Abstract
The objective of this study was to evaluate the feasibility of sentinel lymph node biopsy by using a radiotracer lymphatic mapping technique in patients with squamous cell carcinoma of the oral cavity, and the diagnostic value of this technique. We studied twenty patients with previously untreated squamous cell carcinomas of the oral cavity and N0 necks. After the peritumoral injection of 99mTc filtered tin colloid preop-eratively, lymphoscintigraphy and intraoperative mapping using a gamma detector were performed to localize sentinel nodes. An open biopsy of the sentinel node was followed by complete neck dissection. We identified the sentinel nodes in 19 of 20 patients (95.0%) by lymphoscintigraphy and in all (100%) by intraoperative gamma detector. In all cases, the status of the sentinel node accurately predicted the pathologic status of the neck with the false negative rate being 0%. The negative predictive value for the absence of cervical metastases was 100%. In conclusion, our radio-localization technique of sentinel nodes using 99mTc filtered tin colloid in N0 squamous cell carcinomas of the oral cavity is technically feasible and appears to accurately predict the presence of the occult metastatic disease.
Keywords: Sentinel Lymph Node Biopsy, Lymphatic Metastasis, Mouth Neoplasms, Radionuclide Imaging
INTRODUCTION
Squamous cell carcinoma of the head and neck spreads via the lymphatics to cervical lymph nodes, but controversy remains about the best means to determine the nodal status of these patients. Clinical examination and radiologic imaging techniques are relatively inaccurate for staging the neck, and the nodal status can only be determined accurately following a neck dissection (1-3).
Several authors have shown that if the first draining lymph node has no evidence of micrometastases on histologic examination, the incidence of metastases to the remaining lymphatic basin in cutaneous melanoma and head and neck cancer is less than 5% (4, 5). This has led to sentinel lymph node biopsy (SLNBx) becoming an accepted technique for determining whether metastases are present in the first echelon of draining lymph nodes in patients with head and neck cancer (6). SLNBx has allowed these patients to avoid procedural morbidity while undergoing elective neck dissection for their malignancies.
Recent studies on sentinel lymph node localization in head and neck squamous cell carcinoma have revealed a localization rate of 97% (95% confidence interval, 94-100%), an average micrometastatic rate to sentinel lymph nodes of 31% (95% confidence interval, 23-39%), and a false negative rate of 2.4% (95% confidence interval, 0-5%) (3, 4, 7-10).
The clinical application of this potentially valuable procedure as a sole diagnostic tool to stage the nodal status requires its standardization at each institute, because results reportedly vary according to the scanning techniques, the materials for localization and the method of histological examination (4). Therefore, multi-institutional clinical trials are currently determining the diagnostic accuracy of uniform SLNBx techniques such as 'American College of Surgeons Oncology Group protocol Z0360', and there is a growing consensus that a diagnostic procedure to detect cancer cells in the sentinel lymph node is an effective method for staging lymph node metastasis of head and neck cancers.
The primary objective of this study was to evaluate the feasibility of SLNBx by using a radiotracer lymphatic mapping technique in patients with squamous cell carcinoma of the oral cavity. A secondary objective was to determine whether the histopathologic status of the sentinel node predicts the status of the other regional lymph nodes. This study could serve as the primary protocol of the SLNBx procedure in head and neck cancer for further ongoing clinical trials.
MATERIALS AND METHODS
From 2002 through 2005, we enrolled 20 consecutive patients, 15 men and 5 women, aged from 35 to 68 yr (mean: 53 yr) (Table 1). The eligibility criteria for participation in this prospective study were a diagnosis of previously untreated, biopsy-proven squamous cell carcinoma of the oral cavity and no evidence of cervical lymph node metastasis by clinical and computed tomography evaluation. Primary sites other than the oral cavity were excluded because their relative inaccessibility made precise injections around the primary site difficult. The primary tumor was located in the oral tongue in 19 patients and in the buccal mucosa in 1 patient. The application of the elective neck dissection was directed for a primary tumor depth of greater than 5 mm in stage T1 and any tumor in stage T2 or higher. As for the depth of primary lesion, the authors evaluated the primary lesion of the subjects by two methods: physical examination and radiological evaluation. Careful palpation was routinely performed at each examination and more importantly under general anesthesia before the definite resection. Also we evaluated the lesion by contrast enhanced CT or MRI, which partly provided the information about the depth of primary lesion. When the physical palpation reveals that the lesions of the oral cavity are put on the mucosal layer only, and that the lesions show their movement apart from the submucosal structure such as intrinsic muscles without fixation, the authors regarded the depth of lesion as less than 5 mm. In addition, if the only superficial thickening and superficial enhancement of lesions was noted in the preoperative radiological study (CT or MRI), the depth of lesion was thought to be less than 5 mm. In case of the infiltration to the submucosal tissue, we performed the elective neck dissection for those subjects.
Table 1.
Patient profiles and the results of sentinel lymph node biopsy
SLN, sentinel lymph node; T, tongue; B, buccal mucosa.
All patients underwent selective neck dissection removing levels I - III or I - IV of the cervical lymph node groups after the application of SLNBx prodecure. The difference of the extent of neck dissection (with or without level IV) mainly depended on the results of the preoperative SLNBx procedure. If the sentinel lymph node was found in level IV lymph node groups with the result of SLNBx procedure, the surgeon took out the level IV lymph node group as well as the level I to III (Case number 9, 13, and 19 in Table 1). The SLNBx procedure was explained in detail to all patients, who subsequently provided their informed consent. The Institutional Review Board of Samsung Medical Center approved the protocol for this study.
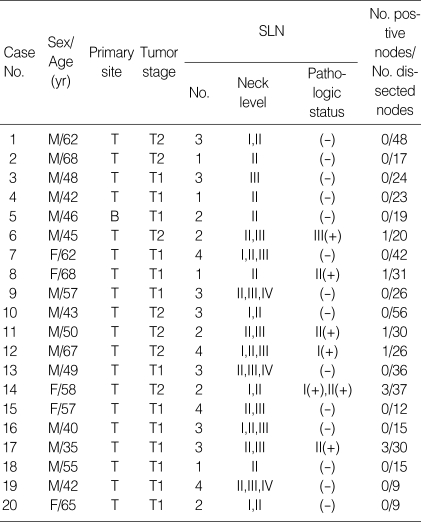
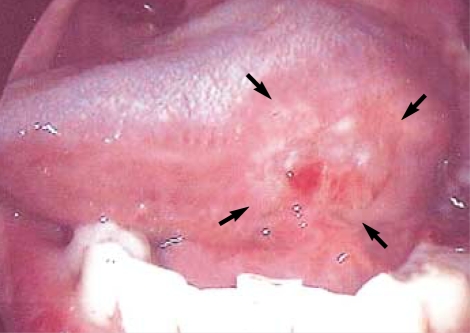
On the day prior to surgery, patients attended the Department of Nuclear Medicine for the peritumoral injection of the radioacitive tracer (Fig. 1). The radioactive tracer used was technetium 99m (99mTc) prepared with tin colloid (Amerscan™ Hepatate II™ agent, Nycomed Amersham Health Inc., London, U.K.) and filtered so that the particles were less than 200 nm. The tracer (5-6 mCi in 0.6 mL) was injected submucosally around the circumference of the primary tumor. For 1 hr after the injection, dynamic lymphoscintigraphy was performed every 5 min in the anterior and lateral views and then until 2 hr prior to surgery at an interval of 3-6 hr, with static images obtained to monitor the residual radioactivity (Fig. 2).
Fig. 1.
The peritumoral injection of the radioacitive tracer. 99mTc filtered tin colloid (5-6 mCi in 0.6 mL) was injected submucosally around the circumference of the primary tumor.
Fig. 2.
Lymphoscintigraphy after the peritumoral injection. For 1 hr after the injection, dynamic lymphoscintigraphy was performed every 5 min in the anterior and lateral views and then until 2 hr prior to surgery at an interval of 3-6 hr, with static images obtained to monitor the residual radioactivity.
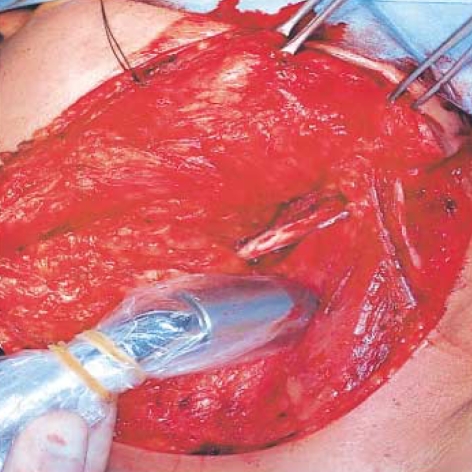
Intraoperatively, all radioactive lymph nodes were identified with a handheld gamma probe (Navigator GPS, Tyco Health Care, Mansfield, MA, U.S.A.) that was used to identify radioactive sentinel nodes (Fig. 3), including those marked preoperatively through the surface by lymphoscintigraphy. To reduce the detection of radiation from the injection site, the primary tumor was removed first with an adequate resection margin according to current accepted treatment standards. After localization on the skin surface, the skin flaps for elective neck dissection were raised and the hand held gamma probe (sterilely wrapped) was used to precisely identify any radioactive nodes (Fig. 4). Lymph nodes with radioactivity counts greater than threefold the background counts were considered the sentinel lymph nodes (4, 9) We also adopted the following criteria for these lymph nodes: 1) the highly radioactive in vivo before incision, 2) the highly radioactive ex vivo, and 3) decreased radioactivity in the lymphatic bed after removal of the highly radioactive node (9). After removal of sentinel lymph nodes, all patients then received elective neck dissection.
Fig. 3.
The handheld gamma probe (Navigator GPS, Tyco Health Care, Mansfield, MA, U.S.A.). The handheld gamma probe was used to identify radioactive sentinel nodes, including those marked preoperatively through the surface by lymphsocintigraphy.
Fig. 4.
The detection of sentinel node using a handheld gamma probe. After localization on the skin surface, the skin flaps for elective neck dissection are raised and the hand held gamma probe (sterilely wrapped) is used to precisely identify any radioactive nodes.
Sentinel nodes were labeled according to their radioactivity and anatomic lymph node level after the radioactivity within the node was confirmed ex vivo (Fig. 5). The sentinel nodes were divided into three equal levels through their longest axis and frozen with Optimal Cutting Temperature compound separately. One section for each block was evaluated using frozen section analysis with hematoxylin-eosin staining.
Fig. 5.
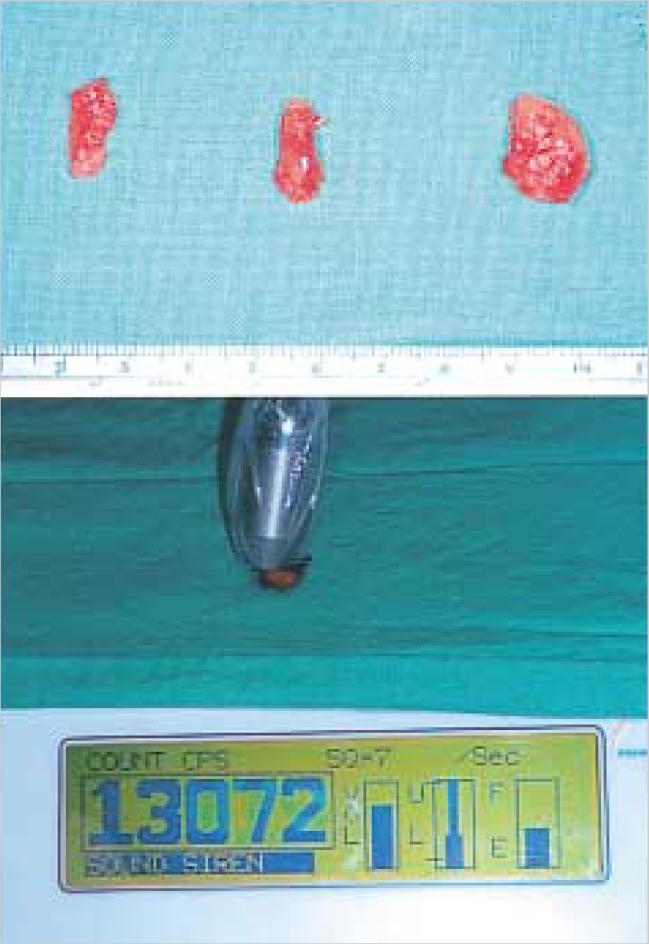
Confirmation of the radioactivity of sentinel nodes ex vivo. Sentinel nodes were labeled according to their radioactivity after the radioactivity within the node was confirmed ex vivo.
After frozen section diagnosis, frozen tissue blocks were melt, fixed in 10% neutral buffered formalin, and embedded in paraffin. These paraffin blocks were serially sectioned at 250 µm, and then six sections from each level within the block were prepared with hematoxylin-eosin stainings and examined for possible metastasis. If the node still appeared free from tumors, immunohistochemical analysis for cytokeratin (AE1/AE3) was performed. Cytokeratin positivity was compared with the adjacent serial section stained with hematoxylin-eosin to confirm that it represented viable tumor cells. The full pathological protocol was used to examine nodes that appeared negative following frozen section hematoxylin-eosin analysis. The incidences of metastases in the sentinel lymph nodes and the remaining lymphadenectomy specimen were compared.
RESULTS
SLNBx with 99mTc tin colloid radiotracer lymphatic mapping in patients with squamous cell carcinoma of the oral cavity identified at least one sentinel lymph node in 19 of 20 cases (95.0%) by lymphoscintigraphy and in all cases (100%) by intraoperative gamma probing.
The number of sentinel nodes in each case ranged from one to four with a mean of 2.55. The size of sentinel nodes ranged from 0.8 to 1.5 cm (0.85±0.33 cm, mean±SD) in the short axis and from 0.5 to 2.0 cm (1.33±0.60 cm, mean±SD) in the long axis.
All sentinel nodes were found in the ipsilateral neck to primary tumors, mostly at cervical lymph node level II (95.0%) followed by level III (55.0%) and level I (35.0%).
Frozen-section histological analyses according to our pathologic protocol accurately revealed the pathologic status of the sentinel lymph node, as confirmed by hematoxylin-eosin staining. Further tumor cells were not detected by cytokeratin AE1/AE3 immunohistochemical staining.
In 6 of 20 cases, the SLNBx procedure revealed the malignant cells in the sentinel lymph nodes (the rate of occult metastasis: 30.0%). Therefore, positive tumor cells in sentinel nodes predicted N(+) accurately.
Moreover, there was no instance in which micrometastatic disease was negative in the sentinel nodes while being positive in the remaining lymph nodes (negative predictive value: 100% and false negative rate: 0%)
DISCUSSION
SLNBx in head and neck cancer is becoming accepted as useful in predicting the cervical nodal status for clinically node negative neck. However, the application of SLNBx in clinical settings requires several problems to be overcome, including proving the technical feasibility of the procedure used at each institute (which varies between institutions) and achieving an acceptable identification rate of the sentinel node and acceptable predictability for cervical nodal status (4).
In the first two cases in our series, we used a combination of blue dye mapping and radiotracer injection. However, we experienced difficulty in determining the precise location of the blue-stained nodes before skin incision when using the blue dye technique, resulting in unnecessary dissection (4, 8, 9). Moreover, the rate of identification and the prediction rate of neck lymph node pathology of sentinel nodes has been reported to be only 50-75%, making it difficult to accurately select all first draining lymph nodes (4, 8) and the passage time of blue dye is relatively too short (about 15 min) to allow the procedure to be completed (4, 8). For these reasons, the blue dye mapping technique was not used after the second cases in our series.
In contrast, radiolocalization of the sentinel lymph node is gaining popularity (11). In recent studies using radiotracers, the diagnostic accuracy and the localization rate reaches almost 100% (9-11). The figures obtained from this study correspond with other reports. At identifying the sentinel nodes in node-negative oral cavity cancer patients, the detection rate ranged from 93.8% to 100% (3, 4, 7, 8, 11) in the literature, which is similar to that of our method. Also the diagnostic accuracy of predicting cervical nodal status by analyzing the sentinel lymph nodes in our study (100%) confirms the high predictability of earlier studies (94-100%) (7, 8, 11).
One problem occurs when the lymphatic bed being surveyed is immediately adjacent to the primary lesion containing injected radionuclide. To avoid this problem, we first excised the primary tumor with an adequate safety margin before identifing sentinel nodes using a hand-held gamma probe, which was easily achieved due to the absence of radioactivity interference from the injected site.
There are several 99mTc labeled radiocolloids reported in the literature, but as the materials licensed for use in Korea are limited, 99mTc filered tin colloid was selected for this study. 99mTc tin colloids were filtered into its relatively regular particle size (≤200 nm), because smaller particle colloids tend to be faster moving, and in case of disease present within the sentinel node, larger particles are less likely to penetrate into the sentinel node, so have a chance not to detect it (8, 11, 12). A literature also reported that smaller sized 99mTc tin colloids were slightly superior to the regular sized 99mTc tin colloids in the identification of sentinel nodes (97.3% vs. 86.5%) (12, 13). We found that 99mTc filtered tin colloid is an alternative radiotracer that produces reliable results.
The assistance of a qualified nuclear medicine physician was essential for the handling of the radioisotope and for interpretation of the lymphoscintigraphic imaging. In this study, we gave some modifications to the injection time and the amount of radiotracer.
On the day prior to surgery, the tracer was injected around the primary tumor, instead of in the morning on the day of surgery. The amount of 99mTc filtered tin colloid radiotracer in this study was 5-6 mCi in contrast to that of other reports (1-2 mCi) (3, 4, 7-9). With its half-life of about 6 hr, 1-1.5 mCi of radiotracer remained in the tissue on the following morning, which is similar to several other reports. Our modifications of a delay of 12-20 hr between the injection of 99mTc filtered tin colloid and the application of SLNBx have a major advantage in giving sufficient time to get more static images, which effectively identified the sentinel lymph node during lymphoscintigraphy and intraoperative gamma probing in all patients.
As discussed by Byers et al., another concern in SLNBx is whether this technique will detect 'skip metastasis' with acceptable identification rate (14). Recently, the concept of 'skip metastasis' is being redefined as skip metastasis may represent non-standard but sequential or multiple drainages of an individual neck in parallel array (9, 15). According to this explanation, the sentinel node will always be the initial site of micrometastasis, and the evaluation of the histological status of the sentinel node may help to predict the incidence of regional micrometastasis in head and neck squamous cell carcinoma.
If a reliable assessment of the sentinel lymph nodes could be made with frozen section biopsy, a decision regarding elective neck dissection would be possibly made at the time of SLNBx and unnecessary surgeries could be avoided with safe oncological results. Frozen section analysis may be a rate-limiting step for SLNBx as the determining factor in concurrent elective neck dissection. We believe that the data regarding frozen sectioning should be added to the ongoing study.
Recent meta-analysis across the world reported that the SLNBx procedure has shown high sensitivity rates for oral squamous cell carcinomas and is reliable and reproducible (16, 17), which we also reconfirmed through this study by the validation of our technique. Now, SLNBx procedure seems to be sufficiently validated, though it requires further refinement of intra-operative frozen section analysis (18, 19). Trial of SLNBx procedure as a sole method for neck management also showed a promising result with a high diagnostic accuracy (83.3%), which could spare unnecessary neck dissection in patients with no evidence of metastasis in the sentinel lymph nodes (19). Through subsequent clinical trials, the authors think that the SLNBx procedure can achieve its position firmly as a useful and effective method for clinically node negative oral cancer.
In conclusion, our techniques of lymphoscintigraphy and SLNBx using 99mTc filtered tin colloid in squamous cell carcinomas of the oral cavity were technically feasible and identified the sentinel node in all cases. The histological evaluation of sentinel nodes according to our protocol provided the exact reflections of cervical nodal status. This study could guide as the primary protocol of SLNBx procedure for clinical trials if the results of further study corroborate the data presented here.
Footnotes
This study was supported by IN-SUNG Foundation for Medical Research, Seoul, Korea.
References
- 1.Don DM, Anzai Y, Lufkin RB, Fu YS, Calcaterra TC. Evaluation of cervical lymph node metastases in squamous cell carcinoma of the head and neck. Laryngoscope. 1995;105:669–674. doi: 10.1288/00005537-199507000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Friedman M, Mafee MF, Pacella BL, Jr, Strorigl TL, Dew LL, Toriumi DM. Rationale for elective neck dissection in 1990. Laryngoscope. 1990;100:54–59. doi: 10.1288/00005537-199001000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Werner JA, Dunne AA, Ramaswamy A, Dalchow C, Behr T, Moll R, Folz BJ, Davis RK. The sentinel node concept in head and neck cancer: solution for the controversies in the N0 neck? Head Neck. 2004;26:603–611. doi: 10.1002/hed.20062. [DOI] [PubMed] [Google Scholar]
- 4.Alex JC. The application of sentinel node radiolocalization to solid tumors of the head and neck: a 10-year experience. Laryngoscope. 2004;114:2–19. doi: 10.1097/00005537-200401000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Morton DL, Thompson JF, Essner R, Elashoff R, Stern SL, Nieweg OE, Roses DF, Karakousis CP, Mozzillo N, Reintgen D, Wang HJ, Glass EC, Cochran AJ. Validation of the accuracy of intraoperative lymphatic mapping and sentinel lymphadenectomy for early-stage melanoma: a multicenter trial. Multicenter Selective Lymphadenectomy Trial Group. Ann Surg. 1999;230:453–463. doi: 10.1097/00000658-199910000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiseman SM, Loree TR, Hicks WL, Jr, Rigual NR. Sentinel lymph node biopsy in SCC of the head and neck: a major advance in staging the N0 neck. Ear Nose Throat J. 2002;81:156–160. 163. [PubMed] [Google Scholar]
- 7.Taylor RJ, Wahl RL, Sharma PK, Bradford CR, Terrell JE, Teknos TN, Heard EM, Wolf GT, Chepeha DB. Sentinel node localization in oral cavity and oropharynx squamous cell cancer. Arch Otolaryngol Head Neck Surg. 2001;127:970–974. doi: 10.1001/archotol.127.8.970. [DOI] [PubMed] [Google Scholar]
- 8.Shoaib T, Soutar DS, Prosser JE, Dunaway DJ, Gray HW, McCurrach GM, Bessent RG, Robertson AG, Oliver R, MacDonald DG. A suggested method for sentinel node biopsy in squamous cell carcinoma of the head and neck. Head Neck. 1999;21:728–733. doi: 10.1002/(sici)1097-0347(199912)21:8<728::aid-hed8>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 9.Alex JC, Sasaki CT, Krag DN, Wenig B, Pyle PB. Sentinel lymph node radiolocalization in head and neck squamous cell carcinoma. Laryngoscope. 2000;110:198–203. doi: 10.1097/00005537-200002010-00003. [DOI] [PubMed] [Google Scholar]
- 10.Mozzillo N, Chiesa F, Botti G, Caraco C, Lastoria S, Giugliano G, Mazzarol G, Paganelli G, Ionna F. Sentinel node biopsy in head and neck cancer. Ann Surg Oncol. 2001;8:103S–105S. [PubMed] [Google Scholar]
- 11.Ross GL, Shoaib T, Soutar DS, MacDonald DG, Camilleri IG, Bessent RG, Gray HW. The first international conference on sentinel node biopsy in mucosal head and neck cancer and adoption of a multicenter trial protocol. Ann Surg Oncol. 2002;9:406–410. doi: 10.1007/BF02573877. [DOI] [PubMed] [Google Scholar]
- 12.Higashi H, Natsugoe S, Uenosono Y, Ehi K, Arigami T, Nakabeppu Y, Nakajo M, Aikou T. Particle size of tin and phytate colloid in sentinel node identification. J Surg Res. 2004;121:1–4. doi: 10.1016/j.jss.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Jinno H, Ikeda T, Matsui A, Kitagawa Y, Kitajima M, Fujii H, Nakamura K, Kubo A. Sentinel lymph node biopsy in breast cancer using technetium-99m tin colloids of different sizes. Biomed Pharmacother. 2002;56(Suppl 1):213s–216s. doi: 10.1016/s0753-3322(02)00222-6. [DOI] [PubMed] [Google Scholar]
- 14.Byers RM, Weber RS, Andrews T, McGill D, Kare R, Wolf P. Frequency and therapeutic implications of "skip metastases" in the neck from squamous carcinoma of the oral tongue. Head Neck. 1997;19:14–19. doi: 10.1002/(sici)1097-0347(199701)19:1<14::aid-hed3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 15.Norman J, Cruse CW, Espinosa C, Cox C, Berman C, Clark R, Saba H, Wells K, Reintgen D. Redefinition of cutaneous lymphatic drainage with the use of lymphoscintigraphy for malignant melanoma. Am J Surg. 1991;162:432–437. doi: 10.1016/0002-9610(91)90255-c. [DOI] [PubMed] [Google Scholar]
- 16.Stoeckli SJ, Pfaltz M, Ross GL, Steinert HC, MacDonald DG, Wittekind C, Soutar DS. The second international conference on sentinel node biopsy in mucosal head and neck cancer. Ann Surg Oncol. 2005;12:919–924. doi: 10.1245/ASO.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 17.Paleri V, Rees G, Arullendran P, Shoaib T, Krishman S. Sentinel node biopsy in squamous cell cancer of the oral cavity and oral pharynx: a diagnostic meta-analysis. Head Neck. 2005;27:739–747. doi: 10.1002/hed.20228. [DOI] [PubMed] [Google Scholar]
- 18.Gallegos-Hernandez JF, Hernandez-Hernandez DM, Flores-Diaz R, Sierra-Santiesteban I, Pichardo-Romero P, Arias-Ceballos H, Minauro-Munoz G, Alvarado-Cabrero I. The number of sentinel nodes identified as prognostic factor in oral epidermoid cancer. Oral Oncol. 2005;41:947–952. doi: 10.1016/j.oraloncology.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Terada A, Hasegawa Y, Goto M, Sato E, Hyodo I, Ogawa T, Nakashima T, Yatabe Y. Sentinel lymph node radiolocalization in clinically negative neck oral cancer. Head Neck. 2006;28:114–120. doi: 10.1002/hed.20305. [DOI] [PubMed] [Google Scholar]