Abstract
This is a retrospective study of 500 patients with spinal cord injury who underwent abdominal ultrasonography as a routine screening test from 2000 to 2003. We analyzed the results according to the different abdominal organ systems. Among the 500 cases, 226 (45.2%) showed abnormal findings. 98 cases of abnormal findings in the liver included 75 of fatty liver and 13 of mass. The 88 cases of abnormal findings in the bladder included 56 of bladder wall thickening, 14 of cystitis and 10 of urinary stone. The 35 cases of abnormal findings in the kidney included 19 of renal cyst and 6 of pelvic dilatation. The 35 cases with gallbladder abnormalities included 19 with gallstones and 11 with biliary sludge. Excluding the cases with bladder wall thickening, there were still 170 cases with abnormal ultrasonographic findings. Abdominal sonography seems to be a useful tool in detecting hidden intraabdominal pathologies in patients with spinal cord injury.
Keywords: Ultrasonography, Spinal Cord Injuries, Gallstones, Cholelithiasis, Bladder
INTRODUCTION
Spinal cord injury (SCI) is a lifelong tragedy that causes dysfunction of the nervous system and many internal organs. SCI patients experience loss of motor and sensory function below the level and dysfunction of bladder and bowel in most cases. Common complications include urinary tract infections, urinary stone, hydronephrosis (1, 2), fecal impaction, gastric ulcer (3) and pressure ulcer. Other pathological conditions known to occur more frequently in SCI patients include gallstone (4), abdominal aortic aneurysms (5), pancreatitis and bladder carcinoma (6). However, it is very difficult to detect these abnormalities in the early stage due to sensory loss below the level of injury.
Abdominal ultrasonography is a useful non-invasive tool for the diagnosis of intraabdominal pathology. There have been only a few studies on the cost-effectiveness of abdominal ultrasonography (1, 2) and sonographic findings of each intraabdominal organ such as the kidney (7) and gallbladder (GB) (4, 8) in SCI patients.
We analyzed the abnormal findings of each organ system and tried to identify if any factors are associated with these abnormal findings.
MATERIALS AND METHODS
Methods
This study reviewed the charts of 500 patients with SCI, who were admitted to Yonsei Rehabilitation Hospital and underwent abdominal ultrasound testing between 2000 and 2003. A trained radiologist performed the abdominal ultrasonography and evaluated the liver, kidney, bladder and GB and other intra-abdominal abnormalities. Thereafter multivariate regression analysis was performed to evaluate the effect of demographic factors such as gender, age, post-injury duration, severity of injury, and level of injury divided by T10 level on the abnormal sonographic findings. The T10 level was chosen to categorize the injury level because SCI patients injured below T10 level have an intact sympathetic nervous supply, whereas those injured above T10 have an impaired one.
Subjects
Among the 500 patients, 356 male (71.2%) and 144 female (28.9%), the mean post-injury duration at admission was 11.14 months (1-264 months) and the mean age was 36.87 yr old (4-87 yr). The level of injury ranged from C2 to L5, with 280 cases of tetraplegia and 220 cases of paraplegia. American Spinal Injury Association (ASIA) injury classifications were 47.5% (237) ASIA A, 15.2% (76) ASIA B, 14.0% (70) ASIA C, and 23.3% (117) ASIA D.
Statistical analyses
Statistical analysis was performed using SPSS version 10.0. Multiple logistic regression analysis was used to evaluate the effects of the demographic factors.
RESULTS
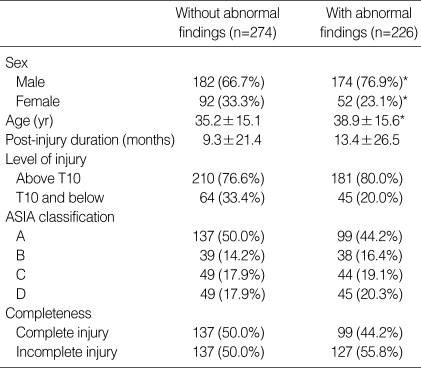
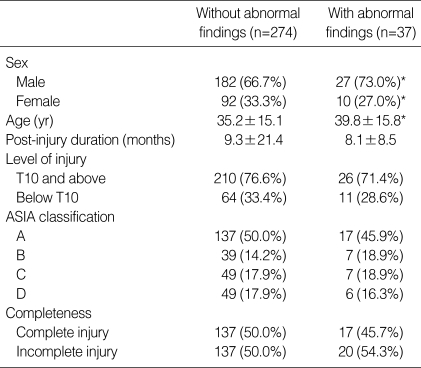
A high incidence of abnormalities was noted throughout the study. Table 1 shows the characteristics of the group with normal findings and of the group (226 cases: 45.2%) with abnormal sonographic findings. Liver abnormalities were observed in 98 patients (19.6%), bladder abnormalities in 88 (17.6%), kidney abnormalities in 41 (8.2%), and GB abnormalities in 37 (1.4%).
Table 1.
Characteristics of the patients
Mean±SD in age and post-injury duration. The other values were number (% in each group).
*p value <0.05.
Liver abnormalities included fatty liver (75 patients: 15.0%), cyst (8 patients: 1.6%), hemangioma (5 patients: 1.0%), diffuse liver disease (4 patients: 0.8%), biliary tract dilatation (3 patients: 0.6%), liver cirrhosis and clonorchiasis (3 patients: 0.6%). The group with abnormal liver findings had significant differences in gender, age, post-injury duration and completeness of injury in comparison with the normal group (Table 2).
Table 2.
Characteristics of the patients with liver abnormalities
Mean±SD in age and post-injury duration.
The other values were number (% in each group).
*p value <0.05.
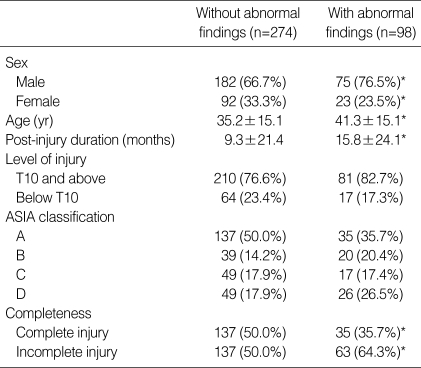
Bladder abnormalities included bladder wall thickening (56 patients: 11.2%), cystitis (14 patients: 2.8%), bladder stone (10 patients: 2.0%), and bladder wall trabeculation (8 patients: 1.8%). The group with abnormal bladder findings had significant differences in age, gender, and post-injury duration in comparison with the normal group (Table 3).
Table 3.
Characteristics of patients with bladder abnormalities
Mean±SD in age and post-injury duration.
The other values were number (% in each group).
*p value <0.05.
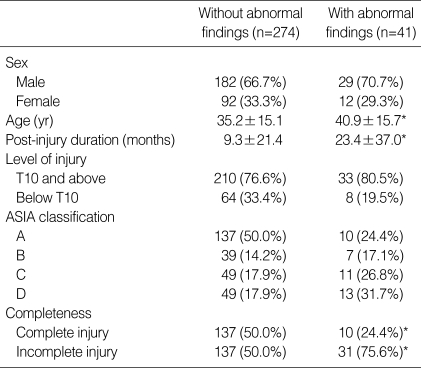
Kidney abnormalities included renal cyst (19 patients: 3.8%), pelvic dilatation (6 patients: 1.2%), chronic renal disease (6 patients: 1.2%), hydronephrosis (5 patients: 1.0%), pyelonephritis (3 patients: 0.6%) and renal stone (2 patients: 0.4%). The group with abnormal kidney findings had significant differences in age, post-injury duration and completeness of injury in comparison with the normal group (Table 4). The patients with a renal cyst were significantly older than those with hydronephrosis (51.1 yr vs. 24.8 yr) (p< 0.05).
Table 4.
Characteristics of patients with kidney abnormalities
Mean±SD in age and post-injury duration.
The other values were number (% in each group).
*p value <0.05.
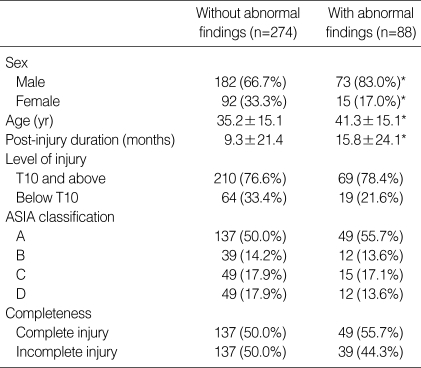
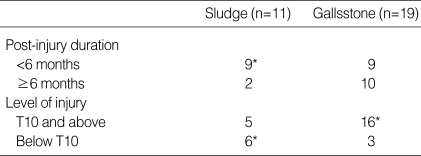
GB abnormalities included gallstone (19 patients: 3.8%), biliary sludge (11 patients: 2.2%), GB cyst (11 patients: 2.2%) and hemangioma (7 patients: 1.4%). The group with abnormal GB findings had significant differences in gender, age, in comparison with the normal group (Table 5). When compared with patients with biliary sludge, gallstone patients had longer post-injury duration and higher level of injury (Table 6). Gallstone and biliary sludge were found more frequently in those patients with an incomplete injury (19 patients) than in those with a complete injury (11 patients) (p< 0.05).
Table 5.
Characteristics of patients with gallbladder abnormalities
Mean±SD in age and post-injury duration.
The other values were number (% in each group).
*p value <0.05.
Table 6.
Findings of gallstone and sludge according to the post-injury duration and level of injury
*p value <0.05.
Eighteen patients had other abnormal sonographic lesions in the abdomen: splenomegaly (5 patients: 1.0%), pleural effusion (4 patients: 0.8%), ascites (3 patients: 0.6%), prostatic cyst (2 patients: 0.4%), pancreatic abnormalities (2 patients: 0.4%), uterine myoma (2 patients: 0.4%).
DISCUSSION
Ultrasonography of the abdomen has several major advantages. It is non-invasive, ionizing radiation is not used and the examination is not time-consuming when performed by experienced hands. In contrast, the main disadvantage of ultrasound imaging is its dependence on the operator's ability. Patients with a marked spinal deformity, gross constipation or immobility are difficult to examine (9). Diseases associated with SCI require an early diagnosis and intervention. A few studies have evaluated abdominal sonography as a screening test. Ozer and Shannon performed a prospective study to determine the cost effectiveness of the renal ultrasound examination in SCI as a screening modality, and concluded that it was effective in patients with hematuria, fever, and changes in the urinary habit (2). Marca et al. reported a retrospective study of abdominal ultrasonography in military veteran SCI patients for approximately 3 yr. The results showed that there were renal abnormalities in 7.1%, liver abnormalities in 76.8%, and pancreatic abnormalities in 20.6%. Liver abnormalities included liver parenchymal disease (45.6%) and fatty liver (19.7%) (1). In contrast, our study showed that fatty liver was more frequently detected than liver abnormalities. This discrepancy may be due to the high rate of liver parenchymal diseases from hepatitis induced by drug and alcohol abuse, which are endemic in the veteran population. Rosman et al. found that there was no association between viral hepatitis and SCI in the cases without intravenous drug abuse (10).
Marca's study showed a high incidence of pancreatitis (20.6%) due to the old age (mean age: 58.0 yr old) and high alcohol consumption, which was endemic in veteran patients (1). However, our data showed a very low incidence of pancreatic abnormalities (0.4%), possibly due to the younger age (mean age: 35.2 yr old).
The incidence of gallstones in the SCI patients ranged from 29 to 49% in several studies (4, 11). The possible reasons considered for this increased incidence of gallstones were a decreased food intake, parenteral nutrition, and an altered metabolic state with peripheral fat mobilization secondary to prolonged immobility and altered GB and gastrointestinal motility (8). The reported incidence of gallstones in the normal population in Korea has ranged from 4.0-4.2% (12, 13), but the incidence of patients with SCI was reported to be as high as 22.5% (14). However, in this study the incidence was 3.8%, which is similar to that of the normal population. This might be due to the longer duration of the post-injury in the previous study (21.2 yr) (14) in comparison to this study (11.1 months).
The reason for the altered GB and gastrointestinal motility has been believed to be an impaired sympathetic nerve supply to GB (which originates from the thoracic spinal segments 7-10) following SCI while the parasympathetic supply via the vagus nerve remained intact (4, 15). The parasympathetic supply induces a GB contraction, while the sympathetic supply induces a GB relaxation during the filling stage (16). Tandon et al. reported that in patients with SCI above T10, the fasting volume and ejection fraction of GB were decreased, but there were no differences in the resting volume and emptying time of GB in SCI (8).
There was a higher incidence of gallstones in patients with SCI below T10 and intact sympathetic nervous supply than in patients with SCI above T10 and impaired sympathetic nervous supply. It is possible that a sympathetic nervous supply could promote sludge formation through its inhibitory effect on intestinal motility following SCI (15). The resulting extension in gut transit time would lead to a relative increase in the deoxycholic acid concentration in the bile acid pool, which might result in sludge formation (16). However, this study did not evaluate the gut motility and further research will be required.
Virgili et al. reported upper urinary tract abnormalities such as hydronephrosis, vesicoureteral reflux and pyelonephritis in approximately 25% of patients with SCI, and the incidence of these abnormalities increased with increasing patient age (7). In this study, the average age of the patients with renal abnormalities was significantly older than that in the normal SCI group. Renal ultrasonography has a relatively high sensitivity in detecting a calyceal dilatation, but a low sensitivity in detecting a renal scar or ureter abnormalities (9).
Hoffberg and Cardenas reported that a bladder trabeculation was found in 31% of SCI patients who had been injured for less than 12 months, and that it was associated with male patients, vesicoureteral reflux, bladder diverticulum and an upper motor type neurogenic bladder (17). Bladder trabeculation and bladder shape deformity such as a pine tree shape and bladder wall thickening were associated with a high pressure of the upper urinary tract (18). In this study, a bladder trabeculation was detected only in 1.8% of patients. This was less than the incidence reported in the study of Hoffberg and Cardenas because the present study did not use excretory urography, cystography and cystoscopy, which are more sensitive in detecting bladder conditions than ultrasonography alone.
References
- 1.Marca L, Irene M, Craig J, Guo X, Chandralapaty SK. Lack of justification for routine abdominal ultrasonography in patients with chronic spinal cord injury. J Rehabil Res Dev. 2004;41:101–108. doi: 10.1682/jrrd.2004.01.0101. [DOI] [PubMed] [Google Scholar]
- 2.Ozer MN, Shannon SR. Renal sonography in asymptomatic persons with spinal cord injury: a cost-effectiveness analysis. Arch Phys Med Rehabil. 1991;72:35–37. [PubMed] [Google Scholar]
- 3.Gore RM, Mintzer RA, Calenoff L. Gastrointestinal complications of spinal cord injury. Spine. 1981;6:538–544. doi: 10.1097/00007632-198111000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Apstein MD, Dalecki-Chipperfield K. Spinal cord injury is a risk factor for gallstone disease. Gastroenterology. 1987;98:966–968. doi: 10.1016/0016-5085(87)90971-1. [DOI] [PubMed] [Google Scholar]
- 5.Gordon IL, Kohl CA, Arefi M, Complin RA, Vulpe M. Spinal cord injury increases the risk of abdominal aortic aneurysm. Am Surg. 1996;62:249–252. [PubMed] [Google Scholar]
- 6.Groah SL, Weitzenkamp DA, Lammertse DP, Whiteneck GG, Lezotte DC, Hamman RF. Excess risk of bladder cancer in spinal cord injury: evidence for an association between indwelling catheter use and bladder cancer. Arch Phys Med Rehabil. 2002;83:346–351. doi: 10.1053/apmr.2002.29653. [DOI] [PubMed] [Google Scholar]
- 7.Virgili G, Finazzi Agro E, Giannantoni A, D'Amico A, Germani S, Petta F, Vespasiani G. Ultrasonography of the upper urinary tract in patients with spinal cord injury. Arch Ital Urol Androl. 2000;72:225–227. [PubMed] [Google Scholar]
- 8.Tandon RK, Jain RK, Garg PK. Increased incidence of biliary sludge and normal gallbladder contractility in patients with high spinal cord injury. Gut. 1997;1:682–687. doi: 10.1136/gut.41.5.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morcos SK, Thomas DG. A comparison of real-time ultrasonography with intravenous urography in the follow up of patients with spinal cord injury. Clin Radiol. 1988;39:49–50. doi: 10.1016/s0009-9260(88)80340-4. [DOI] [PubMed] [Google Scholar]
- 10.Rosman AS, Chinigo AS, Spungen AM, Drexler HJ, Bauman WA. Viral hepatitis in patients with spinal cord injury is explained by known risk factors. J Spinal Cord Med. 1998;21:25–31. doi: 10.1080/10790268.1998.11719507. [DOI] [PubMed] [Google Scholar]
- 11.Moonka R, Stiens SA, Resnick WJ, McDorald JM, Eubank WB, Dominitz JA, Stelzner MG. The prevalence and natural history of gallstones in spinal cord injured patients. J Am Coll Surg. 1999;189:274–281. doi: 10.1016/s1072-7515(99)00143-x. [DOI] [PubMed] [Google Scholar]
- 12.Kim YJ, Kim BG, Park DY, Lee HC, Yoon YB, Song IS, Kim CY. Gallstone development after gastrectomy. Korean J Gastroenterol. 1997;29:506–514. [Google Scholar]
- 13.Jung HW, Chun KS, Kim YS, Kim MH, Choi H. Prevalence of gallstones in Korea. J Korean Acad Family Med. 1992;13:581–591. [Google Scholar]
- 14.Park TH, Byun JH, Suh JH, Jin WJ, Choi YC, Ha SC, Doo CJ, Kim MY. A study on the incidence of gallstones in patients with spinal cord injury. Korean J Gastroenterol. 1998;31:227–232. [Google Scholar]
- 15.Fealey RD, Szurszewski JH, Merritt JL, DiMagno EP. Effect of traumatic spinal cord transection on human upper gastro-intestinal motility and gastric emptying. Gastroenterology. 1984;87:69–75. [PubMed] [Google Scholar]
- 16.Marcus SN, Heaton KW. Intestinal transit deoxycholic acid and the cholesterol saturation of bile; three inter-related factors. Gut. 1986;27:550–558. doi: 10.1136/gut.27.5.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffberg HJ, Cardenas DD. Bladder trabeculation in spinal cord injury. Arch Phys Med Rehabil. 1986;67:750–753. doi: 10.1016/0003-9993(86)90009-2. [DOI] [PubMed] [Google Scholar]
- 18.Ogawa T, Yoshida T, Fujinaga T. Bladder deformity in traumatic spinal cord injury patients. Hinyokika Kiyo. 1988;34:1173–1178. [PubMed] [Google Scholar]