Abstract
Rates of disease progression differ among patients with Alzheimer’s disease, but little is known about prognostic predictors. The aim of the study was to assess whether sociodemographic factors, disease severity and duration, and vascular factors are prognostic predictors of cognitive decline in Alzheimer’s disease progression. We conducted a longitudinal clinical study in a specialized clinical unit for the diagnosis and treatment of dementia in Rome, Italy. A total of 154 persons with mild to moderate Alzheimer’s disease consecutively admitted to the dementia unit were included. All patients underwent extensive clinical examination by a physician at admittance and all follow-ups. We evaluated the time-dependent probability of a worsening in cognitive performance corresponding to a 5-point decrease in Mini-Mental State Examination (MMSE) score. Survival analysis was used to analyze risk of faster disease progression in relation to age, education, severity and duration of the disease, family history of dementia, hypertension, hypercholesterolemia, and type 2 diabetes. Younger and more educated persons were more likely to have faster Alzheimer’s disease progression. Vascular factors such as hypertension and hypercholesterolemia were not found to be significantly associated with disease progression. However, patients with diabetes had a 65% reduced risk of fast cognitive decline compared to Alzheimer patients without diabetes. Sociodemographic factors and diabetes predict disease progression in Alzheimer’s disease. Our findings suggest a slower disease progression in Alzheimer’s patients with diabetes. If confirmed, this result will contribute new insights into Alzheimer’s disease pathogenesis and lead to relevant suggestions for disease treatment.
Keywords: Disease progression, Cognitive decline, Dementia, Diabetes, Education
Introduction
Persons with Alzheimer’s disease (AD) show memory decline that progressively worsens and is accompanied by a parallel decline in other cognitive domains. Patients become completely dependent in activities of daily living and die after 8–10 years from the first diagnosis [15, 16, 42]. The disease is marked by key events such as severe cognitive impairment, the inability to dress, eat, and wash, institutionalization, and death. The time of occurrence of these events is highly variable from patient to patient, and thus it is difficult for clinicians to make prognostic predictions about individual patients. It is important to identify prognostic markers to improve patient care and long-term planning.
A number of sociodemographic factors and vascular risk factors have been found to increase the risk of elderly individuals developing AD [24]. However, little is known about whether such factors also play a role in the progression of the disease itself. Some vascular risk factors and disorders have been found to be associated with a faster progression rate [5, 19, 27], including cerebrovascular accidents [27] and systolic hypertension [19].
In the current study, we aimed to examine whether sociodemographic and vascular factors predict faster cognitive decline in patients with AD, using a clinical sample of AD patients from a specialized dementia clinic in Italy, who were followed for an average of 2 years. Identifying predictors of disease progression in AD might provide new insights into the pathogenic mechanisms of AD and suggest new therapeutic interventions.
Materials and methods
Patients
The cohort of AD patients was enrolled at the Center for Dementia Diagnosis and Treatment, IRCCS Foundation Santa Lucia Hospital in Rome, Italy. The dementia center was set up as part of a country-wide project promoted by the Italian health authorities called “Progetto Cronos” [26], which aims to offer patients with AD and other dementias a multi-disciplinary approach and a prospective treatment plan. Patients are referred to the center, mostly by GPs, for evaluation. After diagnosis, some patients continued their care at the center, but since the Foundation Santa Lucia Hospital is not a primary center for AD, some patients were referred elsewhere depending on, for example, demographic factors. A total of 1,096 patients were consecutively admitted to the clinic between 2000 and 2006. All patients were examined by a neurologist and neuropsychologist. At the first visit to the center, 109 (9.5%) patients were normal, 167 (15.2%) had MCI [10], 377 (34.9%) patients had “pure” AD diagnosed according to the NINCDS-ARDRA criteria [18], 226 (20.6%) had other types of dementia, and 217 (19.8%) had other diagnoses, including Parkinson’s disease, depression, etc. Only the 377 patients with pure AD were eligible for this study. At the center a neurologist followed up the patients and carried out all activities concerned with diagnosis, drug prescription, and treatment monitoring. When necessary, a geriatrician and/or a psychiatrist were consulted. At the first visit a brain MRI examination was performed. Patients whose brain imaging results confirmed cerebrovascular damage that could justify all or part of their cognitive disorders were diagnosed as possible AD. We excluded 220 patients with severe cranial trauma, focal neurological signs, and possible AD, as well as patients who attended the clinic only once. A further four patients were excluded from the analysis due to suspended acetyl-cholinesterase inhibitor treatment because of adverse drug reactions or perceived inefficacy. Thus, the study population consisted of 154 patients with probable AD.
Ethics
Ethical permission was provided by the Ethical Committee of Foundation Santa Lucia, and the study was performed in accordance with the ethical standards of the 1964 Helsinki declaration. Patients and their next-of-kin gave their consent to be included in the study.
Evaluation
Patients underwent extensive examination by a neurologist, and a complete health history was collected from all patients and their relatives. Patients with mildly or moderately severe AD started treatment with an acetyl-cholinesterase inhibitor and were invited to periodic follow-up visits. At the time of enrollment and follow-up examinations, cognitive performance was evaluated with the Mini-Mental State Examination (MMSE) [11] according to age- and education-adjusted scores [10].
The clinical examination included information concerning the maximum number of years of formal education of the patients, age, sex, and family history of dementia. Hypertension, type 2 diabetes, and hypercholesterolemia were defined as (1) a diagnosis and subsequent treatment by a physician at the clinic or (2) a relative’s report of previous and ongoing treatment for the respective condition. Disease duration of AD was defined in months by the examining neurologist based on the clinical exam and anamnesis. Disease duration of AD was categorized into three groups: <1 year, 1–2 years, and >2 years.
Outcome: disease progression
A decrease of 5 points or more on the MMSE since enrollment was considered an indicator of disease progression based on previous research [30]. A 5-point decrease was considered a clinically relevant worsening and too large of a change to be due to the intrinsic limits of test reliability [7]. The date of the visit when the 5-point reduction was recorded marked the time of occurrence of the progression.
Statistical analyses
The occurrence rates of the time-dependent event “disease progression” were evaluated by survival analysis, and survival curves were derived with the Kaplan-Meier’s method [14]. The following variables were considered as possible predictors of disease progression: age, sex, education, MMSE score at enrollment, family history of dementia, disease duration and severity, hypertension, type 2 diabetes, and hypercholesterolemia. The continuous variables (age, education, and MMSE) were categorized according to the tertile distribution. The age categories included: ≤70 years, 71–77 years, and ≥77 years. Education was categorized as follows: ≤5 years, 6–8 years, and ≥8 years. Age- and education-adjusted MMSE scores were divided into three groups corresponding to the following categories: ≤17, 17.1–20.2, and ≥20.3.
As previous research on the topic [19] suggested that various vascular factors may have different roles on the progression of AD, we examined vascular factors separately. First, analyses of survival were carried out with Cox’s proportional hazard models [8] in which variables were entered separately into the model. Second, the analysis of survival was repeated with adjustment for all sociodemographic and vascular factors.
Results
The 154 AD patients fulfilling the inclusion criteria underwent at least one follow-up visit after initial examination. The mean follow-up time was 23 months (SD 15.6), and on average patients had 3.3 (SD 1.6) follow-up visits. The demographic and clinical characteristics of the patients are presented in Table 1. There were twice as many women as men. Mean age was 73 years and mean education 8 years. Severity of AD was mild to moderate with mean disease duration of about 2 years. More than a third of the patients reported having a relative with dementia. Hypertension and hypercholesterolemia were common. The 36 hypertensive patients were all treated; the most common drugs used were ACE inhibitors as monotherapy or with diuretics. None of the women were treated with estrogen replacement therapy. Diabetes was present in the same proportion of men and women. All but one of the diabetic patients had type 2 diabetes and were treated with oral drugs. Of the 20 patients treated with oral drugs 12 were prescribed metformin, 6 sulphonylureas, and the remaining 2 were treated with both sulphonylureas and metformin. The average follow-up duration was about 2 years. During this period, 40% (n = 61) had a fast disease progression, defined as a 5-point decrease in the MMSE.
Table 1.
Demographic and clinical characteristics of Alzheimer disease patients
| Women (n = 101) | Men (n = 53) | Total (n = 154) | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Age (years) | 74 (8.4) | 72 (7.6) | 73 (8.2) |
| Disease duration (months) | 26 (13.7) | 27 (17.0) | 26 (14.7) |
| MMSE | 17 (4.3) | 19 (4.4) | 18 (4.8) |
| Follow-up (months) | 23 (14.5) | 25 (17.7) | 23 (15.6) |
| Education (years) | 7 (3.7) | 11 (4.3) | 8 (4.4) |
| n (%) | n (%) | n (%) | |
| Hypertension | 34 (33.7) | 18 (34.6) | 52 (34.0) |
| Hypercholesterolemia | 19 (18.9) | 9 (17.3) | 28 (18.3) |
| Diabetes | 15 (14.9) | 7 (13.5) | 22 (14.4) |
| Family history for AD | 29 (24.8) | 21 (40.4) | 50 (32.7) |
| MMSE score decrease at follow-up greater than or equal to 5 | 38 (38.6) | 23 (44.2) | 61 (39.9) |
Table 2 shows AD progression rates as well as the crude (predictors entered separately) and multivariable hazard ratios (adjustment for all predictors) of progression according to baseline sociodemographic and vascular factors. More advanced age was associated with reduced risk of progression, i.e., the progression of patients over 70 years of age was almost half that of younger patients. The risk of progression of patients with 6+ years of education was twice that of patients with <5 years of education. Severity of cognitive impairment, as measured by the MMSE, did not influence disease progression. Patients with a 2 year disease duration had reduced risk of progression compared both to patients with shorter or longer disease duration, but this difference was not statistically significant in the crude analysis. Hypertension, hypercholesterolemia, and family history of dementia were not associated with disease progression. On the contrary, disease progression in AD patients with diabetes was about 60% less than that of non-diabetic AD patients.
Table 2.
Progression rates and crude and multivariable hazard ratios of progression according to baseline sociodemographic and vascular factors
| Total | Patients with disease progression of >5 MMSE | |||
|---|---|---|---|---|
| n (%) | n (%) | Crudea | Multivariateb | |
| HR (95% CI) | HR (95% CI) | |||
| Age (years) | ||||
| ≤70 | 49 (31.8) | 28 (57.1) | 1 | 1 |
| 71–77 | 52 (33.8) | 18 (34.6) | 0.48 (0.3–0.9) | 0.54 (0.3–1.1) |
| >77 | 53 (34.4) | 15 (28.3) | 0.48 (0.3–0.9) | 0.50 (0.3–1.0) |
| Sex | ||||
| Women | 101 (65.6) | 38 (37.6) | 1 | 1 |
| Men | 53 (34.4) | 23 (43.4) | 1.1 (0.7–1.9) | 0.79 (0.4–1.5) |
| Education (years) | ||||
| ≤5 | 71 (46.1) | 18 (25.4) | 1 | 1 |
| 6–8 | 31 (20.1) | 17 (54.8) | 2.2 (1.1–4.2) | 2.5 (1.2–5.2) |
| ≥9 | 52 (33.8) | 26 (50.0) | 2.5 (1.3–4.5) | 2.8 (1.4–5.5) |
| Disease duration (years) | ||||
| ≤1 | 53 (34.4) | 23 (43.4) | 1 | 1 |
| 1–2 | 49 (31.8) | 18 (36.7) | 0.67 (0.4–1.3) | 0.46 (0.2–0.9) |
| >2 yrs | 52 (33.8) | 20 (38.5) | 1.2 (0.7–2.2) | 1.0 (0.5–2.0) |
| MMSE at enrollment | ||||
| ≤17 | 51 (33.1) | 16 (31.4) | 1 | 1 |
| 17.0–20.2 | 51 (33.1) | 25 (49.0) | 1.2 (0.6–2.2) | 1.6 (0.8–3.3) |
| ≥20.30 | 52 (33.8) | 20 (38.5) | 1.3 (0.7–2.5) | 1.5 (0.7–3.2) |
| Hypertension | 52 (33.8) | 19 (36.5) | 1.0 (0.6–1.7) | 1.2 (0.7–2.2) |
| Diabetes | 22 (14.3) | 5 (22.7) | 0.38 (0.2–0.9) | 0.36 (0.1–0.9) |
| Hyper-cholesterolemia | 28 (18.2) | 8 (28.6) | 0.73 (0.3–1.5) | 0.58 (0.3–1.3) |
| Family history of dementia | 50 (32.5) | 19 (38.1) | 0.90 (0.6–1.7) | 1.0 (0.6–1.9) |
aCrude hazard ratios: Cox proportional hazard models using single predictors, with 95% confidence intervals (95% CI)
bMultivariate hazard ratios: Cox proportional hazard models with multiple adjustment for all variables in the table, with 95% confidence intervals (95% CI)
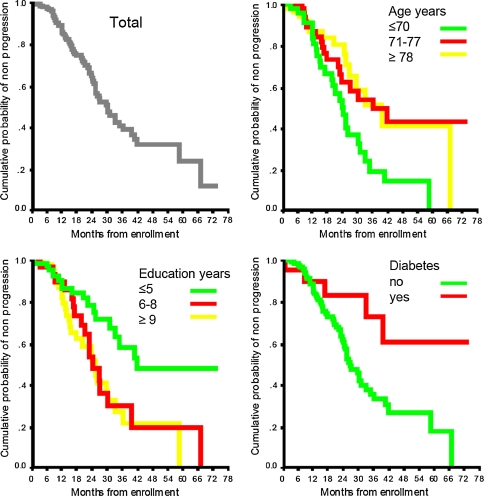
The cumulative time-dependent probabilities of disease progression for the entire cohort and by categories of age, education, and diabetes are presented in Fig. 1. Disease progression was generally similar for the different categories of patients for the first year and then tended to diverge. No clear trend for slower progression with increasing age was apparent, and the main difference was between patients aged ≤70 years and all older patients. The same was true for education where patients with ≤5 years of education had less disease progression than other patients. We conducted a supplemental analysis to investigate whether the association between younger age and disease progression was due to early onset AD cases. Twenty-four patients had AD onset before the age of 65. In early onset AD patients, fast disease progression was observed in 18 (75.0%) subjects, as opposed to 43 (33.1%) of the 130 patients with onset after the age of 65 years. The hazard ratio of fast progression in early compared to late onset AD patients was 2.2 (95% CI: 1.3–3.9, P = 0.007).
Fig. 1.
Cumulative time-dependent probability of AD progression (reduction of 5 points on MMSE) for the whole cohort and by age, education, and presence of diabetes
The multivariable analysis (Table 2) did not introduce any relevant modification of the size or direction of the crude hazard ratio estimates. The reduction in the probability of progression observed in association with disease durations of 2 years became more evident and statistically significant. The hazard of disease progression in diabetic AD patients was slightly lower than the univariate estimates and maintained the statistical significance.
Discussion
In the current study, we followed a clinical cohort of AD patients to examine factors related to disease progression and found that older age, lower education, and type 2 diabetes are associated with slower disease progression in AD patients.
The finding of a worse prognosis in younger AD patients is not unique to the current study, as others have found trends for faster cognitive decline in younger AD patients [6, 21]. Considering that AD is an aging-related disorder, which is present well before symptoms appear, it is reasonable to expect that when the disease is manifest at younger ages it might be also more aggressive and progress more quickly. In our patients, there were 24 people with early onset AD, defined as an onset before age 65. These patients accounted for half of the cases in the age group <71. The higher risk of progression observed in association with younger age was completely explained by these early onset patients. This observation suggests that early onset AD, where hereditary forms of the disease are more frequent, might have a worse prognosis in comparison to sporadic cases.
Lower education has also been found to be associated with slower progression rates in previous studies [22, 33, 36]. It is likely that persons with low education have a reduced cognitive reserve and thus are more vulnerable to the effects of the pathological process of AD, leading to an earlier manifestation of the distinctive signs and symptoms of dementia. If the progression rate of AD pathology is not influenced by cognitive reserve, it is possible that more educated persons experience clinically evident AD for a shorter period of time, and thus their cognitive decline will appear to be faster than less educated patients. In the current study, we were unable to determine whether education levels per se were responsible for the reduced risk of AD progression, or whether education was a proxy for another associated factor, such as sociodemographic status.
We found an association between diabetes and an approximately 65% reduction in risk of fast progression in AD. This association was independent from all the other variables considered as potential prognostic predictors. This finding replicates results reported in another study [19], which found some vascular risk factors and disorders were associated with higher progression rates of the cognitive disturbance in AD patients, yet diabetic AD patients had reduced progression rates. As their study included a very elderly sample of people aged 85+, our findings demonstrate that this pattern of cognitive decline in AD also occurs in younger AD patients. Furthermore, two studies reported less severe AD neuropathology [2] and reduced cognitive decline [41] in association with diabetes medication.
Epidemiological studies have indicated that diabetes increases the risk of dementia both of vascular and neurodegenerative origin [4]. The reasons for this association are unknown, although it has been hypothesized that some characterizing features and complications of diabetes such as micro-vascular damage [17], impaired glucose metabolism [13], and insulin imbalance [9, 32] might play a role.
One potential explanation of the association between diabetes and AD progression is that it is not diabetes per se but the vascular complications of diabetes that lead to neurodegeneration. The association between better AD prognosis and diabetes might be due to the existence of brain vascular damage in these patients that is associated with the cognitive impairment. Indeed, unlike neurodegenerative dementia, where the disturbance is progressive, in dementia of vascular origin cognitive decay tends to occur concomitantly with new cerebrovascular events. It is possible that the better prognosis of diabetic AD patients might be linked to the fact that by treating diabetes the vascular complications of the disease are prevented. However, it is not easy to prevent vascular events with antidiabetic therapy [37], because the vascular damage seems independent of glycemic control. Therefore, we cannot explain the lower AD progression rates of the diabetic patients observed in this study as the result of having prevented cerebrovascular events with antidiabetic drugs. Furthermore, this explanation is contradicted by the fact that the hypertensive patients observed in this, and other studies, did not show any prognostic advantage [24, 27], and with adequate, early control, the risk of cerebrovascular events in the elderly is reduced in hypertensive individuals [35].
Much evidence links type 2 diabetes to neurodegenerative disorders and AD. Pancreatic islet cells producing insulin might evolve from an ancestral insulin-producing neuron [31]. Insulin crosses the blood–brain barrier in animals [1] and, probably, in humans [40]. In the whole brain, neurons and astrocytes express insulin receptors at synapses but insulin binding is prevalent in the olfactory bulb, cerebral cortex, and hippocampus [34] which are among the principal brain areas involved in the pathological process of AD. Indeed, insulin administration has been shown to improve cognitive functioning [3, 20, 28]. Contrary to these observations, chronic hyperinsulinemia and diabetes are associated with higher occurrence of AD and with reduced learning and memory [38, 39]. In diabetic patients, this association does not seem to be mediated by chronic hyperglycemia because cognitive impairment was also evident in subjects with normal levels of glycosylated hemoglobin [38]. These apparently contradictory findings suggest a potentially different role of acute and chronic exposure to insulin [38] on the brain and brain functions. Insulin might promote the intraneuronal release of β-amyloid (Aβ) [12] and insulin, and Aβ peptides are degraded by the insulin degrading enzyme (IDE) which is also able to reduce amyloid plaque formation [25]. Thus, chronic normoglycemic hyperinsulinemia, which characterizes the early phases of type 2 diabetes, might increase the production of Aβ creating a competition for the IDE between Aβ peptides and insulin itself. On the other hand, when diabetes is clinically manifest the insulin levels are reduced due to failure of pancreatic islet cells, and the degradation of Aβ peptides becomes more efficient even in comparison with non-diabetic individuals. This two-phase mechanism, which postulates more efficient degradation of β-amyloid peptides in patients with type 2 diabetes, might explain why reduced AD progression rates are observed in these patients.
Other more complex mechanisms may play a role. For example, insulin presents some analogies with the neuronal growth factor, insulin-like growth factor 1 (IGF 1). Insulin and IGF 1 have specific receptors on neurons, and at high concentrations insulin can cross-react with IGF 1 receptor [23]. Thus, a possible role of insulin on neuronal trophy and on resistance to neurodegenerative processes cannot be excluded.
Another explanation of the better prognosis for diabetic patients with AD might be related to antidiabetic treatment. It has been hypothesized that some drugs that enhance the sensitivity of insulin receptors may be effective in AD. One of these drugs (rosiglitazone) is being studied, but the first results are controversial [29]. All but one patient in our study was treated with antidiabetic oral drugs that increase both the sensitivity of the insulin receptor and the production of insulin by the pancreatic islet cells. Thus, it is possible that the higher levels of insulin induced by these treatments might have a role in explaining our observation of a slower progression rate of AD in diabetic patients. As in other studies [19], it was not possible to determine whether the slower cognitive decline in diabetic AD patients was associated with treatment, as all patients underwent therapy.
There are a few limitations of our study that deserve mention. First, our sample was relatively small, which affected statistical power. However, we were able to follow patients closely. Second, our results may not be generalizable to all populations, particularly as all our patients were treated with acetyl-cholinesterase inhibitors. The strengths of our study include the extensive clinical examination and follow-ups, as well as the inclusion of a wide age range of patients, which verified previous findings in older patients [19].
Identifying factors that will predict progression of AD, will help clinicians estimate disease prognosis, which may help to improve patient care as well as long-term planning for caregivers. Furthermore, identifying factors associated with faster disease progression may help better understand AD disease mechanisms, which will have relevant implications for AD comprehension and treatment. Further studies are needed to replicate the observation of better prognosis of AD patients with type 2 diabetes and to determine the mechanisms behind the association.
Acknowledgment
Dr Palmer was supported by a Marie Curie Fellowship from the European Union.
Conflict of interest statement The authors report no conflict of interest.
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Contributor Information
Massimo Musicco, Phone: +39-02-26422629, FAX: +39-02-26422629, Email: m.musicco@hsantalucia.it.
Katie Palmer, Email: k.palmer@hsantalucia.it.
References
- 1.Banks WA, Kastin AJ (1998) Differential permeability of the blood–brain barrier to two pancreatic peptides: insulin and amylin. Peptides 19:883–889. doi:10.1016/S0196-9781(98)00018-7 [DOI] [PubMed]
- 2.Beeri MS, Schmeidler J, Silverman JM et al (2008) Insulin in combination with other diabetes medication is associated with less Alzheimer neuropathology. Neurology 71(10):750–757. doi:10.1212/01.wnl.0000324925.95210.6d [DOI] [PMC free article] [PubMed]
- 3.Benedict C, Hallschmid M, Hatke A et al (2004) Intranasal insulin improves memory in humans. Psychoneuroendocrinology 29:1326–1334. doi:10.1016/j.psyneuen.2004.04.003 [DOI] [PubMed]
- 4.Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P (2006) Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol 5:64–74. doi:10.1016/S1474-4422(05)70284-2 [DOI] [PubMed]
- 5.Borroni B, Archetti S, Ferrari M, Cesana BM, Padovani A (2007) Relationship of vascular risk to the progression of Alzheimer disease. Neurology 68:1083–1084. doi:10.1212/01.wnl.0000260435.20698.77 author reply 1084 [DOI] [PubMed]
- 6.Buccione I, Perri R, Carlesimo GA et al (2007) Cognitive and behavioural predictors of progression rates in Alzheimer’s disease. Eur J Neurol 14:440–446. doi:10.1111/j.1468-1331.2007.01693.x [DOI] [PubMed]
- 7.Byrne L, Bucks RS, Wilcock GK (2000) Mini mental state examination. Lancet 355:314–315. doi:10.1016/S0140-6736(05)72308-4 [DOI] [PubMed]
- 8.Cox DR (1972) Regression models and life tables. J R Stat Soc Ser A 34:187–220
- 9.Craft S, Watson GS (2004) Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet Neurol 3:169–178. doi:10.1016/S1474-4422(04)00681-7 [DOI] [PubMed]
- 10.Crum RM, Anthony JC, Bassett SS, Folstein MF (1993) Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA 269:2386–2391. doi:10.1001/jama.269.18.2386 [DOI] [PubMed]
- 11.Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198. doi:10.1016/0022-3956(75)90026-6 [DOI] [PubMed]
- 12.Gasparini L, Xu H (2003) Potential roles of insulin and IGF-1 in Alzheimer’s disease. Trends Neurosci 26:404–406. doi:10.1016/S0166-2236(03)00163-2 [DOI] [PubMed]
- 13.Gold SM, Dziobek I, Sweat V et al (2007) Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia 50:711–719. doi:10.1007/s00125-007-0602-7 [DOI] [PubMed]
- 14.Kaplan EL, Meier P (1958) Non parametric estimation from incomplete observations. J Am Stat Assoc 53:457–481. doi:10.2307/2281868 [DOI]
- 15.Larson EB, Shadlen MF, Wang L et al (2004) Survival after initial diagnosis of Alzheimer disease. Ann Intern Med 140:501–509 [DOI] [PubMed]
- 16.Lesser GT (2005) Survival in Alzheimer disease. Arch Neurol 62:689. doi:10.1001/archneur.62.4.689-a author reply 689–690 [DOI] [PubMed]
- 17.Manschot SM, Biessels GJ, de Valk H et al (2007) Metabolic and vascular determinants of impaired cognitive performance and abnormalities on brain magnetic resonance imaging in patients with type 2 diabetes. Diabetologia 50:2388–2397. doi:10.1007/s00125-007-0792-z [DOI] [PMC free article] [PubMed]
- 18.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34:939–944 [DOI] [PubMed]
- 19.Mielke MM, Rosenberg PB, Tschanz J et al (2007) Vascular factors predict rate of progression in Alzheimer disease. Neurology 69:1850–1858. doi:10.1212/01.wnl.0000279520.59792.fe [DOI] [PubMed]
- 20.Moosavi M, Naghdi N, Choopani S (2007) Intra CA1 insulin microinjection improves memory consolidation and retrieval. Peptides 28:1029–1034. doi:10.1016/j.peptides.2007.02.010 [DOI] [PubMed]
- 21.Mungas D, Reed BR, Ellis WG, Jagust WJ (2001) The effects of age on rate of progression of Alzheimer disease and dementia with associated cerebrovascular disease. Arch Neurol 58:1243–1247. doi:10.1001/archneur.58.8.1243 [DOI] [PubMed]
- 22.Ngandu T, von Strauss E, Helkala EL et al (2007) Education and dementia: what lies behind the association? Neurology 69:1442–1450. doi:10.1212/01.wnl.0000277456.29440.16 [DOI] [PubMed]
- 23.Pandini G, Frasca F, Mineo R, Sciacca L, Vigneri R, Belfiore A (2002) Insulin/insulin-like growth factor I hybrid receptors have different biological characteristics depending on the insulin receptor isoform involved. J Biol Chem 277:39684–39695. doi:10.1074/jbc.M202766200 [DOI] [PubMed]
- 24.Patterson C, Feightner JW, Garcia A, Hsiung GY, MacKnight C, Sadovnick AD (2008) Diagnosis and treatment of dementia: 1. Risk assessment and primary prevention of Alzheimer disease. CMAJ 178:548–556. doi:10.1503/cmaj.070796 [DOI] [PMC free article] [PubMed]
- 25.Pérez A, Morelli L, Cresto JC, Castaño EM (2000) Degradation of soluble amyloid beta-peptides 1-40, 1-42, and the Dutch variant 1-40Q by insulin degrading enzyme from Alzheimer disease and control brains. Neurochem Res 25:247–255. doi:10.1023/A:1007527721160 [DOI] [PubMed]
- 26.Raschetti R, Maggini M, Vanacore N (2003) Post-marketing studies: the Italian CRONOS project. Int J Geri Psy 18:962. doi:10.1002/gps.992 author reply 963 [DOI] [PubMed]
- 27.Regan C, Katona C, Walker Z, Hooper J, Donovan J, Livingston G (2006) Relationship of vascular risk to the progression of Alzheimer disease. Neurology 67:1357–1362. doi:10.1212/01.wnl.0000240129.46080.53 [DOI] [PubMed]
- 28.Reger MA, Watson GS, Frey WH et al (2006) Effects of intranasal insulin on cognition in memory-impaired older adults: modulation by APOE genotype. Neurobiol Aging 27:451–458. doi:10.1016/j.neurobiolaging.2005.03.016 [DOI] [PubMed]
- 29.Risner ME, Saunders AM, Altman JF et al (2006) Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer’s disease. Pharmacogenomics J 6:246–254 [DOI] [PubMed]
- 30.Ruitenberg A, Kalmijn S, de Ridder MA et al (2001) Prognosis of Alzheimer’s disease: the Rotterdam study. Neuroepidemiology 20:188–195. doi:10.1159/000054786 [DOI] [PubMed]
- 31.Rulifson EJ, Kim SK, Nusse R (2002) Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science 296:1118–1120. doi:10.1126/science.1070058 [DOI] [PubMed]
- 32.Sabayan B, Foroughinia F, Mowla A, Borhanihaghighi A (2008) Role of insulin metabolism disturbances in the development of Alzheimer disease: mini review. Am J Alzheimers Dis Other Demen 23:192–199. doi:10.1177/1533317507312623 [DOI] [PMC free article] [PubMed]
- 33.Scarmeas N, Albert SM, Manly JJ, Stern Y (2006) Education and rates of cognitive decline in incident Alzheimer’s disease. J Neurol Neurosurg Psychiatry 77:308–316. doi:10.1136/jnnp.2005.072306 [DOI] [PMC free article] [PubMed]
- 34.Schulingkamp RJ, Pagano TC, Hung D, Raffa RB (2000) Insulin receptors and insulin action in the brain: review and clinical implications. Neurosci Biobehav Rev 24:855–872. doi:10.1016/S0149-7634(00)00040-3 [DOI] [PubMed]
- 35.Staessen JA, Thijisq L, Fagard R et al (2004) Effects of immediate versus delayed antihypertensive therapy on outcome in the Systolic Hypertension in Europe Trial. J Hypertens 22:847–857. doi:10.1097/00004872-200404000-00029 [DOI] [PubMed]
- 36.Stern Y, Albert S, Tang MX, Tsai WY (1999) Rate of memory decline in AD is related to education and occupation: cognitive reserve? Neurology 53:1942–1947 [DOI] [PubMed]
- 37.UK Prospective Diabetes Study (UKPDS) Group (1998) Intensive blood–glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352:837–853. doi:10.1016/S0140-6736(98)07019-6 [DOI] [PubMed]
- 38.Vanhanen M, Koivisto K, Kuusisto J et al (1998) Cognitive function in an elderly population with persistent impaired glucose tolerance. Diabetes Care 21:398–402. doi:10.2337/diacare.21.3.398 [DOI] [PubMed]
- 39.Vanhanen M, Koivisto K, Moilanen L et al (2006) Association of metabolic syndrome with Alzheimer disease: a population-based study. Neurology 67:843–847. doi:10.1212/01.wnl.0000234037.91185.99 [DOI] [PubMed]
- 40.Wallum BJ, Taborsky GJ, Porte D et al (1987) Cerebrospinal fluid insulin levels increase during intravenous insulin infusions in man. J Clin Endocrinol Metab 64:190–194 [DOI] [PubMed]
- 41.Wu JH, Haan MN, Liang J, Ghosh D, Gonzalez HM, Herman WH (2003) Impact of antidiabetic medications on physical and cognitive functioning of older Mexican Americans with diabetes mellitus: a population-based cohort study. Ann Epidemiol 13:369–376. doi:10.1016/S1047-2797(02)00464-7 [DOI] [PubMed]
- 42.Xie J, Brayne C, Matthews FE et al (2008) Survival times in people with dementia: analysis from population based cohort study with 14 year follow-up. BMJ 336:258–262. doi:10.1136/bmj.39433.616678.25 [DOI] [PMC free article] [PubMed]