Abstract
In multicellular organisms, cell size is tightly controlled by nutrients and growth factors. Increasing ambient glucose induces enhanced protein synthesis and cell size. Continued exposure of cells to high glucose in vivo, as apparent under pathological conditions, results in cell hypertrophy and tissue damage. We demonstrate that activation of TGF-β signaling has a central role in glucose-induced cell hypertrophy in fibroblasts and epithelial cells. Blocking the kinase activity of the TβRI receptor or loss of its expression prevented the effects of high glucose on protein synthesis and cell size. Exposure of cells to high glucose induced a rapid increase in cell surface levels of the TβRI and TβRII receptors, and a rapid activation of TGF-β ligand by matrix metalloproteinases, including MMP-2 and MMP-9. The consequent autocrine TGF-β signaling in response to glucose led to Akt-TOR pathway activation. Accordingly, preventing MMP-2/MMP-9 or TGF-β-induced TOR activation inhibited high glucose-induced cell hypertrophy.
Introduction
Cell size is highly controlled and its deregulation has been implicated in obesity, diabetes and cancer. Cell growth is defined as increase in cell mass, often associated with increased protein synthesis (Mamane et al., 2006). The best characterized signaling pathway that regulates cell size is defined by the sequential activation of phosphatidylinositol 3-kinase (PI3K), Akt, TOR and S6 kinase. Growth factors that act through tyrosine kinase receptors, such as insulin and IGF-1, activate PI3K, thus enhancing the phosphorylation of Akt. Consequent activation of mTOR results in phosphorylation of S6 kinase and 4E–BP1, leading to enhanced translation (Hay and Sonenberg, 2004; Manning and Cantley, 2007). The contribution of additional signaling pathways that control cell size during homeostasis remains poorly understood.
Glucose is an essential nutrient for cells and provides energy for cell growth. After being transported into the cell by glucose transporters, glucose undergoes a metabolic process known as glycolysis, which generates ATP and NADPH as energy source and regulates the activity of TOR, protein synthesis and cell size (Herman and Kahn, 2006). High glucose induces increased protein synthesis and cell size, and promotes cell hypertrophy in various tissues and organs, including muscle, kidney and heart Elevated levels of blood glucose, i.e. hyperglycemia, consequently increase the risk and complications of diseases such as obesity, diabetes and heart disease (Wolf and Ziyadeh, 1999; Sartorelli and Fulco, 2004; Neubauer, 2007). How glucose induces increased cell size is poorly understood. Increased Akt activity has been shown to stimulate transport and metabolism of glucose and triggers TOR-dependent increases in protein translation (Plas and Thompson, 2005; Manning and Cantley, 2007).
Several observations correlate hyperglycemia to increased activity of transforming growth factor-β (TGF-β). In diabetic patients and rodent models of diabetes, continuous exposure of cells to high glucose has been linked to hypertrophy of proximal tubular and mesangial cells, and accumulation of extracellular matrix proteins and fibrosis (Ziyadeh, 2004). Consistent with the induction of extracellular matrix protein expression by TGF-β and with TGF-β’s role in fibrosis (Zavadil and Bottinger, 2005), TGF-β1 levels were increased in the glomerular and tubular compartments of the kidney in rodent models of diabetes, and Smad3 activation was observed in these cells (Kolm-Litty et al., 1998; Hong et al., 2001; Isono et al., 2002). High glucose was also shown to induce TGF-β expression, leading to production of extracellular matrix proteins (Ziyadeh et al., 1994), and exposure of cells to high glucose can increase the expression of TGF-β1 and/or the TβRII receptor (Hong et al., 2001; Iglesias-de la Cruz et al., 2002). These observations suggest a functional linkage of glucose-stimulated increase of protein synthesis, in particular of extracellular matrix proteins, with increased TGF-β signaling. However, a direct role of TGF-β signaling in the glucose-stimulated increase in cell size has not been revealed.
TGF-β, the prototype of a 33-member TGF-β family, acts through cell surface receptor complexes of two type I and two type II receptors, i.e. TβRI and TβRII. Following ligand binding, the TβRII receptors phosphorylate and activate the TβRI receptors, which C-terminally phosphorylate and thereby activate Smad2 and Smad3. These then form a complex with Smad4, translocate into the nucleus, and regulate the transcription of TGF-β responsive genes (Shi and Massague, 2003; Feng and Derynck, 2005). Smad signaling does not account for other TGF-β responses and, accordingly, non-Smad mechanisms that relay TGF-β signals have been characterized (Derynck and Zhang, 2003; Moustakas and Heldin, 2005). Recent findings revealed that TGF-β can activate PI3K, leading to activation of the PI3K–Akt-TOR-S6 kinase pathway in response to TGF-β. Activation of this pathway by TGF-β was observed in cells undergoing epithelial to mesenchymal transition, and allows TGF-β to directly regulate translation, complementing the Smad-mediated transcription regulation, and to enhance cell size (Lamouille and Derynck, 2007; Das et al., 2008).
We explored the physiological connection between the effects of glucose on cell metabolism and TGF-β signaling, based on our observation that TGF-β can induce increased cell size through activation of Akt-TOR signaling (Lamouille and Derynck, 2007). We found that, in fibroblasts and epithelial cells, glucose-induced increase in cell size was blocked by inhibiting the function of the TβRI kinase, thus blocking TGF-β signaling. Glucose induced a rapid externalization of the TβRII and TβRI receptors at the cell surface, and metalloproteinase-mediated TGF-β activation, thus strongly enhancing autocrine TGF-β signaling, and, in turn, activating the Akt-TOR pathway. Consequently, high glucose-induced cell hypertrophy was also inhibited by preventing matrix metalloprotease-2/9 activation or TGF-β-induced TOR activation. These observations have relevance for the physiology of hyperglycemia-induced pathologies that are associated with tissue hypertrophy, including cancer.
Results
Glucose increases cell size and protein content
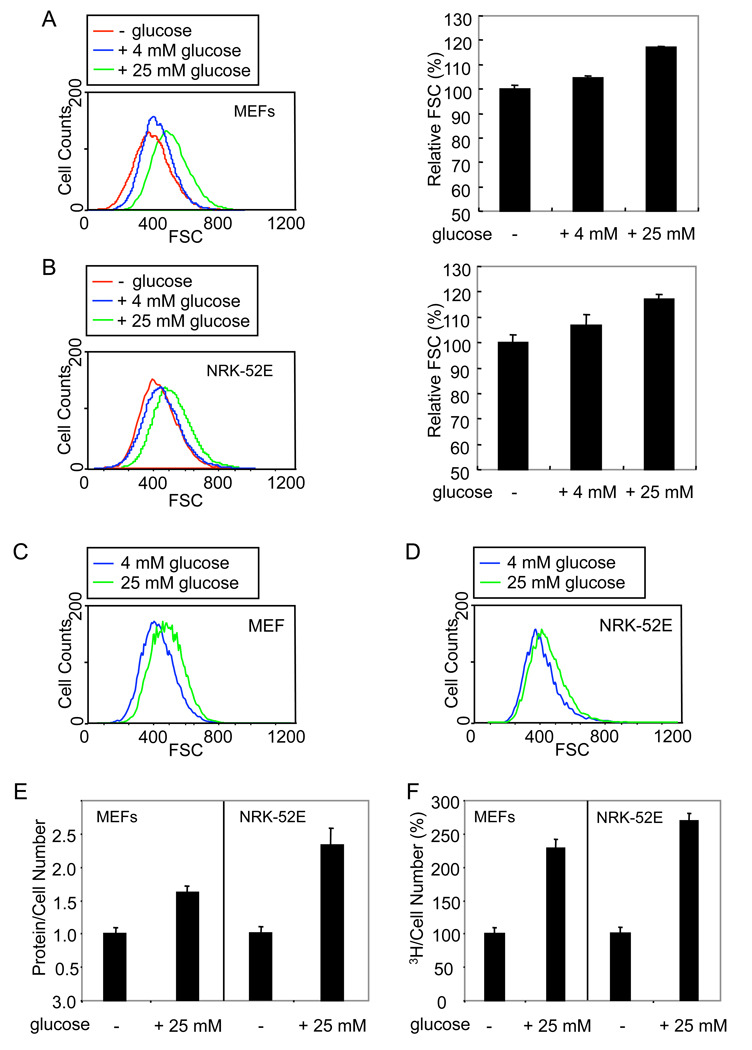
To evaluate the effect of glucose, we analyzed cells by flow cytometry using forward light scatter as parameter indicative of cell size. Since cell size varies with changes in cell cycle (Coelho and Leevers, 2000; Pyronnet and Sonenberg, 2001), we examined the size of only the cells in the G1 phase, although the effects of glucose on cell cycle were minor (data not shown). Mouse embryonic fibroblasts (MEFs) and rat kidney epithelial NRK-52E cells were cultured overnight without glucose and then exposed to medium containing 4 mM (normal) or 25 mM (high) glucose. In these cells, 4 mM glucose induced a 3–10% increase in cell size, and 25 mM glucose induced an increase in cell size of 20–50% after 24 h (Fig. 1A, B). Cells cultured in 4 mM glucose underwent a 5–20% increase in cell size following exposure to 25 mM glucose after 24 h (Fig. 1C, D). The increase in cell size induced by 25 mM glucose was reversible upon withdrawal of glucose or a decrease to 4 mM glucose (Suppl. Fig. 1A–D). As osmolarity control, 25 mM mannose (Tesfamariam et al., 1990) did not increase cell size (Suppl. Fig. 1E). Similarly to MEFs and NRK-52E cells, a shift from 4 mM to 25 mM glucose induced an increase in cell size of human umbilical vein endothelial cells, T4-2 breast carcinoma cells and HepG2 hepatoma cells (Suppl. Fig. 2).
Figure 1. Glucose induces increase in cell size and protein synthesis.
(A, B) MEFs (A) and NRK-52E cells (B) were cultured without glucose for 24 h, and shifted to medium containing 0, 4 or 25 mM glucose for 24 h. Cells in G1 phase were analyzed by flow cytometry using the forward light scatter (FSC) parameter, and relative cell size distribution was determined. (C, D) FSC profiles of MEFs (C) and NRK-52E cells (D) cultured with 4 mM glucose and then shifted to medium with 4 or 25 mM glucose. The FSC profiles of cells in G1 were obtained as in A, B. (E) Protein content of MEFs or NRK-52E cells in G1, cultured without glucose for 24 h, and then shifted to medium without or with 25 mM glucose for 24 h. (F) New protein synthesis in MEFs or NRK-52E cells cultured without glucose for 24 h and then shifted to medium without or with 25 mM glucose for 24 h. Values were normalized for cell number, relative to untreated cells.
Because increased cell size often correlates with increased protein content, we measured the protein content in untreated and glucose-treated cells. Glucose increased the cell protein content by 60–90% in MEFs and 100–170% in NRK-52E cells after 24 h (Fig. 1E). Accordingly, glucose induced increased protein synthesis, measured by incorporation of 3H-labeled leucine into newly synthesized proteins (Fig. 1F). Similarly to MEFs and NRK-52E cells, glucose induced an increase in cell size and protein content in HepG2 carcinoma cells (data not shown). These results indicate that glucose can stimulate increase of protein synthesis and cell size.
TGF-β receptor activity is required for glucose-induced increase of cell size
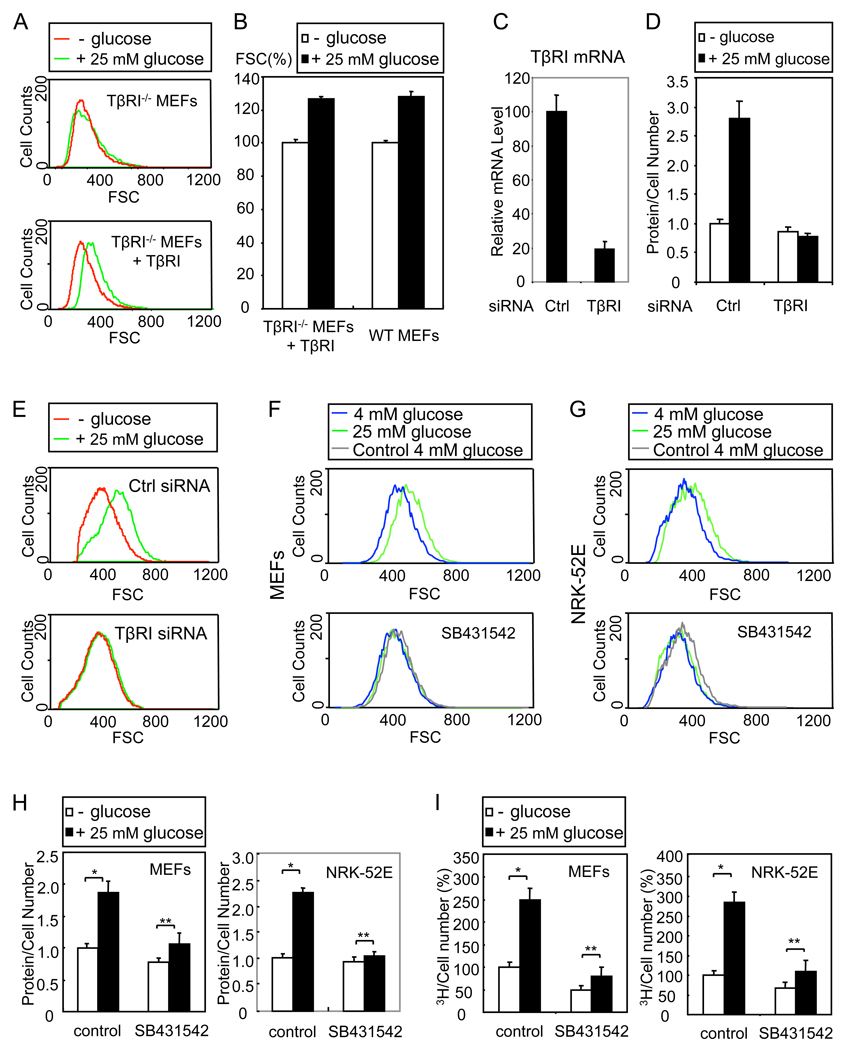
TGF-β induces increased cell size and protein content in cells undergoing epithelial to mesenchymal transition (Lamouille and Derynck, 2007). Since the TβRI receptor activates TGF-β-induced transcription and translation responses, we examined the effect of glucose in TβRI−/− MEFs. In contrast to wild-type MEFs (Fig. 1A), glucose did not significantly increase the size of the TβRI−/− MEFs (Fig. 2A, top). However, ectopic expression of TβRI in the TβRI−/−MEFs restored the glucose-induced increase in cell size (Fig. 2A, bottom, Fig. 2B). We also knocked down the expression of TβRI in MEFs using siRNA for mouse TβRI, which resulted in an 80% decrease in TβRI mRNA level (Fig. 2C) and nearly complete downregulation of TGF-β-induced expression of the TGF-β responsive Smad7 gene (data not shown). Downregulation of TβRI expression blocked the glucose-induced increase of protein content (Fig. 2D) and cell size (Fig. 2E) in MEFs. We obtained similar results in HepG2 cells using siRNA for human TβRI (data not shown). We conclude that the glucose-induced increase of cell size and protein content requires TβRI.
Figure 2. Glucose-induced increase in cell size and protein content requires Tβ RI kinase.
(A) Cell size distribution of TβRI−/− MEFs and TβRI−/− MEFs expressing a reintroduced TβRI (TβRI−/− MEFs + TβRI), cultured without or with 25 mM glucose for 24 h. Only cells in G1 phase were analyzed. (B) FSC profiles of wild-type (WT) MEFs and TβRI−/− MEFs + TβRI in G1, cultured without or with 25 mM glucose for 24 h. The relative mean values with SD (error bars) for 5 replicates are shown as a bar graph. (C) MEFs were transfected with TβRI siRNA or control siRNA (Ctrl). TβRI mRNA was quantified using qRT-PCR. (D) MEFs, co-transfected with green fluorescent siRNA and control or TβRI siRNA, were cultured without or with 25 mM glucose for 24 h. The protein content of green fluorescent cells in G1 phase was measured. (E) Cell size distribution of MEFs in G1, transfected with siRNA and cultured as in D, determined using the FSC parameter. (F, G) Cell size distribution of MEFs (F) or NRK-52E cells (G) in G1, cultured with 4 or 25 mM glucose for 24 h in the absence or presence of SB431542. The grey lines, marked “Control 4 mM glucose”, in the right panels correspond to the 4 mM glucose profiles in the absence of SB431542 that are shown in the left panels. (H) Protein content of MEFs or NRK-52E cells in G1, cultured without or with 25 mM glucose for 24 h in the absence or presence of SB431542. (I) New protein synthesis of MEFs or NRK-52E cells, cultured without or with 25 mM glucose for 24 h in the absence or presence of SB431542. Values were normalized to the cell number relative to untreated cells (*, P < 0.01; **, P > 0.05).
To examine the role of the TβRI kinase in the glucose-induced increase in cell size and protein content, we treated the MEFs and NRK-52E cells with the kinase inhibitor SB431542, which specifically targets the TβRI receptor (Inman et al., 2002). SB431542 inhibited the glucose-induced increase of cell size (Fig. 2F, G) and protein synthesis (Fig. 2H, I) in both cell types, and HepG2 cells (data not shown). The cells in the presence of SB431542 were slightly smaller than those in the absence of SB431542, suggesting a contribution of autocrine TGF-β signaling to cell size (Fig. 2F, G). Similar observations were made in human umbilical vein endothelial cells, T42 breast cancer cells and HepG2 hepatoma cells (Suppl. Fig. 2). These data indicate that the TβRI kinase is essential for the regulation of protein synthesis and cell size by glucose.
TGF-β signaling increases cell size and protein content in glucose-responsive cells
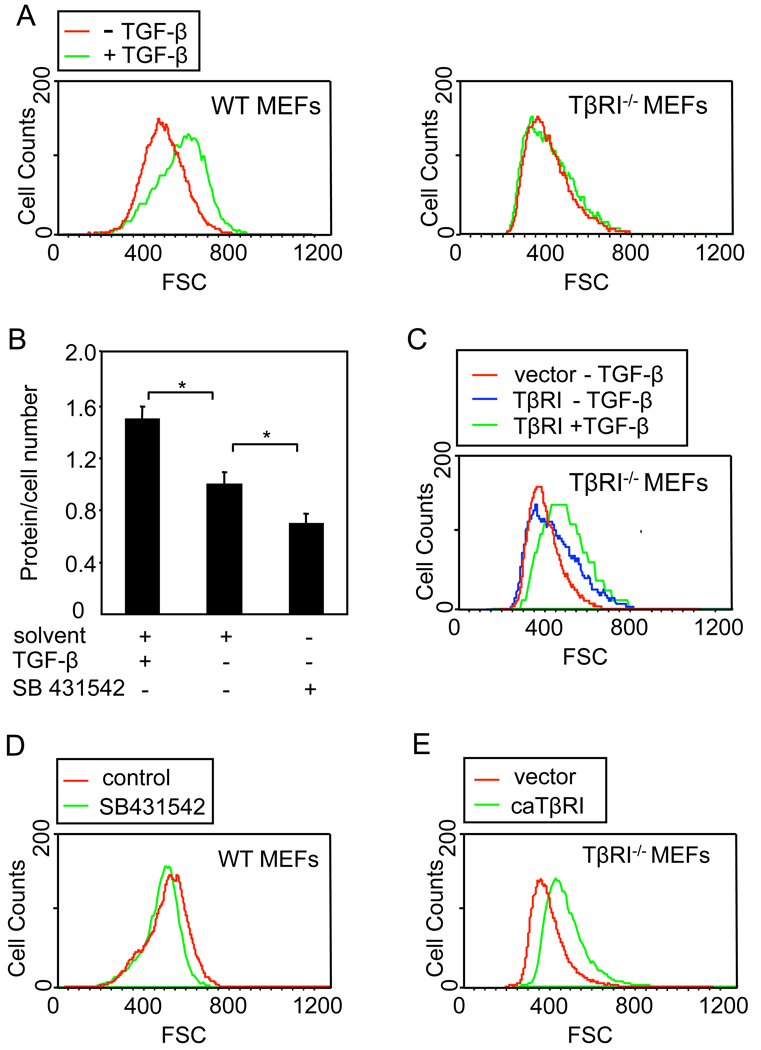
To evaluate whether TGF-β signaling leads to increased cell size in glucose-responsive cells, we treated the MEFs with TGF-β, which had only a minor effect on the cell cycle (data not shown), TGF-β induced an increase in cell size (Fig. 3A) and protein content (Fig. 3B) of wild-type MEFs in G1 phase. In contrast, TGF-β did not affect the cell size of TβRI−/− MEFs (Fig. 3A), but ectopic TβRI expression resulted in increased cell size and restored the stimulatory effect of TGF-β on cell size (Fig. 3C). Conversely, inhibition of autocrine TGF-β signaling with SB431542 resulted in decreased protein content and cell size (Fig. 3B, D). In addition, expression of a mutant TβRI (caTβRI) that activates TGF-β signaling independent of ligand led increased cell size in TβRI−/− MEFs (Fig. 3E). These data indicate that, in these cells, TGF-β signaling leads to increased cell size and protein synthesis. Together with our previous data, we conclude that the glucose-induced increase in cell size and protein synthesis requires functional TGF-β signaling and that activation of TGF-β signaling may mediate the glucose-induced cell size and protein synthesis.
Figure 3. TGF-β induces increases in cell size and protein synthesis.
(A) Cell size distribution of wild-type and TβRI−/− MEFs in G1, treated or not with TGF-β for 24 h. (B) Protein content of MEFs treated with TGF-β, SB431542 or solvent control for 24 h, normalized for cell number relative to untreated cells (*, P < 0.01). (C) Cell size distribution of TβRI−/− MEFs in G1, transfected with a TβRI expression plasmid or control vector, and treated or not with TGF-β for 24 h. (D) Cell size distribution of MEFs in G1, treated for 24 h with SB431542 or solvent control. (E) Cell size distribution of TβRI−/− MEFs in G1, transfected with a plasmid encoding constitutively active (ca) TβRI or control vector.
Glucose induces Smad3 activation and signaling
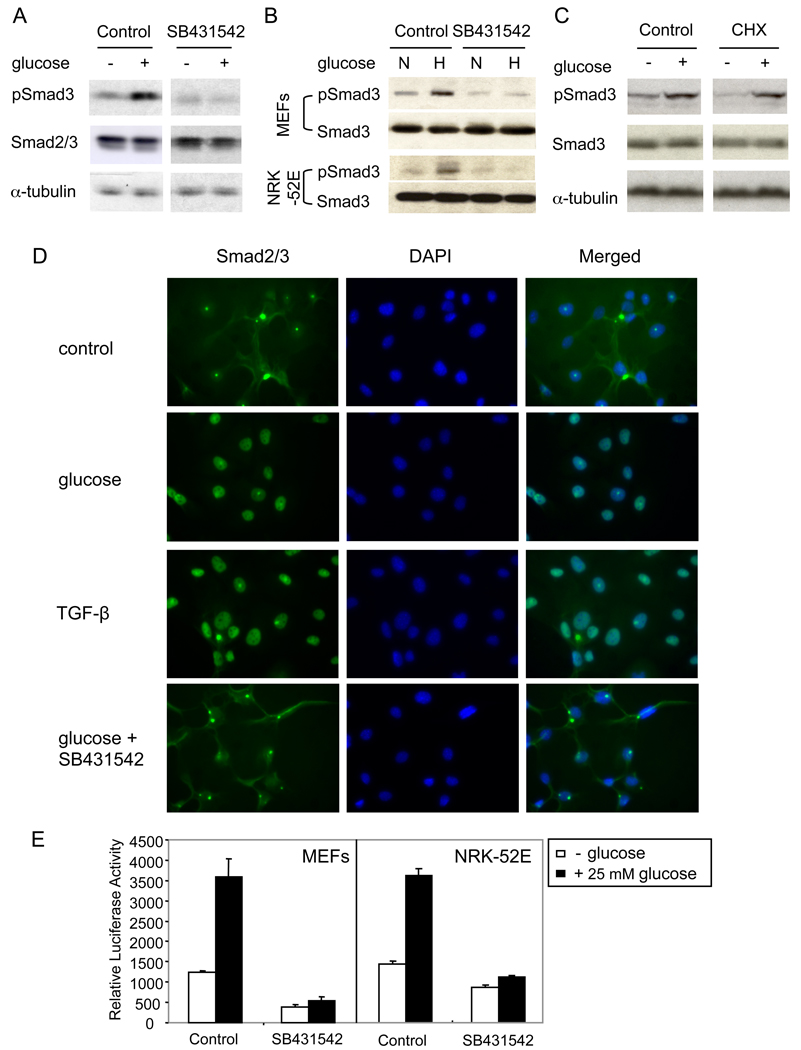
The requirement of TβRI signaling for glucose-induced increase in cell size and protein synthesis raises the possibility that glucose may activate TGF-β signaling. As shown in Fig. 4A, B, shifting the cells from no or 4 mM glucose to 25 mM glucose induced a rapid C-terminal phosphorylation of Smad3, similarly to Smad3 activation in response to TGF-β. Similarly to the effect of glucose on cell size, SB431542 blocked the activation of Smad3 by glucose, decreasing it to a level below the one in the presence of 4 mM glucose (Fig. 4A, B). The rapid activation of Smad3 by glucose suggested that this response does not require new protein synthesis. Accordingly, C-terminal phosphorylation of Smad3 in response to glucose occurred in the presence of the protein synthesis inhibitor cycloheximide (Fig. 4C). The increase in Smad3 activation in response to 25 mM glucose was still apparent after 24 h, and was reversed upon withdrawal of glucose or a decrease to 4 mM glucose (Suppl. Fig. 1F).
Figure 4. Glucose induces Smad phosphorylation and nuclear translocation.
(A) MEFs, cultured for 24 h without glucose, were incubated for 30 min without or with 25 mM glucose in the presence or absence of SB431542, lysed and assayed by western blotting for phospho-Smad3 (pSmad3), Smad2/3 or α-tubulin. (B) MEFs and NRK-52E cells, cultured with 4 mM glucose, were incubated for 30 min with normal (4 mM, N) or high (25 mM, H) glucose, with or without SB431542, lysed and immunoblotted for pSmad3 and Smad3. (C) MEFs, cultured for 24 h without glucose, were incubated for 30 min without or with 25 mM glucose in the absence or presence of cycloheximide (CHX), lysed and immunoblotted for pSmad3, Smad3 or α-tubulin. (D) MEFs were treated with 25 mM glucose, TGF-β or glucose and SB431542 for 30 min, and the subcellular localization of Smad2/3 was visualized. DAPI was used to stain the nuclei. (E) Effect of 25 mM glucose in the absence or presence of SB431542 on transcription from the 4xSBE-lux reporter, transfected into MEFs or NRK-52E cells.
Upon activation by TβRI, Smad2 and 3 undergo nuclear translocation. As shown in Fig. 4D, glucose induced nuclear translocation of Smad2/3, similarly to TGF-β. The glucose-induced nuclear translocation of Smad2 and Smad3 was blocked by SB431542 (Fig. 4D), indicating that it required the TβRI kinase activity.
Finally, we evaluated whether glucose activated the transcription activity of Smad3. As shown in Fig. 4E, glucose enhanced the transcription from the Smad3-responsive 4xSBE-lux promoter (Liberati et al., 2001) in MEFs and NRK-52E cells, indicating increased Smad3 activity. SB431542 blocked the glucose-induced transcription, and decreased the basal activity in this assay, an indication of autocrine TGF-β signaling under basal conditions and an essential role of TβRI activity in this response to glucose. These data demonstrate that glucose activates signaling by TβRI, resulting in Smad3 activation and Smad3-mediated transcription in the absence of added TGF-β.
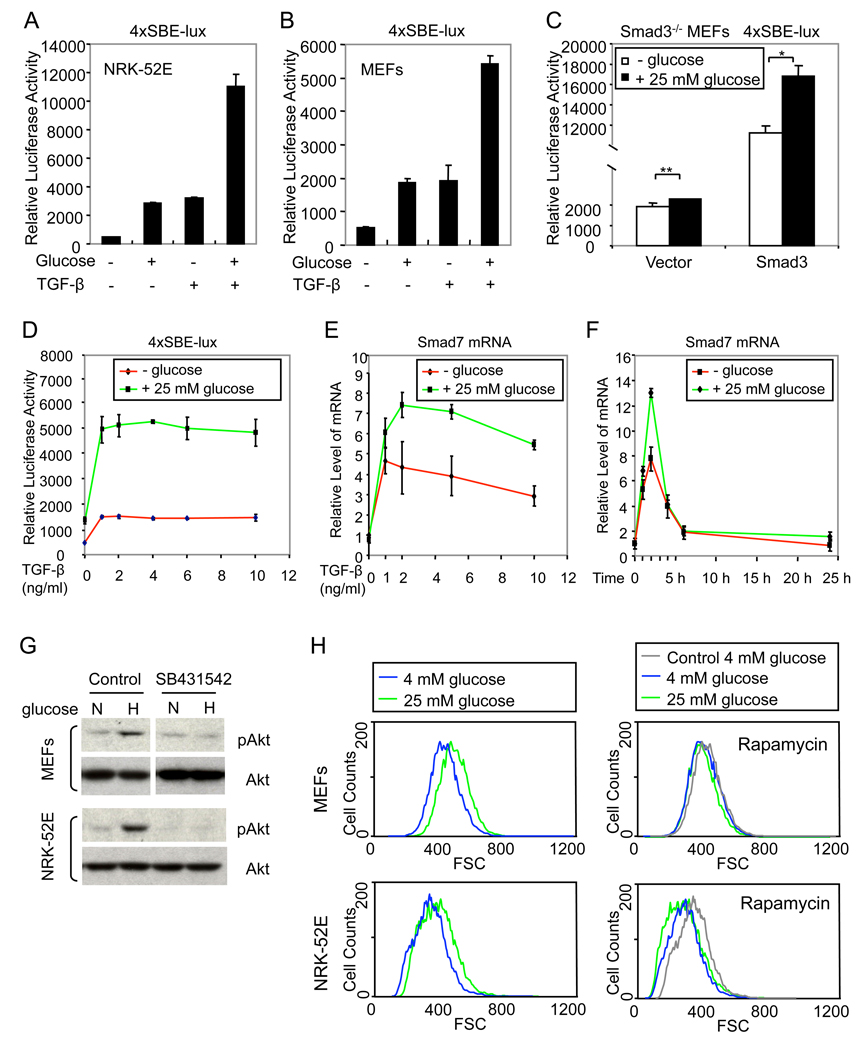
Glucose enhances TGF-β/Smad signaling
Since glucose activates TGF-β signaling, the response to exogenous TGF-β may depend on the level of glucose in the cell culture medium. We examined the effect of glucose on transcription from the Smad3-dependent 4xSBE-lux promoter. Glucose enhanced the basal transcription, which depends on autocrine TGF-β signaling, and the transcription response to activated TβRI in NRK-52E cells and MEFs (Fig. 5A, B). Glucose did not induce transcription from this reporter in Smad3−/− MEFs (Fig. 5C), but expression of Smad3 restored the induction of 4xSBE-dependent transcription and its increase by glucose (Fig. 5C). Furthermore, the TGF-β dose-dependent transcription from the Smad3-responsive 4xSBE promoter was substantially enhanced in the presence of glucose (Fig. 5D). Accordingly, glucose also enhanced the expression of Smad7 mRNA in response to TGF-β (Fig. 5E) without changing the time-dependence of the response (Fig. 5F). Together, these results indicate that glucose enhances the TGF-β-induced, Smad3-mediated transcription activation.
Figure 5. Glucose enhances TGF-β-induced Smad3 activity and Akt/TOR activation.
(A, B) Effect of TGF-β in the absence or presence of 25 mM glucose on transcription from the 4xSBE-lux reporter in NRK-52E cells (A) or MEFs (B). (C) Effect of 25 mM glucose on transcription from 4xSBE-lux in Smad3−/− MEFs transfected with a Smad3 expression plasmid or control vector (*, P < 0.01; **, P > 0.05). (D, E) Effect of increasing concentrations of TGF-β, in the absence or presence of 25 mM glucose, on transcription from 4xSBE-lux (D) and Smad7 mRNA expression, quantified by qRT-PCR (E), in MEFs. (F) Effect of TGF-β, added for different times in the absence or presence of 25 mM glucose, on Smad7 mRNA expression. (G) MEFs and NRK-52E cells were cultured with 4 mM glucose and then shifted for 5 min to normal (4 mM, N) or high (25 mM, H) glucose, with or without SB431542, lysed and immunoblotted for phospho-Akt (pAkt) and total Akt. (H) Cell size distribution of MEFs and NRK-52E cells, cultured with 4 mM glucose and then shifted to 4 mM or 25 mM glucose for 24 h in the absence or presence of rapamycin, was determined by flow cytometry using FSC as parameter. The grey lines, marked “Control 4 mM glucose”, in the right panels correspond to the 4 mM glucose profiles in the absence of rapamycin, shown in the left panels.
Since TGF-β activates Akt-TOR signaling (Lamouille and Derynck, 2007), we examined whether glucose also induces Akt activation in MEFs and NRK-52E cells. As shown in Fig. 5G, switching cells from 4 mM glucose to 25 mM glucose induced a rapid increase of Akt phosphorylation. Similarly to the activation of Smad3, Akt phosphorylation in response to high glucose was blocked by SB431542, indicating that it resulted from activation of TGF-β signaling. Furthermore, rapamycin, an inhibitor of TOR in TOR complex 1, prevented the 25 mM glucose-induced increase of cell size in both MEFs and NRK-52E cells (Fig. 5H), as well as in endothelial and cancer cells (Suppl. Fig. 2). As was seen in response to SB431542 (Fig. 2F, G), the cells in the presence of rapamycin were slightly smaller than those in the absence of rapamycin (Fig. 5H), suggesting a contribution of autocrine TGF-β signaling leading to TOR activation in the control of cell size. These results indicate that glucose induces activation of Akt through induction of TGF-β signaling, and that TGF-β-induced Akt-TOR signaling plays an essential role in glucose-regulated cell size.
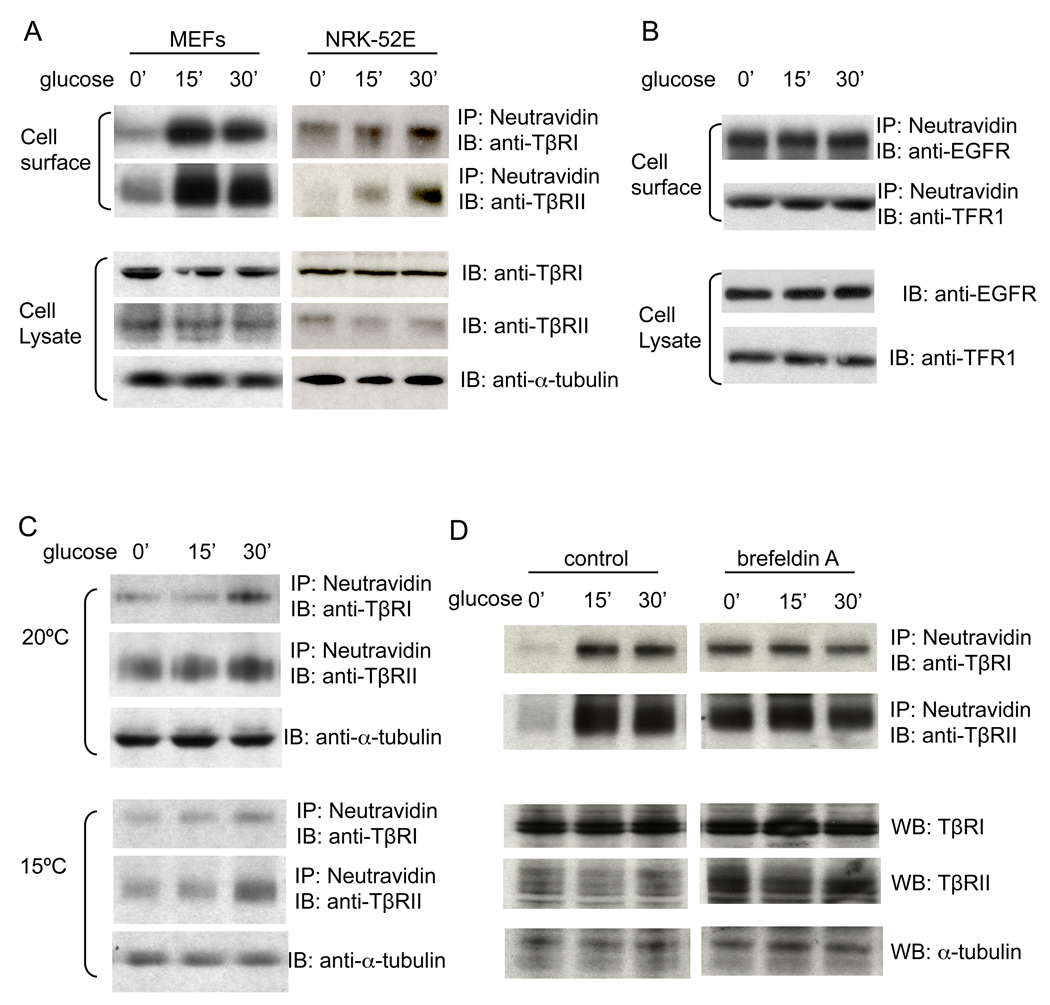
Glucose increases the cell surface expression of TGF-β receptors
The activation of Smad3 by glucose, and the glucose-induced increase in cell size and protein content, dependent upon TβRI, may be explained by an upregulation of the TGF-β signaling system. Since TGF-β signals through a complex of TβRII and TβRI, we investigated whether glucose affected the expression of either receptor. Glucose did not induce rapid changes of TβRII or TβRI mRNA (data not shown) or protein (Fig. 6A, lower panels) levels in MEFs or NRK-52E cells. In contrast, it induced a rapid and strong increase in cell surface levels of both TβRII and TβRI, as assessed by cell surface protein biotin labeling (Fig. 6A, upper panels). In MEFs, the levels of cell surface TGF-β receptors were strongly increased after 15 min of glucose addition, and started to decline after 30 min (Fig. 6A, left, upper), possibly due to endocytosis. In NRK-52E cells, glucose induced a slower increase of the cell surface levels of TβRI and TβRII, which was apparent after 15 min and more pronounced after 30 min (Fig. 6A, right, upper). Glucose did not induce changes in the cell surface levels of the EGF receptor or transferrin receptor 1 (Fig. 6B), suggesting that the effect of glucose on cell surface expression of the TGF-β receptors was specific.
Figure 6. Glucose increases the cell surface levels of TβRI and TβRII.
(A) MEFs and NRK-52E cells were treated with 25 mM glucose for 15 or 30 min. Cell surface proteins were labeled by biotinylation. Western blotting of the biotinylated proteins (upper) or total protein lysates (lower) was performed using the indicated antibodies. (B) MEFs were treated with 25 mM glucose for 15 or 30 min. Cell surface and whole cell lysate proteins were visualized as in A using antibodies for the EGF receptor (EGFR) or transferrin receptor 1 (TFR1). (C) MEFs were incubated at 20ºC or 15ºC for 4 h and then treated with 25 mM glucose for 15 or 30 min. (D) MEFs were pre-treated with brefeldin A or solvent control for 1 h and then treated with 25 mM glucose for 15 or 30 min in the presence or absence of brefeldin A. In C and D, cell surface and whole lysate proteins were visualized as in A.
Vesicular transport in the secretory pathway can be arrested by incubating cells at 15ºC or 20ºC to block exit from the endoplasmic reticulum (ER) or trans-Golgi network (Milgram and Mains, 1994; Saraste et al., 1986). Incubation of the MEFs at 20ºC strongly impaired the glucose-induced cell surface expression of both TβRI and TβRII; no increase in cell surface levels of TβRI or TβRII was apparent at 15 min, and only a modest increase was observed at 30 min (Fig. 6B, upper panels). At 15ºC, which impairs protein transport from the ER to the Golgi, the glucose-induced increase in TβRI and TβRII levels was also inhibited (Fig. 6B, lower panels). These data suggest that glucose rapidly enhances cell surface expression of TβRI and TβRII by stimulating their transport.
We also used brefeldin A (BFA) to block protein transport from the ER to the Golgi. BFA prevented the increase of cell surface TβRI and TβRII levels in response to glucose (Fig. 6D, right). In the absence of glucose treatment (i.e. at 0 min), cell surface levels of TGF-β receptors are higher in BFA-treated cells (right) than in the control group (left). This may be due to effects of BFA on endocytosis (Uhlin-Hansen and Yanagishita, 1995). Together, these data indicate that glucose stimulates the transport of TGF-β receptors from intracellular compartments to the cell surface.
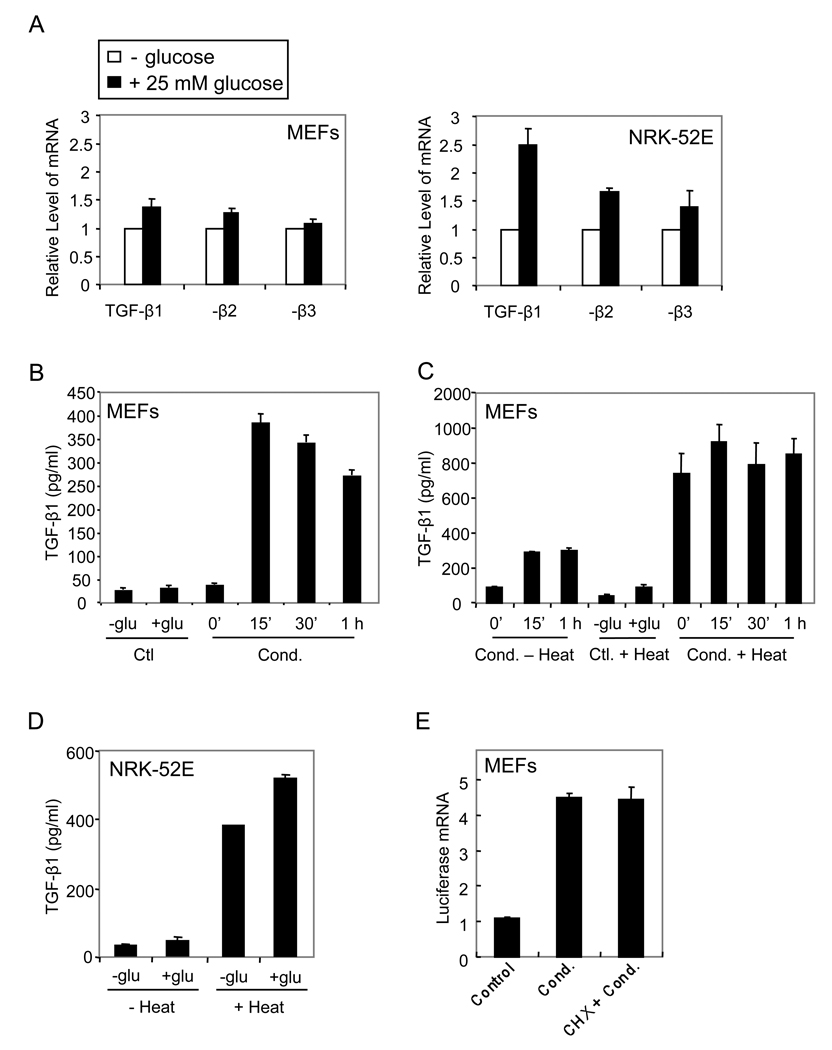
Glucose increases the activation of TGF-β
In addition to enhancing the cell surface levels of TGF-β receptors, glucose may also regulate the TGF-β ligand. Glucose did not affect the TGF-β1, TGF-β2 and TGF-β3 mRNA levels in MEFs, but moderately enhanced their levels in NRK-52E cells (Fig. 7A). We also determined the amount of active TGF-β ligand in the medium using a reporter cell line, TMLC, that scores TGF-β activity through induction of luciferase expression from an integrated promoter segment (Abe et al., 1994). Conditioned media of glucose-treated or untreated cells were assayed and calibrated against a concentration curve of TGF-β1. As shown in Fig. 7B, glucose induced the rapid generation of active TGF-β in MEFs, increasing the levels of active TGF-β 10-fold (from 38.8±4.2 to 390.3±50.0 pg/ml; n=6, p<0.05) at 15 min. No further enhancement of active TGF-β generation was seen at subsequent time points (Fig. 7B).
Figure 7. Glucose increases the production of bioactive TGF-β ligand.
(A) MEFs or NRK-52E cells were treated with 25 mM glucose for 3 h, and TGF-β1, -β2 and -β3 mRNA were quantified using RT-PCR. (B) 25 mM glucose was added to MEFs for different times, and the levels of active TGF-β in the conditioned media (Cond.) were scored using the TMLC reporter cells. Media containing no glucose (-glu) or 25 mM glucose (+glu) were used as controls (Ctl). (C) 25 mM glucose was added to MEFs for different times and the conditioned media were treated or not with heat, prior to the TMLC assay to measure active TGF-β. Media containing no glucose or 25 mM glucose, treated with heat, were used as controls. (D) 25 mM glucose was added to NRK-52E cells for 1 h, and the conditioned media were treated or not with heat prior to the TMLC assay. (E) 25 mM glucose was added to MEFs for 1 h in the absence or presence of cycloheximide (CHX), and the conditioned media (Cond.) or glucose-containing control medium (Ctl) were added to the TMLC reporter cells. RNA was extracted and luciferase mRNA was quantified by qRT-PCR.
Since TGF-β is secreted in a latent complex that requires activation to bind to the receptors (Annes et al., 2003), active TGF-β may be generated from latent TGF-β without changes in TGF-β levels, or through increased release of total TGF-β by the cells. To distinguish between these possibilities, we converted all latent TGF-β in the conditioned medium into active TGF-β using heat treatment, and measured the levels of TGF-β in the TMLC reporter assay. As shown in Fig. 7C, glucose did not enhance the total amount of TGF-β in the medium. Comparison of the active TGF-β levels without and after heat treatment revealed that a third of the total TGF-β was activated in response to glucose. These data indicate that glucose induced a rapid activation of latent TGF-β, without an effect on the total level of secreted TGF-β in MEFs. In NRK-52E cells, the active TGF-β ligand was barely measurable without heat treatment, and the total amount of secreted TGF-β, assessed after heat treatment, was moderately enhanced by glucose after 1 h (Fig. 7D), consistent with the increased TGF-β mRNA levels (Fig. 7A).
The rapid activation of TGF-β in response to glucose suggested that no new protein synthesis was required. To test this, we treated MEFs with glucose in the presence or absence of cycloheximide, added the conditioned media to the TMLC cells, and measured the induction of luciferase mRNA expression. As shown in Fig. 7E, the presence of cycloheximide did not affect the induction of luciferase mRNA. These results indicate that TGF-β activation in response to glucose does not require new protein synthesis, and that the activation of the TGF-β responsive reporter resulted from a direct induction by TGF-β in the medium, rather than from a component that might induce TGF-β expression in the reporter cells. Together, these data indicate that glucose induced a rapid activation of latent TGF-β, without the need for new protein synthesis.
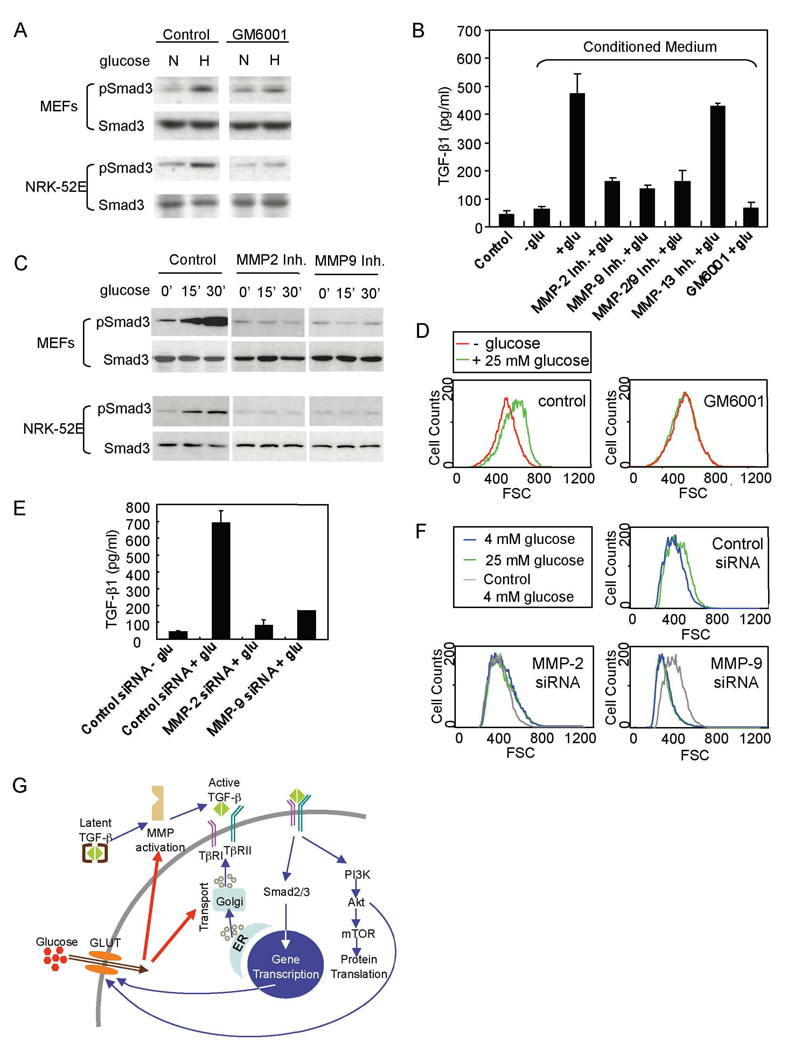
Glucose induces metalloproteinase-mediated activation of latent TGF-β
Various mechanisms have been shown to result in activation of latent TGF-β (Annes et al., 2003). Among these, matrix metalloproteinases, i.e. MMP-2, −3, −9, −13 and −14 have been implicated in TGF-β activation (Maeda et al., 2002; D'Angelo et al., 2001; Yu and Stamenkovic, 2000). Considering the rapid activation of TGF-β in response to glucose, we evaluated the role of MMPs in the glucose-induced activation of TGF-β. The broad spectrum MMP inhibitor GM6001 inhibited glucose-stimulated phosphorylation of Smad3 in MEFs and NRK-52E cells (Fig. 8A), indicating that MMPs mediate the activation of TGF-β signaling. We also examined the effect of MMP inhibitors on glucose-induced activation of TGF-β using the TMLC reporter assay. GM6001 inhibited TGF-β activation in MEFs in response to glucose by ∼90% (Fig. 8B). Since increased MMP-2 and −9 expression and activity have been associated with effects of high glucose in fibroblasts (Polhill et al., 2004; Lee et al., 2007), we examined their roles in glucose-induced activation of TGF-β. Treatment of MEFs with specific MMP-2, MMP-9 or MMP-2/9 inhibitors conferred a 60–70% inhibition of TGF-β activation, whereas inhibition of MMP-13 had no effect (Fig 8B). Furthermore, both MMP-2 and MMP-9 inhibitors blocked the activation of Smad3 by glucose (Fig. 8C). Thus, the activation of TGF-β in response to glucose is largely due to metalloproteinase activation, primarily of the related MMP-2 and −9. We then examined whether MMP-mediated activation of TGF-β plays a role in glucose-induced increase of cell size. As shown in Fig. 8D, GM6001 abolished the effect of glucose on the increase of cell size in NRK-52E cells. To confirm that MMP-2 and MMP-9 mediate the effect of glucose on TGF-β activation and cell size, we silenced the expression of MMP-2 and MMP-9. Transfection of NRK-52E cells with siRNA specific for MMP-2 or MMP-9 resulted in a strongly decreased activation of TGF-β in response to glucose (Fig. 8E). Furthermore, downregulation of MMP-2 or MMP-9 expression blocked the high glucose-induced increase of cell size in NRK-52E cells (Fig. 8F) and endothelial cells (Suppl. Fig. 3). In NRK-52E cells, MMP-9 siRNA conferred a decrease in cell size, when compared to cells at 4 mM glucose (Fig. 8F). Reminiscent of the effect of SB431542, this decrease may reflect the effect of autocrine TGF-β signaling. However, the stronger decrease suggests that additional mediators of cell size may be inhibited, when silencing MMP-9 expression. Inhibition of MMP-9 expression did not affect the basal cell size in endothelial cells, and prevented the high glucose-induced increase in cell size (Suppl. Fig. 3). These results indicate that MMP-2 and MMP-9 are mediators for glucose-induced activation of latent TGF-β.
Figure 8. Metalloproteinases are essential for glucose-induced activation of TGF-β.
(A) MEFs and NRK-52E cells, cultured in 4 mM glucose were shifted to 4 mM (N) or 25 mM (N) glucose for 30 min in the absence or presence of GM6001. Cells were assayed by western blotting for phospho-Smad3 (pSmad3) or Smad3. (B) 25 mM glucose was added to MEFs for 1 h in the absence or presence of MMP inhibitors as shown. Active TGF-β in the conditioned media and glucose-containing control medium was scored using TMLC cells. (C) 25 mM glucose was added to MEFs or NRK-52E cells for 15 or 30 min in the absence or presence of MMP-2 or MMP-9 inhibitor, and the levels of pSmad3 and Smad3 were assayed by western blotting. (D) 25 mM glucose was added to NRK-52E cells for 24 h in the absence or presence of GM6001. The size distribution of cells in G1 was determined by flow cytometry. (E) 25 mM glucose was added for 1 h to NRK-52E cells transfected with control, MMP-2 or MMP-9 siRNA, and the levels of active TGF-β in the conditioned media were scored using the TMLC reporter cells. (F) NRK-52E cells were transfected with MMP-2, MMP-9 or control siRNA. The size distribution of cells cultured with 4 mM or 25 mM glucose was determined by flow cytometry. The grey lines, marked “Control 4 mM glucose”, in the two lower panels correspond to the 4 mM glucose profile in the cells transfected with control siRNA, shown in the top panel. (G) Proposed model of the functional interactions of high glucose with TGF-β signaling resulting in increased cell size.
Discussion
Exposure of cells to high glucose has long been known to increase cell size. We provide evidence for an essential role of autocrine TGF-β signaling in glucose-induced cell hypertrophy. Glucose-induced cell hypertrophy required functional TβRI signaling and glucose rapidly induced TGF-β signaling, leading to activation of the Akt-TOR pathway and, consequently, increased cell size. The TGF-β signaling resulted from a rapid glucose-induced cell surface presentation of TβRII and TβRI, drastically enhancing the receptor levels at the cell surface, and a rapid activation of latent TGF-β by matrix metalloproteinases. These findings have relevance for pathologies associated with high glucose-induced cell hypertrophy, such as diabetes and cancer, and for the physiology of cells in culture, in which adding glucose to media is standard.
Glucose-activated signaling leading to increased cell size
Various extracellular signals induce an increase in protein synthesis and cell size through activation of the PI3K–Akt-TOR pathway. Most attention has focused on insulin and growth factors that act through tyrosine kinase receptors (Hay and Sonenberg, 2004; Plas and Thompson, 2005). TGF-β family proteins act through complexes of dual specificity kinase receptors. In spite of the different nature of these receptors, TGF-β can activate PI3K–Akt-TOR-S6 kinase signaling (Lamouille and Derynck, 2007; Das et al., 2008).
Much less is understood about how nutrients, such as amino acids and glucose, induce increased protein synthesis and cell size, although exposure of cells to amino acids or high glucose activates Akt-TOR signaling (Um et al., 2006). How addition of glucose leads to Akt-TOR signaling has not been well characterized, and it has been proposed that changes in intracellular calcium or indirect activation of Akt by insulin or glucagon-like peptide GLP-1 may be involved (Holz and Chepurny, 2005). Conversely, Akt activation causes increased expression and cell surface localization of the major glucose transporters, GLUT1 and GLUT4, leading to increased glucose uptake and glycolysis (Plas and Thompson, 2005).
Role of TGF-β signaling in glucose-induced cell hypertrophy
Central findings are that the glucose-induced increase in cell size requires functional TGF-β receptors, that glucose induces rapid activation of TGF-β signaling, that glucose-induced Akt-TOR signaling depends on the TβRI kinase, and that adding TGF-β to these cells results in increased protein synthesis and cell size, similarly to glucose. These observations invite the scenario that glucose-induced cell hypertrophy results from activation of autocrine TGF-β signaling, leading to Akt-TOR signaling, or, at a minimum, that TGF-β signaling through Akt-TOR is required component for glucose-induced cell hypertrophy. Accordingly, both TβRI kinase inhibitor and rapamycin inhibited high glucose-induced cell hypertrophy. Our results extend previous findings that linked high glucose to TGF-β. High glucose was shown, in mesangial cells, to increase expression of extracellular matrix proteins as well as TGF-β (Ziyadeh et al., 1994), and the increase in collagen synthesis was reduced in the presence of a neutralizing anti-TGF-β antibody (Sharma et al., 1996; Ziyadeh et al., 2000). Furthermore, TGF-β1 levels were increased in the glomerular and tubular kidney compartments in rodent models of diabetes, and Smad3 activation was observed in these cells (Kolm-Litty et al., 1998; Hong et al., 2001; Isono et al., 2002). Conversely, TGF-β stimulates the expression of the glucose transporter GLUT1 and glucose uptake in some cell types (Kitagawa et al., 1991; Inoki et al., 1999). This finding and our data that glucose rapidly induces TGF-β signaling together suggest that glucose-induced TGF-β signaling may provide a positive feedback mechanism, resulting in increased glucose uptake and increased glycolysis (Fig. 8G).
Finally, our observations that TGF-β induces increased protein synthesis and cell size in MEFs, NRK-52E cells and HepG2 cells, and that high glucose induces cell hypertrophy through TβRI in these cells as well as endothelial and T4-2 carcinoma cells extend our previous finding that TGF-β induces increased protein synthesis in cells undergoing epithelial to mesenchymal transition (Lamouille and Derynck, 2007). Thus, TGF-β-induced cell hypertrophy may be a common response in different cell types.
Glucose rapidly and selectively enhances the cell surface presentation of TGF-β receptors
The rapid activation of autocrine TGF-β signaling in response to high glucose appears to result from increased cell surface levels of TGF-β receptors in combination with activation of TGF-β made by the cells. The rapid and substantial increases in cell surface levels of TβRII and TβRI in response to high glucose strongly enhance the ligand binding capacity and sensitivity of the cells to TGF-β. The rapid presentation of TGF-β receptors at the cell surface was selective, as high glucose did not affect the cell surface levels of the EGF and transferrin receptors. The increased TGF-β receptor levels at the cell surface did not result from increased expression per se, but from increased transport through the ER and Golgi. Altered receptor recycling may contribute to the increased cell surface presentation of the TGF-β receptors. The enhanced cell surface levels of TβRII and TβRI in cells treated with brefeldin A may result in part from decreased receptor internalization, since brefeldin A inhibits endocytosis of certain membrane-bound proteins (Uhlin-Hansen and Yanagishita, 1995). How glucose signals to rapidly and selectively enhance the TβRII and TβRI levels is a question for future research. It is noted that increased TβRII expression was observed in glomerular and tubular kidney cells in diabetic mice (Isono et al., 2000), and that exposure of podocytes to glucose for 14 days resulted in a 50 % increased TβRII expression (Iglesias-de la Cruz et al., 2002). These effects are long term and moderate in comparison to the rapid and drastic increase in cell surface presentation of TβRII and TβRI that does not correlate with increased receptor expression.
Glucose induces rapid activation of TGF-β ligand by matrix metalloproteinases
In addition to the rapid increase in TGF-β receptors at the cell surface, glucose also induced a rapid activation of TGF-β ligand. Ligand activation is an essential event in the regulation of TGF-β, especially since the level of active TGF-β is normally minimal, compared to total TGF-β (Annes et al., 2003). The 10-fold activation of latent TGF-β within 15 min after adding glucose, to the extent that a third of all TGF-β is active, is to our knowledge unprecedented.
MMPs are secreted and cell surface enzymes that process or degrade various extracellular proteins (Egeblad and Werb, 2002). Several MMPs have been proposed to regulate conversion of latent TGF-β. Among these, enhanced activities of MMP-2, −9 and/or −13 have been associated with myocardial or cartilage hypertrophy (Derosa et al., 2007a; D'Angelo et al., 2000), and increased MMP-2 and −9 levels were found in association with diabetes (Derosa et al., 2007b), while high glucose increased MMP-2 expression (Lee et al., 2007). Our data indicate that the rapid activation of TGF-β in response to high glucose is mediated by MMPs and that MMP-2 and/or MMP-9 largely account for the activation of TGF-β in response to glucose. The importance of the MMP-mediated activation of TGF-β is illustrated by the inhibition of high glucose-induced hypertrophy of NRK-52E cells by GM6001, or siRNA to MMP-2 or MMP-9. How glucose signals to activate the MMPs remains to be determined.
High glucose was shown to induce TGF-β expression, leading to increased production of extracellular matrix proteins (Ziyadeh et al., 1994). Accordingly, high glucose induced in NRK-52E cells a moderate increase in TGF-β mRNAs. Increases in TGF-β expression confer a long term response to glucose and can not account for the 10-fold activation of TGF-β within 15 min, which does not require new protein synthesis.
The glucose-TGF-β connection in cell culture, diabetes and cancer
Our observations have relevance for the studies of TGF-β in cell culture. Cells in culture display autocrine TGF-β signaling, consistent with their expression of TGF-β receptors and TGF-β1. Since glucose is a standard component of cell culture media, the cell surface presentation of the TGF-β receptors and activation of autocrine TGF-β signaling in culture may result from the addition of glucose. This complements our previous finding that culturing cells on plastic, i.e. in the absence of extracellular matrix, activates TGF-β1 expression (Streuli et al., 1993). Finally, glucose enhances the responses to exogenous TGF-β, consistent with the activation of autocrine TGF-β signaling by glucose. Accordingly, the TGF-β responses depend on the level of glucose in the medium.
Our results contribute to the understanding of pathologies in which increased cell size has been linked to increased glucose uptake. In diabetes, the prolonged exposure of cells to high glucose has been linked to hypertrophy of proximal tubular and mesangial cells, accumulation of extracellular matrix and fibrosis, leading to renal insufficiency (Ziyadeh, 2004). Increased TGF-β expression, in the context of exposure of cells to high glucose, has also been linked to increased extracellular matrix production and fibrosis. Our results provide a mechanism of rapid activation of TGF-β signaling in response to high glucose, and highlight the central role of TGF-β signaling in glucose-induced cell hypertrophy.
Cancer cells display increased glucose uptake and glycolysis, when compared to normal cells (Gatenby and Gillies, 2004; Deberardinis et al., 2008). In fact, the high glucose utilization by tumors provides the basis for tumor imaging using PET scanning (Gatenby and Gillies, 2004). Specific oncogenic pathways, such as those mediated by PI3K or Myc, were shown to regulate protein synthesis, cell size and glucose uptake (Plas and Thompson, 2005; Ruggero and Sonenberg, 2005; Deberardinis et al., 2008). Therefore, an increase in glucose uptake may augment cell size and contribute to neoplastic transformation. Another hallmark of tumor cells is that they show increased expression and activation of TGF-β, most often TGF-β1 (Derynck et al., 2001), and often have increased levels and activity of metalloproteinases, in particular MMP-2 and MMP-9 (Egeblad and Werb, 2002). Based on our results, it is tempting to link increased glucose uptake by tumor cells to increased MMP-2 and MMP-9 activity, leading to activation of TGF-β. This scenario would connect increased glucose uptake with increased TGF-β activity and increased cell size in the context of cancer progression.
Experimental Procedures
Cell culture and treatments
Wild-type and TβRI−/− mouse embryonic fibroblasts (MEFs) (Larsson et al., 2001) were provided by S. Karlsson (Lund University Hospital, Sweden) and spontaneously immortalized. MEFs from Smad3−/− mice and wild-type littermates (Datto et al., 1999) were obtained from X.-F. Wang (Duke University Medical School). The MEFs were propagated in DMEM with glucose (4.5 g/l) and 10% fetal bovine serum (FBS). NRK-52E cells were cultured in DMEM with glucose (4.5 g/l) and 5% bovine calf serum (BCS). Human T4-2 breast carcinoma cells were obtained from M. J. Bissell (Lawrence Berkeley National Lab), and cultured on collagen (PureCol, Inamed Biomaterials) in 50:50 mix of DMEM and F12 medium supplemented with 5 µg/ml prolactin, 250 ng/ml insulin, 1.4 × 10–6 M hydrocortisone, 10–10 M β-estradiol, 2.6 ng/ml sodium selenite and 10 µg/ml transferrin. Human umbilical vein endothelial cells (HUVECs) were obtained from Cascade Biologics, and propagated in Medium 200 and low Serum Growth Supplement (LSGS) (Cascade Biologics). Human HepG2 hepatocellular carcinoma cells were from ATCC and cultured in DMEM with glucose (4.5 g/l) and 10% fetal bovine serum (FBS).
Effects of glucose or TGF-β in cell culture
To study the effect of glucose, cells were cultured in medium without glucose or with 4 mM D-glucose for 24 h, and then switched to medium with 25 mM glucose for 15 min to 24 h. To study the reversibility of the effect of glucose, the NRK-52E cells that were cultured in medium with 25 mM glucose for 24 h, were washed with PBS and then incubated with DMEM without glucose or with 4 mM glucose for 48 h. For osmolarity control, NRK-52E cells were treated with 25 mM D-mannose (Sigma-Aldrich) for 24 h. To study the effect of TGF-β, cells were treated with 1 to 10 ng/ml TGF-β1 (PeproTech) for 15 min to 24 h. To inhibit the TβRI kinase or TOR activity, 3 µM SB431542 (Sigma-Aldrich) or 100 nM rapamycin (Calbiochem) were added 1 h before the switch to high glucose and during treatment. The solvent DMSO was used as a control for both inhibitors.
Flow cytometry
Cells were trypsinized and resuspended in PBS containing 2% FBS, and incubated with 7 µg/ml Hoechst 33342 (Sigma-Aldrich) for 45 min at room temperature. 1 µg/ml propidium iodide (Sigma-Aldrich) was added prior to flow cytometry, performed using a sorter/analyzer SE three-laser system (FACSVantage; Becton Dickinson). The cell cycle and cell size were analyzed using Flowjo software (Tree Star Inc.).
Protein content and new protein synthesis assays
To measure protein content, cells were trypsinized, and the cell number was determined. The cells were lysed in radioimmunoprecipitation assay buffer with protease inhibitors, and the protein content was quantified using protein assay (Bio-Rad Laboratories) and normalized to cell number. To quantify new protein synthesis, cells were incubated in leucine-free DMEM overnight, and 5 µCi/ml 3H-leucine (Perkin Elmer) was added for 3 h. 3H-leucine incorporation was quantified as described (Franch et al., 1995) using a scintillation counter (LS 3801; Beckman) and normalized against cell number. Unless stated otherwise, all graphs show one out of two experiments, with SD for triplicates.
RNA interference
The mouse TβRI siRNA (Mm_Tgfbr1_4), rat MMP-2 siRNA (Rn_Mmp2_1_HP), rat MMP-9 siRNA (Rn_RGD:621320_1_HP), human MMP-2 siRNA (Hs_MMP2_5), human MMP-9 siRNA (Hs_MMP9_5) and control siRNA were from Qiagen. Transfections of siRNA were performed using LipofectamineTM RNAiMAX (Invitrogen). To study the effect of TβRI, cells were co-transfected with green fluorescent control siRNA (Qiagen, Neg. siRNA AF 488) and TβRI siRNA or control siRNA at a 1:9 ratio followed by indicated assays. To study the effect of MMP-2 or −9, cells were transfected with siRNA for rat or human MMP-2 or MMP-9.
Transfections and luciferase assays
MEFs and NRK-52E cells were starved overnight in serum-free, glucose-depleted DMEM. Transfections, TGF-β treatments, and reporter assays were done as described (Alliston et al., 2005). The total amount of transfected DNA was kept constant by adding control vector DNA as needed. 24 h after transfection, cells were treated with 2 ng/ml TGF-β in the presence or absence of 25 mM glucose for 12–24 h and luciferase activities were measured. Results are shown as luciferase expression values normalized for transfection efficiency.
Immunofluorescence microscopy
Cells were fixed with 4% PFA for 30 min, permeabilized in 2%PFA and 0.2% Triton X-100 for 10 min, and incubated in PBS - 3% BSA blocking solution for 1 h. The slides were incubated for 2 h with anti-Smad2/3 (BD Biosciences), diluted (1:500) in PBS - 3% BSA, and stained for 1 h with FITC-conjugated secondary antibody (1:500; Invitrogen). Slides were incubated with DAPI (1:10,000; Sigma-Aldrich) for 10 min to stain the nuclei. Cells were viewed by epifluorescence microscopy.
Cell surface biotinylation
Cells were washed with ice-cold PBS, and incubated with EZ-link Sulfo-NHS-LC-Biotin (0.5 mg/ml in PBS). Biotinylation reactions were stopped using 0.1 M glycine in PBS, and cells were lysed in MLB lysis buffer (20 mM Tris-Cl, 200 mM NaCl, 10 mM NaF, 1 mM NaV2O4, 1% NP-40, 2 mM Pefabloc, 0.5 mM Leupeptin, 1g/ml Aprotinin, pH 7.5). Cell lysates were incubated overnight with Neutravidin beads (Pierce), and the beads were then washed 3 times with MLB lysis buffer. The adsorbed proteins were analyzed by SDS-PAGE and western blot using anti-TβRI (V-22) (Santa Cruz), anti-TβRII (L-21) (Santa Cruz), anti-EGFR (1005) (Santa Cruz) or anti-TfR1 (13–6800) (Invitrogen) antibodies. These antibodies and anti-α-tubulin antibody (Sigma) were also used in parallel western blot assays of total lysates.
Immunoblotting for TGF-β signaling
To evaluate the activation of TGF-β signaling, cells were washed with PBS and lysed as described above. The total cell proteins were analyzed by SDS-PAGE and western blot using anti-Smad2/3 (#3102), anti-Smad3 (C67H9) (#9523), anti-pSmad3 (Ser423/425) (C25A9) (#9520), anti-pAkt (Ser473) (#9271) or anti-Akt (#9272) from Cell Signaling Technology. The anti-α-tubulin antibody (Sigma) was used as a loading control.
RNA preparation and quantitative real-time PCR
RNA was prepared using the RNeasy mini-kit (Qiagen). Reverse transcriptions were performed using M-MLV reverse transcriptase and random hexamer oligodeoxynucleotides (Invitrogen). Real-time PCR was performed with SYBR Green I Dye (Molecular probes) and the following primers: Smad7, 5’-TGCTGTGAATCTTACGGGAAG-3’ and 5’-AATCCATCGGGGTATCTGGAG-3’; Firefly luciferase, 5’-GCTGGGCGTTAATCAGAGAG-3’ and 5’-GTGTTCGTCTTCGTCCCAGT-3’. Ribosomal protein L19 (rPL19) was used as internal control. The primers for rPL19 were 5’-ATGTATCACAGCCTGTACCTG-3’ and 5’-TTCTTGGTCTCTTCCTC- CTTG-3’.
Temperature Blockade
MEFs were starved overnight in DMEM without glucose (Invitrogen) at 37ºC and 5% CO2. For 20ºC or 15ºC block, the medium was replaced with 2–8ºC glucose-depleted DMEM buffered with 10 mM HEPES and cells were incubated at 20ºC or 15ºC for 4h. Release of the blockade was started by replacing HEPES-buffered DMEM with 37˚C medium without HEPES (Milgram and Mains, 1994; Saraste et al., 1986).
TGF-β bioassay
Active TGF-β was measured using TMLC reporter cells (Abe et al., 1994) provided by D.B. Rifkin (New York University School of Medicine). MEFs or NRK-52E cells were starved with serum-free, glucose-depleted DMEM overnight and stimulated with 25 mM glucose for 15 min to 1 h or left untreated. As needed, MMP inhibitors GM6001, MMP-2 inhibitor I, MMP-9 inhibitor I, MMP-2/MMP-9 inhibitor III or CL-82198 (Calbiochem, CA), were added 1 h before and then during glucose treatment. DMSO solvent was used as a control. Conditioned media were heated at 80°C for 10 min or kept at 4°C until assayed. TMLC cells were treated with conditioned medium or serial dilutions of a TGF-β standard for 12–24 h. Glucose-depleted DMEM or DMEM with 25 mM glucose were used as controls, and assay values were derived as described (Abe et al., 1994). To increase the concentration of TGF-β, the conditioned media from NRK-52E cells were concentrated using Microcon centrifugal filters (Millipore).
Supplementary Material
Acknowledgments
This research was sponsored by grants RO1-CA63101 and PO1-HL60231 (Project III) to R.D. We thank M. Nakamura and Y. Wu for help with flow cytometry, D. Sheppard and M. Mock for the TMLC reporter cells and assay protocol, J. S. Kang for the retroviral TβRI expression vector, C. Liu, L. Choy and S. Lamouille for helpful discussions, and L. Kockel and D. Ruggero for critical review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe M, Harpel JG, Metz CN, Nunes I, Loskutoff DJ, Rifkin DB. An assay for transforming growth factor-β using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal. Biochem. 1994;216:276–284. doi: 10.1006/abio.1994.1042. [DOI] [PubMed] [Google Scholar]
- Alliston T, Ko TC, Cao Y, Liang YY, Feng XH, Chang C, Derynck R. Repression of bone morphogenetic protein and activin-inducible transcription by Evi-1. J. Biol. Chem. 2005;280:24227–24237. doi: 10.1074/jbc.M414305200. [DOI] [PubMed] [Google Scholar]
- Annes JP, Munger JS, Rifkin DB. Making sense of latent TGF-β activation. J. Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- Coelho CM, Leevers SJ. Do growth and cell division rates determine cell size in multicellular organisms? J. Cell Sci. 2000;113(Pt 17):2927–2934. doi: 10.1242/jcs.113.17.2927. [DOI] [PubMed] [Google Scholar]
- D’Angelo M, Billings PC, Pacifici M, Leboy PS, Kirsch T. Authentic matrix vesicles contain active metalloproteases (MMP). a role for matrix vesicle-associated MMP-13 in activation of transforming growth factor-β. J. Biol. Chem. 2001;276:11347–11353. doi: 10.1074/jbc.M009725200. [DOI] [PubMed] [Google Scholar]
- D’Angelo M, Yan Z, Nooreyazdan M, Pacifici M, Sarment DS, Billings PC, Leboy PS. MMP-13 is induced during chondrocyte hypertrophy. J. Cell Biochem. 2000;77:678–693. [PubMed] [Google Scholar]
- Das F, Ghosh-Choudhury N, Mahimainathan L, Venkatesan B, Feliers D, Riley DJ, Kasinath BS, Choudhury GG. Raptor-rictor axis in TGF-β-induced protein synthesis. Cell Signal. 2008;20:409–423. doi: 10.1016/j.cellsig.2007.10.027. [DOI] [PubMed] [Google Scholar]
- Datto MB, Frederick JP, Pan L, Borton AJ, Zhuang Y, Wang XF. Targeted disruption of Smad3 reveals an essential role in transforming growth factor β-mediated signal transduction. Mol. Cell Biol. 1999;19:2495–2504. doi: 10.1128/mcb.19.4.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deberardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Derosa G, Cicero AF, Scalise F, Avanzini MA, Tinelli C, Piccinni MN, Peros E, Geroldi D, Fogari E, D’Angelo A. Metalloproteinase-2 and −9 in diabetic and nondiabetic subjects during acute coronary syndromes. Endothelium. 2007a;14:45–51. doi: 10.1080/10623320601177064. [DOI] [PubMed] [Google Scholar]
- Derosa G, D’Angelo A, Tinelli C, Devangelio E, Consoli A, Miccoli R, Penno G, Del Prato S, Paniga S, Cicero AF. Evaluation of metalloproteinase 2 and 9 levels and their inhibitors in diabetic and healthy subjects. Diabetes Metab. 2007b;33:129–134. doi: 10.1016/j.diabet.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Derynck R, Akhurst RJ, Balmain A. TGF-β signaling in tumor suppression and cancer progression. Nat. Genet. 2001;29:117–129. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- Feng XH, Derynck R. Specificity and versatility in TGF-β signaling through Smads. Annu. Rev. Cell Dev. Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- Franch HA, Shay JW, Alpern RJ, Preisig PA. Involvement of pRB family in TGF β-dependent epithelial cell hypertrophy. J. Cell Biol. 1995;129:245–254. doi: 10.1083/jcb.129.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- Herman MA, Kahn BB. Glucose transport and sensing in the maintenance of glucose homeostasis and metabolic harmony. J. Clin. Invest. 2006;116:1767–1775. doi: 10.1172/JCI29027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz GG, Chepurny OG. Diabetes outfoxed by GLP-1? Sci. STKE. 2005;2005:e2. doi: 10.1126/stke.2682005pe2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SW, Isono M, Chen S, Iglesias-de la Cruz MC, Han DC, Ziyadeh FN. Increased glomerular and tubular expression of transforming growth factor-β1, its type II receptor, and activation of the Smad signaling pathway in the db/db mouse. Am. J. Pathol. 2001;158:1653–1663. doi: 10.1016/s0002-9440(10)64121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias de la Cruz MC, Ziyadeh FN, Isono M, Kouahou M, Han DC, Kalluri R, Mundel P, Chen S. Effects of high glucose and TGF-β1 on the expression of collagen IV and vascular endothelial growth factor in mouse podocytes. Kidney Int. 2002;62:901–913. doi: 10.1046/j.1523-1755.2002.00528.x. [DOI] [PubMed] [Google Scholar]
- Inman GJ, Nicolas FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, Laping NJ, Hill CS. SB-431542 is a potent and specific inhibitor of transforming growth factor-β superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol. Pharmacol. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- Inoki K, Haneda M, Maeda S, Koya D, Kikkawa R. TGF-β1 stimulates glucose uptake by enhancing GLUT1 expression in mesangial cells. Kidney Int. 1999;55:1704–1712. doi: 10.1046/j.1523-1755.1999.00438.x. [DOI] [PubMed] [Google Scholar]
- Isono M, Chen S, Hong SW, Iglesias-de la CruzMC, Ziyadeh FN. Smad pathway is activated in the diabetic mouse kidney and Smad3 mediates TGF-β-induced fibronectin in mesangial cells. Biochem. Biophys. Res. Commun. 2002;296:1356–1365. doi: 10.1016/s0006-291x(02)02084-3. [DOI] [PubMed] [Google Scholar]
- Isono M, Mogyorosi A, Han DC, Hoffman BB, Ziyadeh FN. Stimulation of TGF-β type II receptor by high glucose in mouse mesangial cells and in diabetic kidney. Am. J. Physiol Renal Physiol. 2000;278:F830–F838. doi: 10.1152/ajprenal.2000.278.5.F830. [DOI] [PubMed] [Google Scholar]
- Kitagawa T, Masumi A, Akamatsu Y. Transforming growth factor-β1 stimulates glucose uptake and the expression of glucose transporter mRNA in quiescent Swiss mouse 3T3 cells. J. Biol. Chem. 1991;266:18066–18071. [PubMed] [Google Scholar]
- Kolm-Litty V, Sauer U, Nerlich A, Lehmann R, Schleicher ED. High glucose-induced transforming growth factor β1 production is mediated by the hexosamine pathway in porcine glomerular mesangial cells. J. Clin. Invest. 1998;101:160–169. doi: 10.1172/JCI119875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamouille S, Derynck R. Cell size and invasion in TGF-β-induced epithelial to mesenchymal transition is regulated by activation of the mTOR pathway. J. Cell Biol. 2007;178:437–451. doi: 10.1083/jcb.200611146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson J, Goumans MJ, Sjostrand LJ, van Rooijen MA, Ward D, Leveen P, Xu X, ten DP, Mummery CL, Karlsson S. Abnormal angiogenesis but intact hematopoietic potential in TGF-β type I receptor-deficient mice. EMBO J. 2001;20:1663–1673. doi: 10.1093/emboj/20.7.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Bae SS, Kim KH, Lee WS, Rhim BY, Hong KW, Kim CD. High glucose enhances MMP-2 production in adventitial fibroblasts via Akt1-dependent NF- κ B pathway. FEBS Lett. 2007;581:4189–4194. doi: 10.1016/j.febslet.2007.07.058. [DOI] [PubMed] [Google Scholar]
- Liberati NT, Moniwa M, Borton AJ, Davie JR, Wang X-F. An essential role for Mad homology domain 1 in the association of Smad3 with histone deacetylase activity. J. Biol. Chem. 2001;276:22595–22603. doi: 10.1074/jbc.M010778200. [DOI] [PubMed] [Google Scholar]
- Maeda S, Dean DD, Gomez R, Schwartz Z, Boyan BD. The first stage of transforming growth factor β1 activation is release of the large latent complex from the extracellular matrix of growth plate chondrocytes by matrix vesicle stromelysin-1 (MMP-3) Calcif. Tissue Int. 2002;70:54–65. doi: 10.1007/s002230010032. [DOI] [PubMed] [Google Scholar]
- Mamane Y, Petroulakis E, LeBacquer O, Sonenberg N. mTOR, translation initiation and cancer. Oncogene. 2006;25:6416–6422. doi: 10.1038/sj.onc.1209888. [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milgram SL, Mains RE. Differential effects of temperature blockade on the proteolytic processing of three secretory granule-associated proteins. J. Cell Sci. 1994;107(Pt 3):737–745. doi: 10.1242/jcs.107.3.737. [DOI] [PubMed] [Google Scholar]
- Moustakas A, Heldin CH. Non-Smad TGF-β signals. J. Cell Sci. 2005;118:3573–3584. doi: 10.1242/jcs.02554. [DOI] [PubMed] [Google Scholar]
- Neubauer S. The failing heart--an engine out of fuel. N. Engl. J. Med. 2007;356:1140–1151. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- Plas DR, Thompson CB. Akt-dependent transformation: there is more to growth than just surviving. Oncogene. 2005;24:7435–7442. doi: 10.1038/sj.onc.1209097. [DOI] [PubMed] [Google Scholar]
- Polhill TS, Saad S, Poronnik P, Fulcher GR, Pollock CA. Short-term peaks in glucose promote renal fibrogenesis independently of total glucose exposure. Am. J. Physiol Renal Physiol. 2004;287:F268–F273. doi: 10.1152/ajprenal.00084.2004. [DOI] [PubMed] [Google Scholar]
- Pyronnet S, Sonenberg N. Cell-cycle-dependent translational control. Curr. Opin. Genet. Dev. 2001;11:13–18. doi: 10.1016/s0959-437x(00)00150-7. [DOI] [PubMed] [Google Scholar]
- Ruggero D, Sonenberg N. The Akt of translational control. Oncogene. 2005;24:7426–7434. doi: 10.1038/sj.onc.1209098. [DOI] [PubMed] [Google Scholar]
- Saraste J, Palade GE, Farquhar MG. Temperature-sensitive steps in the transport of secretory proteins through the Golgi complex in exocrine pancreatic cells. Proc. Natl. Acad. Sci. U. S. A. 1986;83:6425–6429. doi: 10.1073/pnas.83.17.6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorelli V, Fulco M. Molecular and cellular determinants of skeletal muscle atrophy and hypertrophy. Sci. STKE. 2004;2004:re11. doi: 10.1126/stke.2442004re11. [DOI] [PubMed] [Google Scholar]
- Sharma K, Jin Y, Guo J, Ziyadeh FN. Neutralization of TGF-β by anti-TGF-β antibody attenuates kidney hypertrophy and the enhanced extracellular matrix gene expression in STZ-induced diabetic mice. Diabetes. 1996;45:522–530. doi: 10.2337/diab.45.4.522. [DOI] [PubMed] [Google Scholar]
- Shi Y, Massague J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Streuli CH, Schmidhauser C, Kobrin M, Bissell MJ, Derynck R. Extracellular matrix regulates expression of the TGF-β1 gene. J. Cell Biol. 1993;120:253–260. doi: 10.1083/jcb.120.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesfamariam B, Brown ML, Deykin D, Cohen RA. Elevated glucose promotes generation of endothelium-derived vasoconstrictor prostanoids in rabbit aorta. J. Clin. Invest. 1990;85:929–932. doi: 10.1172/JCI114521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlin-Hansen L, Yanagishita M. Brefeldin A inhibits the endocytosis of plasma-membrane-associated heparan sulphate proteoglycans of cultured rat ovarian granulosa cells. Biochem. J. 1995;310(Pt 1):271–278. doi: 10.1042/bj3100271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um SH, D’Alessio D, Thomas G. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell Metab. 2006;3:393–402. doi: 10.1016/j.cmet.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Wolf G, Ziyadeh FN. Molecular mechanisms of diabetic renal hypertrophy. Kidney Int. 1999;56:393–405. doi: 10.1046/j.1523-1755.1999.00590.x. [DOI] [PubMed] [Google Scholar]
- Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-β and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- Zavadil J, Böttinger EP. TGF-β and epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764–5774. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- Ziyadeh FN. Mediators of diabetic renal disease: the case for TGF-β as the major mediator. J. Am. Soc. Nephrol. 2004;1(15 Suppl):S55–S57. doi: 10.1097/01.asn.0000093460.24823.5b. [DOI] [PubMed] [Google Scholar]
- Ziyadeh FN, Hoffman BB, Han DC, Iglesias-de la Cruz MC, Hong SW, Isono M, Chen S, McGowan TA, Sharma K. Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-β antibody in db/db diabetic mice. Proc. Natl. Acad. Sci. U. S. A. 2000;97:8015–8020. doi: 10.1073/pnas.120055097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziyadeh FN, Sharma K, Ericksen M, Wolf G. Stimulation of collagen gene expression and protein synthesis in murine mesangial cells by high glucose is mediated by autocrine activation of transforming growth factor-β. J. Clin. Invest. 1994;93:536–542. doi: 10.1172/JCI117004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.