Abstract
We report the characterisation of 27 cardiovascular-related traits in 23 inbred mouse strains. Mice were phenotyped either in response to chronic administration of a single dose of the β-adrenergic receptor blocker atenolol or under a low and a high dose of the β-agonist isoproterenol and compared to baseline condition. The robustness of our data is supported by high trait heritabilities (typically H2>0.7) and significant correlations of trait values measured in baseline condition with independent multistrain datasets of the Mouse Phenome Database. We then focused on the drug-, dose-, and strain-specific responses to β-stimulation and β-blockade of a selection of traits including heart rate, systolic blood pressure, cardiac weight indices, ECG parameters and body weight. Because of the wealth of data accumulated, we applied integrative analyses such as comprehensive bi-clustering to investigate the structure of the response across the different phenotypes, strains and experimental conditions. Information extracted from these analyses is discussed in terms of novelty and biological implications. For example, we observe that traits related to ventricular weight in most strains respond only to the high dose of isoproterenol, while heart rate and atrial weight are already affected by the low dose. Finally, we observe little concordance between strain similarity based on the phenotypes and genotypic relatedness computed from genomic SNP profiles. This indicates that cardiovascular phenotypes are unlikely to segregate according to global phylogeny, but rather be governed by smaller, local differences in the genetic architecture of the various strains.
Introduction
The β-adrenergic system controls cardiac contractility and excitability, heart rate and vascular tone. Pharmacological targeting of β-adrenergic receptors is a well documented first-line therapeutic approach for the management of cardiac arrhythmias, cardio-protection after myocardial infarction, and hypertension [1], [2], while β-agonists are administered in cases of bradycardia, heart block or asthma [3], [4]. A major concern in clinical practice is the marked variability of human responses to such treatments, often leading to unwanted side-effects or showing at best little relief [5], [6].
The purpose of this study is to explore inter-individual variance of cardiovascular-related traits induced by sustained pharmacological perturbations of the β-adrenergic system, using inbred mouse strains as a model. Atenolol (ate) and isoproterenol (iso) were chosen for their antagonist effects on β-adrenergic receptors. Ate is a β-blocker with strong cardio-selectivity for β1-adrenoreceptors. It is widely prescribed to patients with hypertension, coronary heart disease, and arrhythmias. In contrast, iso is a classical β-adrenergic agonist that induces positive cardiac inotropy and chronotropy. It is administered acutely in cases of bradycardia, heart block or pulmonary emergencies. In both humans and mice, ate is mainly eliminated unchanged by the kidneys and the faeces [7], [8], while iso is metabolised within minutes post-administration into inactive metabolites by the liver catechol-O-methyltransferase (COMT) [9].
In humans, only about half of hypertensive individuals respond to ate by a significant reduction of systolic blood pressure [10], whereas acute delivery of iso may induce differential vasodilatory effects in healthy subjects [11]. In rodents and other mammals, sustained activation of the β-adrenergic system by chronic administration of iso is a classical and well characterised model of left ventricular hypertrophy (LVH) independent of blood pressure [12]. LVH is a major risk factor for ventricular dysfunction and heart failure. In its pathological manifestations, it is characterised by increased left ventricular mass and wall thickness, elevated cross-sectional area and dimensions of cardiomyocytes, as well as increased perivascular and interstitial myocardial fibrosis. LVH is further associated with the reactivation of a foetal transcription program as indicated by up-regulation of cardiac genes otherwise expressed in myocardium only during embryonic development [12]. Recent experimental data suggest that, in mice, iso-induced LVH is modulated by strain-specific factors. In particular, extensive phenotypic characterisation revealed that the strain A/J developed greater morphological changes than C57BL/6J when exposed to five consecutive daily injections of 100 mg iso per kg body weight [13]. Similarly, evidence obtained in FVB and C57BL6/SV129 mice challenged with single injections of 1 µg iso indicates that strain-specific genetic variants are also likely to modulate the chronotropic action of iso on heart rate [14].
Here, we report the characterisation of heart rate (HR), systolic blood pressure (SBP), electrocardiograms (ECG) and cardiac weight indices in age-matched males of 23 inbred mouse strains. Rigorous experimental standards were applied so as to minimise the impact of non-inherited factors on trait values and drug responses. In particular, physiological consequences of β-adrenergic perturbations were tested independently of pathological influences, measurements were repeated in an average of ten individuals per strain and per drug condition, and sensitive phenotypes such as HR and SBP were monitored across three consecutive days in each mouse, following a training period of one week. These traits were recorded either in baseline condition (ctr) or in response to sustained administration of iso at 1 (iso1) or 10 (iso10) mg/kg per day or ate at 10 mg/kg per day for two weeks. Mice were chosen in accordance with the recommendations of the Mouse Phenome Database (MPD) so as to cover as much genetic diversity as possible [15], the density of genotypic information available in each strain and their suitability with the experimental design. For these reasons, wild-derived inbred lines were excluded from the panel.
Our study provides a rich dataset of cardiovascular-related phenotypes upon β-adrenergic challenge in different genetic backgrounds. We first focus our analysis on the response of key measurements including HR, SBP, ventricular and atrial weight indices, body weight and weight gain, as well as ECG parameters. We then explore the complete dataset using unsupervised bi-clustering to elucidate and visualise the patterns of correlations between strains, treatments, and phenotypes. We compare strain similarity based on the phenotypes with genotypic relatedness computed from genomic SNP profiles. Information extracted from these analyses is discussed in terms of novelty and biological implications. Finally, we discuss the suitability of using our data to map underlying genetic loci by association studies.
Results
In this study, hereafter abbreviated CV-PGX (for “cardio-vascular pharmaco-genomics”), we have characterised 23 inbred mouse strains for 27 cardiovascular and related phenotypes such as systolic blood pressure (SBP), heart rate (HR), electrocardiogram (ECG) parameters and cardiac weight indices, either under baseline condition (ctr) or in response to chronic administration of isoproterenol at 1 (iso1) or 10 (iso10) mg/kg per day or atenolol at 10 mg/kg per day (ate), as described in Materials and Methods and in Figure 1 . Traits are listed in Table 1 and mean values and standard deviations of ten selected phenotypes across all strains and drug conditions are presented as bar graphs in Figure 2 (see Supplement S1 for all traits). One-way Analyses Of Variance (ANOVA) showed high reproducibility for all phenotypes measured under ctr or any given treatment. In particular, variances were significantly smaller within than between strains, indicating that all phenotypic responses are highly heritable (typically 0.7<H2<1; Supplement S1 ).
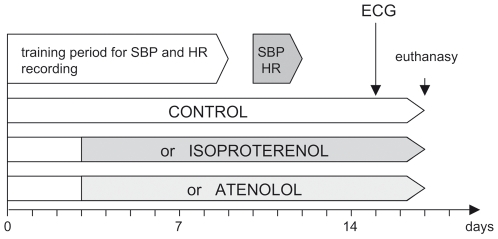
Figure 1. Phenotypic monitoring and timing in inbred mice.
Animals were trained on the Visitech BP-2000 tail-cuff apparatus on a daily basis from days 1 to 5 and 8 to 9. SBP and HR were effectively recorded on days 10, 11, and 12. Osmotic mini-pumps loaded with the appropriate drugs were implanted sub-cutaneously on day 3 under anaesthesia. ECGs were recorded on day 15 under halothane anaesthesia. Mice were sacrificed by decapitation on day 17.
Table 1. Phenotypes and abbreviations.
| condition | abbreviation | phenotype |
| in conscious mice | HR (TC) | heart rate (tail-cuff; beats/min) |
| SBP | systolic blood pressure (tail-cuff; mmHg) | |
| BWS | body weight at start (g) | |
| BWE | body weight at end (g) | |
| BWG | body weight gain (g) | |
| under anaesthesia | HR (ECG) | heart rate (beats/min) |
| Pamp | amplitude of p wave (mV) | |
| Parea | area of p wave (mV*ms) | |
| Pdur | duration of p wave (ms) | |
| PR | PR interval (ms) | |
| RR | RR interval (ms) | |
| Qamp | amplitude of Q wave (mV) | |
| QRS | QRS interval (ms) | |
| QRSarea | area of QRS complex (mV*ms) | |
| QT | QT interval (ms) | |
| QTc | corrected QT interval (ms) | |
| Ramp | amplitude of R wave (mV) | |
| Samp | amplitude of S wave (mV) | |
| ST | ST interval (ms) | |
| after euthanasia | HW | heart weight at end (mg) |
| VW | weight of cardiac ventricles (mg) | |
| VWI | ventricular weight index (ratio VW/BWE in mg/g) | |
| VW/BWS | ventricular weight index (ratio VW/BWS in mg/g) | |
| AW | weight of cardiac atria (mg) | |
| AWI | atrial weight index (ratio AW/BWE in mg/g) | |
| AW/BWS | atrial weight index (ratio AW/BWS in mg/g) | |
| VW/AW | ratio VW/AW |
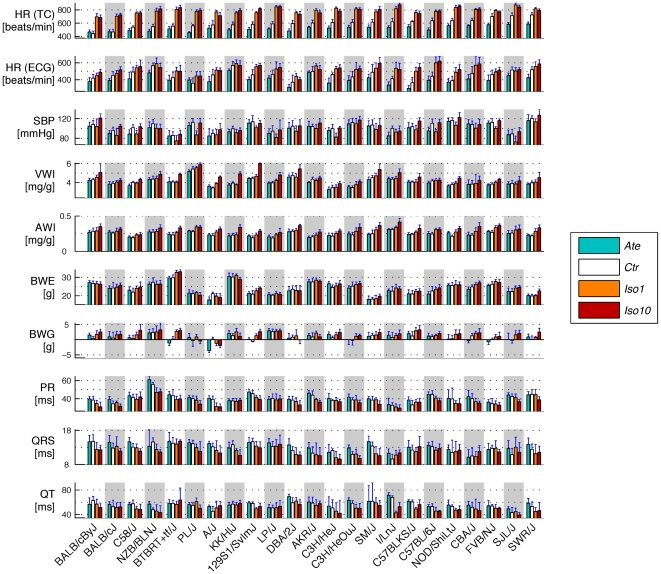
Figure 2. Means and standard deviations of ten selected phenotypes in 23 inbred mouse strains.
White bars: ctr; blue bars: ate; orange bars: iso1; red bars: iso10. Strains are ranked by increasing HR (TC) means of ctr mice. See Table 1 for abbreviations.
Phenotypes measured in baseline condition
We first focused on data recorded in baseline condition. As some of these traits, in particular SBP and HR, are notoriously sensitive to slight perturbations of environmental or experimental conditions, we assessed the consistency of our data with information available from independent experiments and laboratories. To do so, we calculated Pearson's and Spearman's correlations between our mean ctr measurements and values from multistrain records of the MPD [15] across the common strains. These comparisons were restricted to MPD projects using inbred strains and experimental procedures similar with those of the present study. A subset of five such pair-wise comparisons is illustrated in Figure 3 , while more comprehensive results are available in Supplement S1 .
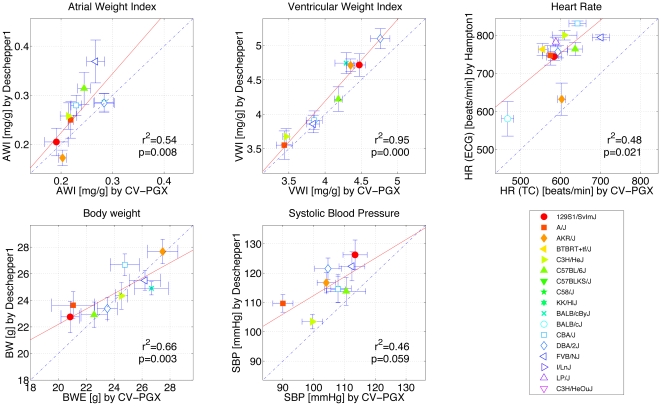
Figure 3. Conservation of baseline phenotypes across independent studies.
Five comparisons of ctr CV-PGX strain means with means of selected MPD projects are illustrated. The dashed line is the diagonal of identity and the red line is the best fit of (x∶y) pairs. Data are presented as means±SD. See Table 1 for abbreviations. r2: squared coefficient of correlation (Pearson); p∶ p-value.
The most robust phenotypes were those related to body and heart weight, with Pearson's correlations r2>0.5 (p<0.01) in at least one third of the comparisons ( Supplement S1 ). This strong conservation is illustrated by the significant correlations of BWE and VWI with the dataset of Deschepper [16], in which ten strains were common with the present study ( Figure 3 ). Results were more divergent for the weight of cardiac atria, as average AWI of most strains was lower in the CV-PGX study than in Deschepper's data ( Figure 3 ). This discordance is most likely related to the small size of the atria and may be a result of subtle differences in the dissection process rather than intrinsic strain variability.
The conservation of baseline HR and SBP strain means across independent studies was much weaker, as illustrated by the two examples in Figure 3 . Yet, the total numbers of significantly correlated CV-PGX vs MPD datasets were higher than expected by chance for both traits ( Supplement S1 ), suggesting that despite marked susceptibility to the environment, blood pressure and heart rate are also controlled in part by genetic determinants. Altogether, these comparisons indicate that ctr measurements are consistent with data from other studies, further supporting the validity of our experimental protocol.
Of note, mean HR values measured by ECG correlated poorly with those obtained (i) by tail-cuff in the same animals (Pearson r 2 = 0.02, p = 0.52; Supplement S1 ) or (ii) by ECGs in independent studies (MPD). Considering that tail-cuff experiments were performed in conscious mice whereas ECGs were recorded under anaesthesia, these discrepancies may point towards strain-specific confounding effects of anaesthesia.
Patterns of trait and strain correlations in baseline condition
Baseline phenotypes were further investigated for patterns of correlations across strains. To this end we normalised phenotypic data into z-scores and analysed them by hierarchical bi-clustering ( Supplement S1 ). In these re-ordered tables (matrices) of z-scores, phenotypes (columns) were clustered according to the absolute values of the similarities across all strains while strains (rows) were clustered according to signed similarities across all phenotypes.
The vast majority of the ctr phenotypes were only mildly correlated across strains (correlations r<0.5, Suppl. Figure 5A in Supplement S1 ) but as expected, HW, AW and VW increased with BWE (correlations r>0.7, p<0.05). Interestingly, these traits also shared good similarity with Pdur (0.4<r<0.7, p<0.05). In contrast, no significant correlation could be identified between HR and SBP (r = 0.25, p = 0.26), between HR and VWI (r = −0.21, p = 0.34) or AWI (r = −0.12, p = 0.59), and between SBP and AWI (r = 0.04, p = 0.87) or VWI (r = 0.27, p = 0.22). In anaesthetised mice, ECG intervals were only mildly correlated to HR. Altogether these data suggest that the genetic components contributing to most baseline phenotypes are largely independent.
Phenotypes measured under atenolol or isoproterenol treatment
We next focused on strain responses to chronic β-adrenergic blockade or activation. For each phenotype p and strain s a signed significance value
was attributed to the effect of each treatment t = ate, iso1, iso10 with respect to the ctr group. We used the Wilcoxon ranksum test, with test statistics 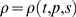 and associated p-value
and associated p-value 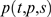 . Signed significances are presented in
Figure 4
for the same parameters and strain order as in
Figure 2
(graphs for all phenotypes are available in
Supplement S1
). Altogether, the amplitudes of responses to drug treatments were strain-specific and high- or low-responders could be identified for each trait. Most phenotypes were affected by (at least the highest dose of) iso with a nominal significance level of p<0.05 in at least one strain, whereas the opposite effect of ate was usually milder. Below, we focus on the results of HR, SBP and cardiac weight indices. Information regarding the effect of ate and iso on ECG parameters and body weight are available in
Supplement S1
.
. Signed significances are presented in
Figure 4
for the same parameters and strain order as in
Figure 2
(graphs for all phenotypes are available in
Supplement S1
). Altogether, the amplitudes of responses to drug treatments were strain-specific and high- or low-responders could be identified for each trait. Most phenotypes were affected by (at least the highest dose of) iso with a nominal significance level of p<0.05 in at least one strain, whereas the opposite effect of ate was usually milder. Below, we focus on the results of HR, SBP and cardiac weight indices. Information regarding the effect of ate and iso on ECG parameters and body weight are available in
Supplement S1
.
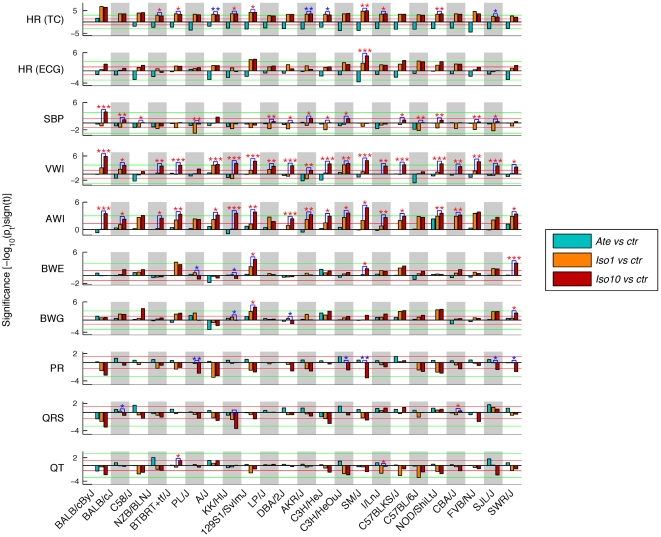
Figure 4. Intra-strain significance of drug treatments.
Data are presented for the phenotypes selected in Figure 2. Intra-strain p-values of phenotypic data recorded in treated vs untreated groups (Wilcoxon ranksum statistics) are presented as bar graphs on a -log10 scale. The threshold of significance is indicated by red lines (p = 0.05) and -log p-values are signed according to the directionality of the effect induced by the drugs. When significant, p-values for testing responses under iso10 vs iso1 are indicated by coloured stars (i.e. *: p<0.05; **: p<0.01; ***: p<0.001; red star: phenotypic mean under iso10> phenotypic mean under iso1; blue star: phenotypic mean under iso10<phenotypic mean under iso1). Blue bars: ate vs ctr; orange bars: iso1 vs ctr; red bars: iso10 vs ctr. P-values smaller than 0.001 (bars extending beyond the green lines or ***) hold up against Bonferroni correction for multiple testing of either all strains for a given phenotype or all phenotypes for a given strain. Strains are ranked as in Figure 2. See Table 1 for abbreviations.
We note that the significance levels in Figure 4 (and Suppl. Figure 4 of Supplement S1 ) have not been corrected for multiple testing. When asking whether a response is significant in the global context of our analysis, the significance threshold has to be adjusted. A simple procedure is the well-known Bonferroni correction, where the nominal significance threshold α = 0.05 is lowered to α' = α/N (N being the number of tests). For example, for a given phenotype (or strain) one corrects for considering simultaneously N = 23 (N = 27) tests, giving α'≈0.002. Thus significance values extending beyond the (green) threshold lines at±3 in Figure 4 clearly hold up against testing all strains or all phenotypes. Yet, this correction is very stringent and over-conservative – in particular when correcting for testing all phenotypes and all strains. Thus we also applied the Benjamini-Hochberg (BH) step-up procedure [17], [18] to correct for multiple hypotheses testing. This procedure controls the false discovery rate yielding adequate, yet significantly milder corrections. We report globally BH-corrected p-values as well as those for any given phenotype or any given strain (correcting for all 23 strains or all 27 phenotypes, respectively) in Supplement S1 .
Heart rate in conscious mice
The most robust effect of the β-adrenergic drugs was the pronounced strain-specific positive cardiac chronotropy induced by both concentrations of iso in all 23 strains ( Figures 2 and 4 ). Under iso10 stimulation, mean HR was increased between 61±43 beats/min in strain SWR/J and 279±33 beats/min in strain BTBRT+ tf/J. The lower concentration of iso induced a similar or even slightly higher (i.e. see strains A/J, AKR/J, C3H/HeJ and SJL/J) acceleration in sixteen strains, while for the seven other lines HR was significantly higher under iso10 than iso1 ( Figures 2 and 4 ). Conversely, the opposite properties of ate on HR were significant in all strains except both Balb/c lines, reaching a maximal reduction of 141±43 beats/min in strain SWR/J.
Overall, there was a marked tendency that stronger responders to β-stimulation had lower baseline HR, while stronger responders to β-blockade had higher ctr HR. In other words in most strains baseline values could be used to predict the magnitude of rate changes in response to pharmacological treatment. These data may reflect the existence of electrical and/or mechanical limits to the pacing capacity of the atrial sinus node. Reaching the upper limit of cardiac pacing would explain the relatively weak iso-mediated positive chronotropy in strains SJL/J and SWR/J, whose pulse was initially high. Accordingly, strain upper limits were attained in at least sixteen lines exposed to iso (i.e. those in which pulse recorded under iso1 was higher or similar as under iso10), ranging from 697±45 beats/min in strain Balb/cByJ to 877±45 beats/min in strain I/LnJ. It is more difficult to draw conclusions regarding the lower limit of pacing automaticity, since a single dose of β-blocker was used. Yet, HR mean values measured in conscious ate-treated mice were never lower than the baseline pulse values of strain Balb/cByJ (i.e. 442±35 beats/min).
r 2 values of Pearson's correlations between pulse rate recorded in resting state or under drug treatment were 0.74 for ate vs ctr (p = 1.4×10−7), 0.54 for iso1 vs ctr (p = 7.3×10−5), and 0.22 for iso10 vs ctr (p = 2.5×10−3; Suppl. Figure 3 of Supplement S1 ). This information is consistent with iso10 producing the strongest perturbations on HR and indicates that the patterns of drug- and dose-dependent HR responses are strain-specific. Similarly, when assessing the impact of the treatments on the correlations with the datasets of the MPD ( Supplement S1 ), Pearson's r 2 values decreased when comparing pulse of drug-treated animals instead of our ctr with that of Hampton1's records [19]. When considering the other MPD projects, correlations were the highest in (CV-PGXate:Svenson), (CV-PGXiso10:Gavras), and (CV-PGXiso10:Jaxwest1HR) pairs ( Supplement S1 ), probably reflecting differences in stress levels across projects.
Systolic blood pressure
Perturbations of the β-adrenergic system resulted in rather subtle effects on blood pressure. Ate tended to slightly decrease SBP, but the effect was below significance in all strains except C57BL/6J ( Figure 4 ). Iso1 also reduced SBP, on average by 10 mmHg, probably as a result of vasodilation. This decrease reached significance in five strains (C3H/HeJ, C57BL/6J, FVB/NJ, PL/J, and SJL/J). As for iso10, it tended to slightly increase SBP in the majority of the strains, perhaps as a consequence of increased cardiac inotropy, but the trend was significant in a single line (i.e. Balb/cByJ). Even though differences between iso-treated and ctr mice were globally minor, the opposite action of iso1 and iso10 on SBP was significant in fourteen strains ( Figure 4 ). Modest hemodynamic changes in response to iso are consistent with previous data obtained in rats [20] and mice [13], confirming that cardiac hypertrophy induced by iso is essentially independent of SBP.
Heart weight and cardiac weight indices
Chronic activation of the β-adrenergic system is a classical trigger of left ventricular hypertrophy [12], [21]. In our study, iso induced strain-, dose- and compartment-specific perturbations of cardiac mass ( Figures 2 , 4 and Supplement S1 ). Under infusion of iso10, the relative increase of VWI reached significance (p<0.05) in nineteen strains, ranging from 1.7% in C57BL/6J mice to 33% in 129S1/SvImJ (i.e. C57BL6/J: 4.3±0.03 mg/g in iso10 vs 4.2±0.04 mg/g in ctr; 129S1/SvImJ: 6.0±0.26 mg/g in iso10 vs 4.9±0.05 mg/g in ctr). By comparison, only three lines differed from the controls in the iso1-treated group, reaching a maximal relative increase of 11% in strain A/J ( Figure 4 ). These three strains were all characterised by a positive dose-response to iso. In contrast to the above, the resistance of strains AKR/J, C57BL/6J, and PL/J to β-stimulation was characterised by similar indexed and non-indexed ventricular weight in treated and untreated mice ( Figures 2 and 4 , and data not shown), whereas the small variation of VWI in C58/J mice was the consequence of parallel increases of VW and BW.
Cardiac atria responded to chronic β-stimulation with the same directionality as the ventricles but exhibited more pronounced sensitivities to iso ( Figures 2 and 4 ): AWI was increased by iso1 in fifteen strains, including those with poor responding ventricles, and by iso10 in all lines (p<0.05). The magnitude of atrial hypertrophy was larger under iso10, extending from a relative increase of AWI of 22% in strain Balb/cByJ to 57% in CBA/J.
Consistent with the fact that ate was administered to healthy mice, β-blockade only modestly affected cardiac weight and indices in the majority of the strains. Three of the four lines resistant to iso responded by a significant reduction of VWI under ate (i.e. AKR/J: −6.4%; C58/J: −7.8%; and C57BL/6J: −5.6%, p<0.05), but a concomitant reduction of VW was significant only in C57BL/6J mice (data not shown). Similarly, the overall impact of ate on cardiac atria was negligible except in strain NOD/ShiLtJ that exhibited a relative increase of AW and AWI of 21% when compared to controls.
Of note, the weak iso-dependent increase of VWI in strain C57BL/6J is consistent with the data of Faulx et al. who investigated cardiovascular changes in 12–15 week-old A/J and C57BL/6J males challenged with five consecutive daily injections of 100 mg/kg iso [22]. In these conditions, the heart weight index (HWI) increased by 2.7% in strain C57BL/6J and by 23% in strain A/J. In contrast, HWI in 8-wk old C57BL/6J males infused with 40 mg/kg iso for seven days increased from 5.0 in ctr to 6.0 mg/g (i.e. a relative increase of 22%) in treated animals [23]. These apparent inconsistencies may relate to the fast biotransformation of iso by liver enzymes [9]. In the latter example, the dose of continuously-administered iso was indeed four-fold higher than the CV-PGX iso10 concentration. While the 100 mg/kg used by Faulx et al. might seem even higher, one should keep in mind that in this case iso was administered in single daily shots. Thus, the total daily amount of drug is likely to be much lower than when delivered in a sustained fashion as most of it would have been rapidly metabolised and eliminated.
Correlations and clustering of phenotypes in the presence of β-adrenergic drugs
Each drug treatment was assessed for its impact on the patterns of correlations across mean trait values or strains (see Suppl. Figures 5 – 7 in Supplement S1 ). The high correlations between HW, AW, VW and BWE were globally maintained also upon perturbations of the β-adrenergic system but were slightly reduced under iso10 as compared to ctr mice (i.e. 0.4<r<0.8; compare Suppl. Figures 6A and 6D in Supplement S1 ). High similarity of HW and VW with Pdur was still detected under β-stimulation (i.e. 0.5<r<0.8) but it was significantly reduced under ate (r = 0.19, p = 0.38). Again and irrespective of the drug condition, no significant correlation could be identified between HR and SBP, between HR and VWI or AWI, and between SBP and AWI or VWI.
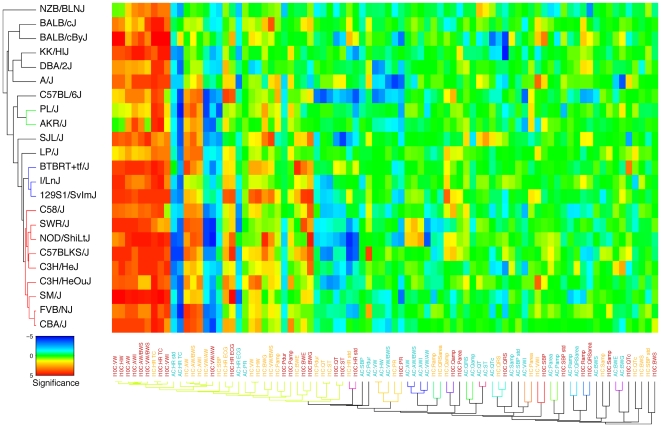
Figure 6. Patterns of phenotype and strain correlations under drug treatment.
For each strain (rows) and combination (columns) of a phenotype and a treatment (ate, iso1, iso10) the significance (signed -log10 value of Wilcoxon ranksum test, as used in Figure 4) of the phenotypic response with respect to the ctr group is shown using a colour code. Rows and columns are clustered according to pattern similarity. The branches of the dendrograms illustrating the clusters are plotted with the same colour as long as the average linkage distance is less than 50% of the maximal distance. AC: ate vs ctr; I1C: iso1 vs ctr; I10C: iso10 vs ctr. See Table 1 for abbreviations. HR std: standard deviation of HR (TC) strain means; SBP std: standard deviation of SBP strain means.
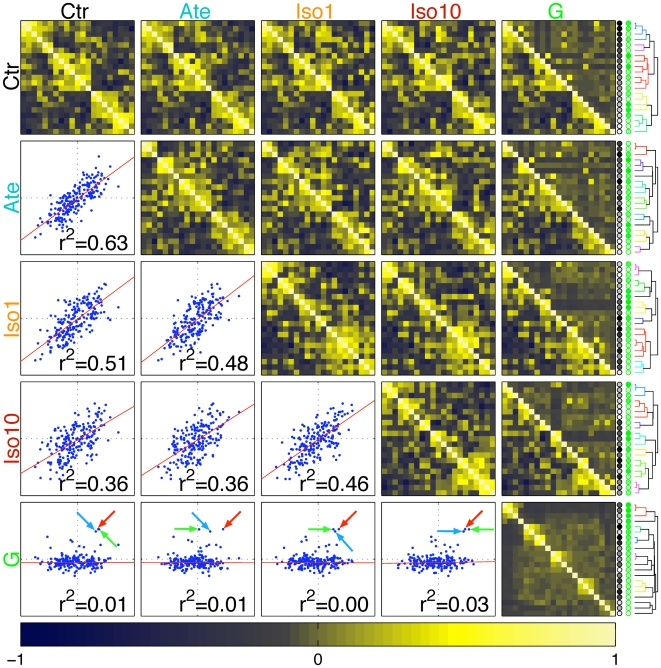
Figure 7. Comparison of strain relatedness based on phenotypes or genotypes.
Along the main diagonal, five clustered correlation matrices 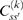 (where k = ctr, ate, iso1, iso10, or G) across all phenoypes or SNPs (k = G) are shown. Similar strains are placed close to each other and their order is indicated by the coloured circles and dendrograms displayed on the right as determined by standard hierarchical clustering. Above these five matrices ten composite matrices are shown whose lower left part consists of the correlation matrix shown on the main diagonal to the left, while their upper right contains the correlations from the dataset indicated on top, using the same order of strains (i.e. the rows of all adjacent correlation matrices correspond to a fixed strain). The degrees of correlations are indicated by a colour code. In the scatter plots below the diagonal, each pair of strains (s,s') is represented by a dot whose coordinates are given by the correlations
(where k = ctr, ate, iso1, iso10, or G) across all phenoypes or SNPs (k = G) are shown. Similar strains are placed close to each other and their order is indicated by the coloured circles and dendrograms displayed on the right as determined by standard hierarchical clustering. Above these five matrices ten composite matrices are shown whose lower left part consists of the correlation matrix shown on the main diagonal to the left, while their upper right contains the correlations from the dataset indicated on top, using the same order of strains (i.e. the rows of all adjacent correlation matrices correspond to a fixed strain). The degrees of correlations are indicated by a colour code. In the scatter plots below the diagonal, each pair of strains (s,s') is represented by a dot whose coordinates are given by the correlations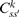 and
and 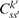 where k and k' are indicated on the left and top, respectively. The correlation r2 between these dots is indicated and a red line shows the best linear fit. Blue arrow: C3H/HeJ vs CBA/J, red arrow: 129S1/SvImJ vs LP/J, green arrow: C57BL76J vs C57BLKS/J.
where k and k' are indicated on the left and top, respectively. The correlation r2 between these dots is indicated and a red line shows the best linear fit. Blue arrow: C3H/HeJ vs CBA/J, red arrow: 129S1/SvImJ vs LP/J, green arrow: C57BL76J vs C57BLKS/J.
Structure of the response to β-adrenergic drugs
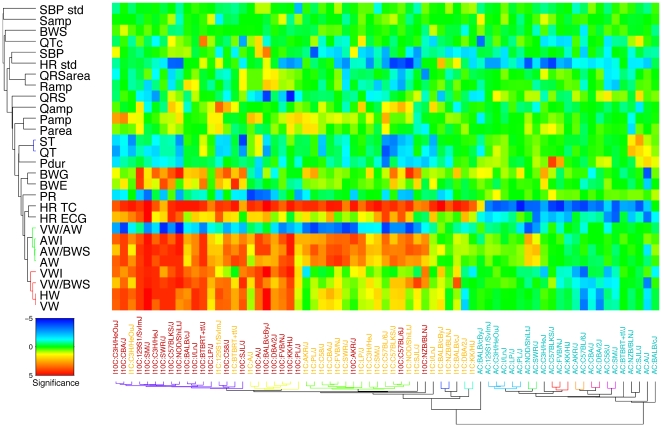
In the previous paragraphs, we focused on the differential behaviour of a selection of traits across strains and treatments, considering one trait and/or one drug condition at a time. Yet, such serial screening is not well suited to fully exploit the rather large set of phenotypic data we generated (i.e. 27 phenotypes measured in 23 strains and under 4 treatment conditions). In order to explore the relationships between the various parameters in a more comprehensive manner and to provide a more global picture on the structure of these data, we performed unsupervised bi-clustering analyses. Specifically, we transformed the three dimensional table 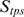 of the signed p-values attributed to the effect of each treatment (t = ate, iso1, iso10) with respect to the ctr group into two two-dimensional tables (matrices). The first matrix Spi (
Figure 5
) has one row for each phenotype p, while each column i refers to a combination (t,s) of a treatment t in strain s. Conversely, the second matrix Ssj (
Figure 6
) has one row for each strain s, while each column j refers to a combination (t,p) of a treatment under which the phenotype p was observed (we chose not to consider a matrix that has one row for each treatment). Hierarchical bi-clustering was applied to determine clusters in these matrices (see
Materials and Methods
for details). Strains were clustered according to (signed) values of similarity (i.e. Pearson correlations between significance profiles across all phenotypes). In contrast, we used absolute correlations as similarity measure between phenotypes (such that inversely related observables, like HR and ECG intervals are clustered together). The reordered matrices together with the similarity dendrograms are shown in colour codes, providing a convenient, unbiased and condensed means to compare subsets (clusters) of related phenotypes or strains based on their response to β-adrenergic drugs.
of the signed p-values attributed to the effect of each treatment (t = ate, iso1, iso10) with respect to the ctr group into two two-dimensional tables (matrices). The first matrix Spi (
Figure 5
) has one row for each phenotype p, while each column i refers to a combination (t,s) of a treatment t in strain s. Conversely, the second matrix Ssj (
Figure 6
) has one row for each strain s, while each column j refers to a combination (t,p) of a treatment under which the phenotype p was observed (we chose not to consider a matrix that has one row for each treatment). Hierarchical bi-clustering was applied to determine clusters in these matrices (see
Materials and Methods
for details). Strains were clustered according to (signed) values of similarity (i.e. Pearson correlations between significance profiles across all phenotypes). In contrast, we used absolute correlations as similarity measure between phenotypes (such that inversely related observables, like HR and ECG intervals are clustered together). The reordered matrices together with the similarity dendrograms are shown in colour codes, providing a convenient, unbiased and condensed means to compare subsets (clusters) of related phenotypes or strains based on their response to β-adrenergic drugs.
Figure 5. Patterns of phenotype and strain correlations under drug treatment.
For each phenotype (rows) and combination (columns) of a strain and a treatment (ate, iso1, iso10) the significance (signed -log10 value of Wilcoxon ranksum test, as used in Figure 4) of the phenotypic response with respect to the ctr group is shown using a colour code. Rows and columns are clustered according to pattern similarity. The branches of the dendrograms illustrating the clusters are plotted with the same colour as long as the average linkage distance is less than 20% of the maximal distance. AC: ate vs ctr; I1C: iso1 vs ctr; I10C: iso10 vs ctr. See Table 1 for abbreviations. HR std: standard deviation of HR (TC) strain means; SBP std: standard deviation of SBP strain means.
In Figure 5 , strain responses to pharmacological challenge (i.e. “treated strains”) clustered mainly according to the type of drug, as the first branching in the tree clearly discriminates ate- from iso-treated lines (with the exception of strain Balb/cByJ under ate). This segregation reflects the opposite response of HR to the two antagonist drugs, indicating that HR reaction to treatments was the most discriminative phenotype of the whole dataset (according to principle component analysis, data not shown). Sub-clustering in iso-mediated responses was essentially driven by the strain-specific and dose-dependent responses of cardiac ventricles and atria to β-stimulation. This resulted in an almost perfect segregation between the iso1- and iso10-treated groups of strains. Five strains were poor responders to iso1 at the level of cardiac atria (when compared to controls) and segregated in three separate clusters (i.e. strains KK/HlJ, DBA/2J, Balb/cJ, NZB/BlNJ, and Balb/cByJ). Of all strains exhibiting increased AW and AWI under iso, a subset of twelve lines exhibited modest changes of HW, VW and VWI (i.e. see green cluster of treated strains in Figure 5 ). This effect was present under iso1 in ten strains (i.e. strains NOD/ShiLtJ, C57BLKS/J, SM/J, C3H/HeJ, LP/J, SWR/J, FVB/NJ, CBA/J, C58/J, and PL/J) and under both concentrations of iso in lines AKR/J and C57BL/6J. The main trends in the remaining strains were essentially (but not exclusively) observed under iso10. They were dominated by concomitant increases of AW, AWI, HW, VW, and VWI. These strong responding strains clustered into two distinct subsets, depending on the effect of sustained β-stimulation on BW and BWG. Thus, both values remained unchanged in six lines (i.e. PL/J, KK/HlJ, FVB/NJ, DBA/2J, Balb/cByJ, and A/J), while they tended to increase in the majority of the fourteen others (i.e. see purple cluster of treated strains in Figure 5 ). While strains of the green sub-cluster tended to exhibit reduced blood pressure under treatment, this trait was either stable or slightly increased in the yellow and purple groups.
Looking at the categorisation under β-blockade, we first note that both Balb/c strains were relatively insensitive to ate, in particular for HR. As such, they did not co-segregate with any of the other lines. The next most informative sub-category correlates best with HR patterns of the ECG data, mirroring differential pulse responses under anaesthesia.
From the same figure, it is also possible to address the segregation of phenotypic modifications across all treatments. More specifically, the first two clusters (i.e. red and green clusters of phenotypic changes, Figure 5 ) include responses of traits related to cardiac weight and indices (correlations across trait responses: 0.5<|r|<1, Suppl. Figure 8A, Supplement S1 ). The structure of these clusters was essentially determined by the differential (and dose-dependent) effects of iso on cardiac ventricles and atria. These responses are in relatively close proximity with those of HR (absolute correlations: 0.5<|r|<0.9), PR (absolute correlations: 0.6<|r|<0.8), BWE (correlations: 0.4<r<0.8) and BWG (correlations: 0.4<r<0.8), whereas the patterns of SBP variations are more discordant (absolute correlations: |r|<0.4). Interestingly, despite the effect of anaesthesia on ECG parameters, significance scores for the two measurements of HR responses are highly correlated across all treated strains (r = 0.79, p = 3×10−7, Suppl. Figure 8A, Supplement S1 ), even though the respective baseline values were only poorly correlated. This is reflected by HR and HR (ECG) appearing co-clustered, while the patterns of PR intervals modifications are slightly more distinct (correlation with HR: r = −0.67, p = 7×10−5). Control phenotypes such as body weight before treatment (BWS) and SBP standard deviation (SBP std) that did not notably vary across strains and drug conditions did not cluster with the other variables. Apart from Pamp, amplitudes and areas of ECG waves, which were poorly affected by the drugs, are also relatively distant from the other traits.
Figure 6 summarises the clustering of inbred strains as determined by their responses to ate and iso. As expected, strains were all positively correlated (i.e. correlations: 0.4<r<1, Suppl. Figure 9A, Supplement S1 ), indicating that they usually differed only in the relative amplitude but not the directionality of the responses. NZB/BlNJ mice were on average the least affected by the treatments and as such the most distant from the others. Three sub-groups seem to emerge from this analysis. The first one comprises fourteen strains organised in two clusters and two separate lines (i.e. red cluster: strains CBA/J, FVB/NJ, SM/J, C3H/HeOuJ, C3H/HeJ, C57BLKS/J, NOD/ShiLtJ, SWR/J, and C58/J; blue cluster: strains 129S1/SvImJ, I/LnJ, and BTBRT+ tf/J; as well as strains LP/J and SJL/J, Figure 6 ; correlations: 0.7<r<1, Suppl. Figure 9A, Supplement S1 ) while the second category includes five strains (i.e. A/J, DBA/2J, KK/HlJ, BALB/cByJ, and BALB/cJ; correlations: 0.6<r<0.9). Three additional strains (i.e. AKR/J, PL/J and C57BL/6J) are in mild proximity with the first sub-group (i.e. correlations: 0.6<r<0.9) and more distant from the second one (i.e. correlations: 0.4<r<0.8). Trends specific to the first sub-group are marked increases of (i) HR (ECG) under iso, (ii) AW, AWI, VW, VWI, and HW under iso10, and (iii) BWG under iso10. The second cluster is characterised by very mild changes of (i) HR under anaesthesia, (ii) AW and AWI under iso1 and (iii) BWG under iso10, whereas HW, VW, and VWI are significantly increased by iso10. The three strains standing between these two groups correlate with very mild variations of VW, HW, and VWI under both concentrations of iso as well as decreased SBP under iso1. It is tempting to speculate that these phenotypic patterns segregate due to specific genetic determinants but further analyses will be required to establish our observations and link them to genetic variation.
On the other axis of Figure 6 , phenotype treatment combinations exhibit no clear clustering hierarchy (except for small groups of trivially related phenotypes). Rather weakly affected traits in response to β-adrenergic perturbations gradually agglomerate towards the right, the more strongly affected traits appearing on the left.
Strain relatedness based on phenotypic or genotypic data
Of the 23 strains characterised above, 21 have been genotyped at a genome-wide density of over 100′000 SNPs [24]. To compare strain proximity based either on the phenotypes observed in each of the four experimental conditions (ctr, ate, iso1 and iso10; Suppl.
Figures 5
and
7
in
Supplement S1
) or on these genotypes (G; Suppl. Figure 10 in
Supplement S1
), we computed the five corresponding correlations matrices 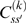 (where k = ctr, ate, iso1, iso10, or G, see
Materials and Methods
for details). In
Figure 7
, these (symmetric) matrices are drawn along the main diagonal of the figure (i.e. from top left to bottom right) with similar strains placed close to each other (i.e. the highest inter-strain correlations tend to appear close to the diagonal of each matrix). Strain order was determined by standard hierarchical clustering and is indicated by the circles and dendrograms displayed on the right of the figure. Specifically, the order based on phenotypic (ctr) relatedness is symbolised by shades inside the circles graded from black to white, whereas the order based on genetic proximity is represented by shades graded from green to white (i.e. each circle represents a single strain and strains with similar shades tend to be closely related). Dendrograms associated to trait-based relatedness are identical to those of Suppl.
Figures 5 and 7 (
Supplement S1
), with the exception that strains Balb/cJ and C3H/HeOuJ, for which genetic information was missing [24], were not taken into account. Above these five matrices we show ten composite matrices. In each of the latter, the lower left part is identical to the correlation matrix shown on the main diagonal on the left, while the upper right contains the correlations within the datasets indicated on top (while keeping the same strain order as in the lower left part). In other words, the matrices in the top row of the figure allow for the successive comparison of the strain proximity as determined by ctr phenotypes with those obtained from traits recorded under ate, iso1 and iso10 or from the genotypes. Accordingly, the second row allows for comparison across treatments or genotypes using the strain order as determined by ate phenotypes, and so on. Below the main diagonal we show scatter plots, where for each pair of strains (s,s') we draw a dot whose coordinates are given by the correlations
(where k = ctr, ate, iso1, iso10, or G, see
Materials and Methods
for details). In
Figure 7
, these (symmetric) matrices are drawn along the main diagonal of the figure (i.e. from top left to bottom right) with similar strains placed close to each other (i.e. the highest inter-strain correlations tend to appear close to the diagonal of each matrix). Strain order was determined by standard hierarchical clustering and is indicated by the circles and dendrograms displayed on the right of the figure. Specifically, the order based on phenotypic (ctr) relatedness is symbolised by shades inside the circles graded from black to white, whereas the order based on genetic proximity is represented by shades graded from green to white (i.e. each circle represents a single strain and strains with similar shades tend to be closely related). Dendrograms associated to trait-based relatedness are identical to those of Suppl.
Figures 5 and 7 (
Supplement S1
), with the exception that strains Balb/cJ and C3H/HeOuJ, for which genetic information was missing [24], were not taken into account. Above these five matrices we show ten composite matrices. In each of the latter, the lower left part is identical to the correlation matrix shown on the main diagonal on the left, while the upper right contains the correlations within the datasets indicated on top (while keeping the same strain order as in the lower left part). In other words, the matrices in the top row of the figure allow for the successive comparison of the strain proximity as determined by ctr phenotypes with those obtained from traits recorded under ate, iso1 and iso10 or from the genotypes. Accordingly, the second row allows for comparison across treatments or genotypes using the strain order as determined by ate phenotypes, and so on. Below the main diagonal we show scatter plots, where for each pair of strains (s,s') we draw a dot whose coordinates are given by the correlations 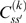 and
and 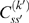 where k and k' are indicated on the left and top, respectively. The correlation r2 between these dots is indicated and a red line shows the best linear fit of the data.
where k and k' are indicated on the left and top, respectively. The correlation r2 between these dots is indicated and a red line shows the best linear fit of the data.
Pair-wise comparisons indicate that the patterns of strain relatedness based on any of the four sets of phenotypic data are positively correlated. The closest profiles are those obtained from traits measured in ctr and ate mice (i.e. correlation r 2 = 0.63, p = 7×10−7), while those resulting from the phenotypes measured in ctr and iso10 or ate and iso10 are significantly more divergent (i.e. r 2 = 0.36, p = 0.001 and r 2 = 0.36, p = 0.001, respectively, Figure 7 ). These differences reflect the strain-specificity of the trait modifications induced by the β-adrenergic drugs, in particular iso10.
Nominal values of inter-strain correlations based on whole-genome genetic profiles are detailed in Suppl. Figure 10 ( Supplement S1 ). In this analysis, the vast majority of the correlations are below 0.2. Four non-overlapping clusters of strains are characterised by correlations above this value, the closest pairs being 129S1/SvImJ vs LP/J, C3H/HeJ vs CBA/J, and C57BL/6J vs C57BLKS/J (i.e. all with correlations r>0.4). This hierarchy agrees well with the known ancestry of laboratory mouse strains [25], [26]. Irrespective of the drug condition, we observe little concordance when comparing overall strain phylogeny based on phenotypes with relatedness computed from the genomic SNP-profiles ( Figure 7 ). Yet, it is interesting to note that the three genetically closest pairs of strains tend to segregate in the same or adjacent trait-based clusters (see arrows in Figure 7 and related clusters in Suppl. Figure 7 of Supplement S1 ). This is true for strains 129S1/SvImJ and LP/J in all conditions and for strains CBA/J and C3H/HeJ as well as C57BL/6J and C57BLKS/J in ctr, iso1, and iso10 conditions. Conversely, we have already highlighted divergence between strains C57BL/6J and A/J or C57BL/6J and 129S1/SvImJ for traits such as VWI under β-stimulation. Accordingly, when considering all phenotypes, trait-based relatedness is consistently relatively low between these latter pairs of strains (Suppl. Figure 7 in Supplement S1 ). Nevertheless, globally the correlation between relatedness based on our cardiovascular phenotypes and whole-genome SNP profiles is low. This suggests that these phenotypes are likely to be governed by smaller, local differences in the genomes of the various strains.
Discussion
Inter-individual differences in response to medication are well-established clinical issues, the study of which is a large field of research in itself. Yet, pharmacogenetic studies in humans are hampered by several important concerns such as compliance issues, environmental confounders and limitations to non-invasive measurements. In this context laboratory mice, which have already played a key role for dissecting the genetic basis of disease susceptibility and providing key support for many current concepts of disease pathogenesis and treatment strategies, are also likely to become instrumental for complementing and furthering human pharmacogenetics [27]. Indeed, several recent studies already addressed the genetic basis of strain-specific susceptibility to carcinogens [28], [29] and analgesics [30], [31] or the metabolism of warfarin, testosterone and irinotecan [32], [33] in inbred mice.
Here we undertook a similar approach focusing on the cardiovascular system. Our large survey assessed the suitability of using a large panel of inbred mouse strains to investigate physiological responses to β-adrenergic treatments. To the best of our knowledge, it provides the first large-scale standardised characterisation of heart rate, systolic blood pressure, ECG and cardiac weight indices in response to sustained infusion of atenolol and isoproterenol in such a panel.
The robustness of our experimental standards is supported by several essential validation steps: first, mean strain values measured in ctr condition correlated well with independent datasets of the Mouse Phenome Database. In agreement with the notion that morphological parameters are highly heritable and least affected by environment, traits related to body and cardiac weight were the most consistent with independent studies. Even though conservation across independent datasets was much reduced for SBP and HR, the number of significant between-projects correlations was higher than expected by chance for both traits. As discussed by others [16], [34], [35], this confirms that despite marked susceptibility to environmental factors, a significant portion of HR and SBP variance observed in inbred mouse strains is genetically determined. Second, phenotypic variances were generally smaller within than between strains, indicative of elevated trait heritabilities (i.e. typically H2>0.7). H2 values were indeed significantly higher than in human populations, in particular for SBP, HR and ECG intervals (see for instance [36], [37] and [38]), where H2 estimates typically vary around 0.6 in twin studies and around 0.25 in nuclear families. This further emphasises the potential of using our mouse model instead of traditional human cohorts for downstream genome-wide association scans. Third and regardless of the treatment conditions, strain means for all phenotypes had uni-modal distributions, consistent with the idea that they are complex traits under the control of multiple genetic loci.
We found that cardiovascular responses to drug exposure were trait-, drug-, and dose-specific. In order to obtain a comprehensive and unbiased overview of the structure of the phenotypic data with respect to the relationships across strains, treatments, and phenotypes, we performed a comprehensive comparative investigation. Our unsupervised bi-clustering analyses facilitated processing the large phenotypic data in order to extract key information regarding strain proximity and drug-dependent phenotypic perturbations. For instance our clustered matrices of significance score made immediately apparent that phenotypic changes were usually more significant under β-stimulation than β-blockade (consistent with iso and ate being administered to healthy animals housed in a reduced stress environment). When considering all experimental conditions, HR was the most, and SBP and QTc the least affected phenotypes in response to both pharmacological compounds. Strain responses of phenotypes related to body and heart weight (i.e. HW, AW, AWI, VW, VWI, BWE, BWG) were well correlated with drug-induced changes of heart rate and ECG intervals (in particular PR, Pdur, QT and ST), but not with the responses of systolic blood pressure and ECG wave amplitudes or areas. Trait-specific patterns of sensitivity to iso ranged from maximal response under treatment with iso1 for HR to negligible effects under either concentrations of iso for SBP. We detected compartmental and strain-specific cardiac sensitivity to iso, with atria responding at lower concentrations than ventricles in the majority of the strains. At this stage, the biological mechanisms underlying differential sensitivities to chronic β-stimulation are not known but might reflect distinct and strain-specific distributions of atrial and ventricular β-adrenergic receptors and/or differential downstream signalling pathways. Altogether, these data suggest that responses to β-adrenergic drugs by themselves are determined by complex genetic architectures. Beyond the specific context of the present study, it is worth mentioning that the unsupervised bi-clustering analyses we employed in this study could also be useful for dissecting other large datasets of complex phenotypic traits, which are likely to become more and more frequent.
Due to their broad use to generate transgenic or knock-out models, the lines C57BL/6J and 129S1/SvImJ are of particular interest. Our comprehensive analyses as well as other reports [16], [34], [35] have clearly highlighted their differential behaviours for baseline phenotypes such as SBP, atrial and ventricular size, heart rate, heart rate variability and cardiac metabolism. Recently, Barrick et al. further emphasised on the specificity of 129S1/SvImJ and C57BL/6J responses to prolonged trans-aortic constriction (TAC), another model of left ventricular hypertrophy associated with increased hemodynamic load and sustained β-adrenergic stimulation [39]. Upon aortic banding, C57BL/6J mice had an earlier onset and more pronounced impairment in contractile function, with corresponding left and right ventricular dilatation, fibrosis, change in expression of hypertrophy markers, and increased liver weights at five weeks post-constriction. In contrast, 129S1/SvImJ mice had delayed transition to decompensated heart failure, with relatively mild alterations in histology and markers of hypertrophy at five weeks post-TAC and preserved systolic function until eight weeks post-TAC, suggesting that 129S1/SvImJ genetic modifiers might protect against the earlier and more severe pathological changes seen in C57BL/6J mice [39]. In contrast, in our study, iso10-mediated increase of VWI was marked in strain 129S1/SvImJ while C57BL/6J mice were significantly more resistant. This indicates that different signalling pathways may lead to cardiac hypertrophy, depending on the type of upstream trigger (i.e. pressure overload vs sustained β-adrenergic activation). These observations put an additional emphasis on the need for detailed characterising of these and other strains, both in terms of baseline cardiovascular phenotypes and in response to pressure overload or β-adrenergic challenge.
Future work will aim at associating the rich phenotypic data of this study with genetic markers. Indeed the vast majority of the strains of our CV-PGX panel has been extensively genotyped [24]. Early studies already suggested that the genome of laboratory mice is a mosaic of regions of distinct but limited sub-specific origins [40]. More recent resequencing efforts have refined this picture by cataloguing over 8 million SNP alleles across 16 inbred strains [41] and genetic maps of similar densities were further imputed in 49 strains [42]. These invaluable resources allow for inferring the ancestry of most of the genome with good confidence and provide mapping resolution usually higher than in other model organisms. Thus, it is now becoming feasible to map trait variation in inbred mouse strains by testing association to loci of inferred ancestry [24], [43], [44]. Here we already used genomic SNP profiles to show that overall genetic similarity of the strains exhibits little concordance with their phenotypic relatedness in terms of the collection of cardio-vascular traits we measured. This indicates that cardiovascular phenotypes are unlikely to segregate according to global phylogeny, but rather be governed by smaller, local differences in the genomes of the various strains. Thus, given the significant heritability of many of these traits, we are confident that association studies using our phenotypic resource have good chances to reveal new candidate loci related to differential cardiovascular responses under treatments.
Materials and Methods
Ethics Statement
All animal procedures have been approved by the “Service vétérinaire cantonal vaudois” (authorisation n° 1649) and were performed in accordance with the National Institutes of Health (NIH) guidelines for the care and use of laboratory animals.
Mice
Inbred mouse strains were selected from the priority strains list of the Mouse Phenome Database (MPD: http://www.jax.org/phenome [15]), based on their genealogy and the density of SNPs characterised in each strain. Mice were purchased in two to six lots per strain, either from Charles River, France (strains Balb/cByJ, C3H/HeOuJ, CBA/J, and DBA/2J) or from the Jackson Laboratory at Bar Harbor, Maine, USA (strains 129S1/SvImJ, A/J, AKR/J, BALB/cJ, BTBRT+ tf/J, C3H/HeJ, C57BL6/J, C57BLKS/J, C58/J, FVB/NJ, I/LnJ, KK/HlJ, LP/J, NOD/ShiLtJ, NZB/BlNJ, PL/J, SJL/J, SM/J, and SWR/J). Only males, aged ten to twelve weeks on average, were included in the experimental protocol. Mice were housed at our local animal research facility under conditions of 14 hours light, 10 hours darkness, ambient temperature of 25°C, and relative humidity of 30–60%.
Pharmacological agents and treatments
Ate and iso (Sigma-Aldrich) were administered to mice chronically for two weeks ( Figure 1 ). Osmotic mini-pumps (Alzet, model 2002, Charles River Laboratories) were implanted sub-cutaneously under anaesthesia and set to deliver ate at 10 mg/kg per day and iso at 1 (iso1) and 10 (iso10) mg/kg per day. Based on the results of a pilot comparison of four C57BL/6J mice implanted with minipumps loaded with 0.9% NaCl vs six non-implanted isogenic animals, ctr mice were not implanted with minipumps.
Phenotypic characterisation
A general outline of the phenotyping process is presented in Figure 1 . In brief, heart rate (HR) and systolic blood pressure (SBP) were monitored in conscious animals by the tail-cuff (TC) method, using a Visitech BP-2000 blood pressure analysis system. All animals were trained for one week prior to recording effective values on days 10, 11, and 12 ( Figure 1 ) and all measurements were taken at a similar time of the day (2–5 pm). Electrocardiograms (ECG) were recorded in halothane-anaesthetised mice using the IOX 1.7.0 and ECG-Auto 1.5.7 softwares (EMKA Technologies). On day 16 or 17, mice were weighted and sacrificed by decapitation. Hearts were rapidly excised from the animals, rinsed in ice-cold phosphate buffered saline (PBS) solution, and blotted dry. Cardiac atria and ventricles were dissected and weighted separately. Cardiac tissues were frozen in liquid nitrogen and stored at −70°C. Phenotypes recorded in the present project are listed in Table 1 . Individual phenotypes were assessed in multiple series of independent experiments. In each strain, an average of ten individuals per experimental condition (ctr, ate, iso1 and iso10) were monitored across all phenotypes.
Data management and statistics
Heritability
The heritability H2 of each phenotype was calculated separately for each treatment over all strains. It is defined as:
where Var(G) is the genetic (i.e. the inter-strain) variance, and Var(E) is the environmental (i.e. the intra-strain) variance of the considered phenotype.
Strain and phenotype clustering
Bi-Clustering was performed using the Matlab® standard clustering routine. Similarity between phenotype p and p' was computed as 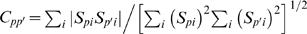 . Similarity between strains s and s' was computed as
. Similarity between strains s and s' was computed as 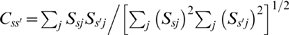 . See main text for the definitions of
. See main text for the definitions of 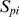 and
and 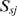 . Note that both expressions are invariant under an inverse transformation of any phenotype.
. Note that both expressions are invariant under an inverse transformation of any phenotype.
Phenotypic versus genotypic relatedness
We use Pearson correlations 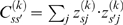 both for phenotypic (k = ctr, ate, iso1, iso10) and genotypic (k = G) similarity. For the latter we first normalised the genotype of each SNP by taking the z-score across all strains. Here
both for phenotypic (k = ctr, ate, iso1, iso10) and genotypic (k = G) similarity. For the latter we first normalised the genotype of each SNP by taking the z-score across all strains. Here 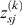 refers to the z-scores (the deviation from the mean value in units of standard deviation) for phenotypic (j runs across all phenotypes) or genotypic (j runs across all SNPs) profiles, respectively.
refers to the z-scores (the deviation from the mean value in units of standard deviation) for phenotypic (j runs across all phenotypes) or genotypic (j runs across all SNPs) profiles, respectively.
Supporting Information
Supplementary Results.
(1.57 MB PDF)
Acknowledgments
The authors would like to thank Drs Eleazar Eskin and Toby Johnson for their valuable feedback and comments, and Mrs Christine Perregaux for technical help.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the Centre Hospitalier Universitaire Vaudois and the University of Lausanne, Switzerland, the Swiss National Science Foundation (grants nb 310000-112552, JSB, HA, TP, and FM and nb 3100AO-116323/1, SB), the Giorgi-Cavaglieri Foundation (SB) and the European Framework Project 6 (through the EuroDia, AnEuploidy and Hypergenes projects, SB). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hollenberg NK. The role of beta-blockers as a cornerstone of cardiovascular therapy. Am J Hypertens. 2005;18:165S–168S. doi: 10.1016/j.amjhyper.2005.09.010. 10.1016/j.amjhyper.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 2.Chrysant SG, Chrysant GS, Dimas B. Current and future status of beta-blockers in the treatment of hypertension. Clin Cardiol. 2008;31:249–252. doi: 10.1002/clc.20249. 10.1002/clc.20249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gillies M, Bellomo R, Doolan L, Buxton B. Bench-to-bedside review: Inotropic drug therapy after adult cardiac surgery – a systematic literature review. Crit Care. 2005;9:266–279. doi: 10.1186/cc3024. 10.1186/cc3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prenner BM. Role of long-acting beta2-adrenergic agonists in asthma management based on updated asthma guidelines. Curr Opin Pulm Med. 2008;14:57–63. doi: 10.1097/MCP.0b013e3282f27121. 10.1097/MCP.0b013e3282f27121. [DOI] [PubMed] [Google Scholar]
- 5.Wilkinson GR. Drug metabolism and variability among patients in drug response. N Engl J Med. 2005;352:2211–2221. doi: 10.1056/NEJMra032424. [DOI] [PubMed] [Google Scholar]
- 6.Muszkat M, Stein CM. Pharmacogenetics and response to beta-adrenergic receptor antagonists in heart failure. Clin Pharmacol Ther. 2005;77:123–126. doi: 10.1016/j.clpt.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Fitzgerald JD, Ruffin R, Smedstad KG, Roberts R, McAinsh J. Studies on the pharmacokinetics and pharmacodynamics of atenolol in man. Eur J Clin Pharmacol. 1978;13:81–89. doi: 10.1007/BF00609750. [DOI] [PubMed] [Google Scholar]
- 8.Reeves PR, Barnfield DJ, Longshaw S, McIntosh DA, Winrow MJ. Disposition and metabolism of atenolol in animals. Xenobiotica. 1978;8:305–311. doi: 10.3109/00498257809060955. [DOI] [PubMed] [Google Scholar]
- 9.Morgan DJ. Clinical pharmacokinetics of beta-agonists. Clin Pharmacokinet. 1990;18:270–294. doi: 10.2165/00003088-199018040-00002. [DOI] [PubMed] [Google Scholar]
- 10.Materson BJ, Reda DJ, Cushman WC, Massie BM, Freis ED, et al. Single-drug therapy for hypertension in men. A comparison of six antihypertensive agents with placebo. The Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. N Engl J Med. 1993;328:914–921. doi: 10.1056/NEJM199304013281303. [DOI] [PubMed] [Google Scholar]
- 11.Kirstein SL, Insel PA. Autonomic nervous system pharmacogenomics: a progress report. Pharmacol Rev. 2004;56:31–52. doi: 10.1124/pr.56.1.2. [DOI] [PubMed] [Google Scholar]
- 12.Osadchii OE. Cardiac hypertrophy induced by sustained beta-adrenoreceptor activation: pathophysiological aspects. Heart Fail Rev. 2007;12:66–86. doi: 10.1007/s10741-007-9007-4. 10.1007/s10741-007-9007-4. [DOI] [PubMed] [Google Scholar]
- 13.Faulx MD, Ernsberger P, Vatner D, Hoffman RD, Lewis W, et al. Strain-dependent beta-adrenergic receptor function influences myocardial responses to isoproterenol stimulation in mice. Am J Physiol Heart Circ Physiol. 2005;289:H30–H36. doi: 10.1152/ajpheart.00636.2004. [DOI] [PubMed] [Google Scholar]
- 14.Shusterman V, Usiene I, Harrigal C, Lee JS, Kubota T, et al. Strain-specific patterns of autonomic nervous system activity and heart failure susceptibility in mice. Am J Physiol Heart Circ Physiol. 2002;282:H2076–H2083. doi: 10.1152/ajpheart.00917.2001. [DOI] [PubMed] [Google Scholar]
- 15.Grubb SC, Maddatu TP, Bult CJ, Bogue MA. Mouse phenome database. Nucleic Acids Res. 2009;37:D720–D730. doi: 10.1093/nar/gkn778. 10.1093/nar/gkn778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deschepper CF, Olson JL, Otis M, Gallo-Payet N. Characterization of blood pressure and morphological traits in cardiovascular-related organs in 13 different inbred mouse strains. J Appl Physiol. 2004;97:369–376. doi: 10.1152/japplphysiol.00073.2004. [DOI] [PubMed] [Google Scholar]
- 17.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57:289–300. [Google Scholar]
- 18.Yekutieli D, Benjamini Y. Resampling-based false discovery rate controlling multiple test procedures for correlated test statistics. Journal of Statistical Planning and Inference. 1999;82:171–196. [Google Scholar]
- 19.Chu V, Otero JM, Lopez O, Morgan JP, Amende I, et al. Method for non-invasively recording electrocardiograms in conscious mice. BMC Physiol. 2001;1:6. doi: 10.1186/1472-6793-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shizukuda Y, Buttrick PM, Geenen DL, Borczuk AC, Kitsis RN, et al. beta-adrenergic stimulation causes cardiocyte apoptosis: influence of tachycardia and hypertrophy. Am J Physiol. 1998;275:H961–H968. doi: 10.1152/ajpheart.1998.275.3.H961. [DOI] [PubMed] [Google Scholar]
- 21.Kudej RK, Iwase M, Uechi M, Vatner DE, Oka N, et al. Effects of chronic beta-adrenergic receptor stimulation in mice. J Mol Cell Cardiol. 1997;29:2735–2746. doi: 10.1006/jmcc.1997.0508. [DOI] [PubMed] [Google Scholar]
- 22.Faulx MD, Chandler MP, Zawaneh MS, Stanley WC, Hoit BD. Mouse strain-specific differences in cardiac metabolic enzyme activities observed in a model of isoproterenol-induced cardiac hypertrophy. Clin Exp Pharmacol Physiol. 2007;34:77–80. doi: 10.1111/j.1440-1681.2007.04531.x. 10.1111/j.1440-1681.2007.04531.x. [DOI] [PubMed] [Google Scholar]
- 23.Errami M, Galindo CL, Tassa AT, Dimaio JM, Hill JA, et al. Doxycycline attenuates isoproterenol- and transverse aortic banding-induced cardiac hypertrophy in mice. J Pharmacol Exp Ther. 2008;324:1196–1203. doi: 10.1124/jpet.107.133975. 10.1124/jpet.107.133975. [DOI] [PubMed] [Google Scholar]
- 24.Wade CM, Daly MJ. Genetic variation in laboratory mice. Nat Genet. 2005;37:1175–1180. doi: 10.1038/ng1666. [DOI] [PubMed] [Google Scholar]
- 25.Beck JA, Lloyd S, Hafezparast M, Lennon-Pierce M, Eppig JT, et al. Genealogies of mouse inbred strains. Nat Genet. 2000;24:23–25. doi: 10.1038/71641. [DOI] [PubMed] [Google Scholar]
- 26.Petkov PM, Ding Y, Cassell MA, Zhang W, Wagner G, et al. An efficient SNP system for mouse genome scanning and elucidating strain relationships. Genome Res. 2004;14:1806–1811. doi: 10.1101/gr.2825804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cotsapas C. Identifying genetic components of drug response in mice. Pharmacogenomics. 2008;9:1323–1330. doi: 10.2217/14622416.9.9.1323. 10.2217/14622416.9.9.1323. [DOI] [PubMed] [Google Scholar]
- 28.Fenske TS, McMahon C, Edwin D, Jarvis JC, Cheverud JM, et al. Identification of candidate alkylator-induced cancer susceptibility genes by whole genome scanning in mice. Cancer Res. 2006;66:5029–5038. doi: 10.1158/0008-5472.CAN-05-3404. 10.1158/0008-5472.CAN-05-3404. [DOI] [PubMed] [Google Scholar]
- 29.Liu P, Wang Y, Vikis H, Maciag A, Wang D, et al. Candidate lung tumor susceptibility genes identified through whole-genome association analyses in inbred mice. Nat Genet. 2006;38:888–895. doi: 10.1038/ng1849. 10.1038/ng1849. [DOI] [PubMed] [Google Scholar]
- 30.Liang DY, Liao G, Wang J, Usuka J, Guo Y, et al. A Genetic Analysis of Opioid-induced Hyperalgesia in Mice. Anesthesiology. 2006;104:1054–1062. doi: 10.1097/00000542-200605000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith SB, Marker CL, Perry C, Liao G, Sotocinal SG, et al. Quantitative trait locus and computational mapping identifies Kcnj9 (GIRK3) as a candidate gene affecting analgesia from multiple drug classes. Pharmacogenet Genomics. 2008;18:231–241. doi: 10.1097/FPC.0b013e3282f55ab2. 10.1097/FPC.0b013e3282f55ab2. [DOI] [PubMed] [Google Scholar]
- 32.Guo Y, Weller P, Farrell E, Cheung P, Fitch B, et al. In silico pharmacogenetics of warfarin metabolism. Nat Biotechnol. 2006;24:531–536. doi: 10.1038/nbt1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo Y, Lu P, Farrell E, Zhang X, Weller P, et al. In silico and in vitro pharmacogenetic analysis in mice. Proc Natl Acad Sci U S A. 2007;104:17735–17740. doi: 10.1073/pnas.0700724104. 10.1073/pnas.0700724104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsukahara C, Sugiyama F, Paigen B, Kunita S, Yagami K. Blood pressure in 15 inbred mouse strains and its lack of relation with obesity and insulin resistance in the progeny of an NZO/HILtJ x C3H/HeJ intercross. Mamm Genome. 2004;15:943–950. doi: 10.1007/s00335-004-2411-3. [DOI] [PubMed] [Google Scholar]
- 35.Howden R, Liu E, Miller-DeGraff L, Keener HL, Walker C, et al. The genetic contribution to heart rate and heart rate variability in quiescent mice. Am J Physiol Heart Circ Physiol. 2008;295:H59–H68. doi: 10.1152/ajpheart.00941.2007. 10.1152/ajpheart.00941.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bochud M, Bovet P, Elston RC, Paccaud F, Falconnet C, et al. High heritability of ambulatory blood pressure in families of East African descent. Hypertension. 2005;45:445–450. doi: 10.1161/01.HYP.0000156538.59873.86. 10.1161/01.HYP.0000156538.59873.86. [DOI] [PubMed] [Google Scholar]
- 37.Seidlerova J, Bochud M, Staessen JA, Cwynar M, Dolejsova M, et al. Heritability and intrafamilial aggregation of arterial characteristics. J Hypertens. 2008;26:721–728. doi: 10.1097/HJH.0b013e3282f4d1e7. 10.1097/HJH.0b013e3282f4d1e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pilia G, Chen WM, Scuteri A, Orru M, Albai G, et al. Heritability of cardiovascular and personality traits in 6,148 Sardinians. PLoS Genet. 2006;2:e132. doi: 10.1371/journal.pgen.0020132. 10.1371/journal.pgen.0020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barrick CJ, Rojas M, Schoonhoven R, Smyth SS, Threadgill DW. Cardiac response to pressure overload in 129S1/SvImJ and C57BL/6J mice: temporal- and background-dependent development of concentric left ventricular hypertrophy. Am J Physiol Heart Circ Physiol. 2007;292:H2119–H2130. doi: 10.1152/ajpheart.00816.2006. 10.1152/ajpheart.00816.2006. [DOI] [PubMed] [Google Scholar]
- 40.Wade CM, Kulbokas EJ, III, Kirby AW, Zody MC, Mullikin JC, et al. The mosaic structure of variation in the laboratory mouse genome. Nature. 2002;420:574–578. doi: 10.1038/nature01252. [DOI] [PubMed] [Google Scholar]
- 41.Frazer KA, Eskin E, Kang HM, Bogue MA, Hinds DA, et al. A sequence-based variation map of 8.27 million SNPs in inbred mouse strains. Nature. 2007;448:1050–1053. doi: 10.1038/nature06067. 10.1038/nature06067. [DOI] [PubMed] [Google Scholar]
- 42.Szatkiewicz JP, Beane GL, Ding Y, Hutchins L, Pardo-Manuel d V, et al. An imputed genotype resource for the laboratory mouse. Mamm Genome. 2008;19:199–208. doi: 10.1007/s00335-008-9098-9. 10.1007/s00335-008-9098-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J, Liao G, Usuka J, Peltz G. Computational genetics: from mouse to human? Trends Genet. 2005;21:526–532. doi: 10.1016/j.tig.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 44.Kang HM, Zaitlen NA, Wade CM, Kirby A, Heckerman D, et al. Efficient control of population structure in model organism association mapping. Genetics. 2008;178:1709–1723. doi: 10.1534/genetics.107.080101. 10.1534/genetics.107.080101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Results.
(1.57 MB PDF)