Abstract
Monosensitization differs both immunologically and clinically from polysensitization, and specific immunotherapy is more effective in patients sensitized only to a single pollen than in multiple-pollen sensitized patients. To further examine the differences between monosensitized and polysensitized allergies, allergic indices were examined in 68 monosensitized and 62 polysensitized patients with childhood asthma. Measurements included symptom scores, eosinophil counts, skin prick tests, serum total and specific IgE levels, and IL-10 levels, and were used to compare allergic indices between the two groups. Patients were followed for 18 months following immunotherapy to examine the effectiveness of the treatment. Symptom scores and total IgE levels were significantly higher in the polysensitized group than those in the monosensitized group (p<0.05). The levels of skin test response decreased significantly in both groups following immunotherapy. In the monosensitized group, symptom scores and specific IgE levels were significantly reduced after immunotherapy (p<0.05). In the polysensitized group, symptom scores were reduced after immunotherapy (p<0.05), but the degree of reduction was less than that of the monosensitized group (p<0.05). Moreover, in the polysensitized group, specific IgE levels after immunotherapy did not differ from that before immunotherapy. Serum IL-10 levels were not significantly increased after immunotherapy in either group. In conclusion, polysensitized patients tend to show higher allergic indices and immunotherapy might be less effective for these patients.
Keywords: Asthma, Child, Immunotherapy, Immunoglobulin E
INTRODUCTION
IgE-mediated allergies are extremely common in adults and children with asthma. An allergen-based treatment is therefore of great importance for the management of allergic asthma (1). Specific immunotherapy is thought to be the only treatment that may alter the natural course of allergic disease (2). However, it has been reported that immunotherapy for asthma does not provide benefits beyond those achievable with alternative remedies (3).
Some people are sensitized to only one class of allergens (monosensitization), whereas others are sensitized to more than one class of allergens (polysensitization) (4, 5). Why individuals become sensitized to one or more classes of allergens is not well understood, although a number of genetic and environmental factors have been implicated (6). Recently there has been an increase in antigen sensitivity, and the number of asthma patients who have become sensitive to several antigens is also increasing (7). In addition, polysensitization has been reported to be associated with a lower health-related quality of life in intermittent asthma patients (8). Furthermore, there has also been a suggestion that the specific and total serum IgE levels in multi-antigen-sensitive patients are high, and immunotherapy is not as effective in these patients as in those who are sensitive to a single antigen. However, serum specific IgG levels have been reported to be significantly increased after immunotherapy in both types of patients (8).
The present study was undertaken to compare allergic indices and the efficacy of immunotherapy in patients with typical allergic asthma who are sensitive to one or more antigens.
MATERIALS AND METHODS
Subjects
Among patients who were diagnosed with asthma at the pediatric allergy clinic at Severance hospital, 130 patients who underwent specific immunotherapy for more than 18 months were included in this study.
Asthma was defined as recurrent wheezing or cough, in the absence of a cold, in the preceding 12 months, with a physician's diagnosis (at any time in the patient's history), of bronchial hyperresponsiveness upon methacholine challenge (PC20≤16 mg/mL) and at least 12% reversibility of FEV1 after inhalation of β2 agonist, per criteria of the American Thoracic Society (ATS) (10-12).
Subjects sensitized to only the house dust mite class of allergens were defined as monosensitized, and those sensitized to two to more classes of allergens, including house dust mites, were defined as polysensitized. Because it was considered that atopic subjects are frequently sensitized to more than one allergen belonging to clusters of allergen classes (13, 14).
In order to compare allergic indices between the two groups, symptom scores, total serum IgE levels, specific IgE levels, eosinophil counts, IL-10 levels, and allergic skin test response levels were examined before and after immunotherapy. Informed, written consent was obtained from all participants before inclusion in the study, which was previously approved by the Severance Hospital Institutional Review Board.
Allergic skin prick tests
Skin prick tests were performed with 32 allergens including: i) house dust mites (house dust, Dermatophagoides pteronyssinus, D. farinae), ii) animal dander (cat hair and dander, dog hair, rabbit hair), iii) pollens (sagebrush, ragweed, oak, birch, etc.), iv) molds (Aspergillus, Alternaria, etc.), and v) cockroach (American cockroach, German cockroach) (Torii & Co., Tokyo, Japan), together with negative (saline) and positive (0.5% histamine HCl) controls. Wheal size was measured after 15 min (15). A positive reaction was defined as a wheal larger than 3 mm (16).
The degree of skin test response was expressed as a ratio obtained by dividing the wheal size from house dust mite (D. farinae) by that from the positive control.
Spirometry and methacholine challenge test
Lung function was measured by spirometry (Vmax encore; VIASYS Healthcare Inc., Conshohocken, U.S.A.) performed according to American Thoracic Society standards with the children standing. Bronchial hyperresponsiveness was assessed in all subjects by the methacholine challenge test. Children were eligible if they could perform reproducible spirometry and had an FEV1 of at least 70% of the predicted value (17). The concentration of methacholine causing a 20% decrease in FEV1 (PC20) was determined, and a challenge was considered positive if the PC20 was 16 mg/mL or less. Anti-inflammatory preparations and bronchodilators were withheld for 24 hr before the testing.
Symptom scores
Symptoms such as dyspnea, wheezing, and cough, which indicate asthma, were scored as 0-2, respectively, through interview with the subjects and their parents (18).
Measurement of blood eosinophils, serum total IgE, specific IgE and IL-10 levels
After peripheral blood samples were obtained from the subjects, the total IgE levels and levels of IgE against house dust mites (D. farinae) were measured using the CAP system FEIA (Pharmacia & Upjohn Diagnostic AB, Uppsala, Sweden). The NE-8000 system (Sysmax, Japan) was used to automatically count eosinophils in peripheral blood. Serum IL-10 levels were measured according to the sandwich ELISA method. The minimum detectable dose of human IL-10 is typically less than 0.5 pg/mL (R & D systems Inc., Minneapolis, Minnesota, U.S.A.).
Immunotherapy
The first stage of treatment included administration of minimal doses of vaccines, after which the dose was increased weekly until reaching the maximum dose that the patient could tolerate. The second stage of immunotherapy was the maintenance stage, in which the maximum dose of antigen was maintained monthly for up to 18 months (19). Monosensitized subjects received immunotherapy with house dust mite vaccines and polysensitized subjects with mixed vaccines depending on the sensitivity of the subjects. Both groups had the same dosage of house dust mite antigen in the vaccines.
Statistical analysis
All values are expressed as average±SD. Using the SPSS 11.0 statistical package program, statistical difference was determined between the two groups according to paired t-tests. p values less than 0.05 were considered to be significant.
RESULTS
Comparison of clinical characteristics and allergy indices between monosensitized and polysensitized groups
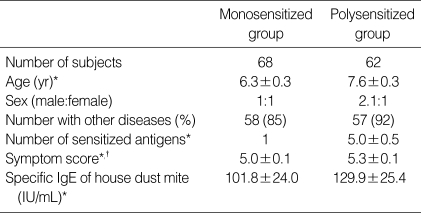
Among 130 total subjects, 68 patients belonged to the monosensitized group and 62 patients to the polysensitized group. The average ages of patients in the two groups were 6.3±0.3 yr and 7.6±0.3 yr, respectively. The male to female ratio was 1:1 in the monosensitized group and 2.1:1 in the polysensitized group. Allergic diseases other than asthma were found in 58 subjects in the monosensitized group (85%) and 57 subjects in the polysensitized group (92%). The most frequently accompanying allergic disease other than asthma was allergic rhinitis, followed by allergic conjunctivitis and atopic dermatitis, and no significant difference existed between the two groups with regard to the presence of these allergic diseases. The average number of positive antigens according to the allergic skin test was 5.0±0.5 in the polysensitized group, and the most frequently seen antigen was the house dust mite, followed by Alternaria and cockroach. The symptom scores of polysensitized subjects were significantly higher than those of monosensitized subjects (p<0.05). The level of IgE specific to house dust mites was increased in the polysensitized group, but this increase was not statistically significant (Table 1). The total serum IgE levels in the polysensitized group were significantly increased compared with that of the monosensitized group, both before and after immunotherapy (p<0.05). The eosinophil counts were also high in the polysensitized group compared with the monosensitized group; however, this difference was also not significant (Table 2).
Table 1.
Comparison of clinical characteristics and allergy indices between monosensitized and polysensitized groups
*Data are expressed as mean±SD, †p<0.05.
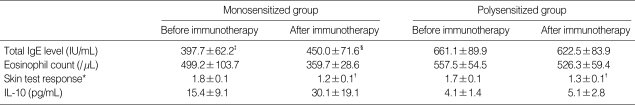
Table 2.
Comparison of allergy indices between monosensitized and polysensitized groups before and after immunotherapy
Data are expressed as mean±SD.
*A/H ratio of wheal of allergen and histamine; †p<0.05, compared to the level of skin test before immunotherapy; ‡p<0.05, compared to total IgE level of polysensitized group before immunotherapy; §p<0.05, compared to total IgE level of polysensitized group after immunotherapy.
Efficacy of immunotherapy
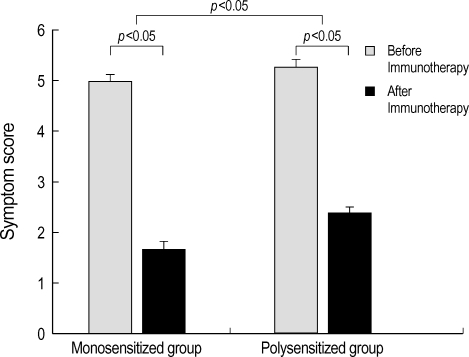
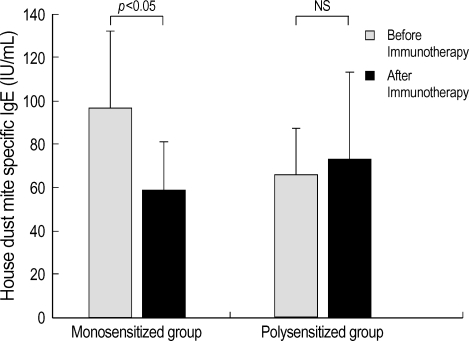
The results of tests conducted before and after immunotherapy showed that changes in the total serum IgE levels, eosinophil counts and serum IL-10 levels were not statistically different between the monosensitized group and polysensitized group (Table 2). In the monosensitized group, symptom scores were decreased from 5.0±0.1 before immunotherapy to 1.7±0.1 after immunotherapy (p<0.05) (Fig. 1). The levels of house dust mite-specific IgE were decreased significantly from 97.3±34.2 IU/mL before immunotherapy to 59.2±21.9 IU/mL after immunotherapy (p<0.05) (Fig. 2). The levels of skin test response were also decreased significantly after immunotherapy (p<0.05) (Table 2). In the polysensitized group, symptom scores were decreased from 5.3±0.1 before immunotherapy to 2.4±0.1 after immunotherapy (p<0.05), less of a decrease than the monosensitized group (p<0.05). The levels of house dust mite-specific IgE actually increased from 65.8±21.6 IU/mL before immunotherapy to 73.2±40.2 IU/mL after immunotherapy (Fig. 2). However, the degree of skin test response was decreased significantly after immunotherapy (p<0.05) (Table 2).
Fig. 1.
Symptom scores differ significantly between monosensitized and polysensitized groups. Symptom scores also decrease significantly after immunotherapy in both groups. Error bars represent the standard deviation.
Fig. 2.
The level of IgE specific to house dust mite (D. farinae) decreases significantly after immunotherapy in the monosensitized group (p<0.05), but not in the polysensitized group. Error bars represent standard deviation. NS, not significant.
DISCUSSION
The results from this study showed differences in allergic indices between monosensitized and polysensitized groups. The symptom scores and levels of total IgE were higher in the polysensitized group compared with the monosensitized group. The specific IgE levels and eosinophil counts were high in the polysensitized group; however, these increases were not significant, in concordance with the previous studies in which the total and specific IgE levels were higher in polysensitized groups (9, 20).
Pene et al. (20) reported that the reason for different IgE immune response between monosensitized and polysensitized groups lies in the difference in IL-4 secretion. Therefore, the subjects could be divided into high IgE responders and low IgE responders. Based on these studies, we could consider two genetic mechanisms involved in controlling the allergic response. In the case of the monosensitized group (low IgE responders), allergy sensitization has a close relationship with the HLA-DR or DP molecule, whereas patients in the polysensitized group (high IgE responders) showed allergy sensitization regardless of HLA-DR or DP, suggesting that epitopes binding with MHC were degenerated (21, 22).
Although there still exists controversy over the value of immunotherapy, studies on its efficacy have been continued (3, 23). In the present study, we found that the symptom scores and the levels of skin test response were decreased after immunotherapy, however, the total IgE levels and eosinophil counts showed no statistically significant changes. Koker et al. (24) reported that total IgE levels and eosinophil counts were decreased after immunotherapy, however, Jarolim et al. (25) reported increase of IgG1, IgG4 and total IgE levels, and Turner et al. (26) and McHugh et al. (27) reported no change in specific IgE levels after immunotherapy. Bousquet et al. (8) stated that the efficacy of immunotherapy is expressed differently between the monosensitized group and the polysensitized group, and that the efficacy of immunotherapy was decreased in the polysensitized group when symptom scores, PGD2, skin test response levels, and specific IgG levels were evaluated. In the present study, the symptom scores and the specific IgE levels were significantly decreased in the monosensitized group, compared with the polysensitized group, indicating a difference in the efficacy of immunotherapy. As described earlier, this decreased efficacy in immunotherapy in the polysensitized group was likely due to an increased IgE response against antigens in the polysensitized group so that these subjects probably did not respond to the immunotherapy, which was composed of optimal allergen doses required in the monosensitized group. Furthermore, antigen mixtures used in the polysensitized group were shown to be unstable compared with the single antigen solutions in a study using an antigen mixture composed of house dust mite, cat hair, or ragweed (28). One limitation in our study may be that we did not evaluate the efficacy of immunotherapy between the groups according to the severity of asthma. It is postulated that the efficacy of immunotherapy may be influenced by the severity of asthma, and thus it may have been better for future reference if the efficacy was evaluated independently in patient groups subdivided according to the level of severity of the asthma. However, it should be noted that in our study, there was a significant difference in the degree of improvement in symptom scores and specific IgE levels between our two groups, but not in the scores or levels themselves.
Human IL-10 inhibits the secretion of IFN-γ and IL-2 from Th1 cells, affects Th2 cells to inhibit the secretion of IL-4 and IL-5, and inhibits the secretion of IL-6 and TNF-α from mononuclear phagocytes (29). Therefore, it is thought that IL-10 functions as an anti-inflammatory cytokine in the body by inhibiting the secretion of IgE and the proliferation and activation of eosinophils to decrease allergic inflammation responses. It is also thought to inhibit the non-specific cellular inflammation responses of mononuclear phagocytes and Th1 cells (30). Borish et al. (30) reported a relatively decreased ability to secrete IL-10 in bronchoalveolar lavage in asthma patients, and Hobbs et al. (31) reported dysfunction in the transcription of the IL-10 gene in allergy patients. In the present study, although not statistically significant, we found an increased level of IL-10 in monosensitized patients, and believe that the IL-10 levels were increased following immunotherapy. These results may indicate that IL-10 function, which might affect allergic asthma, was recovered through immunotherapy.
In conclusion, monosensitized groups and polysensitized groups are immunologically different, and these differences are most likely controlled by the secretion pattern of various cytokines, as allergic indices are more severe and immunotherapy might be less effective in the polysensitized group. Based on these conclusions, to optimize the treatment course of the polysensitized patients, further study will be necessary and the efficacy of immunotherapy in this group requires further evaluation as well.
References
- 1.Bousquet J. Immunotherapy is clinically indicated in the management of allergic asthma. Am J Respir Crit Care Med. 2001;164:2139–2140. doi: 10.1164/ajrccm.164.12.2110107a. [DOI] [PubMed] [Google Scholar]
- 2.Durham SR, Walker SM, Varga EM, Jacobson MR, O'Brien F, Noble W, Till SJ, Hamid QA, Nouri-Aria KT. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999;341:468–475. doi: 10.1056/NEJM199908123410702. [DOI] [PubMed] [Google Scholar]
- 3.Adkinson NF. Immunotherapy is not clinically indicated in the management of allergic asthma. Am J Respir Crit Care Med. 2001;164:2140–2142. doi: 10.1164/ajrccm.164.12.2110107b. [DOI] [PubMed] [Google Scholar]
- 4.Croner S, Kjellman N-IM. Development of atopic disease in relation to family history and cord blood IgE levels. Eleven-year follow-up in 1654 children. Pediatr Allergy Immunol. 1990;1:14–20. [Google Scholar]
- 5.Silvestri M, Oddera S, Rossi GA, Crimi P. Sensitization to airborne allergens in children with respiratory symptoms. Ann Allergy Asthma Immunol. 1996;76:239–244. doi: 10.1016/s1081-1206(10)63433-3. [DOI] [PubMed] [Google Scholar]
- 6.Ownby DR. Environmental factors versus genetic determinants of childhood inhalant allergies. J Allergy Clin Immunol. 1990;86:279–287. doi: 10.1016/s0091-6749(05)80088-0. [DOI] [PubMed] [Google Scholar]
- 7.Cirillo I, Vizzaccaro A, Klersy C, Baiardini I, Marseglia GL, Canonica GW, Tosca MA, Ciprandi G. Quality of life and polysensitization in young men with intermittent asthma. Ann Allergy Asthma Immunol. 2005;94:640–643. doi: 10.1016/S1081-1206(10)61321-X. [DOI] [PubMed] [Google Scholar]
- 8.Bousquet J, Becker WM, Hejjaoui A, Chanal I, Lebel B, Dhivert H, Michel FB. Differences in clinical and immunologic reactivity of patients allergic to grass pollens and to multiple-pollen species. II. Efficacy of a double-blind, placebo-controlled, specific immunotherapy with standardized extracts. J Allergy Clin Immunol. 1991;88:43–53. doi: 10.1016/0091-6749(91)90299-4. [DOI] [PubMed] [Google Scholar]
- 9.Kim CW, Lee JH, Jung HW, Choi SR, Cheong JW, Park JW, Hong CS. Changing pattern of skin reactivity to inhalant allergens in asthmatic patients. J Asthma Allergy Clin Immunol. 2001;21:205–215. [Google Scholar]
- 10.Global Initiative for Asthma. Global strategy for asthma management and prevention. NIH National Heart Lung and Blood Institute; 2004. NIH Publication No. 04-3659. [Google Scholar]
- 11.Sedgwick JB, Vrtis RF, Jansen KJ, Kita H, Bartemes K, Busse WW. Peripheral blood eosinophils from patients with allergic asthma contain increased intracellular eosinophil-derived neurotoxin. J Allergy Clin Immunol. 2004;114:568–574. doi: 10.1016/j.jaci.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 12.American Thoracic Society. Guidelines for Methacholine and Exercise Challenge Testing-1999. Am J Respir Crit Care Med. 2000;161:309–329. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- 13.Storm van's Gravesande K, Moseler M, Kuehr J. The most common phenotypes of sensitization to inhalant allergens in childhood. Clin Exp Allergy. 1997;27:646–652. doi: 10.1046/j.1365-2222.1997.d01-558.x. [DOI] [PubMed] [Google Scholar]
- 14.Zwick H, Popp W, Jager S, Wagner C, Reiser K, Horak F. Pollen sensitization and allergy in children depend on the pollen load. Allergy. 1991;46:362–366. doi: 10.1111/j.1398-9995.1991.tb00599.x. [DOI] [PubMed] [Google Scholar]
- 15.Backman A. Skin tests for epidemiologic studies. Allergy. 1994;49:493–494. doi: 10.1111/j.1398-9995.1994.tb01118.x. [DOI] [PubMed] [Google Scholar]
- 16.Host A, Andrae S, Charkin S, Diaz-Vazquez C, Dreborg S, Eigenmann PA, Friedrichs F, Grinsted P, Lack G, Meylan G, Miglioranzi P, Muraro A, Nieto A, Niggemann B, Pascual C, Pouech MG, Rance F, Rietschel E, Wickman M. Allergy testing in children: why, who, when and how? Allergy. 2003;58:559–569. doi: 10.1034/j.1398-9995.2003.00238.x. [DOI] [PubMed] [Google Scholar]
- 17.Yoon KA, Lim HS, Kim H, Koh YY. Normal predicted values of pulmonary function test in Korean school-aged children. J Korean Pediatr Soc. 1993;36:25–37. [Google Scholar]
- 18.Koh YY. Effect of EPA/DCHA supplementation on the clinical course and the airway responses to methacholine and allergen in the asthmatic children. Pediatr Allergy Respir Dis. 1993;3:32–40. [Google Scholar]
- 19.Lee KY, Kim KE. Diagnosis and management of allergic diseases. 1st ed. Seoul: Korea Medical Press; 2001. Immunotherapy; pp. 651–722. [Google Scholar]
- 20.Pene J, Rivier A, Lagier B, Becker WM, Michel FB, Bousquet J. Differences in IL-4 release by PBMC are related with heterogeneity of atopy. Immunology. 1994;81:58–64. [PMC free article] [PubMed] [Google Scholar]
- 21.Cookson WO, Sharp PA, Faux JA, Hopkin JM. Linkage between immunoglobulin E responses underlying asthma and rhinitis and chromosome 11q. Lancet. 1989;1:1292–1295. doi: 10.1016/s0140-6736(89)92687-1. [DOI] [PubMed] [Google Scholar]
- 22.Marsh DG, Zwollo P, Huang SK, Ansari AA. Molecular genetics of human immune responsiveness to allergens. Ciba Found Symp. 1989;147:171–183. doi: 10.1002/9780470513866.ch11. [DOI] [PubMed] [Google Scholar]
- 23.Bousquet J, Michel FB. Specific immunotherapy in asthma: is it effective? J Allergy Clin Immunol. 1994;94:1–11. doi: 10.1016/0091-6749(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 24.Koker O, Guneser S, Altintas D, Kozanoglu M. Effect of specific immunotherapy in Dermatophagoides pteronyssinus allergic children. Acta Paediatr Jpn. 1994;36:150–152. doi: 10.1111/j.1442-200x.1994.tb03151.x. [DOI] [PubMed] [Google Scholar]
- 25.Jarolim E, Poulsen LK, Stadler BM, Mosbech H, Oesterballe O, Kraft D, Weeke B. A long-term follow-up study of hyposensitization with immunoblotting. J Allergy Clin Immunol. 1990;85:996–1004. doi: 10.1016/0091-6749(90)90043-4. [DOI] [PubMed] [Google Scholar]
- 26.Turner MW, Yalcin I, Soothill JF, Price JF, Warner JO, Hey EN, Chapman MD, Platts-Mills TA. In vitro investigations in asthmatic children undergoing hyposensitization with tyrosine-adsorbed Dermatophagoides pteronyssinus antigen. Clin Allergy. 1984;14:221–231. doi: 10.1111/j.1365-2222.1984.tb02201.x. [DOI] [PubMed] [Google Scholar]
- 27.McHugh SM, Lavelle B, Kemeny DM, Patel S, Ewan PW. A placebo-controlled trial of immunotherapy with two extracts of Dermatophagoides pteronyssinus in allergic rhinitis, comparing clinical outcome with changes in antigen-specific IgE, IgG, and IgG subclasses. J Allergy Clin Immunol. 1990;86:521–531. doi: 10.1016/s0091-6749(05)80208-8. [DOI] [PubMed] [Google Scholar]
- 28.Muthiah R, Kagen S. Allergens as enzymes: degradation of cat and ragweed allergens by house dust mite allergen extract [Abstract] J Allergy Clin Immunol. 1990;85:151. [Google Scholar]
- 29.Del Prete G, De Carli M, Almerigogna F, Giudizi MG, Biagiotti R, Romagnani S. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J Immunol. 1993;150:353–360. [PubMed] [Google Scholar]
- 30.Borish L, Aarons A, Rumbyrt J, Cvietusa P, Negri J, Wenzel S. Interleukin-10 regulation in normal subjects and patients with asthma. J Allergy Clin Immunol. 1996;97:1288–1296. doi: 10.1016/s0091-6749(96)70197-5. [DOI] [PubMed] [Google Scholar]
- 31.Hobbs K, Negri J, Klinnert M, Rosenwasser LJ, Borish L. Interleukin-10 and transforming growth factor-beta promoter polymorphisms in allergies and asthma. Am J Respir Crit Care Med. 1998;158:1958–1962. doi: 10.1164/ajrccm.158.6.9804011. [DOI] [PubMed] [Google Scholar]