Abstract
Background
Effective therapies for the secondary prevention of coronary heart disease–related events are significantly underused, and attempts to improve adherence have often yielded disappointing results. Elimination of patient out-of-pocket costs may be an effective strategy to enhance medication use. We sought to estimate the incremental cost-effectiveness of providing full coverage for aspirin, β-blockers, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, and statins (combination pharmacotherapy) to individuals enrolled in the Medicare drug benefit program after acute myocardial infarction.
Methods and Results
We created a Markov cost-effectiveness model to estimate the incremental cost-effectiveness of providing Medicare beneficiaries with full coverage for combination pharmacotherapy compared with current coverage under the Medicare Part D program. Our analysis was conducted from the societal perspective and considered a lifetime time horizon. In a sensitivity analysis, we repeated our analysis from the perspective of Medicare. In the model, post–myocardial infarction Medicare beneficiaries who received usual prescription drug coverage under the Part D program lived an average of 8.21 quality-adjusted life-years after their initial event, incurring coronary heart disease–related medical costs of $114 000. Those who received prescription drug coverage without deductibles or copayments lived an average of 8.56 quality-adjusted life-years and incurred $111 600 in coronary heart disease–related costs. Compared with current prescription drug coverage, full coverage for post–myocardial infarction secondary prevention therapies would result in greater functional life expectancy (0.35 quality-adjusted life-year) and less resource use ($2500). From the perspective of Medicare, full drug coverage was highly cost-effective ($7182/quality-adjusted life-year) but not cost saving.
Conclusions
Our analysis suggests that providing full coverage for combination therapy to post–myocardial infarction Medicare beneficiaries would save both lives and money from the societal perspective.
Keywords: cost-benefit analysis, drugs, epidemiology, insurance, myocardial infarction, prevention
Practice guidelines recommend that all acute myocardial infarction (MI) patients receive treatment with a β-blocker, a lipid-lowering agent, an angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB), and aspirin1 unless a contraindication exists. Taken in combination, these drugs have been estimated to reduce the relative risk of coronary heart disease (CHD) mortality by 80% compared with placebo.2 Although the rates of prescribing these medications at hospital discharge after acute MI have improved substantially,3 subsequent longer-term adherence to therapy continues to be poor.4 For example, only 46% of patients with CHD report consistent β-blocker use within 1 year of an acute MI,5 and only 50% of patients are adherent to their prescribed statin.6 Fewer than 20% of MI patients use all 4 of the recommended agents (S.E. Ramsay, personal communication, 2006).5
The cost of prescription drugs borne by patients is a central reason for medication underuse.7–11 Even with the advent of prescription drug coverage under Medicare, patients face substantial cost sharing through tiered copayments and a coverage gap or “doughnut hole,”12 and these out-of-pocket costs may reduce the use of prescribed medications.13,14 Thus, eliminating these costs may be a simple and effective strategy to increase adherence and to improve clinical outcomes. Moreover, the cost savings from clinical events that are averted by improved adherence may more than offset the incremental cost of full drug coverage (ie, the elimination of cost sharing), as we have demonstrated in a preliminary analysis of post-MI patients who receive health and drug coverage through employer-sponsored commercial insurance.15
It is unknown whether these results are applicable to Medicare, which provides coverage for ≈400 000 MI patients annually (Hospital Intervention QIOSC based on Center for Medicare and Medicaid Services Discharge Claims Data Warehouse, personal communication, 2006, Oklahoma City, OK).
Medicare offers more modest levels of drug coverage under the Part D prescription drug benefit than is typically offered by commercial insurers16; thus, the tradeoff between the cost savings from improved post-MI outcomes and the cost increase from higher prescription drug expenditures may be substantially different for this large, publicly funded national health insurer. In addition, Medicare beneficiaries are generally enrolled for the remainder of their lives, far longer than is seen in commercially insured populations in which patients frequently change insurers and insurers often cannot benefit financially from long-term health benefits accrued. Our earlier analysis considered only a limited number of post-MI events and assessed a short time horizon from the perspective of a typical health insurer. Accordingly, we performed a cost-effectiveness analysis using a Markov state transition model that assessed the incremental value of providing post-MI Medicare beneficiaries with full compared with current levels of prescription drug coverage for their cardiovascular medications over their lifetimes from both the societal and Medicare perspectives.
Methods
Analytic Model
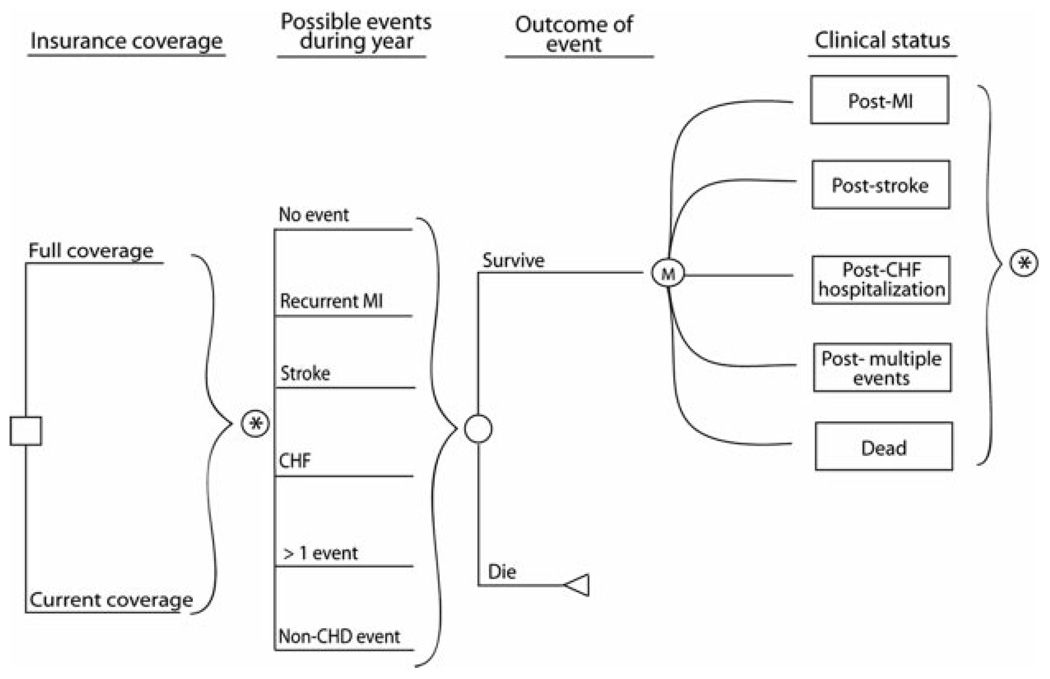
We created a Markov model to evaluate the incremental costs and quality-adjusted life expectancy that would result from providing aspirin, a β-blocker, an ACEI or ARB, and a statin (combination pharmacotherapy) to post-MI Medicare beneficiaries without any out-of-pocket costs (full coverage) compared with the typical coverage provided by the Medicare Part D program. We simulated a cohort of patients 65 years of age who were discharged alive after an acute MI as they transitioned, in 1-year cycles, through a series of post-MI health states over the course of their lifetimes (Figure 1). In the model, each year a patient could remain well, have a fatal or nonfatal reinfarction or stroke, be hospitalized for congestive heart failure (CHF) with the potential of dying of this condition, or die of other causes.
Figure 1.
Cost-effectiveness model structure.□ Represents the choice between full coverage and current coverage. In each 1-year cycle, patients may experience 6 possible events (*) from which they may survive or die. At the end of the cycle, patients are left in 1 of 5 possible Markov states. In the subsequent cycle, patients may once again experience ≥1 event.
The TreeAge Pro 2006 software package (TreeAge Software, Williamstown, Mass) was used to perform our analyses. We discounted quality-adjusted life-years (QALYs) and future costs at a rate of 3%. Our base-case analysis was conducted from the societal perspective.
Model Inputs
Current and Expected Medication Adherence
We obtained the current rates of ACEI/ARB, β-blocker, and statin use and the proportion of patients taking all combinations of these drugs from a contemporary cohort of Medicare patients enrolled in the Pennsylvania Pharmaceutical Assistance Contract for the Elderly program who were discharged from hospital after acute MI (International Classification of Diseases, ninth edition, clinical modification [ICD-9CM] code 410.x excluding 410.92) in 2004. Aspirin use and adherence were obtained from Newby et al.5
Under usual coverage, patients in the cohort were assumed to be receiving prescription drug benefits through the Medicare Part D program, with 37% of their drug costs covered by Medicare.16 In the hypothetical full coverage scenario, we assumed that Medicare paid 100% of drug costs.
The impact of providing full coverage on medication adherence was calculated by applying expected changes in adherence from given changes in coverage (ie, price elasticity for prescription drugs) to current patterns of use. The proportion of patients who would remain fully adherent as a consequence of full coverage was calculated for each drug separately. These incremental changes were applied equally to all groups of patients currently nonadherent to the particular drug (ie, those taking 0, 1, 2, or 3 of the other drugs). For example, the incremental increase in statin use from full coverage was applied equally across patients currently adherent to no drugs, aspirin alone, β-blocker alone, ACEI/ARB alone, aspirin and β-blocker, aspirin and ACEI/ARB, β-blocker and ACEI/ARB, and all 3 (aspirin, β-blocker, and ACEI/ARB). We used conservative estimates of price elasticity (−0.16)17 for our base-case analysis.
Post-MI Event Rates
Because event rates vary as a function of medication use, we calculated post-MI event rates for patients taking 0, 1, 2, 3, and all 4 components of combination therapy. Contemporary estimates of post-MI events in the absence of treatment (ie, taking no aspirin, β-blocker, ACEI/ARB, or statin) do not exist because event rates in post-MI registries or the control arms of clinical trials reflect the use of at least some components of combination pharmacotherapy. Therefore, we calculated baseline (untreated) event rates by decomposing current event rates into the rates among treated and untreated patients as follows: current event rate=(event rate in untreated patients×proportion of patients who are untreated [ie, nonadherent])+(event rate in treated patients×proportion of patients who are treated [ie, adherent]), where the event rate in treated patients is the event rate in untreated patients times the relative risk of outcome with treatment. We estimated the relative risk reductions resulting from combination pharmacotherapy using the peer-reviewed literature,2,18 and we calculated post-MI event rates by analyzing claims for all Medicare beneficiaries discharged from a hospital in 2002 with a primary diagnosis of MI (ICD-9CM 410.x excluding 410.92) (n=301 263) (Hospital Intervention QIOSC based on Center for Medicare and Medicaid Services Discharge Claims Data Warehouse, personal communication, 2006, Oklahoma City, OK).
We estimated the incremental effect of taking 1, 2, 3, or all 4 drugs by multiplying the relative risks compared with placebo of each component of treatment based on Wald and Law’s multiplicative assumption.18 We used averaged risk reductions for all possible combinations of 1, 2, and 3 drugs in our analysis because patients who are not fully adherent may take any combination of the components of combination pharmacotherapy and because each combination has a different efficacy. We conservatively assumed that aspirin and statins did not reduce the risk of CHF hospitalization. Because the use of multiplicative models to estimate treatment effects has been debated, 19,20 we varied the effect of combination pharmacotherapy on CHD outcomes extensively in our sensitivity analyses.
We obtained age-specific mortality rates for post-MI events from the Agency for Healthcare Research and Quality’s nationwide inpatient sample,21 which provides estimates that are consistent with the literature.22 We estimated out-of-hospital death rates from MI, stroke, and CHF by multiplying inpatient mortality rates by ratios of inpatient to outpatient mortality rates obtained from the Centers for Disease Control.23 We calibrated our model to data on life expectancies for subjects with CHD obtained from publications by the Framingham Heart Study24 and the Saskatchewan Health database.25 To account for temporal variation in the risk of post-MI events (ie, event rates are highest immediately after an MI and then decline), we calculated yearly event rates separately for each of the first 4 post-MI years. Thereafter, event rates were assumed to be equivalent to those observed in the fourth post-MI year.
Other Causes of Death
We obtained noncardiovascular mortality rates from US life table data.26 Death resulting from stroke, MI, and CHF was removed from age-specific total mortality by subtracting the rate of death resulting from these causes by age from the overall mortality rate.27
Drug Costs Under Medicare Part D and Full Coverage
Drug costs were obtained from a major online pharmacy28 and calculated on the basis of the distribution of specific drugs within each class used by post-MI Medicare beneficiaries enrolled in the Pennsylvania Pharmaceutical Assistance Contract for the Elderly program. Because no drug costs are incurred for unfilled prescriptions, we calculated medication expenditures based on current and projected levels of adherence. In the base-case analysis, increased costs under full coverage reflect only increased adherence because drug costs are covered entirely by society (ie, patients and/or Medicare) under both current and full coverage.
Cost of Post-MI Events and Ongoing Care
The cost of post-MI events (ie, reinfarction, stroke, and hospitalization for CHF) was calculated as a weighted average of Medicare diagnosis-related group hospital payments for these events. Because single events are associated with multiple diagnosis-related groups, we estimated diagnosis-related group frequencies from the nationwide inpatient sample and weighted event costs accordingly. Post–acute care costs (ie, for rehabilitation and long-term care), which are not included in diagnosis-related group payments, were estimated from the distribution of discharge dispositions observed in the nationwide inpatient sample. Physician costs were estimated from lengths of stay, inpatient procedure frequencies, and the Medicare physician fee schedule. The cost of a non-CHD death was obtained from an analysis of costs in the last month of life conducted by the Centers for Medicare and Medicaid Services.29
Costs for ongoing care in years in which no post-MI event occurred were calculated from the hierarchical condition categories model used by the Centers for Medicare and Medicaid Services to adjust Medicare capitation payments to private healthcare plans.30 Average per diem costs were added for the proportion of patients assumed to be ongoing long-term care residents.31–33 The cost of informal caregiving for patients who had a stroke but were not admitted to a long-term care facility was estimated from the literature.34,35
All costs are presented in 2006 US dollars. When current estimates were not available, costs were standardized using the medical care component of the US Consumer Price Index.
Utilities
Health state utilities were obtained from the published literature.36–38 Consistent with the literature, for subjects with multiple conditions, the utilities for the associated conditions were multiplied together. This assumes that a stroke, for example, reduces a subject’s quality of life by the same percentage regardless of whether CHF also is present.
Sensitivity Analyses
To assess the degree to which the model parameter estimates influenced our results, we performed a series of 1-way analyses varying base-case inputs using the ranges presented in Table 1.
Table 1.
Baseline Assumptions
| Parameter | Base-Case Estimates | Sensitivity Range Tested |
|---|---|---|
| Proportion of patients adherent to different No. of medications under current coverage | ||
| 0 drugs | 0.07 | * |
| 1 drugs | 0.27 | |
| 2 drugs | 0.32 | |
| 3 drugs | 0.25 | |
| 4 drugs | 0.09 | |
| Proportion of patients adherent to different No. of medications under full coverage | ||
| 0 drugs | 0.001 | 0–0.07 |
| 1 drugs | 0.07 | 0–0.27 |
| 2 drugs | 0.29 | 0.08–0.32 |
| 3 drugs | 0.42 | 0.25–0.44 |
| 4 drugs | 0.22 | 0.09–0.48 |
| Annual event rates in patients receiving no drug therapy | ||
| Reinfarction | 0.4–0.06† | 25%–200% of base-case estimates |
| Stroke | 0.02–0.03 | |
| CHF | 0.10–0.23 | |
| Event rate ratios with drug treatment | ||
| Reinfarction | 0.65–0.16‡ | 25%–200% of base-case estimates |
| Stroke | 0.26–0.76 | |
| CHF | 0.43–0.83 | |
| Ratio of outpatient to inpatient CHD deaths§ | 1.4 | … |
| Non-CHD background mortality§ | 0.01 | … |
| Excess mortality rate associated with CHD | 0.02 | … |
| Event-related mortality rates§ | ||
| Reinfarction | 0.05 | 25%–200% of base-case estimates |
| Stroke | 0.04 | |
| CHF | 0.03 | |
| Average cost for 1-year supply of 1 drug,$ | 212 | 50–1000 |
| Proportion of drug cost faced by patients under usual coverage | 0.63 | 0.1–0.9 |
| Event costs, $ | ||
| MI (nonfatal) | 16 563 | 25%–200% of base-case estimates |
| MI (fatal) | 14 494 | |
| Stroke (nonfatal) | 13 878 | |
| Stroke (fatal) | 9414 | |
| CHF admission (nonfatal) | 10 241 | |
| CHF admission (fatal) | 8782 | |
| Non-CHD death | 10 063 | |
| Subsequent CHD-related care costs, $‖ | ||
| Baseline§ | 2231 | … |
| Incremental cost associated with MI history | 2430 | |
| Incremental cost associated with stroke history | 14 011 | |
| Incremental cost associated with CHF | 3507 | |
| Utility weights | ||
| MI | 0.88 | 0.78–0.90 |
| Stroke | 0.64 | 0.39–0.85 |
| CHF | 0.80 | 0.71–0.89 |
| Discount rate | 0.03 | 0–0.05 |
Not tested.
Varies by year. See text for details.
Varies by number of drugs used. Increasing drug use results in small rate ratios. See text for details.
Vary by age. Values presented are for a 65-year-old patient.
Costs are additive in the case of multiple conditions.
In a secondary analysis, we evaluated full coverage from the perspective of Medicare. Under usual coverage, Medicare was assumed to cover 37% of drug costs for those patients who were adherent to therapy. Under full coverage, Medicare was assumed to pay for 100% of drug costs for those patients adherent to therapy. Therefore, the cost of medications under full coverage was assumed to increase compared with usual coverage because of both the increased proportion of costs faced by Medicare and increases in adherence. In this model, per diem long-term care costs and the cost of informal caregiving were ignored because they are not incurred by Medicare. In addition, the cost of non-CHD mortality was reduced to reflect only those costs incurred by Medicare.29 Thus, event and post–acute care costs were lower in this model than in the base-case models.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Base-Case Analysis
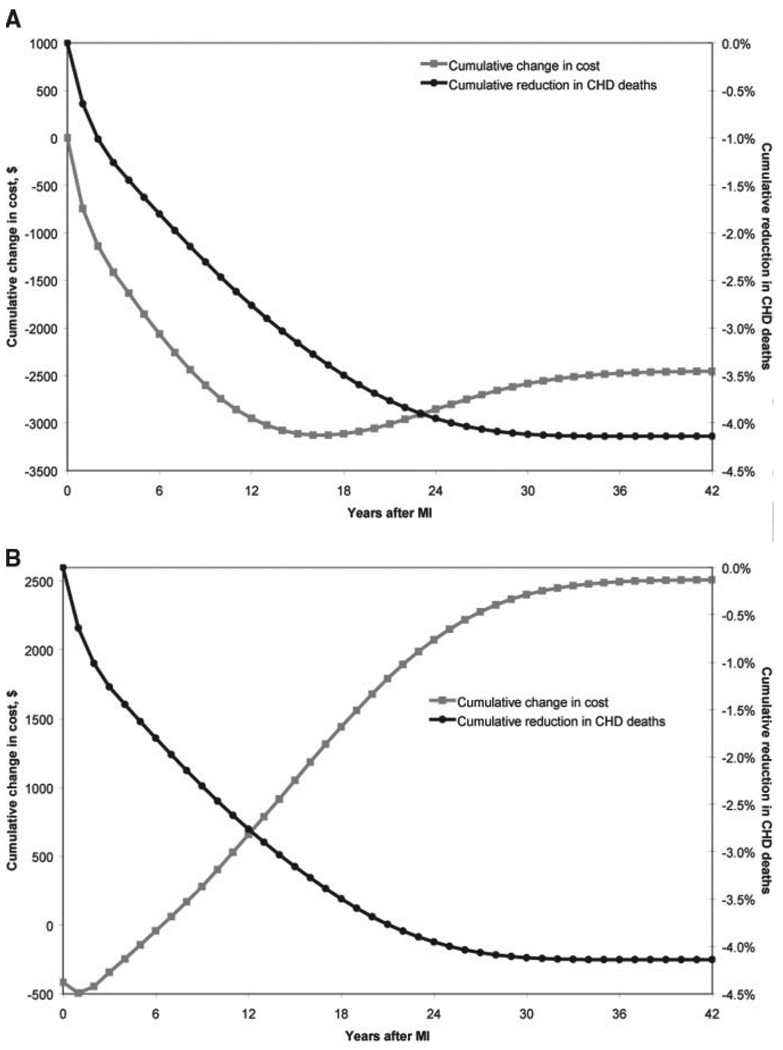
The model predicted that post-MI Medicare beneficiaries 65 years of age who received usual prescription drug coverage under the Part D program would live a average of 8.21 discounted QALYs after their initial event, incurring healthcare costs of $114 000 (Table 2). Those who received full prescription drug coverage lived an average of 8.56 QALYs and incurred $111 600 in costs. The model indicated that compared with current prescription drug coverage, full coverage for post-MI secondary prevention therapies saved both lives (0.35 QALY) and money ($2500). The cost savings from full coverage were attributable to reductions in nondrug healthcare expenditures, most notably the costs related to subsequent hospitalizations (Table 2). Cumulative cost savings and improvements in survival occurred within 1 year after the initial MI (Figure 2A).
Table 2.
Overall Base-Case and Medicare Perspective Results
| Base Case | Medicare Perspective | |||||
|---|---|---|---|---|---|---|
| Current Coverage | Full Coverage | Difference | Current Coverage | Full Coverage | Difference | |
| Costs,*$ | ||||||
| Prescription drug costs | 4595 | 6450 | 1855 | 1700 | 6450 | 4750 |
| Healthcare costs | 109 411 | 105 103 | −4308 | 92 883 | 90 644 | −2239 |
| Total | 114 006 | 111 553 | −2453 | 94 583 | 97 094 | 2512 |
| Effectiveness, QALY | 8.21 | 8.56 | 0.35 | 8.21 | 8.56 | 0.35 |
| Incremental cost-effectiveness ratio, $/QALY | … | … | Dominant† | … | … | 7182 |
All future costs and QALYs were discounted at a rate of 3%/y.
A dominant strategy saves lives and money.
Figure 2.
Cumulative changes in healthcare costs and survival for full coverage vs current drug coverage from the societal (A) and Medicare (B) perspectives. Data are presented as the change in CHD costs and mortality for patients receiving full drug coverage vs current levels of drug coverage.
Sensitivity Analyses
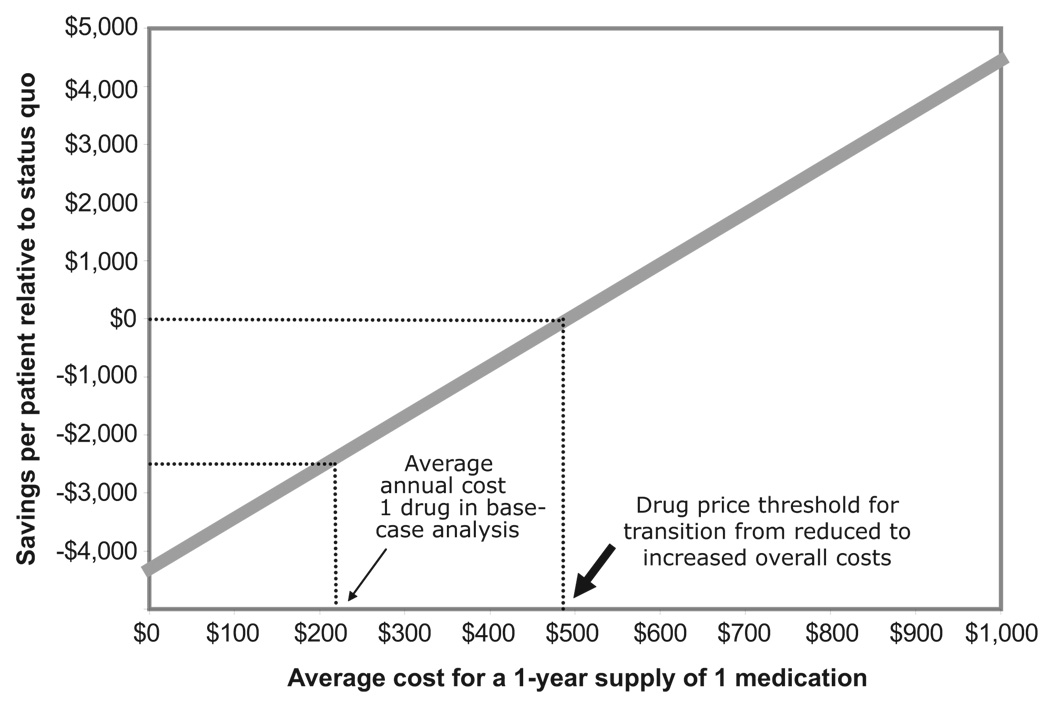
Our base-case results were robust to variation of all model inputs. Full coverage remained cost saving from a societal perspective as long as adherence increased even by 1% compared with coverage under Medicare Part D, and the average cost of a 1-year supply of 1 drug is less than $492 (Figure 3). Full coverage also was cost saving as long as the relative risk reduction from combination pharmacotherapy is at least 53% of base-case estimates (ie, combination pharmacotherapy reduces the risk of reinfarction, stroke, and heart failure by at least 45%, 39%, and 30%, respectively), If combination pharmacotherapy were assumed to be only 25% as effective as base-case values (ie, to result in relative reductions in the risk of reinfarction, stroke, and heart failure of 22%, 20%, and 15%, respectively), the incremental cost-effectiveness ratio of full coverage would be $12 640/QALY. The models were not sensitive to any other parameters, including patient age, baseline event rates, and the probability of death for patients who experience events.
Figure 3.
Impact of annual drug costs on cost savings from full drug coverage. The thin arrow indicates the average drug cost ($212 per drug per year) used in the base-case analysis. The thick arrow indicates the threshold cost ($492) at which full drug coverage transitions from reduced to increased total costs. All future costs are discounted at a rate of 3%.
Repeating our analysis from the perspective of Medicare, the Part D program incurred a relatively larger increase in prescription drug costs under full coverage than society did in the base-case analysis because cost shifting between patients and Medicare is ignored from the societal perspective (Table 2). In contrast, full coverage resulted in smaller incremental savings in other healthcare costs from the Medicare perspective because only those costs incurred by Medicare were included in this analysis. Cumulatively, full coverage results in increased costs after 6 years (Figure 2B), and overall, full coverage would cost Medicare an additional $2512 per beneficiary. Because changes in QALYs were as observed in the base-case model, the incremental cost-effectiveness ratio for full coverage from the perspective of Medicare was $7182/QALY. If the average cost of a 1-year supply of 1 drug were less than $90 or if event-related costs were 80% greater than those used in our base-case analysis (eg, the cost of a fatal MI was $26 000), this strategy would be cost saving from the Medicare perspective.
Discussion
This cost-effectiveness analysis of a hypothetical cohort of Medicare beneficiaries demonstrates that compared with the current Part D drug benefit, the elimination of patient cost sharing for medications commonly prescribed to post-MI patients would both improve health and save money from the societal perspective. The magnitude of observed changes in health outcomes is large and substantially greater than that observed for other interventions widely used to improve post-MI outcomes such as dual antiplatelet therapy39 or early invasive risk stratification strategies.40 In addition, average cost reductions of $2500 per beneficiary would save society $1 billion for the ≈400 000 Medicare beneficiaries who have an acute MI every year (Hospital Intervention QIOSC based on Center for Medicare and Medicaid Services Discharge Claims Data Warehouse, personal communication, 2006, Oklahoma City, OK).
As such, our results strongly support the hypothesis that post-MI Medicare beneficiaries should receive statins, ACEIs/ARBs, and β-blockers without cost sharing and emphasize the urgent need for prospective clinical studies to confirm the effectiveness of this approach.
Our results were robust to many of the model assumptions. In particular, full coverage will dominate current coverage (ie, be both more effective and less costly) as long as more patients remain adherent to therapy than would have otherwise. In addition, although the analysis conducted from the perspective of Medicare did not indicate that full coverage would be cost saving, the incremental cost-effectiveness ratio of this strategy ($7182/QALY) is well below accepted thresholds for commonly used therapies.
The present analysis extends our prior work that evaluated the cost-effectiveness of providing 3 years of full coverage for combination pharmacotherapy to post-MI patients from the perspective of a typical commercial insurance company.15 In contrast to our previous results, full coverage was cost-effective but not cost saving from the Medicare perspective. There are several reasons for this difference: We used Medicare hospitalization reimbursement rates, which are lower than those paid by commercial insurers; we assumed that the amount of patient cost sharing for Part D beneficiaries is more than that for privately insured patients (63% versus 32% of drug costs); we assumed a smaller increase in adherence from full coverage than we did previously; and we considered a much longer time frame. Repeating this analysis using event costs similar to those used in previous analyses of full coverage15,41 resulted in full coverage being a dominant, cost-saving strategy. Lowering the average cost of a 1-year supply of 1 medication to $90 also makes full coverage cost saving from the Medicare perspective. This scenario is easily plausible given the availability of $4/mo ($48/y) generic medications at many retail pharmacies and the likelihood that Medicare prescription drug plans receive even greater discounts than those available to individual consumers.
Overall, our results help make the “business case” for Medicare to expand current levels of prescription drug coverage provided under the Part D program for post-MI secondary prevention therapies. There are many interventions designed to improve medication adherence,42 although those shown to be effective are resource intensive. In contrast, the selective reduction of cost sharing for medications of proven efficacy is administratively less complex and may be scaled to large numbers of patients. Moreover, there is precedent in Medicare for selectively offering benefits to patients with particular conditions (ie, individuals <65 years of age with end-stage renal or Lou Gehrig’s disease) and for particular medications (eg, injectable medications under Part B). The challenge with this adherence improvement strategy, and value-based insurance designs more generally,43 is identifying the medications and associated health conditions for which reducing copayments for prescription drugs will simultaneously improve health and reduce costs, as we have demonstrated for post-MI secondary prevention medications. ACEIs for diabetics,41 statins for high-risk primary and secondary prevention of CHD,44 and antirejection drugs for kidney transplant recipients45 all have promise in this regard. Accordingly, large employers and insurers have begun experimenting with the selective reduction of copayments for patients with a variety of conditions and have found favorable short-term economic returns.46,47
Our analysis is subject to several limitations. First, our results are based on a cost-effectiveness model that was built using estimates from the literature rather than the results of a prospectively conducted trial designed to compare full and current levels of prescription drug coverage. Moreover, because many of our model parameters such as the true benefit of combination pharmacotherapy or the effect of completely removing out-of-pocket costs on medication adherence have not been rigorously tested, our results should be considered hypothesis generating. It is reassuring that our findings were robust to our assumptions. For example, we found full coverage to be cost saving as long as combination pharmacotherapy reduced the relative risk of reinfarction by at least 45%. By way of comparison, this is less than the relative risk reduction over placebo expected from statin therapy alone, without considering the other constituents of combination therapy based on conservative estimates of the trial literature. 2 Nevertheless, a high-quality trial in actual practice is needed to validate the results of our analysis.
Second, we did not evaluate the impact of cost-sharing reductions for other commonly used post-MI medications such as clopidogrel and higher-potency statins. Although these drugs provide incremental benefit over the regimen we evaluated, they do so with significant increases in expense, and their long-term role remains economically undefined. On the basis of our previous analysis,15 providing these medications in the short run appears to be an economically viable strategy. Finally, we did not evaluate the impact of providing insurance coverage to those currently without insurance. Although doing so would result in significant increases in drug costs faced by insurers, it also would likely induce a significantly greater number of currently nonadherent patients to begin using these drugs than among the currently insured population we assessed.
Conclusions
This Markov model–based analysis suggests that compared with the current Part D drug benefit, providing full coverage for combination therapy to post-MI Medicare beneficiaries would both improve clinical outcomes and reduce costs from a societal perspective. Our results extend the growing evidence that reducing patient cost sharing for essential medications is an effective strategy to enhance medication adherence in the face of rising healthcare costs and suggest that ongoing efforts to implement this strategy in the private sector should be carefully evaluated and potentially extended to large public insurers such as Medicare.
CLINICAL PERSPECTIVE
Effective therapies for the secondary prevention of coronary heart disease–related events are significantly underused, and attempts to improve adherence have often yielded disappointing results. Elimination of patient out-of-pocket costs may be an effective strategy to enhance medication use. We created a Markov cost-effectiveness model to estimate the incremental cost-effectiveness of providing Medicare beneficiaries with full coverage for combination pharmacotherapy compared with current coverage under the Medicare Part D program. We found that compared with current prescription drug coverage, full coverage for post–myocardial infarction secondary preventive therapies would result in greater functional life expectancy (0.35 quality-adjusted life-year) and less resource use ($2500). The elimination of patient cost sharing for medications commonly prescribed to post–myocardial infarction patients would therefore both improve health and save money from the societal perspective. The magnitude of observed changes in health outcomes is large and substantially greater than that observed for other interventions widely used to improve post–myocardial infarction outcomes. From the standpoint of Medicare, full drug coverage was highly cost-effective ($7182/quality-adjusted life-year) but not cost saving. Our results strongly support the hypothesis that post–myocardial infarction Medicare beneficiaries should receive statins, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, and β-blockers without cost sharing and emphasize the urgent need for prospective clinical studies to confirm the effectiveness of this approach.
Reprints: Information about reprints can be found online at http://www.lww.com/reprints
Disclosures
Dr Antman is a member of the Thrombolysis in Myocardial Infarction Study Group, which receives grant support for clinical trials from Pfizer, BMS, Merck, COR, Schering-Plough, and Sanofi-Aventis. The other authors report no conflicts.
References
- 1.Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, Hochman JS, Krumholz HM, Kushner FG, Lamas GA, Mullany CJ, Ornato JP, Pearle DL, Sloan MA, Smith SC, Jr, Alpert JS, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Gregoratos G, Halperin JL, Hiratzka LF, Hunt SA, Jacobs AK, Ornato JP. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction) J Am Coll Cardiol. 2004;44:E1–E211. doi: 10.1016/j.jacc.2004.07.014. [DOI] [PubMed]
- 2.Wald NJ, Law MR. A strategy to reduce cardiovascular disease by more than 80% BMJ. 2003;326:1419. doi: 10.1136/bmj.326.7404.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGovern PG, Jacobs DR, Jr, Shahar E, Arnett DK, Folsom AR, Blackburn H, Luepker RV. Trends in acute coronary heart disease mortality, morbidity, and medical care from 1985 through 1997: the Minnesota Heart Survey. Circulation. 2001;104:19–24. doi: 10.1161/01.cir.104.1.19. [DOI] [PubMed] [Google Scholar]
- 4.Stafford RS, Radley DC. The underutilization of cardiac medications of proven benefit, 1990 to 2002. J Am Coll Cardiol. 2003;41:56–61. doi: 10.1016/s0735-1097(02)02670-0. [DOI] [PubMed] [Google Scholar]
- 5.Newby LK, LaPointe NM, Chen AY, Kramer JM, Hammill BG, DeLong ER, Muhlbaier LH, Califf RM. Long-term adherence to evidence-based secondary prevention therapies in coronary artery disease. Circulation. 2006;113:203–212. doi: 10.1161/CIRCULATIONAHA.105.505636. [DOI] [PubMed] [Google Scholar]
- 6.Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA. 2002;288:462–467. doi: 10.1001/jama.288.4.462. [DOI] [PubMed] [Google Scholar]
- 7.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 8.Federman AD, Adams AS, Ross-Degnan D, Soumerai SB, Ayanian JZ. Supplemental insurance and use of effective cardiovascular drugs among elderly Medicare beneficiaries with coronary heart disease. JAMA. 2001;286:1732–1739. doi: 10.1001/jama.286.14.1732. [DOI] [PubMed] [Google Scholar]
- 9.Tamblyn R, Laprise R, Hanley JA, Abrahamowicz M, Scott S, Mayo N, Hurley J, Grad R, Latimer E, Perreault R, McLeod P, Huang A, Larochelle P, Mallet L. Adverse events associated with prescription drug cost-sharing among poor and elderly persons. JAMA. 2001;285:421–429. doi: 10.1001/jama.285.4.421. [DOI] [PubMed] [Google Scholar]
- 10.Roblin DW, Platt R, Goodman MJ, Hsu J, Nelson WW, Smith DH, Andrade SE, Soumerai SB. Effect of increased cost-sharing on oral hypoglycemic use in five managed care organizations: how much is too much? Med Care. 2005;43:951–959. doi: 10.1097/01.mlr.0000178216.23514.b7. [DOI] [PubMed] [Google Scholar]
- 11.Goldman DP, Joyce GF, Escarce JJ, Pace JE, Solomon MD, Laouri M, Landsman PB, Teutsch SM. Pharmacy benefits and the use of drugs by the chronically ill. JAMA. 2004;291:2344–2350. doi: 10.1001/jama.291.19.2344. [DOI] [PubMed] [Google Scholar]
- 12.Cubanski J, Neuman P. Status report on Medicare Part D enrollment in 2006: analysis of plan-specific market share and coverage. Health Aff (Millwood) 2007;26:w1–w12. doi: 10.1377/hlthaff.26.1.w1. [DOI] [PubMed] [Google Scholar]
- 13.Goldman DP, Joyce GF, Zheng Y. Prescription drug cost sharing: associations with medication and medical utilization and spending and health. JAMA. 2007;298:61–69. doi: 10.1001/jama.298.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu J, Price M, Huang J, Brand R, Fung V, Hui R, Fireman B, Newhouse JP, Selby JV. Unintended consequences of caps on Medicare drug benefits. N Engl J Med. 2006;354:2349–2359. doi: 10.1056/NEJMsa054436. [DOI] [PubMed] [Google Scholar]
- 15.Choudhry NK, Avorn J, Antman EM, Schneeweiss S, Shrank WH. Should patients receive secondary prevention medications for free after a myocardial infarction? An economic analysis. Health Aff (Millwood) 2007;26:186–194. doi: 10.1377/hlthaff.26.1.186. [DOI] [PubMed] [Google Scholar]
- 16.Actuarial Research Corporation, The Henry J. Kaiser Family Foundation. [Accessed March 20, 2006];Estimates of Medicare beneficiaries’ out-of-pocket drug spending in 2006: modeling the impact of the MMA. Available at: www.kff.org/medicare/7201.cfm.
- 17.Contoyannis P, Hurley J, Grootendorst P, Jeon SH, Tamblyn R. Estimating the price elasticity of expenditure for prescription drugs in the presence of non-linear price schedules: an illustration from Quebec, Canada. Health Econ. 2005;14:909–923. doi: 10.1002/hec.1041. [DOI] [PubMed] [Google Scholar]
- 18.Hippisley-Cox J, Coupland C. Effect of combinations of drugs on all cause mortality in patients with ischaemic heart disease: nested case-control analysis. BMJ. 2005;330:1059–1063. doi: 10.1136/bmj.330.7499.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White C. “Polypill” to fight cardiovascular disease: summary of rapid responses. BMJ. 2003;327:809. [Google Scholar]
- 20.Combination pharmacotherapy for cardiovascular disease. Ann Intern Med. 2005;143:593–599. doi: 10.7326/0003-4819-143-8-200510180-00010. [DOI] [PubMed] [Google Scholar]
- 21.2003 Nationwide Inpatient Sample. Rockville, Md: Agency for Healthcare Research and Quality; 2003. [Google Scholar]
- 22.Smith SC, Jr, Gilpin E, Ahnve S, Dittrich H, Nicod P, Henning H, Ross J., Jr Outlook after acute myocardial infarction in the very elderly compared with that in patients aged 65 to 75 years. J Am Coll Cardiol. 1990;16:784–792. doi: 10.1016/s0735-1097(10)80322-5. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control National Center for Health Statistics. [Accessed May 1, 2006];Deaths from 39 selected causes by place of death, status of decedent when death occurred in hospital or medical center, and age: United States, 2002. Available at: http://www.cdc.gov/nchs/data/dvs/MortFinal2002_WorkTable307.pdf.
- 24.Peeters A, Mamun AA, Willekens F, Bonneux L. A cardiovascular life history: a life course analysis of the original Framingham Heart Study cohort. Eur Heart J. 2002;23:458–466. doi: 10.1053/euhj.2001.2838. [DOI] [PubMed] [Google Scholar]
- 25.Mahoney EM, Mehta S, Yuan Y, Jackson J, Chen R, Gabriel S, Lamy A, Culler S, Caro J, Yusuf S, Weintraub WS. Long-term cost-effectiveness of early and sustained clopidogrel therapy for up to 1 year in patients undergoing percutaneous coronary intervention after presenting with acute coronary syndromes without ST-segment elevation. Am Heart J. 2006;151:219–227. doi: 10.1016/j.ahj.2005.02.044. [DOI] [PubMed] [Google Scholar]
- 26.Arias E. [Accessed May 1, 2006];United States life tables, 2000. Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr51/nvsr51_03.pdf. [PubMed]
- 27.Kochanek KD, Murphy SL, Anderson RN, Scott C. [Accessed May 1, 2006];Deaths: final data for 2002. Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr53/nvsr53_05.pdf. [PubMed]
- 28. [Accessed October 3, 2006];Drugstore.com. Available at: www.drugstore.com.
- 29.Centers for Medicare and Medicaid Services. [Accessed May 1, 2006];Last year of life expenditures. Available at: http://www.cms.hhs.gov/mcbs/downloads/issue10.pdf.
- 30.Pope GC, Kautter J, Ellis RP, Ash AS, Ayanian JZ, Lezzoni LI, Ingber MJ, Levy JM, Robst J. Risk adjustment of Medicare capitation payments using the CMS-HCC model. Health Care Financing Rev. 2004;25:119–141. [PMC free article] [PubMed] [Google Scholar]
- 31.Retchin SM, Brown RS, Yeh SC, Chu D, Moreno L. Outcomes of stroke patients in Medicare fee for service and managed care. JAMA. 1997;278:119–124. [PubMed] [Google Scholar]
- 32.Ahmed A, Allman RM, DeLong JF. Predictors of nursing home admission for older adults hospitalized with heart failure. Arch Gerontol Geriatr. 2003;36:117–126. doi: 10.1016/s0167-4943(02)00063-8. [DOI] [PubMed] [Google Scholar]
- 33. [Accessed June 25, 2007];Genworth Financial 2006 Cost of Care Survey: nursing homes, assisted living facilities and home care providers. Available at: http://www.aahsa.org/advocacy/assisted_living/reports_data/documents/Genworth_cost_study.pdf.
- 34.Bureau of Labor Statistics. [Accessed June 25, 2006];National occupational employment and wage estimates. Available at: http://www.bls.gov/oes/current/oes_nat.htm#b31-0000.
- 35.Hickenbottom SL, Fendrick AM, Kutcher JS, Kabeto MU, Katz SJ, Langa KM. A national study of the quantity and cost of informal care-giving for the elderly with stroke. Neurology. 2002;58:1754–1759. doi: 10.1212/wnl.58.12.1754. [DOI] [PubMed] [Google Scholar]
- 36.Tsevat J, Goldman L, Soukup JR, Lamas GA, Connors KF, Chapin CC, Lee TH. Stability of time-tradeoff utilities in survivors of myocardial infarction. Med Decis Making. 1993;13:161–165. doi: 10.1177/0272989X9301300210. [DOI] [PubMed] [Google Scholar]
- 37.Mathias SD, Bates MM, Pasta DJ, Cisternas MG, Feeny D, Patrick DL. Use of the Health Utilities Index with stroke patients and their caregivers. Stroke. 1997;28:1888–1894. doi: 10.1161/01.str.28.10.1888. [DOI] [PubMed] [Google Scholar]
- 38.Stinnett AA, Mittleman MA, Weinstein MC. Cost-effectiveness of dietary and pharmacologic therapy for cholesterol reduction in adults. In: Gold MR, Siegel JE, Russel LB, Weinstein MC, editors. Cost-Effectiveness in Health and Medicine. New York, NY: Oxford University Press; 1996. [Google Scholar]
- 39.Schleinitz MD, Heidenreich PA. A cost-effectiveness analysis of combination antiplatelet therapy for high-risk acute coronary syndromes: clopidogrel plus aspirin versus aspirin alone. Ann Intern Med. 2005;142:251–259. doi: 10.7326/0003-4819-142-4-200502150-00007. [DOI] [PubMed] [Google Scholar]
- 40.for the Tactics-TIMI Investigators. Mahoney EM, Jurkovitz CT, Chu H, Becker ER, Culler S, Kosinski AS, Robertson DH, Alexander C, Nag S, Cook JR, Demopoulos LA, DiBattiste PM, Cannon CP, Weintraub WS. Cost and cost-effectiveness of an early invasive vs conservative strategy for the treatment of unstable angina and non-ST-segment elevation myocardial infarction. JAMA. 2002;288:1851–1858. doi: 10.1001/jama.288.15.1851. [DOI] [PubMed] [Google Scholar]
- 41.Rosen AB, Hamel MB, Weinstein MC, Cutler DM, Fendrick AM, Vijan S. Cost-effectiveness of full medicare coverage of angiotensin-converting enzyme inhibitors for beneficiaries with diabetes. Ann Intern Med. 2005;143:89–99. doi: 10.7326/0003-4819-143-2-200507190-00007. [DOI] [PubMed] [Google Scholar]
- 42.Kripalani S, Yao X, Haynes RB. Interventions to enhance medication adherence in chronic medical conditions: a systematic review. Arch Intern Med. 2007;167:540–549. doi: 10.1001/archinte.167.6.540. [DOI] [PubMed] [Google Scholar]
- 43.Chernew ME, Rosen AB, Fendrick AM. Value-based insurance design. Health Aff (Millwood) 2007;26:w195–w203. doi: 10.1377/hlthaff.26.2.w195. [DOI] [PubMed] [Google Scholar]
- 44.Goldman DP, Joyce GF, Karaca-Mandic P. Varying pharmacy benefits with clinical status: the case of cholesterol-lowering therapy. Am J Manag Care. 2006;12:21–28. [PubMed] [Google Scholar]
- 45.Yen EF, Hardinger K, Brennan DC, Woodward RS, Desai NM, Crippin JS, Gage BF, Schnitzler MA. Cost-effectiveness of extending Medicare coverage of immunosuppressive medications to the life of a kidney transplant. Am J Transplant. 2004;4:1703–1708. doi: 10.1111/j.1600-6143.2004.00565.x. [DOI] [PubMed] [Google Scholar]
- 46.Mahoney JJ. Reducing patient drug acquisition costs can lower diabetes health claims. Am J Manag Care. 2005;11:S170–S176. [PubMed] [Google Scholar]
- 47.Fuhrmans V. New tack on copays: cutting them. Wall Street Journal. 2007 May 8;:D1. [Google Scholar]