Abstract
We report a rare case of progressive myelopathy caused by intracranial dural arteriovenous fistula with venous drainage into the spinal perimedullary veins. A 45-yr-old man developed urinary and fecal incontinence and muscle weakness in the lower limbs. Magnetic resonance imaging revealed brainstem edema and dilated veins of the brainstem and spinal cord. Cerebral angiography showed a dural arteriovenous fistula fed by the neuromeningeal branch of the left ascending pharyngeal artery. Occlusion of the fistula could be achieved by embolization after a diagnostic and subsequent therapeutic delay. There was no improvement in clinical condition. For the neurologic outcome of these patients it is important that fistula must be treated before ischemic and gliotic changes become irreversible.
Keywords: Fistula; Arteriovenous, Fistula; Spinal Cord Diseases; Myelopathy
INTRODUCTION
Intracranial dural arteriovenous fistulas (DAVFs) are considered to be an acquired abnormality, probably as a result of revascularization of a previously thrombosed sinus (1). The clinical presentation of the patients with DAVFs are highly variable; some patients can show intracranial haemorrhage, neurologic deficit and signs of intracranial hypertension; others can present with bruit, cranial nerve paresis and ocular signs (2-4). The pattern of venous drainage of DAVFs is the most important determinant of their clinical presentation. This is the reason why DAVFs have been classified according to their venous drainage (3). We report a case who has intracranial DAVF with venous drainage into the spinal perimedullary veins. These vascular lesions have been classified as type V DAVFs in the classification scheme proposed by Cognard et al. (3). This classification gives us useful data to determine the risk with each DAVF. In a series reported by Cognard et al., type V DAVFs produced progressive myelopathy in 50% of cases (3).
Diagnosis may be difficult because the clinical and radiological findings are similar with the much more frequent spinal DAVFs and this type of DAVF is very rare (5-10). When a spinal cord syndrome has no detectable cause, such lesions must be taken into consideration. If the possibility of this diagnosis is considered and cranial angiography is performed without delay, neurological outcome of these patients will be good. Otherwise, it may lead to permanent neurologic deficit which was the case in our patient.
CASE REPORT
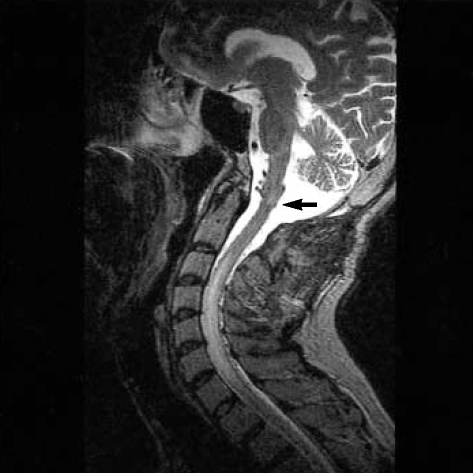
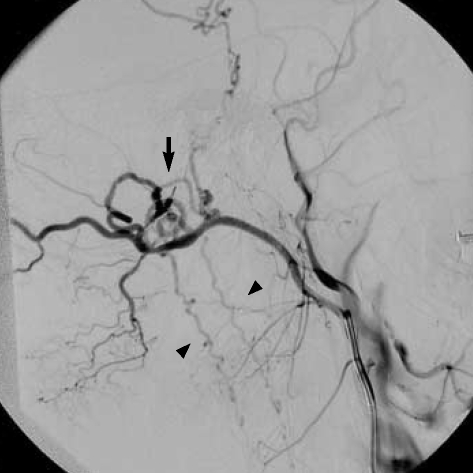
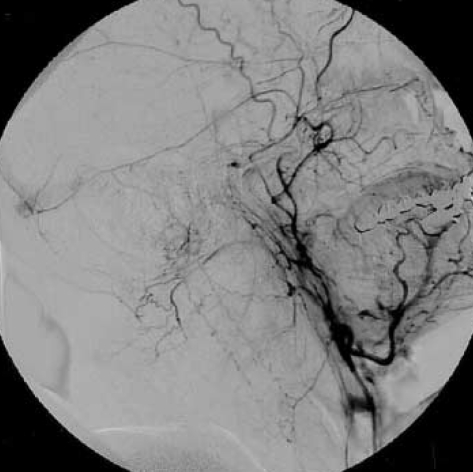
A 45-yr-old man presented to our department with the complaints of urinary and fecal incontinence and muscle weakness in the lower limbs. He had been admitted to another hospital two months ago, with occipital headaches, nausea and vomiting. He had developed urinary and fecal incontinence and muscle weakness in the lower limbs during the subsequent days. Craniocervical magnetic resonance imaging (MRI) study had been performed and ischemia or myelitis in the upper cervical cord and lower brainstem was considered. The patient was treated with conservative medications with no clinical benefit. Subsequently, he was referred to our hospital two months later. On admission to our department, he was able to walk with a cane. Upper extremity muscle strength was completely normal, but for the lower extremities it was graded for musculus iliopsoas and musculus quadriceps femoris 3/5 on the left side and 4/5 on the right side. Craniocervical MRI at that time revealed a diffuse pontomedullary lesion, which was hyperintense on T2-weighted images most likely corresponding to edema. Prominent flow voids of perimedullary blood vessels were identified as well (Fig. 1). Cerebral and spinal angiography was scheduled because of the presumable vascular malformation. Angiography showed a DAVF fed by the occipital artery of the left external carotid artery. Interestingly, the venous drainage was toward the perimedullary veins (Fig. 2). The fistula site was catheterized by a commercially available microcatheter and the DAVF was occluded by Histoacryl mixed with iodized oil at a concentration of 25%. Control angiography showed disappearance of the fistula (Fig. 3).
Fig. 1.
T2-weighted sagittal MRI shows hyperintense lesions consistent with edema in the lower brainstem (arrow) and cervical spinal cord.
Fig. 2.
Selective right external carotid artery angiogram (lateral projection) shows a dural arteriovenous (arrow) fistula fed by the branches of the occipital artery, and draining into the perimedullary veins (arrowheads) around the brainstem and cervical spinal cord.
Fig. 3.
Postembolization right external carotid artery angiogram. There is no evidence of arteriovenous fistula, nor opasification of the perimedullary veins.
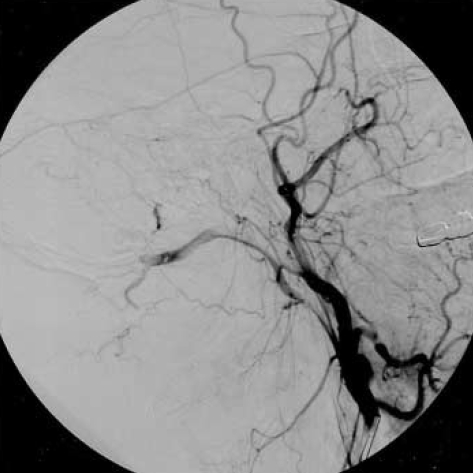
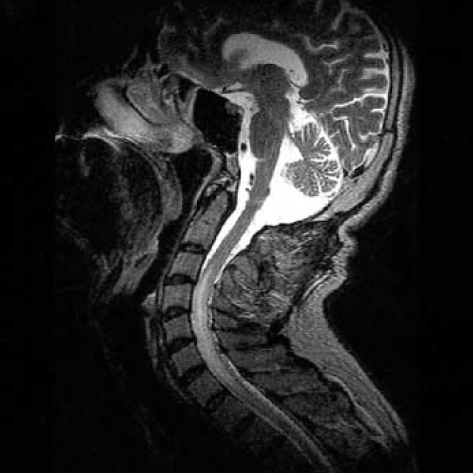
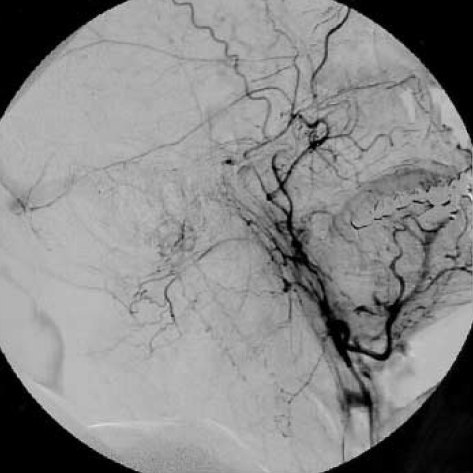
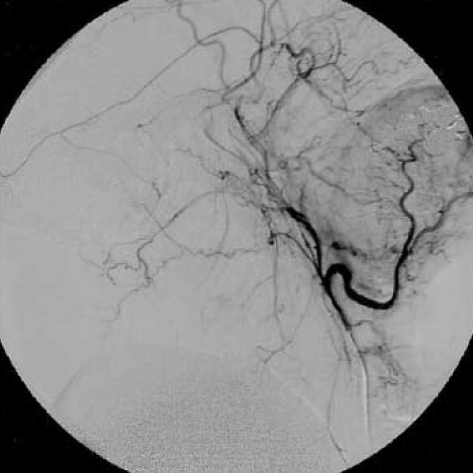
Unfortunately, on clinical follow-up, there was no clinical improvement after treatment. On urodynamic examination, overactive detrusor was detected. The patient was given anticholinergic drug with clean intermittent catheterization and needed to use bilateral cane for walking 3 months after discharge from the hospital. The neurologic examination showed spastic paraparesis and bilateral clonus of the ankles. The patient was hospitalized again for rehabilitation. MRI at that time showed disappearance of the oedema in the brainstem but pathologic signal still persisted in the midportion of the cervical spinal cord (Fig. 4). Cerebral angiography showed a small recurrence of the DAVF (Fig. 5). Superselective angiography showed the neuromeningeal branch of the ascending pharyngeal artery as the feeder of the recurrent fistula (Fig. 6). This fistula was embolized with glue at the 25% concentration. Control angiography showed occlusion of the fistula (Fig. 7). There was no improvement in the clinical condition. Knee-ankle foot orthoses was prescribed for the right lower extremity. However, 6 months later he came back on a wheelchair. He had no caregiver support in the home and had not been doing his exercises which led to an increase in his spasticity. He had also developed depression which made him reluctant to keep up his self mobility. He was readmitted to the hospital and after treatment with antidepressives and physical therapy for one month, he was able to walk with his orthoses. However, he was unwilling to use orthoses and found it more practical for himself to move around in a wheelchair.
Fig. 4.
Three months after endovascular therapy, T2-weighted MRI shows disappearance of the brainstem edema. High-signal region in the midportion of the cervical cord is probably due to gliotic changes.
Fig. 5.
Repeat right external carotid artery angiogram shows faint opacification of the fistulous perimedullary veins.
Fig. 6.
Lateral common carotid angiography shows reappearance of the fistula draining into the perimedullary veins.
Fig. 7.
After embolization of the recurrent fistula, the DAVF is no longer visible on right external carotid artery angiogram.
DISCUSSION
Intracranial DAVFs with perimedullary venous drainage are rare lesions. Since they were first described in 1982 (11), only a small number of cases have been reported in the literature (3, 5-10, 12-17).
Such kind of lesions generally presents with myelopathy. The pathophysiologic mechanism of the progressive spinal cord deficits is due to spinal cord venous hypertension (3). Fistulas localized in the intracranial dura mater can give rise to congestion of the spinal venous system via the anastomotic channels between the veins of the posterior cranial fossa and the spinal perimedullary veins (10).
Intracranial DAVF with spinal venous drainage generally presents with myelopathy but rarely cause hemorrhage (17). In a series reported by Cognard et al., type V DAVFs produced progressive myelopathy in 50% of cases (3). It was reported in the literature that only a few patients of intracranial DAVF with spinal venous drainage induced a hemorrhage (14, 18). In these cases, a cervical medullary-radicular vein, which drained the arterialized flow from DAVF into the epidural space, was found. This kind of drainage prevented the spinal cord venous hypertension and myelopathy.
In a study to investigate the relation between clinical presentation and venous drainage of intracranial DAVFs with spinal venous drainage, it was found that the patients without myelopathy had venous drainage limited to the cervical spinal cord, whereas the patients who had myelopathy had extensive spinal venous drainage descending toward the lower thoracic spinal cord (18). In accordance with this result, our patient had also extensive spinal venous drainage descending toward the lower thoracic spinal cord.
Patients with intracranial DAVF with spinal venous drainage can generally present with ascending myelopathy as the sole symptom according to the several reports (3, 7, 8, 10, 12, 13, 15, 19). MRI and spinal angiography are generally performed in these cases. When symptoms suggest a spinal DAVF and the result of the spinal angiography is normal, the angiographic studies should be extended to include the cranial and pelvic tributaries (17).
Although these types of fistulas are rare, it is crucial that the diagnosis not be delayed for the neurologic outcome of these patients. It is important that such lesions must be considered and cerebral angiography be performed without delay. Our patient referred to our hospital for advanced examination and rehabilitation 2 months after the onset of his neurologic symptoms. He presented with symptoms suggestive of spinal rather than intracranial origin like most of the patients with the same diagnosis. The diagnostic and subsequent therapeutic delay is responsible for the persistant neurologic deficit in our patient. Wiesmann et al. demonstrated that even paraplegia might be reversible if the fistula is diagnosed and treated early enough before ischemic and gliotic changes have been irreversible (17). Early interruption of a cranial DAVF draining into spinal veins permits recovery of the myelopathy. Endovascular therapy, which is a safe and effective method in the treatment of these fistulas, should be tried first (8).
In conclusion, in patients who present with clinical symptoms of spinal cord syndrome who appear to have a MRI picture of "vascular" myelopathy and who exhibit negative spinal angiography, a four-vessel cerebral angiography should be undertaken, aiming at the recognition of an intracranial DAVF (5). The most important thing is to consider the possibility of this diagnosis and perform cerebral and spinal angiography without delay to have a good neurological outcome in these patients. Otherwise, the clinical outcome may be poor as in the present patient.
References
- 1.Mullan S. Reflections upon the nature and management of intracranial and intraspinal vascular malformations and fistulae. J Neurosurg. 1994;80:606–616. doi: 10.3171/jns.1994.80.4.0606. [DOI] [PubMed] [Google Scholar]
- 2.Halbach VV, Higashida RT, Hieshima GB, Rosenblum M, Cahan L. Treatment of dural arteriovenous malformations involving the superior sagittal sinus. Am J Neuroradiol. 1988;9:337–343. [PMC free article] [PubMed] [Google Scholar]
- 3.Cognard C, Gobin YP, Pierot L, Bailly AL, Houdart E, Casasco A, Chiras J, Merland JJ. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995;194:671–680. doi: 10.1148/radiology.194.3.7862961. [DOI] [PubMed] [Google Scholar]
- 4.Awad IA, Little JR, Akarawi WP, Ahl J. Intracranial dural arteriovenous malformations: factors predisposing to an aggressive neurological course. J Neurosurg. 1990;72:839–850. doi: 10.3171/jns.1990.72.6.0839. [DOI] [PubMed] [Google Scholar]
- 5.Bret P, Salzmann M, Bascoulergue Y, Guyotat J. Dural arteriovenous fistula of the posterior fossa draining into the spinal medullary veins-an unusual cause of myelopathy: case report. Neurosurgery. 1994;35:965–968. [PubMed] [Google Scholar]
- 6.Pierot L, Chiras J, Meder JF, Rose M, Rivierez M, Marsault C. Dural arteriovenous fistulas of the posterior fossa draining into subarachnoid veins. Am J Neuroradiol. 1992;13:315–323. [PMC free article] [PubMed] [Google Scholar]
- 7.Versari PP, D'Aliberti G, Talamonti G, Branca V, Boccardi E, Collice M. Progressive myelopathy caused by intracranial dural arteriovenous fistula: report of two cases and review of the literature. Neurosurgery. 1993;33:914–918. doi: 10.1227/00006123-199311000-00023. [DOI] [PubMed] [Google Scholar]
- 8.Gobin YP, Rogopoulos A, Aymard A, Khayata M, Reizine D, Chiras J, Merland JJ. Endovascular treatment of intracranial dural arteriovenous fistulas with spinal perimedullary venous drainage. J Neurosurg. 1992;77:718–723. doi: 10.3171/jns.1992.77.5.0718. [DOI] [PubMed] [Google Scholar]
- 9.Gaensler EH, Jackson DE, Jr, Halbach VV. Arteriovenous fistulas of the cervicomedullary junction as a cause of myelopathy: radiographic findings in two cases. Am J Neuroradiol. 1990;11:518–521. [PMC free article] [PubMed] [Google Scholar]
- 10.Wrobel CJ, Oldfield EH, Di Chiro G, Tarlov EC, Baker RA, Doppman JL. Myelopathy due to intracranial dural arteriovenous fistulas draining intrathecally into spinal medullary veins. Report of three cases. J Neurosurg. 1988;69:934–939. doi: 10.3171/jns.1988.69.6.0934. [DOI] [PubMed] [Google Scholar]
- 11.Woimant F, Merland JJ, Riche MC, de Liege P, Bertrand HJ, Bacri D, Haguenau M, Pepin B. Bulbospinal syndrome related to a meningeal arteriovenous fistula of the lateral sinus draining into spinal cord veins. Rev Neurol (Paris) 1982;138:559–566. [PubMed] [Google Scholar]
- 12.Mascalchi M, Scazzeri F, Prosetti D, Ferrito G, Salvi F, Quilici N. Dural arteriovenous fistula at the craniocervical junction with perimedullary venous drainage. Am J Neuroradiol. 1996;17:1137–1141. [PMC free article] [PubMed] [Google Scholar]
- 13.Partington MD, Rufenacht DA, Marsh WR, Piepgras DG. Cranial and sacral dural arteriovenous fistulas as a cause of myelopathy. J Neurosurg. 1992;76:615–622. doi: 10.3171/jns.1992.76.4.0615. [DOI] [PubMed] [Google Scholar]
- 14.Rivierez M, Gazengel J, Chiras J, Dorwling-Carter D, Debussche D, Dubbs M, Kanaan H. Vertebro-dural arteriovenous fistulae of the foramen magnum with perimedullary venous drainage. Neurochirurgie. 1991;37:179–184. [PubMed] [Google Scholar]
- 15.Willinsky R, TerBrugge K, Lasjaunias P, Montanera W. The variable presentations of craniocervical and cervical dural arteriovenous malformations. Surg Neurol. 1990;34:118–123. doi: 10.1016/0090-3019(90)90107-z. [DOI] [PubMed] [Google Scholar]
- 16.Brunet E, Tache R, Lafitte F, Piotin M, Miaux Y, Martin-Duverneuil N, Chiras J. Intracranial dural arteriovenous fistula with perimedullary venous drainage. J Neuroradiol. 1998;25:103–110. [PubMed] [Google Scholar]
- 17.Wiesmann M, Padovan CS, Pfister HW, Yousry TA. Intracranial dural arteriovenous fistula with spinal medullary venous drainage. Eur Radiol. 2000;10:1606–1609. doi: 10.1007/s003300000382. [DOI] [PubMed] [Google Scholar]
- 18.Brunereau L, Gobin YP, Meder JF, Cognard C, Tubiana JM, Merland JJ. Intracranial dural arteriovenous fistulas with spinal venous drainage: relation between clinical presentation and angiographic findings. Am J Neuroradiol. 1996;17:1549–1554. [PMC free article] [PubMed] [Google Scholar]
- 19.Trop I, Roy D, Raymond J, Roux A, Bourgouin P, Lesage J. Craniocervical dural fistula associated with cervical myelopathy: angiographic demonstration of normal venous drainage of the thoracolumbar cord does not rule out diagnosis. Am J Neuroradiol. 1998;19:583–586. [PMC free article] [PubMed] [Google Scholar]