Abstract
Ras-related, estrogen-regulated, and growth-inhibitory gene (RERG) is a novel gene that was first reported in breast cancer. However, the functions of RERG are largely unknown in other tumor types. In this study, RERG expression was analyzed in hepatocellular carcinomas of human patients using reverse transcriptase PCR analysis. In addition, the possible regulation of RERG expression by histone deacetyltransferases (HDACs) was studied in several cell lines. Interestingly, the expression of RERG gene was increased in hepatocellular carcinoma (HCC) of male patients (57.9%) but decreased in HCC of females (87.5%) comparison with paired peri-tumoral tissues. Moreover, RERG gene expression was increased in murine hepatoma Hepa1-6 cells, human breast tumor MDA-MB-231 cells, and mouse normal fibroblast NIH3T3 cells after treated by HDAC inhibitor, trichostatin A. Our results suggest that RERG may function in a gender-dependent manner in hepatic tumorigenesis and that the expression of this gene may be regulated by an HDAC-related signaling pathway.
Keywords: Ras-Related Estrogen-Regulated and Growth-Inhibitory Gene (RERG); Carcinoma, Hepatocellular; Sex; Histone Acetyltransferases
INTRODUCTION
Ras-related, estrogen-regulated, and growth-inhibitory gene (RERG) is a novel gene that was first reported in breast cancer (1). Like Ras, RERG protein exhibits intrinsic GDP/GTP binding and GTP hydrolysis activity. However, unlike Ras proteins, RERG lacks a known recognition signal for COOH-terminal prenylation and is localized primarily in the cytoplasm. RERG mRNA expression is induced rapidly in MCF-7 breast carcinoma cells stimulated with beta-estradiol, whereas it is repressed by tamoxifen treatment. The expression of RERG protein in these cells resulted in significant inhibition of both anchorage-dependent and anchorage-independent growth in vitro and inhibited tumor formation in nude mice. Moreover, RERG expression was decreased or lost in a significant percentage of primary human breast tumors with poor clinical prognosis. Importantly, high levels of RERG expression correlate with the expression of a set of genes that define an estrogen receptor-positive breast tumor subtype and are associated with a slow rate of tumor cell proliferation and a favorable prognosis for these cancer patients. The study suggested that the loss of RERG expression contributes to breast tumorigenesis. However, there have been no further reports about this gene, and its functions in tumors of other cell types are largely unknown.
Hepatocellular carcinoma (HCC) is the one of the world's most common malignancies (2). A general characteristic of HCC across different geographical areas is the striking male prevalence (3, 4). In addition, animal studies have indicated that male rodents are more susceptible to hepatocarcinogenesis that either occurs spontaneously or is chemically induced, as well as being more susceptible to chronic viral infection in experimental models (5, 6). Therefore, there appears to be a gender-dependent regulation of common molecular mechanisms that leads to a predominance of HCC in human and rodent males. The mechanisms responsible for this gender difference are not known. In addition, the genetic basis of hepatocarcinogenesis is still poorly understood (7).
Histone deacetyltransferases (HDACs) participate in a variety of cellular processes, including transcription, DNA replication, and cell cycle progression. It has been suggested that these enzymes are associated with proliferative diseases such as cancer (8). Consequently, HDACs have recently attracted interest as novel targets for anti-tumor therapies. HDAC inhibitors (HDACIs) have been shown to induce growth arrest, differentiation, and apoptosis of cancer cells in vitro and in vivo (9). In addition, few or no side effects were observed in animal experiments and clinical trials of HDACIs within the therapeutic range studied, which enhances the potential use of these agents for therapeutic applications (10). However, the changes in the genetic profile that are induced by HDACs remain to be investigated.
In this study, the expression of the RERG gene in hepatic tumorigenesis and the regulation of gene expression by HDACs were studied. Gene expression was analyzed by RT-PCR of human and mouse liver tumors as well as of several tumor cell lines treated with the HDACI, trichostatin A (TSA).
MATERIALS AND METHODS
Patients
Primary HCC tumors and adjacent peri-tumoral tissues were obtained from 27 patients (8 females and 19 males) at the Catholic University of Korea (Department of Internal Medicine, Seoul, Korea), Korea Institute of Radiological and Medical Sciences (Laboratory of Molecular Oncology, Seoul, Korea), School of Medicine Ajou University (Department of Surgery, Suwon, Korea), College of Medicine, Chungnam National University (Department of Pathology, Daejeon, Korea), and College of Medicine, Yeungnam University (Department of Biochemistry & Molecular Biology, Daegu, Korea). The tissue samples were snap-frozen and stored in liquid nitrogen until RNA extraction. In all, 54 samples were analyzed.
Mouse hepatic tumor samples
Transgenic animals expressing the H-ras12V oncogene, which specifically induced the formation and growth of hepatic tumors, have been previously described (11). In the present study, hepatic tumoral tissues and adjacent peri-tumoral tissues from male (9 months of age) and female (14 months of age) transgenic mice were analyzed for RERG gene expression. For control experiments, wild-type male and female littermates were used. Tissue samples were snap-frozen and stored in liquid nitrogen until RNA extraction.
Cell culture and treatment
The role of HDACs in regulating RERG gene expression was investigated in murine hepatoma Hepa1-6 cells, human breast tumor MDA-MB-231 cells, and mouse normal fibroblast NIH3T3 cells. All cell lines were obtained from the American Type Cell Culture (ATCC, Rockville, MD, U.S.A.) and were maintained in Dulbecco's modified Eagle's medium (DMEM) (Gibco, Carlsbad, CA, U.S.A.) containing 10% fetal bovine serum (FBS). Almost all HDACs have approximately the same sensitivity to different HDACIs, and TSA (Sigma, St. Louis, MO, U.S.A.) is one of the most efficient HDACIs identified thus far (10). For this reason, TSA (0, 0.5 µM, or 1 µM) (Sigma, St. Louis, MO, U.S.A.) was used in the present study to inhibit HDAC activity. Before treatment, the cells were re-plated and cultured for 24 hr. Subsequently, the mediumwas replaced with fresh medium containing the specified concentration of TSA. After treatment for 24 hr, the medium was washed out, and total RNA was immediately extracted.
Mice hepatectomy
Hepatectomy was performed according to previously described methods (12), using the C57BL/6J inbred mouse as the experimental animal model. After hepatectomy, regenerating liver tissues were sampled at 0-4, and 5 days. The hepatectomy experiments were performed independently for 3 times and 6 mice were hepatectomized for the 6 sample time points at each time. The samples were snap-frozen and stored in liquid nitrogen until RNA extraction.
RT-PCR analysis
Gene expression was analyzed by RT-PCR. Total RNA was isolated from tissues or TSA-treated cells using the TRIzol reagent (Invitrogen Life Technologies Inc., Carlsbad, CA, U.S.A.). RT-PCR was carried out using a reverse transcription system (Promega Corp., Madison, WI, U.S.A.) according to the manufacturer's instructions. The primers used to detect RERG expression were: the sense primer, 5'-ATGGCTAAAAGTGCGGAGGT-3', and the anti-sense primer, 5'-CTAACTACTGATTTT GGTGA-3', for the human samples and the sense primer, 5'-ATGGCTAAGAGCGCAGAGGTCAAG-3', and the anti-sense primer, 5'-CTAACTACTGATTTTGGTGAGCATC-3', for the mouse samples. The primers used for detecting variant ER-α are shown in Table 2. As a loading control, GAPDH expression was measured in a separate RT-PCR experiment. The production of a single band of the expected size on RT-PCR was recognized as amplification of the target genes.
Table 2.
The primers for variant type ER-α determination
Wt, wild type; Vt, variant type; ER, estrogen receptor.
Statistical analysis
The differences between the experimental groups were tested for statistical significance using the chi-squared test. p-values <0.05 were considered to be significant.
RESULTS
Gender-dependent expression of the RERG gene in human HCC
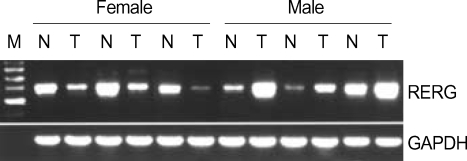
To determine whether the pattern of RERG expression is the same in HCC as in the case of breast cancer (1), 27 paired samples of tumoral and peritumoral tissue were collected from human HCC patients, and RERG gene expression was examined by RT-PCR. In seven of eight (87.5%) female patients, gene expression was decreased in hepatic tumor samples compared with the expression in peri-tumoral tissues (Table 1, Fig. 1). This result was consistent with the pattern of RERG expression reported in breast cancer. However, the level of RERG expression was increased compared with the level in peri-tumoral tissues in 11 of 19 (57.9%) hepatic tumor tissues from males. In 7 of 19 (36.8%) patients, there was no difference in the expression level of RERG between tumoral and peri-tumoral tissues (Table 1). This result was in contrast to the expression pattern of RERG reported in breast cancer. The RERG expression patterns in hepatic tumors of males and females were significantly different (Table 1, Fig. 1). These results indicate that RERG has different, gender-dependent functions in hepatic tumorigenesis.
Table 1.
The expression level of RERG gene in human HCC patients by RT-PCR
N, peri-tumoral tissue; T, tumoral tissue; N/T+, the expression level of RERG was higher in tumoral tissue thue than its peri-tumoral tissue; N/T, the expression level of RERG was same in tumoral tissue and peri-tumoral tissue; N+/T, the expression level of RERG was higher in peri-tumoral tissue than its tumoral tissue. *Indicated significant difference compared within the same column.
Fig. 1.
RERG expression in human hepatocellular carcinoma. RERG expression was analyzed by RT-PCR. GAPDH was used as a quantitative control.
M, size marker; N, adjacent peri-tumoral tissues; T, tumoral tissues.
Expression of the estrogen receptor variant ER-α in HCC tissues of human patients
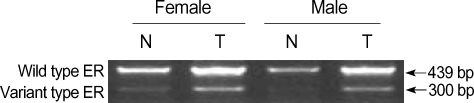
The estrogen receptor (ER) variant ER-α has been suggested to play important roles in breast cancer (13) and hepatocarcinogenesis (14). As RERG is an estrogen-regulated gene involved in breast cancer (1), the expression of the mutant ER-α may influence the regulation of RERG expression in HCC. This hypothesis was tested by measuring ER-α expression in tumors of HCC patients. The results show that, while ER-α was commonly expressed in the tumor and in peri-tumoral tissues of HCC patients, the expression levels were usually higher in the tumor tissues (Fig. 2). In addition, no significant difference was found in ER-α expression between females and males (Table 3).
Fig. 2.
Expression of the estrogen receptor variant ER-α in human HCC. ER-α expression was analyzed by RT-RCR.
T, tumoral tissues; N, adjacent peri-tumoral tissues.
Table 3.
Comparison of the expression of ER-α variant type by sex
hepatocellular carcinoma.
RERG expression is increased in hepatic tumors of male but not female H-ras12V transgenic mice
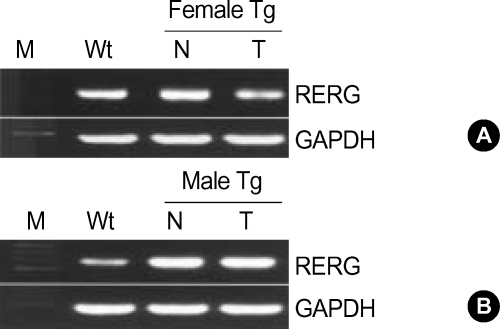
To find out whether the pattern of RERG expression in murine HCC resembles that in human HCC, the expression of the gene was measured in hepatic tumors of H-ras12V transgenic mice. The H-ras12V transgenic mice express the H-ras12V oncogene and develop tumors in liver tissues, thereby providing an animal model of HCC. Furthermore, hepatic tumors in the transgenic mice occur predominately in males (11). The results show that RERG expression in the peritumoral tissues of transgenic females did not differ significantly from that of control littermates, while the levels in tumor tissues were slightly lower (Fig. 3A). In males, two of three transgenic mice showed increased expression of the RERG gene in peri-tumoral liver and tumor tissues, while the levels in the third mouse did not differ from those in normal littermates (Fig. 3B). These results indicate that RERG expression in murine hepatic tumorigenesis is sex-dependent in our H-ras12V transgenic mice.
Fig. 3.
RERG expression in liver tissues of and H-ras12V transgenic mice. RERG expression was analyzed by RT-PCR. Liver or tumor tissues from 9-month-old male (A) and 14-month-old female (B) H-ras12V transgenic mice were sampled.
RERG expression levels do not change during the normal proliferation of hepatocytes
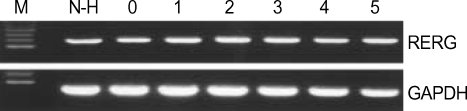
To examine the importance of RERG in normal hepatocyte proliferation, the gene expression was measured in liver tissues of hepatectomized mice. After hepatectomy, the remaining hepatocytes undergo rapid proliferation in order to restore the liver to its original size. Thus, proliferating liver tissue is an excellent system in which to study the proliferation process and the expression of related genes. RERG expression was measured in regenerating liver tissues on days 0-4 and 5 after hepatectomy. However, no differences in expression level were detected (Fig. 4) after hepatectomy. This result indicated that RERG is not primarily involved in the proliferation of normal hepatocytes.
Fig. 4.
RERG expression in liver tissues of hepatectomized mice. RT-PCR analysis of RERG expression in liver tissues on days 0-4, and 5 after hepatectomy. Three independent hepatectomy experiments were performed. GAPDH was used as a quantitative control. M, size marker; Wt, normal control littermate; N, peri-tumoral liver tissues; T, liver tumor tissues; Tg, H-ras12V transgenic mice; N-H, non-hepatectomized mice.
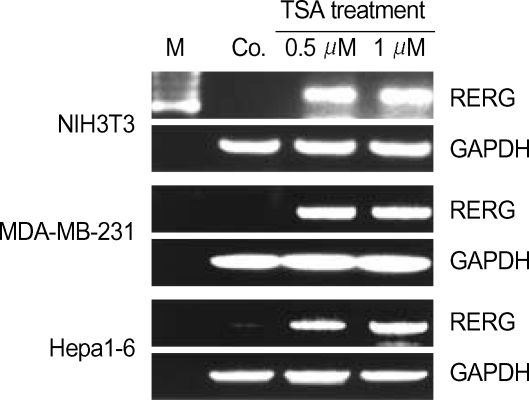
RERG gene expression is regulated by a HDAC-related signaling pathway
HDACs are important in proliferation diseases, owing largely to the functions of these enzymes in regulating gene expression (8). It was therefore of interest to determine whether the RERG gene is under the regulation of HDACs. Several cell lines were treated with the HDAC inhibitor TSA, and RERG expression was subsequently analyzed by RT-PCR. The results show that RERG expression was regulated by HDACs in NIH3T3, MDA-MB-231, and Hepa1-6 cells (Fig. 5). We therefore propose that RERG plays an important role in the inhibition of cell proliferation in several tumor cell types, as has been reported in vitro and in vivo in breast cancer cells.
Fig. 5.
RERG expression in trichostatin A (TSA)-treated cells. Expression of the gene was analyzed by RT-PCR. NIH3T3, MDA-MD0-231, and Hepa1-6 cells were treated with 0.5 µM or 1 µm of the histone deacetyltransferases (HDAC) inhibitor TSA. GAPDH was used as a quantitative control. M, size marker.
Previous reports have also shown that TSA treatment significantly inhibits tumor cell proliferation (15, 16). We obtained similar results in the tumor cell lines MDA-MB-231 and Hepa1-6, whereas no effect was observed in the normal cell line NIH3T3 (data not shown). Thus, it is likely that the inhibition of cell proliferation in response to TSA depends on several factors.
DISCUSSION
RERG is novel gene that was first reported in breast cancer (1). Although RERG is thought to inhibit cell proliferation, its detailed biological functions are largely unknown. In this study, the gender-dependent expression of RERG in HCC and its regulation by HDACs were studied, yielding novel insights into the function of this gene.
The expression pattern of the RERG gene in female HCC patients is consistent with that previously described in breast cancer (1). Like the breast, the liver is a hormone-sensitive organ, and sex hormones are known to affect many functions of the mammalian liver (17, 18). For example, both male and female livers contain androgen receptors (19-21) as well as high-affinity, low-capacity estrogen receptors (22, 23). Furthermore, variant type ER-α was expressed in all female HCC tissues. This variant has been suggested to play an important role in breast cancer and hepatocarcinogenesis (12, 13). As estrogen-dependent regulation of the RERG gene has been suggested (1), it seems likely that the presence of the mutant ER-α in these two tumor types is related to the decrease in RERG gene expression. Conversely, tumor cells must be able to overcome an important barrier, given that RERG has been suggested to play a role in inhibiting the proliferation of breast cancer cells. Taken together, the results suggest that the RERG has the same growth-inhibiting function in female hepatocarcinogenesis as in breast cancer.
By contrast, in male HCC patients, RERG expression in tumor tissues was significantly increased, suggesting that the gene does not inhibit but rather promotes hepatocarcinogenesis in males. Studies of the variant ER-α showed that there was no difference in the expression of the mutant receptor between males and females. We therefore propose that RERG has different, gender-dependent functions; however, the mechanisms explaining this difference remain to be elucidated.
The remaining hepatocytes of hepatectomized mice proliferate rapidly within 1 week in order to restore the liver its original size. Thus, it is an excellent animal model to study normal proliferation processes in vivo. Experiments measuring RERG expression in hepatectomized mice were aimed at determining the function of RERG in normal cellular proliferation, based on the reported ability of this gene to inhibit tumor cell proliferation. It was surprising that RERG expression remained constant during liver regeneration in hepatectomized mice. This observation suggests that the RERG may be not involved in regulating normal cell proliferation. However, the protein level of RERG remained to be examined to find out the possible regulation in post-transcription step.
In vitro experiments showed that RERG expression was significantly increased in normal NIH3T3 cells treated with TSA, an inhibitor of HDACs; however, the growth of these cells was not inhibited. By contrast, in tumor cells treated with TSA, growth was inhibited and RERG expression was significantly increased. These results suggest that RERG mainly functions in tumor cells, perhaps as a monitor that responds to the abnormal status of the cells. This feature of RERG may make it a target for tumor therapy in the future. The results of the in vitro experiments also suggest that RERG gene expression is regulated by a HDAC-related signaling pathway that is distinct from the ER-regulated pathway. These results provide new insight into the function of RERG. The regulation of the gene by multiple pathways adds further support for an important role of RERG in cells.
In conclusion, while the detailed mechanisms remain to be elucidated, our study offers new insights into the function of RERG. Our results are the first to show that RERG is regulated by a HDAC-related signaling pathway. In addition, in hepatic tumorigenesis, the function of RERG may be gender dependent. These unique features of RERG in tumorigenesis may make it a new target for tumor therapy.
Footnotes
This work was supported by the 21st century Frontier Program in the Functional Human Genome Project of Korea (Grant HGC0300324) and the Ministry of Health and Welfare of Korea (Grant 02-PJ2-PG1-CH12-0002).
References
- 1.Finlin BS, Gau CL, Murphy GA, Shao H, Kimel T, Seitz RS, Chiu YF, Botstein D, Brown PO, Der CJ, Tamanoi F, Andres DA, Perou CM. RERG is a novel ras-related, estrogen-regulated and growth-inhibitory gene in breast cancer. J Biol Chem. 2001;276:42259–42267. doi: 10.1074/jbc.M105888200. [DOI] [PubMed] [Google Scholar]
- 2.Joo M, Chi JG, Lee H. Expressions of HSP70 and HSP27 in hepatocellular carcinoma. J Korean Med Sci. 2005;20:829–834. doi: 10.3346/jkms.2005.20.5.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim YS, Um SH, Ryu HS, Lee JB, Lee JW, Park DK, Kim YS, Jin YT, Chun HJ, Lee HS, Lee SW, Choi JH, Kim CD, Hyun JH. The prognosis of liver cirrhosis in recent years in Korea. J Korean Med Sci. 2003;18:833–841. doi: 10.3346/jkms.2003.18.6.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanaka K, Sakai H, Hashizume M, Hirohata T. Serum testosterone: estradiol ratio and the development of hepatocellular carcinoma among male cirrhotic patients. Cancer Res. 2000;60:5106–5110. [PubMed] [Google Scholar]
- 5.Firminger HI, Reuber MD. Influence of adrenocortical, androgenic, and anabolic hormones on the development of carcinoma and cirrhosis of the liver in A x C rats fed N-2-fluorenyldicetamide. J Natl Cancer Inst. 1961;27:559–595. [PubMed] [Google Scholar]
- 6.Kemp CJ, Leary CN, Drinkwater NR. Promotion of murine hepatocarcinogenesis by testosterone is androgen receptor-dependent but not cell autonomous. Proc Natl Acad Sci USA. 1989;86:7505–7509. doi: 10.1073/pnas.86.19.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okuda K. Hepatocellular carcinoma. J Hepatol. 2000;32:225–237. doi: 10.1016/s0168-8278(00)80428-6. [DOI] [PubMed] [Google Scholar]
- 8.Gray SG, Teh BT. Histone acetylation/deacetylation and cancer: an "open" and "shut" case? Curr Mol Med. 2001;1:401–429. doi: 10.2174/1566524013363537. [DOI] [PubMed] [Google Scholar]
- 9.Gui CY, Ngo L, Xu WS, Richon VM, Marks PA. Histone deacetylase (HDAC) inhibitor activation of p21WAF1 involves changes in promoter-associated proteins, including HDAC1. Proc Natl Acad Sci USA. 2004;101:1241–1246. doi: 10.1073/pnas.0307708100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J. 2003;370(Pt 3):737–749. doi: 10.1042/BJ20021321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greene AK, Puder M. Partial hepatectomy in the mouse: technique and perioperative management. J Invest Surg. 2003;16:99–102. [PubMed] [Google Scholar]
- 12.Villa E, Camellini L, Dugani A, Zucchi F, Grottola A, Merighi A, Buttafoco P, Losi L, Manenti F. Variant estrogen receptor messenger RNA species detected in human primary hepatocellular carcinoma. Cancer Res. 1995;55:498–500. [PubMed] [Google Scholar]
- 13.Villa E, Grottola A, Colantoni A, De Maria N, Buttafoco P, Ferretti I, Manenti F. Hepatocellular carcinoma: role of estrogen receptors in the liver. Ann N Y Acad Sci. 2002;963:37–45. [PubMed] [Google Scholar]
- 14.Wang AG, Moon HB, Lee MR, Hwang CY, Kwon KS, Yu SL, Kim YS, Kim M, Kim JM, Kim SK, Lee TH, Moon EY, Lee DS, Yu DY. Gender-dependent hepatic alterations in H-ras12V transgenic mice. J Hepatol. 2005;43:836–844. doi: 10.1016/j.jhep.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 15.Alao JP, Lam EW, Ali S, Buluwela L, Bordogna W, Lockey P, Varshochi R, Stavropoulou AV, Coombes RC, Vigushin DM. Histone deacetylase inhibitor trichostatin A represses estrogen receptor alpha-dependent transcription and promotes proteasomal degradation of cyclin D1 in human breast carcinoma cell lines. Clin Cancer Res. 2004;10:8094–8104. doi: 10.1158/1078-0432.CCR-04-1023. [DOI] [PubMed] [Google Scholar]
- 16.Herold C, Ganslmayer M, Ocker M, Hermann M, Geerts A, Hahn EG, Schuppan D. The histone-deacetylase inhibitor Trichostatin A blocks proliferation and triggers apoptotic programs in hepatoma cells. J Hepatol. 2002;36:233–240. doi: 10.1016/s0168-8278(01)00257-4. [DOI] [PubMed] [Google Scholar]
- 17.Johnson PJ. Sex hormones and the liver. Clin Sci (Lond) 1984;66:369–376. doi: 10.1042/cs0660369. [DOI] [PubMed] [Google Scholar]
- 18.Eagon PK, Porter LE, Francavilla A, DiLeo A, Van Thiel DH. Estrogen and androgen receptors in liver: their role in liver disease and regeneration. Semin Liver Dis. 1985;5:59–69. doi: 10.1055/s-2008-1041758. [DOI] [PubMed] [Google Scholar]
- 19.Roy AK, Milin BS, McMinn DM. Androgen receptor in rat liver: hormonal and developmental regulation of the cytoplasmic receptor and its correlation with the androgen-dependent synthesis of alpha 2u-globulin. Biochim Biophys Acta. 1974;354:213–232. doi: 10.1016/0304-4165(74)90008-7. [DOI] [PubMed] [Google Scholar]
- 20.Sato N, Ota M, Obara K. Presence of binding component(s) for testosterone in rat liver cytosol. Endocrinol Jpn. 1980;27:315–319. doi: 10.1507/endocrj1954.27.315. [DOI] [PubMed] [Google Scholar]
- 21.Nagasue N, Ito A, Yukaya H, Ogawa Y. Androgen receptors in hepatocellular carcinoma and surrounding parenchyma. Gastroenterology. 1985;89:643–647. doi: 10.1016/0016-5085(85)90463-9. [DOI] [PubMed] [Google Scholar]
- 22.Porter LE, Elm MS, Van Thiel DH, Dugas MC, Eagon PK. Characterization and quantitation of human hepatic estrogen receptor. Gastroenterology. 1983;84:704–712. [PubMed] [Google Scholar]
- 23.Rossini GP, Baldini GM, Villa E, Manenti F. Characterization of estrogen receptor from human liver. Gastroenterology. 1989;96:1102–1109. doi: 10.1016/0016-5085(89)91629-6. [DOI] [PubMed] [Google Scholar]