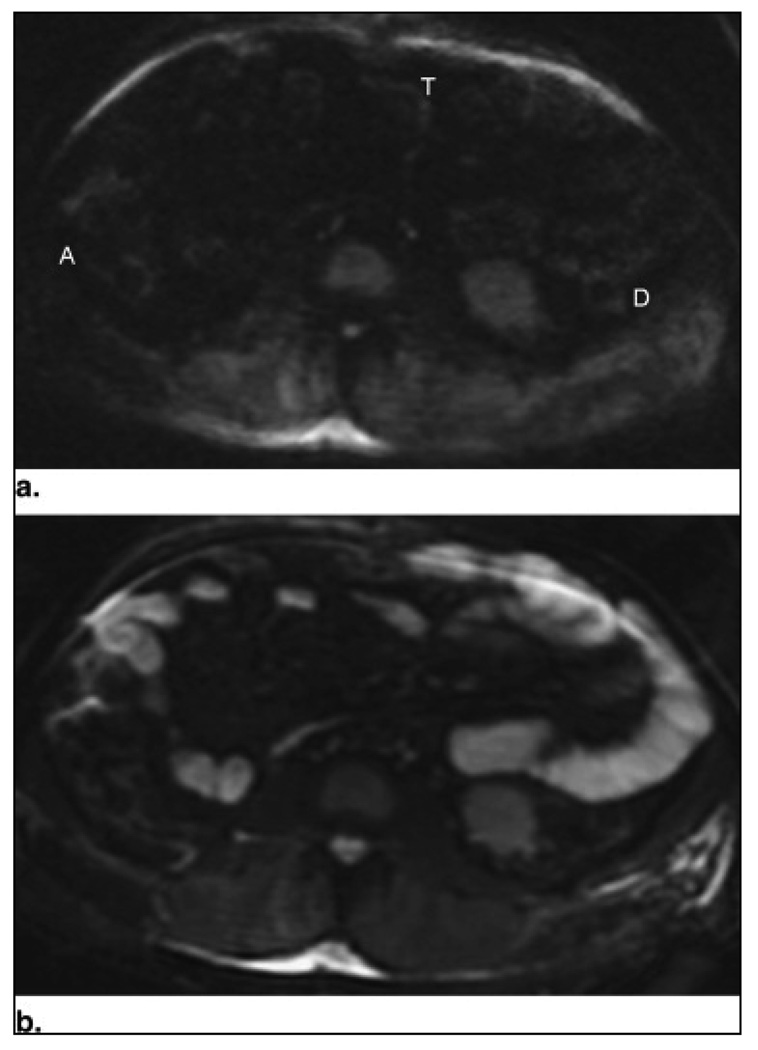
Figure 1.
Patient 7: axial diffusion-weighted images at b = 0 s/mm2 (a) and b = 600 s/mm2 (b) through the midabdomen in a 54-year-old man with suspected Crohn's disease. No increased signal in the walls of the ascending (A), transverse (T), and descending (D) colon segments is seen in the diffusion-weighted image at b = 600 s/mm2 (b). Colonoscopy confirmed the normal findings.
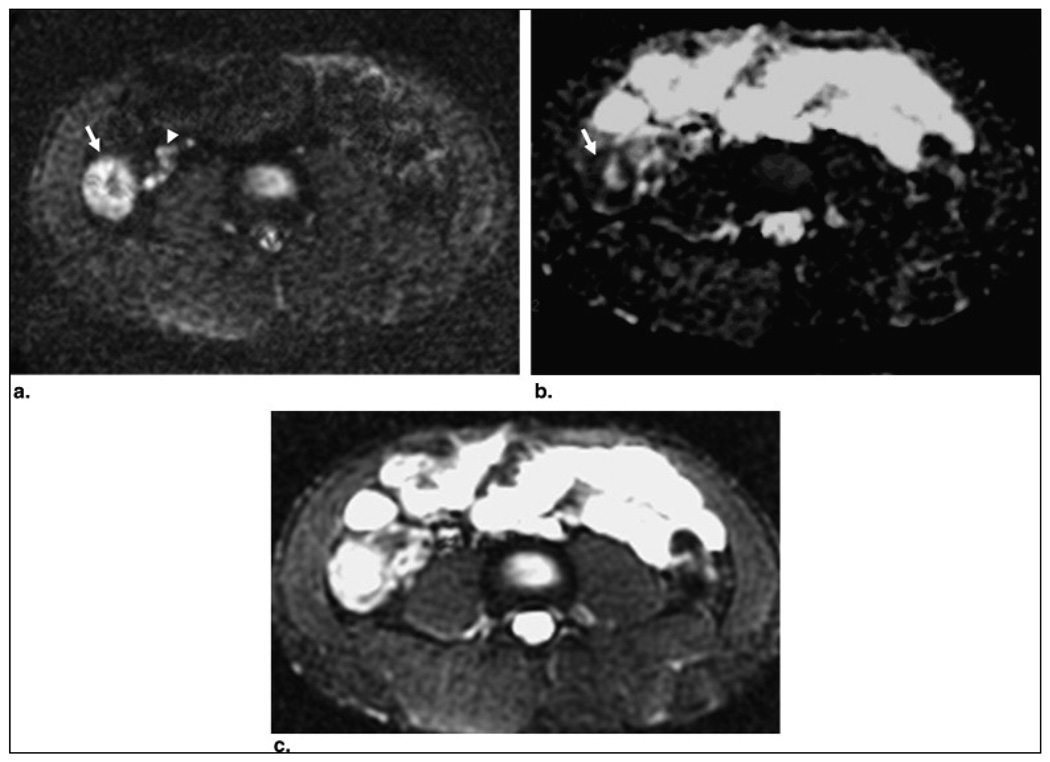
Figure 2.
Patient 11: a 21-year-old man with known Crohn's disease. Axial diffusion-weighted image through the lower abdomen (b = 600 s/mm2) (a) shows increased signal and thickening of the cecal wall (arrow) with associated high-signal pericecal lymph nodes (arrowheads). Note the normal dark signal of the other bowel segments. The cecal wall (arrow) and the lymph nodes demonstrate low signal on the apparent diffusion coefficient map (b), consistent with restricted diffusion. (c) Diffusion-weighted image at b = 0 s/mm2 for comparison. Colonoscopy showed patchy active colitis with ulceration in the cecum.
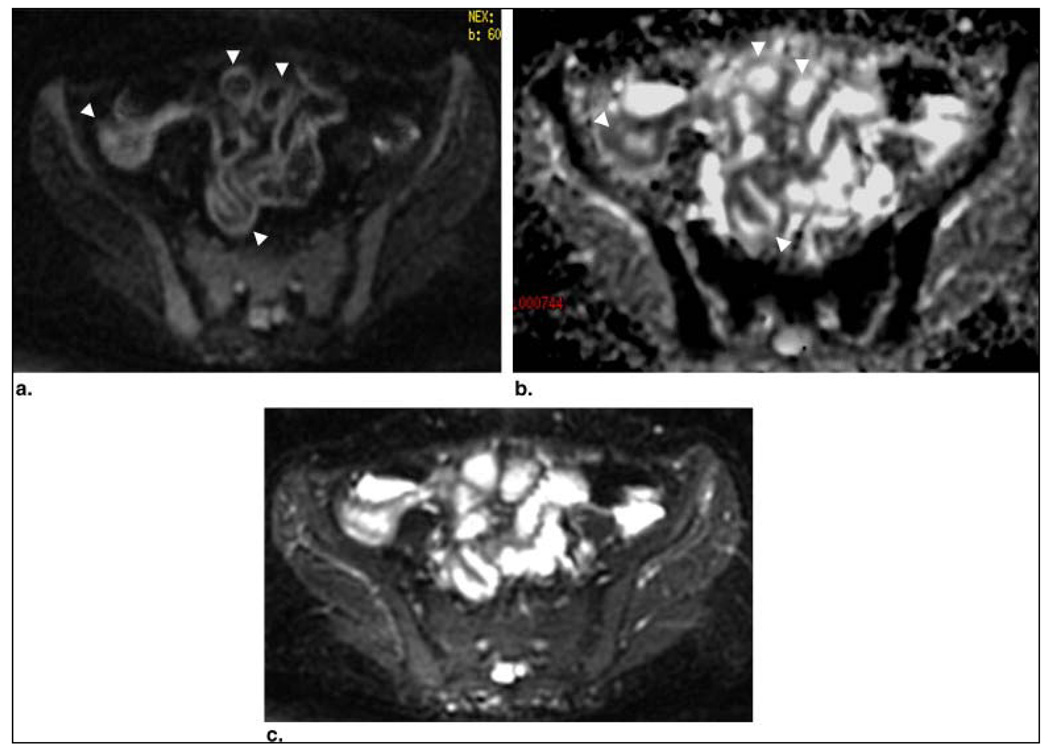
Figure 3.
Patient 10: a 34-year-old woman with known Crohn's disease and history of ileal resection and right hemicolectomy. Axial diffusion-weighted image through the pelvis (b = 600 s/mm2) (a) and corresponding apparent diffusion coefficient map (b) showed multiple small-bowel loops in the pelvis demonstrating restricted diffusion in their walls (arrowheads), including the most distal ileum anastomosed to the colon. (c) Diffusion-weighted image at b = 0 s/mm2 for comparison. Colonoscopic biopsy of the distal small-bowel loops showed active inflammation with ulcers.
Quantitative Analysis
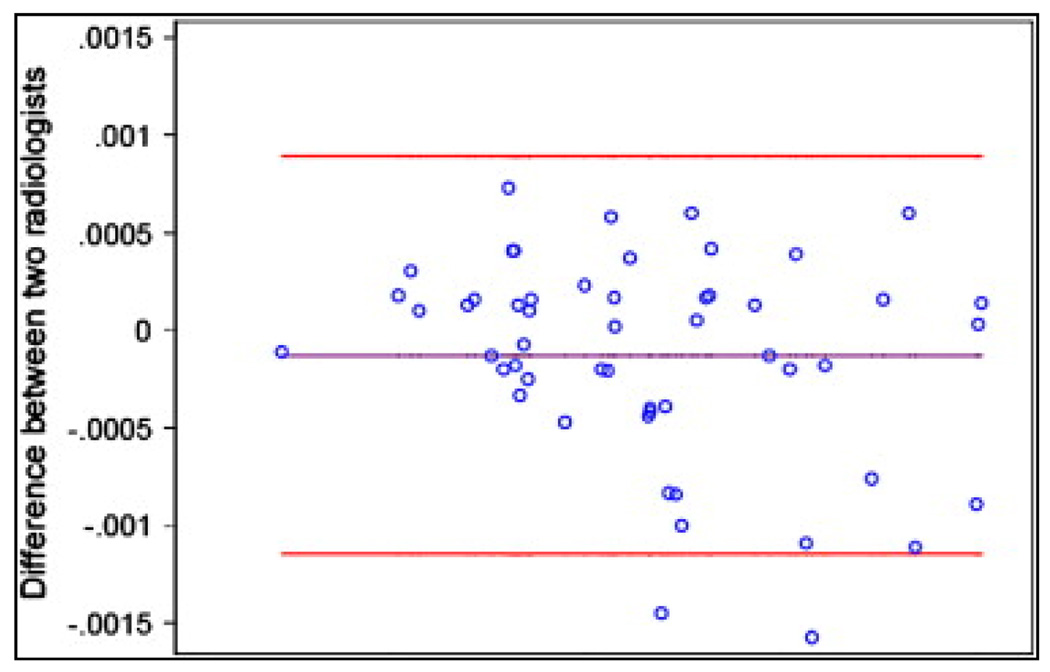
The intra- and interobserver agreement in ADC measurements are shown in Figure 4. The Bland-Altman concordance correlation coefficient was 0.81 for the two measurements by two radiologists, and the mean difference was 0.13 × 10−3 mm2/s (P > .05). These data suggest that both intra-and interobserver reliability were very good.
Figure 4.
Bland-Altman 95% limits of agreement in apparent diffusion coefficient (ADC): interobserver agreement.
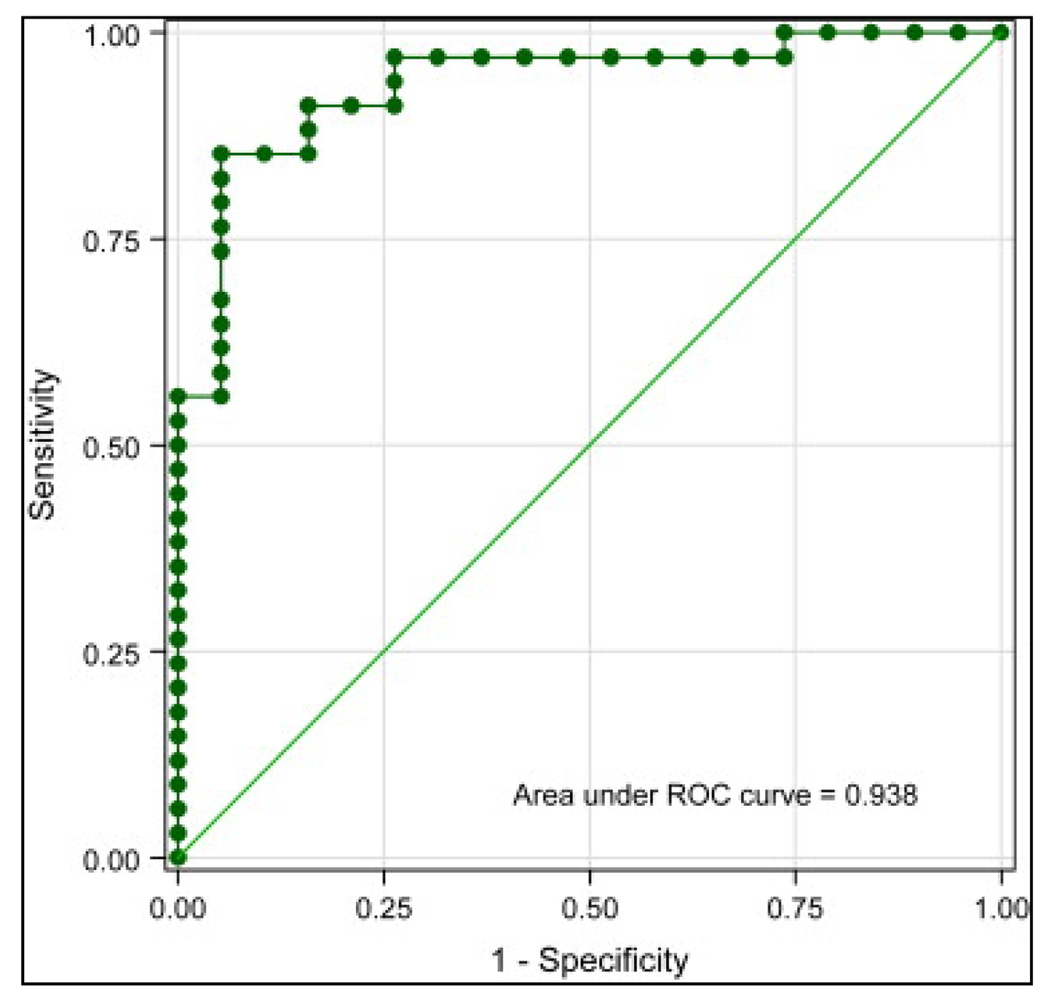
The mean ADC value of proven inflamed bowels was 1.59 ± 0.45 × 10−3 mm2/s (range, 0.46–2.50 × 10−3 mm2/s), compared to 2.74 ± 0.68 × 10−3 mm2/s (range, 1.44–4.03 × 10−3 mm2/s) in normal bowel segments (P < .0001). The area under the receiver-operating characteristic curve was 0.938 (95% confidence interval, 0.873–1.000). Using 2.0 × 10−3 mm2/s as the cutoff point, the sensitivity of low ADC values for detecting inflamed bowels was 84%, and the specificity of high ADC values for ruling out inflamed bowels was 91% (Fig 5).
Figure 5.
Receiver-operating characteristic (ROC) curve for apparent diffusion coefficient.
Discussion
Our results indicate that inflammation of the bowel wall causes restricted diffusion and that DWI yields both qualitative (increased signal intensity) and quantitative (decreased ADC values) information that can be helpful in the evaluation of bowel inflammation. To our knowledge, DWI findings of bowel inflammation in patients with CD have not been previously published.
Previous experience with DWI of the bowel is limited to the detection of colorectal adenocarcinoma. Ichikawa et al (5) reported high sensitivity and specificity (91% and 100%, respectively) for the detection of adenocarcinomas in the colon using DWI with a b value of 1000 s/mm2. In another series of 42 patients with sigmoid and rectal carcinoma, all tumors were clearly depicted from the normal bowel wall and the lumen, which were always hypointense on DWI (6). Hosonuma et al (7) reported 100% sensitivity (15 of 15) for rectal cancer detection on DWI, but in their control group of 20 patients, there were seven false-positive results. The exact reasons for the restricted diffusion in colorectal cancer are still unknown, but increased tissue cellularity and cell density are believed to play a significant role, as well as altered intracellular content of tumor cells (5).
Explanation of the reason for restricted diffusion in the inflamed bowel is a challenging issue that warrants further studies, and at this point, we can only speculate about possible mechanisms. Restricted diffusion has been reported in a variety of inflammatory processes in the brain, including Rasmussen encephalitis, viral and bacterial encephalitis, cerebritis, and cerebral abscess formation (8) and (9). Jaggi et al (8) suggested that the restriction of water may be related to the high viscosity and cellularity of pus. Verswijvel et al (10) reported focal restricted diffusion in pyelonephritis and renal abscesses in a small series. ADC values in the liver were reported to be inversely proportional to the degree of inflammation in patients with viral hepatitis (2). In early acute CD, the lamina propria and submucosa are infiltrated by inflammatory cells (11). Aphtoid ulcers, characteristic early lesions of active CD, are also strongly associated with lymphoid aggregates. These lymphoid aggregates have restricted diffusion within themselves because of increased cell density, as well as further limiting the diffusion by narrowing the limited space in the bowel wall. In addition to the increased number of inflammatory cells, dilated lymphatic channels, hypertrophied neuronal tissue, and the development of granulomas in the bowel wall can further narrow the extracellular space and therefore contribute to the restricted diffusion of water molecules. Accompanying intracellular changes within both the epithelial and inflammatory cells may also have an effect on the changes in diffusion. In the later stages of CD, fibrosis may develop within the bowel wall. Taouli et al (2) showed that increased fibrosis causes a decrease in the ADC values of the liver, reflecting restriction in diffusion. A similar effect of fibrosis may also be possible in the bowel wall. Further studies are needed to investigate the diffusion restriction at different stages of bowel inflammation.
In patients with suspected or known CD, the clinically important issues are the diagnosis of the extent and severity of disease, the differentiation of active inflammation from fibrosis, and the monitoring of response to treatment. A variety of research and clinical scoring tools (such as the Crohn's Disease Activity Index, biologic indexes, and endoscopic and imaging studies) have all been used to answer these questions, but there remains no established gold standard that accurately provides all this information (12). We believe that DWI of the bowel may provide information relevant to all of these important clinical questions. The ability to calculate quantitative parameters such as ADC values may lead to a more objective evaluation of the disease. Larger series investigating these specific aims are needed to better understand the role of DWI in patients with inflammatory bowel disease. DWI sequences have already been added to the routine body MR protocols in many institutions and do not require significant prolongation of the examination or reading time. As DWI techniques have been used in neuroradiologic applications for a long time, technologists are familiar with the technique, and analysis software is widely available. Therefore, logistically, they are not difficult to implement.
Recent advances in sequences, gradient amplitudes, multichannel coils, and parallel imaging have enabled DWI to be increasingly used in the abdomen and pelvis (13). However, the high susceptibility of echoplanar imaging sequences to magnetic field inhomogeneity, local susceptibility gradients, chemical shift, and motion still limit the use of DWI in the abdomen. In our series, artifacts were not a significant problem affecting diagnostic quality. We believe that the use of glucagon (decreasing peristalsis) and low-density oral barium contrast (decreasing the amount of air in the bowel) helped minimize motion and distortion artifacts. The use of DWI with parallel imaging (ASSET factor, 2) also diminished susceptibility, chemical shift, and motion artifacts by shortening the echoplanar imaging train and decreasing the filling time of the k-space. The use of a non-breath-hold DWI technique with multiple signal acqusitions and signal averaging over a longer duration may have improved the signal-to-noise and contrast-to-noise ratios (14). We preferred a relatively high b value (b = 600 s/mm2), which minimized the effect of perfusion on DWI and also reduced the effect of intravoxel incoherent motion not related to diffusion.
Our study had several limitations due to its retrospective design and small patient population. Our study population was a small selected group of patients with a high pre-test probability of CD. The analysis was confined to the colon and terminal ileum, where endoscopic and surgical correlation was available. The rest of the small bowel was not included in the study. Qualitative analysis was performed by consensus decision of two radiologists. Therefore, interobserver variability could not be determined. We preferred consensus reading because the DWI findings of bowel inflammation were not previously described, and one of our aims was to determine these findings. Although we made our best effort by magnifying images and using oval regions of interest to try to exclusively cover the bowel wall, we cannot completely exclude the possibility of a partial volume effect on ADC measurements, especially from normal bowel wall. Very good interobserver agreement and significant difference between normal and abnormal bowel are also suggestive of relatively low contamination from a partial volume effect. Second, we arbitrarily selected a b value of 600 s/mm2. In the future, further studies investigating DWI findings using different b values may determine their role in the detection of bowel inflammation.
In conclusion, DWI with parallel imaging allows the detection of inflammation in patients with CD, and ADC values are decreased in the inflamed bowel segments, indicating restricted diffusion. This small pilot study shows sufficient promise to merit larger clinical investigations. Further studies with larger populations are needed to support our findings and to better define the role of DWI in the clinical management of these patients.
References
- 1.Fidler J. MR imaging of the small bowel. Radiol Clin North Am. 2007;45:317–331. doi: 10.1016/j.rcl.2007.03.012. Abstract | View Record in Scopus | Cited By in Scopus (12) [DOI] [PubMed] [Google Scholar]
- 2.Taouli B, Tolia AJ, Losada M, et al. Diffusion-weighted MRI for quantification of liver fibrosis: preliminary experience. AJR Am J Roentgenol. 2007;189:799–806. doi: 10.2214/AJR.07.2086. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (5) [DOI] [PubMed] [Google Scholar]
- 3.Matsuki M, Inada Y, Nakai G, et al. Diffusion-weighed MR imaging of pancreatic carcinoma. Abdom Imaging. 2007;32:481–483. doi: 10.1007/s00261-007-9192-6. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (14) [DOI] [PubMed] [Google Scholar]
- 4.Zhang J, Tehrani YM, Wang L, Ishill NM, Schwartz LH, Hricak H. Renal masses: characterization with diffusion-weighted MR imaging—a preliminary experience. Radiology. 2008;247:458–464. doi: 10.1148/radiol.2472070823. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (4) [DOI] [PubMed] [Google Scholar]
- 5.Ichikawa T, Erturk SM, Motosugi U, et al. High-B-value diffusion-weighted MRI in colorectal cancer. AJR Am J Roentgenol. 2006;187:181–184. doi: 10.2214/AJR.05.1005. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (19) [DOI] [PubMed] [Google Scholar]
- 6.Nasu K, Kuroki Y, Kuroki S, Murakami K, Nawano S, Moriyama N. Diffusion-weighted single shot echo planar imaging of colorectal cancer using a sensitivity-encoding technique. Jpn J Clin Oncol. 2004;34:620–626. doi: 10.1093/jjco/hyh108. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (29) [DOI] [PubMed] [Google Scholar]
- 7.Hosonuma T, Tozaki M, Ichiba N, et al. Clinical usefulness of diffusion-weighted imaging using low and high b-values to detect rectal cancer. Magn Reson Med Sci. 2006;5:173–177. doi: 10.2463/mrms.5.173. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (4) [DOI] [PubMed] [Google Scholar]
- 8.Jaggi RS, Husain M, Chawla S, Gupta A, Gupta RK. Diagnosis of bacterial cerebellitis; diffusion imaging and proton magnetic resonance spectroscopy. Pediatr Neurol. 2005;32:72–74. doi: 10.1016/j.pediatrneurol.2004.06.019. Article | PDF (141 K) | View Record in Scopus | Cited By in Scopus (8) [DOI] [PubMed] [Google Scholar]
- 9.Kiroglu Y, Calli C, Yunten N, et al. Diffusion-weighted MR imaging of viral encephalitis. Neuroradiology. 2006;48:875–880. doi: 10.1007/s00234-006-0143-7. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (5) [DOI] [PubMed] [Google Scholar]
- 10.Verswijvel G, Vandecaveye V, Gelin G, et al. Diffusion-weighted MR imaging in the evaluation of renal infection: preliminary results. JBR-BTR. 2002;85:100–103. View Record in Scopus | Cited By in Scopus (8) [PubMed] [Google Scholar]
- 11.Riddell RH. Pathology of idiopathic inflammatory bowel disease. In: Kirsner JB, editor. Inflammatory bowel disease. 5th ed. Philadephia, PA: W.B. Saunders; 2000. pp. 427–452. [Google Scholar]
- 12.Sostegni R, Daperno M, Scaglione N, Lavagna A, Rocca R, Pera A. Review article: Crohn's disease: monitoring disease activity. Aliment Pharmacol Ther. 2003;17:11–17. doi: 10.1046/j.1365-2036.17.s2.17.x. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (32) [DOI] [PubMed] [Google Scholar]
- 13.Koh D, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol. 2007;188:1622–1635. doi: 10.2214/AJR.06.1403. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (36) [DOI] [PubMed] [Google Scholar]
- 14.Takahara T, Imai Y, Yamashita T, et al. Diffusion weighted whole body imaging with background body signal suppression (DWIBS): technical improvement using free breathing, STIR and high resolution 3D display. Radiat Med. 2004;22:275–282. View Record in Scopus | Cited By in Scopus (113) [PubMed] [Google Scholar]