Abstract
The Ras-related GTPases Rap1a and 1b have been implicated in multiple biological events including cell adhesion, free radical production and cancer. To gain a better understanding of Rap1 function in mammalian physiology we deleted the Rap1a gene. Although loss of Rap1a expression did not initially affect mouse size or viability, upon backcross into C57Bl/6J mice some Rap1a −/− embryos died in utero. T cell, B cell or myeloid cell development was not disrupted in Rap1a −/− mice. However, macrophages from Rap1a null mice exhibited increased haptotaxis on fibronectin and vitronectin matrices that correlated with decreased adhesion. Chemotaxis of lymphoid and myeloid cells in response to CXCL12 or CCL21 was significantly reduced. In contrast, an increase in Fc receptor-mediated phagocytosis was observed. Since Rap1a was previously copurified with the human neutrophil NADPH oxidase, we addressed whether GTPase loss affected superoxide production. Neutrophils from Rap1a −/− mice had reduced fMLP-stimulated superoxide production as well as a weaker initial response to phorbol ester. These results suggest that, despite 95% amino acid sequence identity, similar intracellular distribution, and broad tissue distribution, Rap1a and 1b are not functionally redundant but rather differentially regulate certain cellular events.
Keywords: Transgenic/knockout mice, signal transduction, neutrophil, macrophage, chemotaxis
The small GTPase Ras is an important regulator of cell growth and differentiation (1). It operates as a molecular switch, alternating between inactive GDP and active GTP-bound states and is the prototype for over130 Ras-related proteins that regulate a myriad of biological functions (1). Rap1a/Krev-1/Smg p21 was originally identified due to homology with Ras (2), by purification of GTP binding proteins (3, 4), copurification with the neutrophil NADPH oxidase (5) and by its ability to revert the phenotype of Ras-transformed cells (6). A closely related protein, Rap1B, sharing 95% amino acid identity but encoded by a separate gene, also exists (7) in addition to three Rap2 proteins that share ~65% identity with Rap1 (2, 8, 9). Like Ras, these GTPases are activated by guanine nucleotide exchange factors (GEFs) and turned off by GTPase activating proteins (GAPs). The activation of Raps by a large number of diversely-regulated GEFs suggests that they play ubiquitous roles in cell biology triggered by multiple extracellular stimuli and associated signaling pathways, reviewed in (10–12). While Rap1 and 2 are regulated by the same GEFs and GAPs, until very recently little was known about their biological functions. Even less is known about the functional redundancy of Rap1A and 1B.
It was originally reported that Rap1 operates as an antagonist of Ras, competing with it for downstream effectors such as c-Raf-1. While this can occur in certain cell types (13), Rap1 has more recently been found to play a number of additional roles. These include activation of the ERK/MAP kinase pathway via B-Raf, promotion of cell-matrix adhesion through inside-out activation of integrins via RAPL and RIAM, and regulation of cell-cell junctions (reviewed in (12, 14, 15)). Over-activation of Rap1 has also been recently associated with hematopoietic malignancy (16, 17). Loss of the only rap1 gene in Drosophila is embryonic lethal (18). Similarly, loss of C3G/RapGEF1, the most ubiquitously expressed Rap exchange factor is lethal to mice (19, 20). However, because C3G also acts on additional Ras family proteins, it was not clear if this lethality was solely due to loss of Rap1 activity (21–24).
To better understand the role of Rap1 in mammalian cell biology and to address possible redundancy between the 1a and 1b proteins, we generated a mouse lacking the rap1a gene. rap1A−/− mice initially exhibited no overt phenotype. However, upon backcrossing mice into the C57Bl/6J strain, some embryonic lethality was observed. Due to the high abundance of Rap1a in human platelets (25) and Rap1a in human neutrophils (26) where it associates with the phagocyte NADPH oxidase (5, 27), we examined several properties of Rap1a-null white blood cells. Although there were no obvious defects in blood cell development, changes in macrophage adhesion, haptotaxis, and phagocytosis were observed and neutrophils produced less superoxide in response to formylated peptide and phorbol ester challenge despite the presence of Rap1b in these cells. These data support the role of Rap1 in cell adhesion and migration, provide a unique model for studying Rap1 function and suggest that the Rap1a and 1b proteins have both redundant and unique biological functions.
Materials and Methods
Generation of rap1A targeting vector
An approximately 19 kb partial Rap1a genomic clone isolated from a 129Sv/J mouse genomic library (28) was sequenced to generate a restriction map and identify exons. The clone was found to encode exons 3 through 6 of rap1A that encode residues 43–184. To generate the targeting vector, a 0.95 kb PvuII-NdeI fragment, located upstream of exon 4, was blunted and inserted into the SalI site of the pBluescript II/NeoTK vector (Fig. 1A) (29). This vector contains the phosphoglycerokinase promoter driving the neo gene and the herpes simplex virus thymidine kinase gene both being expressed in the opposite direction to the Rap1a sequence. An 8.6 kb NdeI-StuI fragment downstream of exon 4 was inserted at the XhoI site of the vector. By this strategy, exon 4 (codons 62–108) and surrounding sequence was replaced by the neomycin resistance gene. The targeting vector was linearized using NotI and electroporated into embryonic stem (ES) cells.
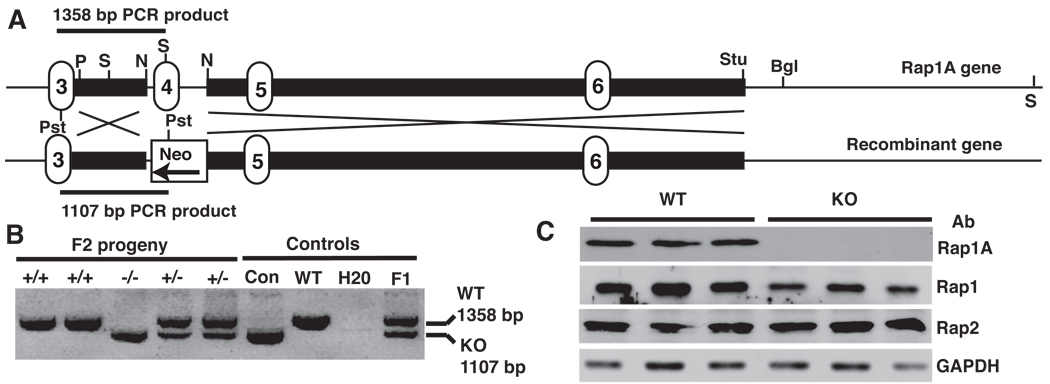
FIGURE 1. Rap1a deletion strategy and detection.
A. Knockout strategy showing replacement of exon 4 with neo resistance gene. Positions of genotyping primers are shown as well restriction enzymes used in gene manipulation or analysis. Bgl, BglII; N, NdeI; Pst, PstI; P, PvuII; S, SpeI; Stu, StuI. Numbered regions indicate location of exons, solid black bars show short and long arms used in homologous recombination.
B. PCR analysis of Rap1a null mice. Using a common primer 5’ of the targeted sequence (in exon 3) and 3’ primers complementary to either exon 4 or the neoR gene, PCR reactions detected Rap1a +/+ (upper band only) −/− (lower band only) or +/− (both bands) mice. Con, control plasmid template; F1, chimeric founder mouse.
C. Western blot of Rap expression in mouse neutrophils. Cells were blotted for Rap1a, total Rap1 or Rap2 expression using specific antibodies. Glyceraldehyde 3 phopshate dehydrogenase (GAPDH) was used as an internal loading control. Data are shown for 3 separate Rap1a +/+ and −/− mice.
Generation of rap1A knockout mice
Culture of ES cells and isolation of homologous recombinants were performed according to standard protocols (30). Screening of 261 G418/gancyclovir double resistant ES clones for homologous recombinants was performed by PCR methods as described (30) using 5'-TCTATCGCCTTCTTGACGAGTTC-3' (ADPR266; in the neo gene) and 5’-CTTGCTCTCCTGTTACCTATAGGTGCC-3’ (Rap1a 5’ KO; in the Rap1a gene) as primers (Fig. 1). Five positive clones were identified and subjected to Southern blot analysis to confirm the structure of the targeted region. ES cell genomic DNA was digested with SpeI and hybridized with an external probe located 3’ of the long arm using a 1.1 kb StuI-BglII fragment as template to confirm long arm structure and correct integration 3’ to the recombination site. PstI digested ES cell genomic DNA was hybridized with a neo probe using a XhoI-SalI fragment from the neomycin resistance gene to confirm correct integration 5’ to the recombination site. Four ES cell clones were confirmed to contain the targeted disruption and used to generate Rap1a knockout mice by standard procedures (30) utilizing C57BL/6J blastocysts and pseudopregnant females as foster mothers. Chimeric mice were successfully generated from just one clone. These were screened by PCR and by Southern blotting for the presence of the disrupted Rap1a allele and mated with C57BL/6J mice to produce F1 heterozygous mice. Heterozygotes for the disrupted gene were identified by PCR using digit samples along with three primers, the common primer Rap1a 5’KO, the neo specific primer ADPR266, and the wild type allele specific primer RapExon (5’-CCGTAAAATCTGTTCTCTC AAGTC-3’). Confirmed heterozygous F1 mice were mated with siblings to generate null mutant mice. Mice were backcrossed 6 times with C57BL/6J male and female mice to create F7 generation. F2 generation mice were used for characterization except where indicated. All mice were maintained in temperature and humidity-controlled conditions with a 12-h light-12-h dark cycle and were allowed food and water ad libitum. All procedures described were approved by the Indiana University School of Medicine animal care and use committee.
Southern blot analysis
Genomic DNA from ES cells was prepared with the Puregene DNA isolation kit (Gentra). Genomic DNA was isolated from mouse tails by the proteinase K digestion method. Mouse tail pieces were clipped and digested in a solution containing 100 mM NaCl, 10 mM Tris-HCl (pH 7.8), 1% SDS, 200 µg of proteinase K per ml at 57°C overnight. After two chloroformisoamyl alcohol (24:1) extractions, the DNA was precipitated with ethanol. Genomic DNA (10 to 20 µg) digested with SpeI or PstI (~10 U/µg) was resolved by electrophoresis in 0.5% agarose gels and transferred to nitrocellulose membranes (BA-85; Shleicher & Schuell). DNA fragment probes were radiolabeled with 32P using the Megaprime DNA labeling system (GE Biosciences).
Flow cytometry
All antibodies were purchased from BD Biosciences (San Diego, CA). Surface expression on the indicated cell populations was analyzed by flow cytometry (FACScan or FACScaliber, Becton Dickinson). The percent positive population was evaluated by the CellQuest program (BD Biosciences).
Blood cell proliferation assays
Cells were isolated from spleens of the indicated genotypes of mice. Cells were then plated in triplicate at 5 × 104 cells/well in a round-bottomed 96-well plate in RPMI 1640 medium supplemented with 10% heat-inactivated FBS (Hyclone, Logan, UT) and stimulated with 2.5 µg/ml plate bound anti-CD3 (for T cells) or 2.5 µg/ml anti-IgM ±5 ng/ml IL-4 (for B cells) for 72 hrs. Cells were pulsed for the last 16–18 hrs with 1µCi per well of [3H]-thymidine. The incorporation of 3H-thymidine was measured with a liquid scintillation counter.
T helper cell differentiation assay
CD4+ cells were purified from spleen and lymph node cells by MiniMacs positive selection and plated in RPMI 1640 + 10% FBS. Cells were plated at 1×106/ml stimulated with 4 µg/ml plate bound anti-CD3, 0.5 µg/ml anti-CD28 in either Th1 (2 ng/ml IL-12 + 10 µg/ml anti-IL-4) or Th2 (10 ng/ml IL-4 + 10 µg/ml anti-IFN-γ) culture conditions. After 5 days in culture, cells were washed and restimulated with 2 µg/ml plate bound anti-CD3 alone. Supernatants were collected after 24 hours and tested for levels of IL-4, IL-5, IL-13 and IFN-γ.
Macrophage isolation, cell migration and phagocytosis adhesion assays
Macrophages were prepared as previously described (31). Cell migration assays were performed on polycarbonate membranes using Transwell migration chambers (pore size 8 µm; Costar Corp., Cambridge, MA) as previously (31). The underside of the membrane to which cells migrate was coated with 20 µg/ml of either vitronectin, or an α4β1 integrin-binding fragment of fibronectin, H296, in PBS for 1 h at 37 °C. Surfaces were subsequently blocked with heat-denatured BSA. Cell adhesion assays were performed using flat bottomed 96-well polystyrene plates coated with 20 µg/ml of either vitronectin or the H296 fibronectin peptide. Adherent cells were fixed with 3.5% formaldehyde and stained with 0.1% crystal violet. The stain was eluted with 10% acetic acid, and absorbance was determined at 600 nm with a microplate reader (31). Recombinant human fibronectin peptide H296 was obtained from Collaborative Biomedical (Bedford, MA). Vitronectin was purified as described previously (31). Phagocytosis of serum opsinized-zymosan or IgG-coated sheep red blood cells was performed as described (32).
Chemotaxis
Chemotaxis assays were performed using 96-well chemotaxis chambers (NeuroProbe, Gaithersburg, MD) in accordance with the manufacturer’s instructions as described previously (33)
Superoxide anion measurement
Purification of mature neutrophils from the bone marrow of 11–16 wk old mice was performed by centrifugation through a Percoll gradient and Histopaque-1119 essentially as described (34). Superoxide response to 10 µM fMetLeuPhe was measured using chemiluminesence essentially as described (35). Superoxide production in response to phorbol ester (200 ng/ml phorbol myristate acetate) was determined by both reduction of cytochrome C and by chemiluminescence (34, 35).
Detection of Rap1 proteins in mouse BM neutrophils
Neutrophils were suspended in PBS to no greater than 5 × 107/ml. 2.7 mM Diisopropyl fluorophosphates (DFP), a serine protease inhibitor, was added and incubated for 10 min on ice. After two washes in PBS, 5×107 cells were lysed in 1ml IPB buffer [20 mM Tris, pH 8.0, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 20 ng/µl chymostatin, 2 mM PMSF, 10 nM leupeptin, 1 mM 4-c2-aminoethyl benzenesulfomyl fluoride] with 30 min incubation on ice. Cell debris were centrifuged down at 16,000 × g for 2 min at 4°C. Cleared lysates were mixed with 2× sample buffer and boiled for 5 min. 30 µg of lysate protein was loaded onto SDS-PAGE gels. Rap1a protein was detected with mAb T22, a generous gift of Dr. Yoshimi Takai, Osaka, Japan (26), and total Rap1a/1b by anti-Rap1 R195 antibody. Rap2 protein was detected with anti-Rap2 from BD Biosciences.
Results
Generation of Rap1a−/− mice
To explore the biological role of Rap1a, a targeting construct that replaces the fourth exon of the murine GTPase with a neomycin resistance gene cassette was developed and used to disrupt the Rap1a gene in ES cells by homologous recombination. Proper integration of the cassette was established by Southern blot (YL and LAQ, unpublished observations) and PCR analysis (Fig. 1B). Embryonic stem cells were injected into mouse blastocysts. Chimeric mice were then bred to obtain germ line transmission of the disrupted allele. Heterozygous mice were further bred to obtain Rap1a-deficient mice. Purified neutrophils were blotted for Rap1a expression using an isoform-specific monoclonal antibody (26). Rap1a was not detected in these cells (Fig. 1C), or in various organs derived from −/− mice (YL and LAQ, unpublished observations). Furthermore, a ~ 16 kDa protein that could potentially have arisen from splicing of exons 3 and 5 was not detected. This was consistent with the lack of Rap1 detection from an engineered Rap1a cDNA, missing the equivalent 47 codons encoded by exon 4, upon transfection of 293T cells (residues 62–108; YL and LAQ, unpublished observations). Western blotting with a pan Rap1 antibody that detects Rap1a and 1b, but not Rap2 indicated a ~25% decrease in total Rap1 protein expressed in neutrophils following deletion of Rap1A. Only modest changes in neutrophil Rap2 were detected (Fig. 1C).
Rap1a−/− mouse viability depended on backgound strain
Rap1A−/− mice were viable, healthy and fertile suggesting that the Rap1a gene is not required for mouse development or breeding. Segregation of the mutant Rap1a allele followed Mendelian inheritance (24%:51%:25% ratio of +/+:+/−:−/− from 446 mice born from heterozygote mating) with +/+ and −/− mice weight and the organ/body weight ratio of their brain, heart, liver, and lungs being indistinguishable between genotypes (YL and LAQ, unpublished observations). These mice were used for initial characterization of Rap1a function. However after backcrossing for 6 generations into the C57BL/6J mouse strain, the number of Rap1a −/− pups generated from heterozygote crosses was significantly reduced (30.3:54.8:14.9% ratio of +/+:+/−:−/− from 241 mice) with no sex bias. Dissection of pregnant Rap1a −/− mice at E9.5-14.5 indicated that the lethality occurred much earlier than E9.5 due to no trace of embryos in the deciduas (HC and WS, unpublished observations).
Normal T cell development and function in the absence of Rap1a
Rap1 is highly expressed in various cells of hematopoeitic lineage (13, 26, 36, 37). Therefore, we next examined if the loss of Rap1a impacted T- or B-cell function. Rap1 is activated in T cells by T cell receptor engagement variably resulting in decreased intracellular signals and enhanced integrin-mediated adhesion (13, 37–39). However the role of Rap1 in T cell development had not been examined. To determine if Rap1a is required for normal T cell development, we first analyzed thymocytes from wild-type and Rap1a−/− mice. Thymi from both genotypes had similar cellularity and flow cytometry revealed similar percentages of cells in the CD4 and CD8 double-negative, double positive and single positive populations (Fig. 2A). Within the double negative population, there were similar percentages of cells expressing CD25 and CD44 (HC and MHK, unpublished observations). These results suggest that Rap1-adeficiency does not adversely affect T cell development.
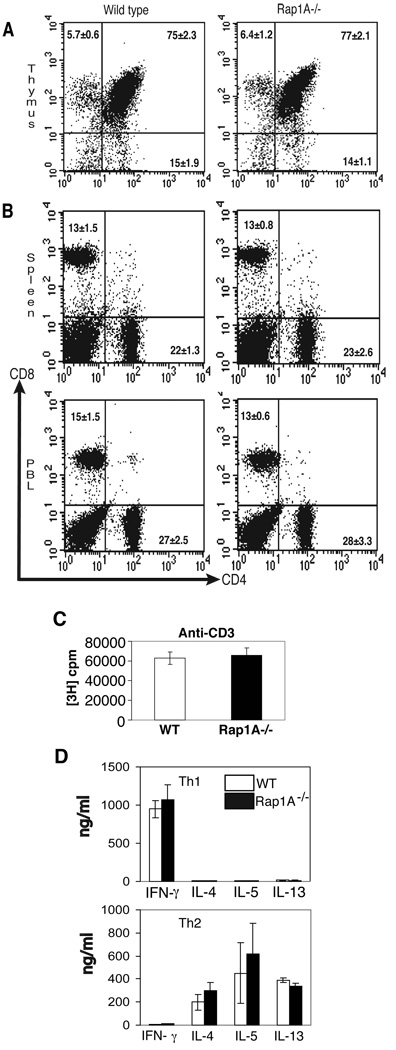
FIGURE 2. Analysis of T cell development and function.
A. Flow cytometric analysis of CD4 and CD8 expression on thymocytes from wild-type and Rap1a-deficient mice.
B. Flow cytometric analysis of CD4 and CD8 expression on wild-type and Rap1a-deficient spleen (top) and peripheral blood (bottom).
C. Splenocytes from wild-type and Rap1a-deficient mice were stimulated with 2.5 µg/ml anti-CD3 in microwells. Cultures were pulsed with [3H]thymidine for the last 18 hours of a 72 hour incubation.
D. CD4+ cells were purified from wild-type or Rap1a-deficient spleen and stimulated with 4 µg/ml plate bound anti-CD3, 0.5 µg/ml anti-CD28 or 10 ng/ml IL-4 + 10 µg/ml anti-IFN-γ, for Th2 cultures or 2 ng/ml IL-12 + 10 µg/ml anti-IL-4 for Th1 cultures. After 5 days in culture, cells were re-stimulated with anti-CD3 alone and supernatants were recovered after 24 hours for cytokine testing by ELISA. Data represent mean ±SEM from 6 +/+ and 5 −/− mice.
Analysis of peripheral blood and spleen by flow cytometry also revealed similar percentages of CD4+ and CD8+ cells with similar cellularity observed in lymph node, peripheral blood and spleen between wild-type and Rap1a−/− mice (Fig. 2B and HC and MHK unpublished observations). To determine if T cells were functional, wild-type and Rap1a−/− splenocytes were stimulated with 2.5 µg/ml anti-CD3 for 48 hours and proliferation was assessed by [3H]thymidine incorporation. As shown in Fig. 2C, both wild-type and Rap1a-deficient T cells proliferated to a similar extent in response to anti-CD3. To further test T cell function, CD4+ T cells were purified from wild-type and Rap1a-deficient mice and differentiated to Th1 and Th2 phenotypes using cytokine and antibody cocktails as described in Methods. The resulting populations were then washed, counted and restimulated with anti-CD3 alone. Wild-type and Rap1a-deficient Th1 cells secreted similar levels of IFN-γ and minimal levels of Th2 cytokines (Fig. 2D). Conversely, Th2 cells derived form both genotypes secreted similar levels of the Th2 cytokines IL-4, IL-5 and IL-13 with little IFN-γ (Fig. 2D). Together, these results indicate that Rap1a is not required for T cell development, migration to peripheral lymphoid organs, proliferation or T helper cell differentiation.
Normal B cell development and function in Rap1a-deficient mice
Rap1 is also activated by B cell receptor engagement (40). To determine if Rap1a is required for B cell development, we first analyzed B cells in the bone marrow of wild-type and Rap1a-deficient mice. Percentages of B220+ and B220+CD43+ populations were indistinguishable between the two genotypes (Fig. 3A). Similarly, the percentages of B220+ cells in the spleen, lymph nodes and peripheral blood of Rap1a-deficient mice did not indicate any defect in B cell development or migration into the periphery (Fig. 3B and unpublished observations).
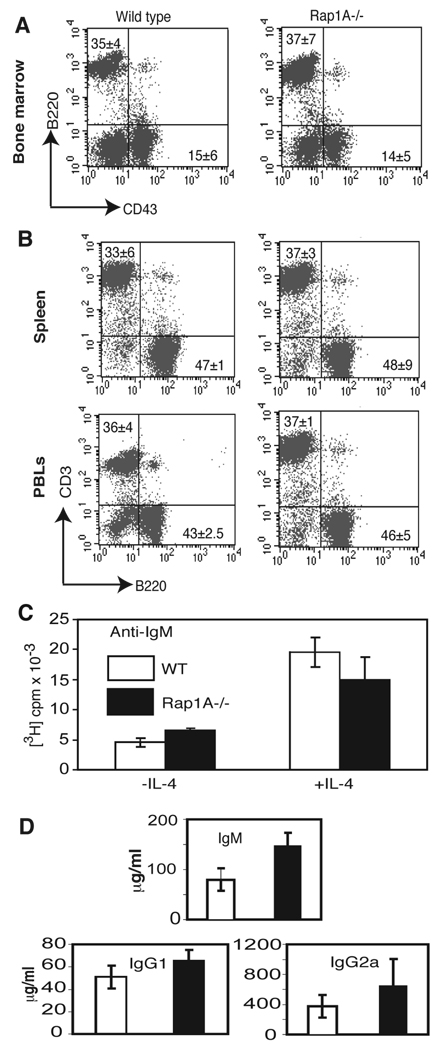
FIGURE 3. Analysis of B cell development and function.
A. Flow cytometric analysis of B220 and CD43 expression in bone marrow from wild-type and Rap1a-deficient mice.
B. Flow cytometric analysis of B220 and CD3 expression on spleen and peripheral blood cells from wild-type and Rap1a-deficient mice.
C. Splenocytes from wild-type and Rap1a-deficient mice were stimulated with 2.5 µg/ml anti-IgM in the presence or absence of 5 ng/ml IL-4 in microwells. Cultures were pulsed with tritiated thymidine for the last 18 hours of a 72 hour incubation.
D. Serum was isolated from wild-type and Rap1a-deficient mice. Concentrations of individual immunoglobulin isotypes were tested by ELISA.
To test the function of Rap1a-deficient B cells, splenocytes from wild-type and Rap1A−/− mice were stimulated in vitro with anti-IgM in the presence or absence of IL-4. Similar levels of proliferation were observed in wild-type and Rap1A-deficient cultures stimulated with anti-IgM or anti-IgM + IL-4 (Fig. 3C). Serum levels of IgM, IgG1 and IgG2a were also indistinguishable between wild-type and Rap1A-deficient cells (Fig. 3D). Together, these data indicate that Rap1A is not required for B cell development or function.
Normal macrophage and granulocyte development in Rap1a-deficient mice
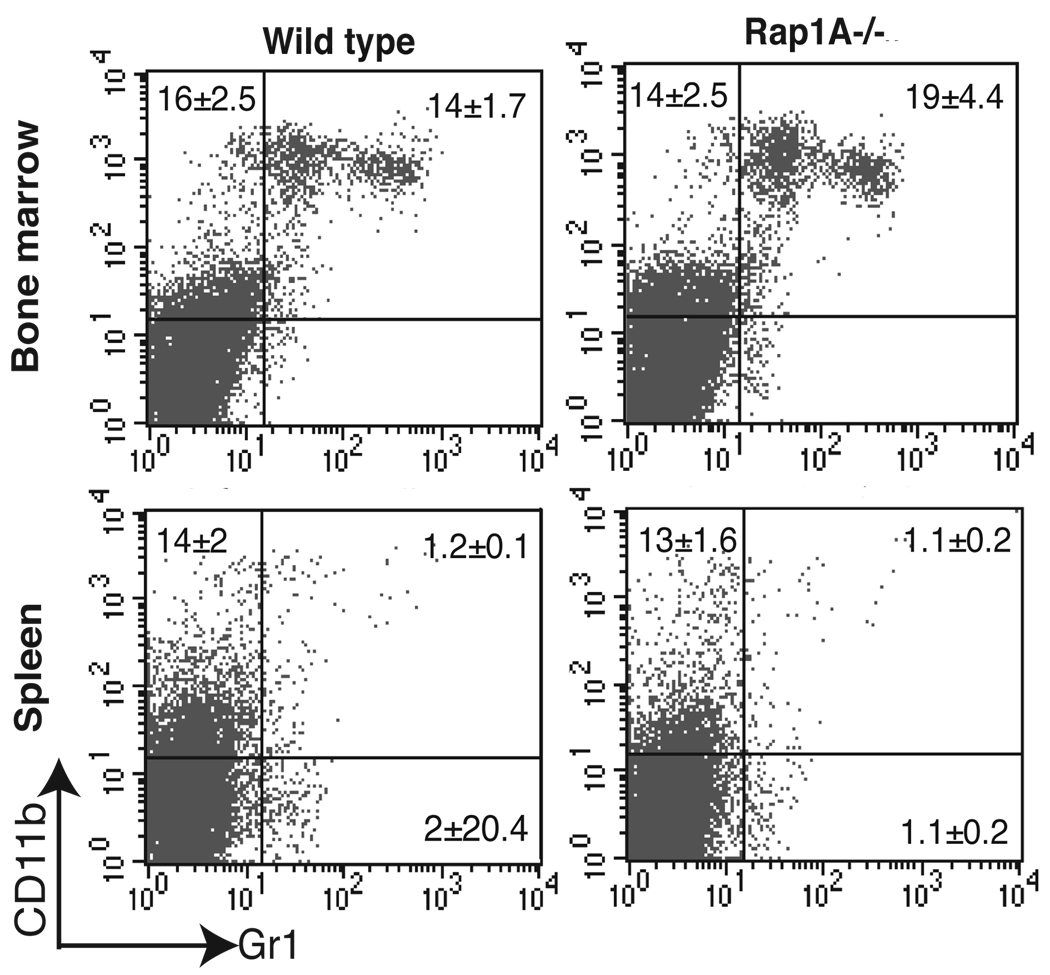
Monocytes and polymorphonuclear leukocytes are stimulated by several factors that activate Rap1 and thus Rap1a may be required for development or function (41). To examine the content of these cells in marrow and spleen, we analyzed the expression of CD11b and Gr-1 on cells in the bone marrow and spleen of wild-type and Rap1a-deficient mice. As shown in Fig. 4, the percentages of CD11b+ and CD11b+Gr-1+ cells are similar between these genotypes, suggesting that Rap1a is not required for normal production of these cells.
FIGURE 4.
Analysis of macrophage and granulocyte development. Flow cytometric analysis of CD11b and Gr-1 expression on bone marrow and spleen cells from wild-type and Rap1a-deficient mice.
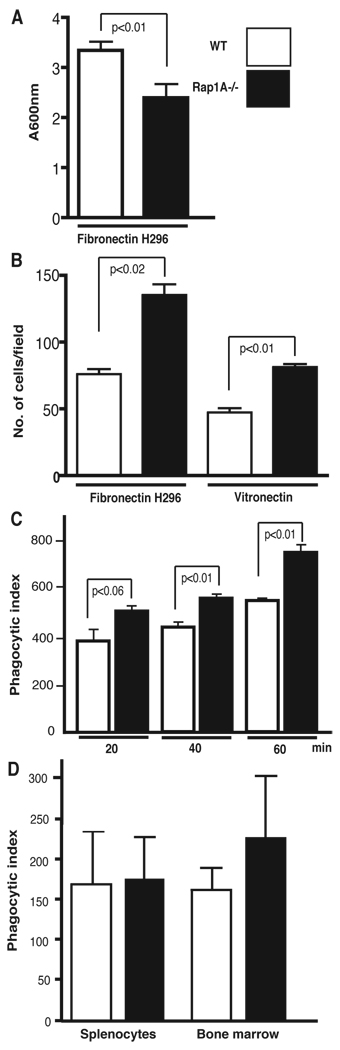
Macrophages from Rap1a −/− mice exhibited increased haptotaxis, migration and phagocytosis
Rap1 has recently been implicated in inside-out signaling to regulate integrin-mediated adhesion (12, 14, 42–45). To address whether Rap1a was required for macrophage adhesion, isolated bone marrow-derived cells were incubated in fibronectin peptide H296-coated 96-well plates for 4 hr and bound cells measured. This is a measure of α4β1 integrin-dependent adhesion. As seen in Fig. 5A, Rap1a null cells bound to this fibronectin fragment less effectively. Similar results were found with adhesion to vitronectin, an αv-specific extracellular matrix protein (PD and DLD, unpublished observations). The levels of Rap1a and total Rap1 were measured using appropriate antibodies. While Rap1a, as expected, was absent from the cells, total Rap1 levels were similar to that of wild type mice (YL and LAQ, unpublished observations). These findings suggest Rap1a and 1b play differential roles in signaling pathways required for provisional integrin dependent migration.
FIGURE 5. Loss of Rap1a affects macrophage haptotaxis, adhesion and phagocytosis.
A. Adhesion of wild-type and Rap1a−/− macrophages to the (α4β1 integrin binding) fibronectin H296 peptide or vitronectin-coated cell culture wells was determined 90 min post plating following fixing and staining of adherent cells.
B. Haptotaxis of wild-type and Rap1a−/− macrophages through fibronectin H296 peptide-coated Transwell filters was measured at indicated time points.
C. Phagocytosis of IgG-coated sheep red blood cells by bone marrow-derived macrophages was measured at the indicated time points after exposure.
D. Phagocytosis of C3bi-opsonized sheep RBCs by macrophages was measured 30 min after exposure.
All data are mean +/− SD for cells derived from 3 mice/genotype and are representative of at least 4 independent experiments (2 experiments using a total of 4 mice were pooled for 5D).
To further address the role of Rap1a in integrin mediated events, we generated mouse embryo fibroblasts (MEFs) from E13.5 embryos and immortalized them using SV40-T (46). A modest but statistically significant reduction in adhesion was noted (30% reduction in adhesion to fibronectin and collagen I, p<0.001).
Because macrophages function by migrating to and phagocytosing foreign material within the body, we examined whether the modest reduction in macrophage adhesion noted above affected their ability to function. Bone marrow-derived (BMD) and splenic macrophages were isolated and random movement (haptotaxis) examined following plating on 8 µm porous filters. As shown in Fig. 5B, BMD macrophages from Rap1a−/− mice migrated considerably faster than those obtained from Rap1a +/+ littermates. Similar results were obtained using either vitronectin or the H296 fibronectin-derived peptide (Fig. 5B) and were qualitatively similar in splenic cells (PD and DLD, not shown). This suggests that the reduced adhesion to matrix may have permitted cells to migrate more effectively. Using flow cytometric analysis, surface expression levels of αV (CD51), α5 (CD49e), Mac-1 (CD11b), β2 (CD18), and α4 (CD49d) integrins or the macrophage marker F4/80 were not significantly different between WT and Rap1a −/− cells (JY, VM and RK, unpublished observations) suggesting that altered integrin levels were not responsible for the reduced adhesion.
Rap1 has been implicated in phagocytosis in both Dicteostelium and macrophages and localized to endocytic vesicles as well as the specific granules of the neutrophil (47–50). Therefore we next compared the ability of macrophages from WT and Rap1a −/− mice to take up IgG-coated sheep red blood cells (SRBCs). The phagocytic index (number of ingested particles/100 macrophages) was greatly increased in Rap1a null cells implicating Rap1a in receptor signaling and/or phagocytosis (Fig. 5C). There was no difference noted in the percentage of macrophages forming SRBC rosettes nor the number of SRBCs bound per spleenor bone marrow-derived macrophage between Rap1a −/− and wild type mice (PD, DLD, unpublished observations). The above macrophage phagocytic activity had been measured in response to Fcγ receptor stimulation that is mediated by Rac-and Cdc42-induced engulfment of particles (51). To examine the response to integrin activation, which is alternatively mediated by Rho (51), we further examined the ability of macrophages to engulf C3bi-opsonized sheep RBCs. Surprisingly, there was no significant difference in the phagocytosis of C3bi-opsonized cells in macrophages derived from Rap1a +/+ versus −/− mice, neither in terms of the percentage of cells taking up RBCs nor the number of RBC taken up by individual macrophages (Fig. 5D). Cell surface expression of FcγRII/III (CD16/32), as measured by fluorescence staining intensity, was not significantly different between Rap1a +/+ and −/− macrophages. Furthermore, all the above macrophage studies yielded comparable data using 2nd or 7th generation backcross into the C57BL/6J mouse strain.
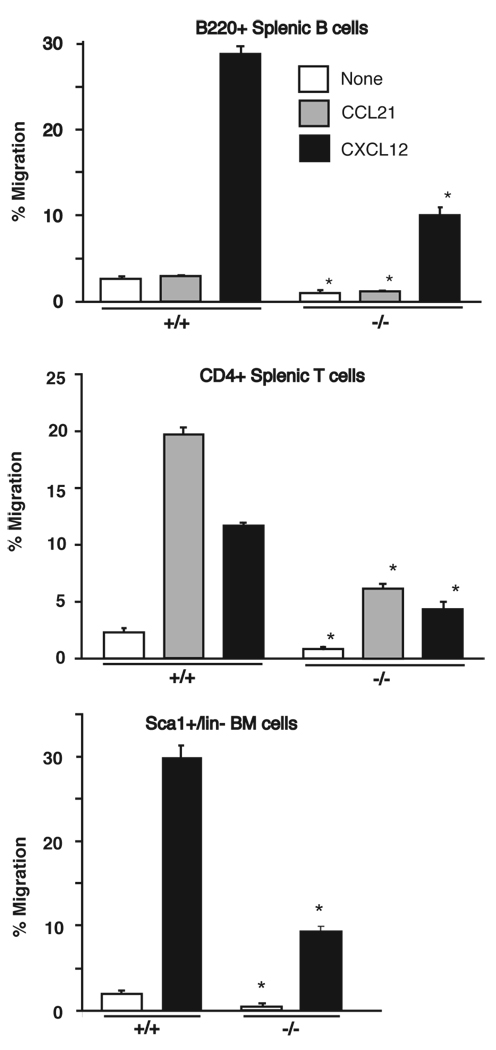
Loss of Rap1a resulted in decreased chemotaxis
Inside-out signaling involves the activation of integrin-extracellular matrix binding from within the cell. Several reports have implicated Rap1 in promoting increased affinity and/or avidity of integrins depending on the system studied (12). G-protein-coupled chemokine receptors promote integrin activation and Rap1 has been implicated as a downstream effector in several G protein-mediated events (44, 52, 53). We therefore examined the ability of T-, B-, and myeloid cells to migrate towards SDF1/CXCL12 and CCL21 chemokines. As seen in Fig 6, a decrease in basal and chemokines-induced migration was observed upon loss of Rap1a. A similar decrease in chemotaxis was seen in Rap1a−/− bone-marrow-derived T and B cells as well as macrophages (KWC, JY and LAQ, unpublished observations). This suggests that the Rap1a isoform may be responsible for coupling chemokine signals to integrin activation. Similar findings were observed with F2 and F7 generation C57BL/6J backcrossed mice. Thus despite alterations in embryonic development, the examined leukocyte functions remained unaltered.
FIGURE 6. Loss of Rap1a influences chemotaxis of leukocytes.
The ability of B220+ splenic B cells (upper), CD4+ splenic T cells (middle), or Sca1+/lin- bone marrow hematopoeitic stem cells (lower) from wild-type and Rap1a−/− mice to migrate towards CCL21 or CXCL12 chemokines was determined using a Transwell assay. Data represent mean +/− SEM for cells derived from 6 mice (4 for bone marrow) *p<0.05 with a two-tailed students t-test.
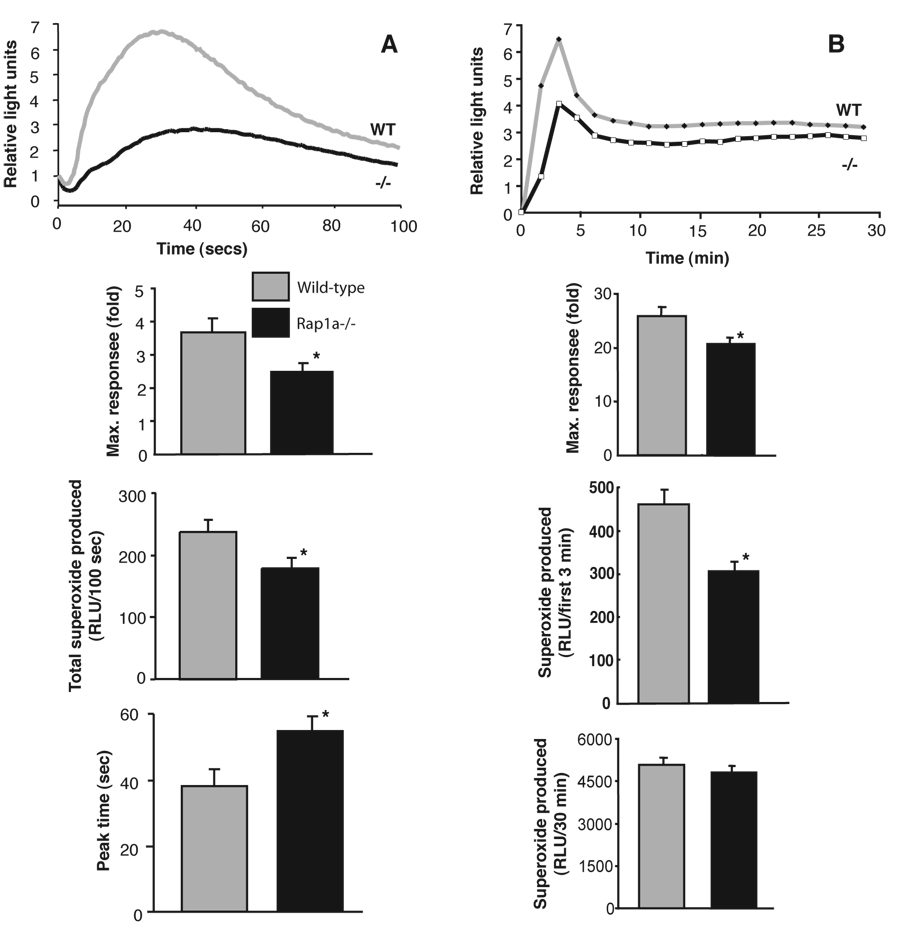
Rap1a was required for an efficient oxidative burst
Rap1a is abundantly expressed in human neutrophils and co-purifies with the p22 subunit of the NADPH oxidase (3, 5, 26). It was previously reported that both activating and inhibitory Rap1 mutants influence the activity of the phagocyte NADPH oxidase (54, 55). We therefore examined the effect of Rap1a loss on oxidase activity of bone marrow-derived neutrophils. Following stimulation with fMLP, cells from WT mice rapidly produced O2.−, peaking at 30 seconds and slowly returned to baseline (Fig. 7A). In contrast, neutrophils from knockout mice had a slower, milder response. The onset of neutrophil superoxide production in response to PMA is typically slower but overall NADPH oxidase activity is much more pronounced and sustained. Although the sustained induction of superoxide production was not statistically distinguishable between WT and Rap1a −/− neutrophils, the initial rate of superoxide production was significantly reduced in Rap1a −/− mice (Fig 7B). This was seen using isoluminol or cytochrome C assays to measure superoxide production. These data support previous findings for a role of Rap1 in NADPH oxidase regulation but indicate that, unlike Rac2 (56–58), Rap1A is not an essential component of the enzyme complex. Analysis of Rap1a and total Rap1 indicated that there was still a significant amount of Rap1b expressed in rap1A−/− mouse neutrophils (Fig. 1C).
FIGURE 7. Loss of Rap1a diminishes the oxidative burst of bone marrow-derived neutrophils.
A. The ability of 10 µM fMLP to stimulate superoxide production in neutrophils derived from wild-type or Rap1a−/− mice was determined using HRP catalyzed activation of isoluminol. Data in upper panel show time courses of superoxide production from cells derived from a typical control and least responsive −/− mouse cells. The maximum response (second panel) and total superoxide produced (third panel) were reduced while the time to reach maximum response was delayed (lower panel) upon averaging data (mean +/− SE) from 12 mice/genotype (data obtained from 4 independent experiments). *p<0.02 (t-test) for comparisons between +/+ and −/− mouse-derived cells.
B. Stimulation with 200 ng/ml PMA promoted superoxide production in cells derived from wild-type or Rap1a−/− mice measured by isoluminol chemiluminescence. Upper panel shows a typical time course. Pooled data in second panel shows a weaker overall maximal response in Rap1a−/− neutrophils. The third panel shows a weaker initial (first 3 min) response to PMA. The lower panel shows a similar amount of superoxide was produced over 30 min in response to phorbol ester stimulation. Mean +/− SE, n=12 per genotype, *p<0.01 to phorbol ester stimulation.
Discussion
Rap1 has been implicated in a number of biological events and is regulated by a broad spectrum of biological stimuli and pathways (10, 12–14). Furthermore, over-activation of Rap1 by knockout of the Spa1 Rap GAP or a RasGRP2 translocation or expression of a mutant transgene, has been recently reported to promote several myeloproliferative disorders (16, 17, 59). Loss of the single Rap1 gene in Drosophila was lethal at the larval stage, suggesting its fundamental role in development (18). However, mammals have two copies of Rap1 (1a and 1b) and, although there is data indicating some differential expression, most notably high Rap1b expression in platelets (25), little was known about the specific functions of the two isoforms. Therefore, to better understand the biological functions of mammalian Rap1, we deleted the mouse Rap1a gene. The results of this study demonstrated that loss of Rap1a affected cell adhesion, migration, phagocytosis and the oxidative burst of leukocytes. This occurred in cells that expressed significant levels of Rap1b protein, suggesting that the two Rap1 isoforms are not redundant. Additionally, defects in early embryonic development were observed that were strain dependent and exhibited only partial penetrance.
The Mendelian ratio of mice born from heterozygote crosses of mixed lineage (C57Bl/6J:129Sv) animals was normal, consistent with the findings of a recent independent report of a Rap1a −/− mouse (60). However, our findings of embryonic lethality in C57Bl/6J back-crossed mice suggests that levels or timing of e.g. Rap1b expression may compensate for loss of Rap1a in the129Sv but not the C57Bl/6J strain. 129Sv-Rap1A−/− mice were not established from chimeric founders. Loss of mice during early development is consistent with embryonic morphogenesis defects in Drosophila (18) and may be related to the role of Rap1 in cell-cell and/or cell-matrix adhesion (14, 15). Interestingly, the non-canonical Wnt pathway was recently demonstrated to regulate zebrafish and Xenopus gastrulation by activating casein kinase ε. This kinase then bound and phosphorylated the Rap GAP, Spa1, resulting in Rap1-GTP accumulation (61). It is therefore possible that loss of Rap1a in mice resulted in insufficient Rap1 activity during gastrulation to mediate Wnt-regulated morphogenesis.
During the completion of this study, Chrzanowska-Wodnicka et al reported that Rap1b knockout resulted in 85% embryonic and perinatal lethality (62). This difference from Rap1a null mice may, at least in part, be due to abnormal bleeding associated with reduced platelet function following Rap1b loss (62). Using the Rap1a −/− mice described in the current study, it was established that Rap1A-null platelets have normal aggregation responses (62), again supporting differential roles for the Rap1a and 1b isoforms in higher eukaryotes. Six of the nine residues that are divergent between Rap1a and 1b are located close to the C-terminus. This is reminiscent of Rac 1 and 2 where 50% of divergent residues occur in the hypervariable domain adjacent to the prenylated CAAX cysteine. C-terminal tail swaps between Rac1 and 2 have demonstrated that these residues confer unique properties to these proteins (63, 64). Whether, like Rac1 versus Rac2, these differences between Rap1a and 1b are due to their divergent C termini remains to be established. Because the hypervariable region can influence the subcellular locale of Ras proteins (65–67), one might anticipate unique subcellular distribution. However, our findings expressing GFP-fusion proteins in Cos7 cells (YL, JY and LAQ, unpublished observations) and those of Pizon et al (48) suggest that the two proteins have similar intracellular localization. This may however differ in specialized cells or situations. Most Rap1a or 1b specific antibodies are of poor quality, detect only unprocessed protein or are no longer available (68–70) making differential expression and localization of endogenous proteins difficult to monitor. We are currently attempting to generate isoform specific anti-sera to address such issues.
Analysis of blood cells revealed that Rap1a was not required for development, migration to peripheral organs, or many mature functions of T, B, or myeloid cells. This is consistent with a recent study by Duchniewicz et al (60) who independently knocked out Rap1a. Duchniewicz et al reported that primary hematopoietic cells isolated from spleen or thymus had a diminished adhesive capacity on ICAM and fibronectin substrates. In addition, polarization of T cells from Rap1a −/− mice after CD3 stimulation was impaired. These cumulative data suggest that Rap1 isoforms are, at least in part, redundant. Total levels of Rap1 were only reduced by 25% in Rap1A−/− neutrophils, suggesting that Rap1b either is the major isoform or is upregulated to compensate for loss of Rap1A. In support of the former notion, PCR analysis of reverse transcribed neutrophil mRNA (YL and LAQ, unpublished observations) suggested that there was at least a two-fold excess of Rap1b over Rap1a message. Therefore it is all the more interesting that there were defects in the ability of Rap1a −/− cells to adhere and migrate. This finding further supports the notion that Rap1 isoforms serve distinct as well as common functions. Upon crossing Rap1a−/− mice with the Rap1b+/− mice generated by Chrzanowska-Wodnicka et al (62), Rap1a/1b double knockout was more lethal than loss of either single isoform; no surviving null offspring were obtained (NCP and LAQ, unpublished observations). However, this finding can support either common or divergent function for Rap1 isoforms.
Since Rap1 has been demonstrated to activate various integrins, the reduction in macrophage binding to fibronectin and vitronectin was logical and consistent with reduced adhesion of MEFs from C3G knockout mice (19, 20). Although C3G can also regulate Rap1b, Rap2, TC10 and RRas (21–24), our data suggest that the effect of GEF loss was at least in part due to decreased Rap1a activation. It is likely that the modest observed decrease in adhesion in the current study was responsible for the enhanced macrophage haptotaxis as was observed with C3G−/− fibroblasts (19, 20). This finding is supported by the decreased adhesive capacity of primary hematopoietic cells in this study and impaired polarization of T cells from Rap1A−/− mice reported by Duchniewicz et al (60). Similar binding of cell surface integrins was observed on macrophages isolated from Rap1a +/+ and −/− mice in the current study suggesting that altered adhesion was not due to changes in integrin composition.
In contrast to non-directional haptotaxis, chemotaxis involves directed migration in response to a chemical gradient. Since Rap1 signaling has been found downstream of G protein coupled receptors (52, 53) and to couple CXCR4 to integrins (44), the reduced chemotactic response to CXCL12 or CCL21 was anticipated and was consistent with our findings. That the chemokines still elicited a significant response is presumably due to the continued presence of Rap1b. Alternatively, Rap2 has also been implicated in chemokine signaling (71). The decreased basal migration on plastic in Fig. 6 can not be attributed to increased adhesion as Rap1a−/− macrophages bound slightly less than WT cells to tissue culture plastic (10% difference, p<0.005) but still exhibited reduced basal and chemokine-induced migration (JY, unpublished observation).
Since Rap1 controls integrin-mediated events through inside-out signaling (14) and specifically can control functional activation of the integrin-type CR3 receptor (αMβ2) (47), we anticipated that loss of Rap1a would impair phagocytosis of complement-coated particles. Since no effect of Rap1a deletion was observed, it is possible that Rap1a and 1b act redundantly in this process or that it is preferentially mediated by Rap1b. FcγR-mediated phagocytosis of immunoglobulin-coated particles is a Cdc42/Rac2-dependent process in macrophages (72). Since we have previously implicated Rap1 in Rac activation (73), it was again anticipated that loss of Rap1a might attenuate uptake of IgG-coated red cells. Interestingly, Rap1a-null macrophages exhibited enhanced FcγR-mediated phagocytosis. We and others have found that activation of Rap1 by its upstream GEFs is spatially regulated, resulting in selective activation of downstream targets at specific intracellular locales (74–78). It is tempting to speculate that in the absence of Rap1a, Rap1b can be more effectively activated in a locale that leads to phagocytosis. For example, activation of Rac by Vav2 at the cell periphery to promote cell spreading is dependent on Rap1-GTP (73). Drosophila macrophage function is dependent on the Rap GEF “Dizzy” (79). Since the localization of the mammalian homolog of Dizzy, PDZ-GEF1/RapGEF2, is regulated by its Rap1-GTP product (75), loss of Rap1a might lead to PDZ-GEF redistribution to more effectively activate Rap1b and enhance phagocytosis. The development of Rap1 isoform-specific antibodies will help address this possibility. Since FcγR signaling in myeloid cells and αv integrin-mediated haptotaxis in macrophages requires Rac2 and the Syk tyrosine kinase, but not Rac1 (31), preferential interaction of Rap1a versus 1b with the Syk-Rac2 signaling axis in myeloid cells might occur. Future experiments will examine the impact of Rap1a loss on integrin induced actin polymerization and macrophage polarization.
Despite Rap1a identification within the phagocyte NADPH oxidase (5, 27), the GTP-dependent component of this enzyme complex was later determined to be Rac2 (56). Here we show that, unlike Rac2, there is not an absolute requirement for Rap1a in order for neutrophils to respond to formylated peptide or phorbol ester. Since only the transient fMLP response and the early stages of the PMA response were attenuated by Rap1a loss, Rap1 may serve a function in enhancing complex assembly, such as Vav recruitment (73) and/or Rac2 activation or specific granule trafficking. Although Rap1a is abundant in human neutrophils (26), there was considerable Rap1b detectable in the Rap1a −/− mouse phagocytes. Interestingly, despite a dependence on Rac2 for NADPH oxidase regulation (58), mouse neutrophils express considerably more Rac1 vs Rac2 compared to humans (35). A similar scenario may exist with Rap1 isoforms in these species.
In summary, we have observed changes in blood cell adhesion, migration, and phagocytosis as well as embryogenesis in Rap1a −/− mice. Our findings are supportive of both novel and redundant functions for Rap1 isoforms. The use of these mice should enable a better understanding of Rap1 function at both the cellular and whole animal level. Additionally, since Rap1 activation has been implicated in the development of several leukemias (16, 17) and preventing solid tumor metastasis (80), it will be interesting to establish if there is any Rap1 isoform specificity in the development of these diseases that are not discernable using dominant inhibitory mutants or Rap GAP over-expression.
Acknowledgements
We are grateful to Dr Yoshimi Takai, Osaka University for generously providing the last 20 µl of Rap1a-specific antibody, Dr Magda Chrzanowska-Wodnicka, Blood Research Center, Milwaukee for Rap1b+/− mice, and Bill Carter/members of IU transgenic core facility for generating Rap1a +/− mice.
Footnotes
Source of support:
LAQ was supported by an Indiana University School of Medicine Biomedical Research Grant, the Indiana Genomics Initiative (INGEN®) of Indiana University that is supported in part by Lilly Endowment Inc. and by NIH grants CA108647 and HL69974. YL was supported by American Heart Association predoctoral fellowship 0110245Z.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol. Rev. 2001;81:153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- 2.Pizon V, Chardin P, Lerosey I, Olofsson B, Tavitian A. Human cDNAs rap1 and rap2 homologous to the Drosophila gene Dras3 encode proteins closely related to ras in the 'effector' region. Oncogene. 1988;3:201–204. [PubMed] [Google Scholar]
- 3.Bokoch GM, Parkos CA. Identification of novel GTP-binding proteins in the human neutrophil. FEBS Lett. 1988;227:66–70. doi: 10.1016/0014-5793(88)81415-7. [DOI] [PubMed] [Google Scholar]
- 4.Kawata M, Matsui Y, Kondo J, Hishida T, Teranishi Y, Takai Y. A novel small molecular weight GTP-binding protein with the same putative effector domain as the ras proteins in bovine brain membranes. Purification, determination of primary structure, and characterization. J. Biol. Chem. 1988;263:18965–18971. [PubMed] [Google Scholar]
- 5.Quinn MT, Parkos CA, Walker L, Orkin SH, Dinauer MC, Jesaitis AJ. Association of a Ras-related protein with cytochrome b of human neutrophils. Nature. 1989;342:198–200. doi: 10.1038/342198a0. [DOI] [PubMed] [Google Scholar]
- 6.Kitayama H, Sugimoto Y, Matsuzaki T, Ikawa Y, Noda M. A ras-related gene with transformation suppressor activity. Cell. 1989;56:77–84. doi: 10.1016/0092-8674(89)90985-9. [DOI] [PubMed] [Google Scholar]
- 7.Pizon V, Lerosey I, Chardin P, Tavitian A. Nucleotide sequence of a human cDNA encoding a ras-related protein (rap1B) Nucleic Acids Res. 1988;16:7719. doi: 10.1093/nar/16.15.7719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohmstede CA, Farrell FX, Reep BR, Clemetson KJ, Lapetina EG. RAP2B: a RAS-related GTP-binding protein from platelets. Proc. Natl. Acad. Sci. U. S. A. 1990;87:6527–6531. doi: 10.1073/pnas.87.17.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paganini S, Guidetti GF, Catricala S, Trionfini P, Panelli S, Balduini C, Torti M. Identification and biochemical characterization of Rap2C, a new member of the Rap family of small GTP-binding proteins. Biochimie. 2005;88:285–295. doi: 10.1016/j.biochi.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Quilliam LA, Rebhun JF, Castro AF. A growing number of guanine nucleotide exchange factors is responsible for activation of ras family GTPases. Progress in Nucleic Acid Research and Molecular Biology. 2002;71:391–444. doi: 10.1016/s0079-6603(02)71047-7. [DOI] [PubMed] [Google Scholar]
- 11.Stork PJ. Does Rap1 deserve a bad Rap? Trends Biochem. Sci. 2003;28:267–275. doi: 10.1016/S0968-0004(03)00087-2. [DOI] [PubMed] [Google Scholar]
- 12.Bos JL. Linking Rap to cell adhesion. Curr. Opin. Cell Biol. 2005;17:123–128. doi: 10.1016/j.ceb.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Stork PJ, Dillon TJ. Multiple roles of Rap1 in hematopoietic cells: complementary versus antagonistic functions. Blood. 2005;106:2952–2961. doi: 10.1182/blood-2005-03-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caron E. Cellular functions of the Rap1 GTP-binding protein: a pattern emerges. J Cell Sci. 2003;116:435–440. doi: 10.1242/jcs.00238. [DOI] [PubMed] [Google Scholar]
- 15.Kooistra MR, Dube N, Bos JL. Rap1: a key regulator in cell-cell junction formation. J Cell Sci. 2007;120:17–22. doi: 10.1242/jcs.03306. [DOI] [PubMed] [Google Scholar]
- 16.Dupuy AJ, Morgan K, von Lintig FC, Shen H, Acar H, Hasz DE, Jenkins NA, Copeland NG, Boss GR, Largaespada DA. Activation of the Rap1 guanine nucleotide exchange gene, CalDAG-GEF I, in BXH-2 murine myeloid leukemia. J. Biol. Chem. 2001;276:11804–11811. doi: 10.1074/jbc.M008970200. [DOI] [PubMed] [Google Scholar]
- 17.Ishida D, Kometani K, Yang H, Kakugawa K, Masuda K, Iwai K, Suzuki M, Itohara S, Nakahata T, Hiai H, Kawamoto H, Hattori M, Minato N. Myeloproliferative stem cell disorders by deregulated Rap1 activation in SPA-1-deficient mice. Cancer Cell. 2003;4:55–65. doi: 10.1016/s1535-6108(03)00163-6. [DOI] [PubMed] [Google Scholar]
- 18.Asha H, de Ruiter ND, Wang MG, Hariharan IK. The Rap1 GTPase functions as a regulator of morphogenesis in vivo. EMBO J. 1999;18:605–615. doi: 10.1093/emboj/18.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohba Y, Ikuta K, Ogura A, Matsuda J, Mochizuki N, Nagashima K, Kurokawa K, Mayer BJ, Maki K, Miyazaki Ji J, Matsuda M. Requirement for C3G-dependent Rap1 activation for cell adhesion and embryogenesis. EMBO J. 2001;20:3333–3341. doi: 10.1093/emboj/20.13.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voss AK, Gruss P, Thomas T. The guanine nucleotide exchange factor C3G is necessary for the formation of focal adhesions and vascular maturation. Development. 2003;130:355–367. doi: 10.1242/dev.00217. [DOI] [PubMed] [Google Scholar]
- 21.Gotoh T, Niino Y, Tokuda M, Hatase O, Nakamura S, Matsuda M, Hattori S. Activation of R-Ras by Ras-guanine nucleotide-releasing factor. J. Biol. Chem. 1997;272:18602–18607. doi: 10.1074/jbc.272.30.18602. [DOI] [PubMed] [Google Scholar]
- 22.Mochizuki N, Ohba Y, Kobayashi S, Otsuka N, Graybiel AM, Tanaka S, Matsuda M. Crk activation of JNK via C3G and R-Ras. J. Biol. Chem. 2000;275:12667–12671. doi: 10.1074/jbc.275.17.12667. [DOI] [PubMed] [Google Scholar]
- 23.Ohba Y, Mochizuki N, Matsuo K, Yamashita S, Nakaya M, Hashimoto Y, Hamaguchi M, Kurata T, Nagashima K, Matsuda M. Rap2 as a slowly responding molecular switch in the Rap1 signaling cascade. Mol. Cell. Biol. 2000;20:6074–6083. doi: 10.1128/mcb.20.16.6074-6083.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiang SH, Baumann CA, Kanzaki M, Thurmond DC, Watson RT, Neudauer CL, Macara IG, Pessin JE, Saltiel AR. Insulin-stimulated GLUT4 translocation requires the CAP-dependent activation of TC10. Nature. 2001;410:944–948. doi: 10.1038/35073608. [DOI] [PubMed] [Google Scholar]
- 25.Torti M, Lapetina EG. Structure and function of rap proteins in human platelets. Thromb Haemost. 1994;71:533–543. [PubMed] [Google Scholar]
- 26.Quilliam LA, Mueller H, Bohl BP, Prossnitz V, Sklar LA, Der CJ, Bokoch GM. Rap1A is a substrate for cyclic AMP-dependent protein kinase in human neutrophils. Journal of Immunology. 1991;147:1628–1635. [PubMed] [Google Scholar]
- 27.Bokoch GM, Quilliam LA, Bohl BP, Jesaitis AJ, Quinn MT. Inhibition of Rap1A binding to cytochrome b558 of NADPH oxidase by phosphorylation of Rap1A. Science. 1991;254:1794–1796. doi: 10.1126/science.1763330. [DOI] [PubMed] [Google Scholar]
- 28.Dower NA, Seldin MF, Pugh S, Stone JC. Organization and chromosomal locations of Rap1a/Krev sequences in the mouse. Mamm Genome. 1992;3:162–167. doi: 10.1007/BF00352461. [DOI] [PubMed] [Google Scholar]
- 29.Askew GR, Doetschman T, Lingrel JB. Site-directed point mutations in embryonic stem cells: a gene-targeting tag-and-exchange strategy. Mol. Cell. Biol. 1993;13:4115–4124. doi: 10.1128/mcb.13.7.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hogen BR, Beddington CF, Lacy E. Manipulating the mouse embryo: a laboratory manual. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- 31.Pradip D, Peng X, Durden DL. Rac2 specificity in macrophage integrin signaling: potential role for Syk kinase. J. Biol. Chem. 2003;278:41661–41669. doi: 10.1074/jbc.M306491200. [DOI] [PubMed] [Google Scholar]
- 32.Yamauchi A, Kim C, Li S, Marchal CC, Towe J, Atkinson SJ, Dinauer MC. Rac2-deficient murine macrophages have selective defects in superoxide production and phagocytosis of opsonized particles. J Immunol. 2004;173:5971–5979. doi: 10.4049/jimmunol.173.10.5971. [DOI] [PubMed] [Google Scholar]
- 33.Christopherson KW, 2nd, Hangoc G, Broxmeyer HE. Cell surface peptidase CD26/dipeptidylpeptidase IV regulates CXCL12/stromal cell-derived factor-1 alpha-mediated chemotaxis of human cord blood CD34+ progenitor cells. J Immunol. 2002;169:7000–7008. doi: 10.4049/jimmunol.169.12.7000. [DOI] [PubMed] [Google Scholar]
- 34.Kim C, Dinauer MC. Rac2 is an essential regulator of neutrophil nicotinamide adenine dinucleotide phosphate oxidase activation in response to specific signaling pathways. J Immunol. 2001;166:1223–1232. doi: 10.4049/jimmunol.166.2.1223. [DOI] [PubMed] [Google Scholar]
- 35.Li S, Yamauchi A, Marchal C, Molitoris J, Quilliam LA, Dinauer MC. Chemoattractant-stimulated Rac activation in wild type and Rac2-deficient murine neutrophils: Preferential activation of Rac2 and correlation of Rac2 gene dosage with chemotaxis and superoxide production. Journal of Immunology. 2002;169:5043–5051. doi: 10.4049/jimmunol.169.9.5043. [DOI] [PubMed] [Google Scholar]
- 36.Matsui Y, Kikuchi A, Kawata M, Kondo J, Teranishi Y, Takai Y. Molecular cloning of smg p21B and identification of smg p21 purified from bovine brain and human platelets as smg p21B. Biochem. Biophys. Res. Commun. 1990;166:1010–1016. doi: 10.1016/0006-291x(90)90911-6. [DOI] [PubMed] [Google Scholar]
- 37.Reedquist KA, Bos JL. Costimulation through CD28 suppresses T cell receptor-dependent activation of the Ras-like small GTPase Rap1 in human T lymphocytes. J. Biol. Chem. 1998;273:4944–4949. doi: 10.1074/jbc.273.9.4944. [DOI] [PubMed] [Google Scholar]
- 38.Boussiotis VA, Freeman GJ, Berezovskaya A, Barber DL, Nadler LM. Maintenance of human T cell anergy: blocking of IL-2 gene transcription by activated Rap1. Science. 1997;278:124–128. doi: 10.1126/science.278.5335.124. [DOI] [PubMed] [Google Scholar]
- 39.Katagiri K, Hattori M, Minato N, Kinashi T. Rap1 functions as a key regulator of T-cell and antigen-presenting cell interactions and modulates T-cell responses. Mol. Cell. Biol. 2002;22:1001–1015. doi: 10.1128/MCB.22.4.1001-1015.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McLeod SJ, Ingham RJ, Bos JL, Kurosaki T, Gold MR. Activation of the Rap1 GTPase by the B cell antigen receptor. J. Biol. Chem. 1998;273:29218–29223. doi: 10.1074/jbc.273.44.29218. [DOI] [PubMed] [Google Scholar]
- 41.M'Rabet L, Coffer P, Zwartkruis F, Franke B, Segal AW, Koenderman L, Bos JL. Activation of the small GTPase rap1 in human neutrophils. Blood. 1998;92:2133–2140. [PubMed] [Google Scholar]
- 42.Reedquist KA, Ross E, Koop EA, Wolthuis RM, Zwartkruis FJ, van Kooyk Y, Salmon M, Buckley CD, Bos JL. The small GTPase, Rap1, mediates CD31-induced integrin adhesion. J. Cell. Biol. 2000;148:1151–1158. doi: 10.1083/jcb.148.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katagiri K, Hattori M, Minato N, Irie S, Takatsu K, Kinashi T. Rap1 is a potent activation signal for leukocyte function-associated antigen 1 distinct from protein kinase C and phosphatidylinositol-3-OH kinase. Mol. Cell. Biol. 2000;20:1956–1969. doi: 10.1128/mcb.20.6.1956-1969.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shimonaka M, Katagiri K, Nakayama T, Fujita N, Tsuruo T, Yoshie O, Kinashi T. Rap1 translates chemokine signals to integrin activation, cell polarization, and motility across vascular endothelium under flow. J. Cell. Biol. 2003;161:417–427. doi: 10.1083/jcb.200301133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katagiri K, Maeda A, Shimonaka M, Kinashi T. RAPL, a Rap1-binding molecule that mediates Rap1-induced adhesion through spatial regulation of LFA-1. Nat. Immunol. 2003;4:741–748. doi: 10.1038/ni950. [DOI] [PubMed] [Google Scholar]
- 46.Williams DA, Rosenblatt MF, Beier DR, Cone RD. Generation of murine stromal cell lines supporting hematopoietic stem cell proliferation by use of recombinant retrovirus vectors encoding simian virus 40 large T antigen. Mol. Cell. Biol. 1988;8:3864–3871. doi: 10.1128/mcb.8.9.3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caron EA, Self J, Hall A. The GTPase Rap1 controls functional activation of macrophage integrin alphaMbeta2 by LPS and other inflammatory mediators. Curr. Biol. 2000;10:974–978. doi: 10.1016/s0960-9822(00)00641-2. [DOI] [PubMed] [Google Scholar]
- 48.Pizon V, Desjardins M, Bucci C, Parton RG, Zerial M. Association of Rap1a and Rap1b proteins with late endocytic/phagocytic compartments and Rap2a with the Golgi complex. J Cell Sci. 1994;107:1661–1670. doi: 10.1242/jcs.107.6.1661. [DOI] [PubMed] [Google Scholar]
- 49.Seastone DJ, Zhang L, Buczynski G, Rebstein P, Weeks G, Spiegelman G, Cardelli J. The small Mr Ras-like GTPase Rap1 and the phospholipase C pathway act to regulate phagocytosis in Dictyostelium discoideum. Mol. Biol. Cell. 1999;10:393–406. doi: 10.1091/mbc.10.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quinn MT, Mullen ML, Jesaitis AJ, Linner JG. Subcellular distribution of the Rap1A protein in human neutrophils: colocalization and cotranslocation with cytochrome b559. Blood. 1992;79:1563–1573. [PubMed] [Google Scholar]
- 51.Olazabal IM, Caron E, May RC, Schilling K, Knecht DA, Machesky LM. Rho-kinase and myosin-II control phagocytic cup formation during CR, but not FcgammaR, phagocytosis. Curr. Biol. 2002;12:1413–1418. doi: 10.1016/s0960-9822(02)01069-2. [DOI] [PubMed] [Google Scholar]
- 52.Meng J, Casey PJ. Activation of Gz attenuates Rap1-mediated differentiation of PC12 cells. J. Biol. Chem. 2002;277:43417–43424. doi: 10.1074/jbc.M204074200. [DOI] [PubMed] [Google Scholar]
- 53.Jordan JD, Carey KD, Stork PJ, Iyengar R. Modulation of rap activity by direct interaction of Galpha(o) with Rap1 GTPase-activating protein. J. Biol. Chem. 1999;274:21507–21510. doi: 10.1074/jbc.274.31.21507. [DOI] [PubMed] [Google Scholar]
- 54.Gabig TG, Crean CD, Mantel PL, Rosli R. Function of wild-type or mutant Rac2 and Rap1a GTPases in differentiated HL60 cell NADPH oxidase activation. Blood. 1995;85:804–811. [PubMed] [Google Scholar]
- 55.Maly FE, Quilliam LA, Dorseuil O, Der CJ, Bokoch GM. Activated or dominant inhibitory mutants of Rap1A decrease the oxidative burst of Epstein-Barr virus-transformed human B lymphocytes. J. Biol. Chem. 1994;269:18743–18746. [PubMed] [Google Scholar]
- 56.Knaus UG, Heyworth PG, Evans T, Curnutte JT, Bokoch GM. Regulation of phagocyte oxygen radical production by the GTP-binding protein Rac 2. Science. 1991;254:1512–1515. doi: 10.1126/science.1660188. [DOI] [PubMed] [Google Scholar]
- 57.Roberts AW, Kim C, Zhen L, Lowe JB, Kapur R, Petryniak B, Spaetti A, Pollock JD, Borneo JB, Bradford GB, Atkinson SJ, Dinauer MC, Williams DA. Deficiency of the hematopoietic cell-specific Rho family GTPase Rac2 is characterized by abnormalities in neutrophil function and host defense. Immunity. 1999;10:183–196. doi: 10.1016/s1074-7613(00)80019-9. [DOI] [PubMed] [Google Scholar]
- 58.Dinauer MC. Regulation of neutrophil function by Rac GTPases. Curr Opin Hematol. 2003;10:8–15. doi: 10.1097/00062752-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 59.Sebzda E, Bracke M, Tugal T, Hogg N, Cantrell DA. Rap1A positively regulates T cells via integrin activation rather than inhibiting lymphocyte signaling. Nat. Immunol. 2002;3:251–258. doi: 10.1038/ni765. [DOI] [PubMed] [Google Scholar]
- 60.Duchniewicz M, Zemojtel T, Kolanczyk M, Grossmann S, Scheele JS, Zwartkruis FJ. Rap1A-deficient T and B cells show impaired integrin-mediated cell adhesion. Mol. Cell. Biol. 2006;26:643–653. doi: 10.1128/MCB.26.2.643-653.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsai IC, Amack JD, Gao ZH, Band V, Yost HJ, Virshup DM. A Wnt-CKIvarepsilon-Rap1 pathway regulates gastrulation by modulating SIPA1L1, a Rap GTPase activating protein. Dev Cell. 2007;12:335–347. doi: 10.1016/j.devcel.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chrzanowska-Wodnicka M, Smyth SS, Schoenwaelder SM, Fischer TH, White GC., 2nd Rap1b is required for normal platelet function and hemostasis in mice. J. Clin. Invest. 2005;115:680–687. doi: 10.1172/JCI22973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamauchi A, Marchal CC, Molitoris J, Pech N, Knaus U, Towe J, Atkinson SJ, Dinauer MC. Rac GTPase Isoform-specific Regulation of NADPH Oxidase and Chemotaxis in Murine Neutrophils in Vivo: ROLE OF THE C-TERMINAL POLYBASIC DOMAIN. J. Biol. Chem. 2005;280:953–964. doi: 10.1074/jbc.M408820200. [DOI] [PubMed] [Google Scholar]
- 64.Filippi MD, Harris CE, Meller J, Gu Y, Zheng Y, Williams DA. Localization of Rac2 via the C terminus and aspartic acid 150 specifies superoxide generation, actin polarity and chemotaxis in neutrophils. Nat. Immunol. 2004;5:744–751. doi: 10.1038/ni1081. [DOI] [PubMed] [Google Scholar]
- 65.Hancock JF. Ras proteins: different signals from different locations. Nat. Rev. Mol. Cell. Biol. 2003;4:373–384. doi: 10.1038/nrm1105. [DOI] [PubMed] [Google Scholar]
- 66.Chan AY, Coniglio SJ, Chuang YY, Michaelson D, Knaus UG, Philips MR, Symons M. Roles of the Rac1 and Rac3 GTPases in human tumor cell invasion. Oncogene. 2005;24:7821–7829. doi: 10.1038/sj.onc.1208909. [DOI] [PubMed] [Google Scholar]
- 67.Bivona TG, Quatela SE, Bodemann BO, Ahearn IM, Soskis MJ, Mor A, Miura J, Wiener HH, Wright L, Saba SG, Yim D, Fein A, Perez de Castro I, Li C, Thompson CB, Cox AD, Philips MR. PKC regulates a farnesyl-electrostatic switch on K-Ras that promotes its association with Bcl-XL on mitochondria and induces apoptosis. Mol Cell. 2006;21:481–493. doi: 10.1016/j.molcel.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 68.Reszka AA, Halasy-Nagy J, Rodan GA. Nitrogen-bisphosphonates block retinoblastoma phosphorylation and cell growth by inhibiting the cholesterol biosynthetic pathway in a keratinocyte model for esophageal irritation. Mol. Pharmacol. 2001;59:193–202. doi: 10.1124/mol.59.2.193. [DOI] [PubMed] [Google Scholar]
- 69.Klinz FJ, Seifert R, Schwaner I, Gausepohl H, Frank R, Schultz G. Generation of specific antibodies against the rap1A, rap1B and rap2 small GTP-binding proteins. Analysis of rap and ras proteins in membranes from mammalian cells. Eur. J. Biochem. 1992;207:207–213. doi: 10.1111/j.1432-1033.1992.tb17039.x. [DOI] [PubMed] [Google Scholar]
- 70.Winegar DA, Ohmstede CA, Chu L, Reep B, Lapetina EG. Antisera specific for rap 1 proteins distinguish between processed and nonprocessed rap 1b. J. Biol. Chem. 1991;266:4375–4380. [PubMed] [Google Scholar]
- 71.McLeod SJ, Li AH, Lee RL, Burgess AE, Gold MR. The Rap GTPases regulate B cell migration toward the chemokine stromal cell-derived factor-1 (CXCL12): potential role for Rap2 in promoting B cell migration. J Immunol. 2002;169:1365–1371. doi: 10.4049/jimmunol.169.3.1365. [DOI] [PubMed] [Google Scholar]
- 72.Caron E, Hall A. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science. 1998;282:1717–1721. doi: 10.1126/science.282.5394.1717. [DOI] [PubMed] [Google Scholar]
- 73.Arthur WT, Quilliam LA, Cooper JA. Rap1 promotes cell spreading by localizing Rac guanine nucleotide exchange factors. J. Cell. Biol. 2004;167:111–122. doi: 10.1083/jcb.200404068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li Y, Asuri S, Rebhun JF, Castro AF, Paranavitana NC, Quilliam LA. The RAP1 guanine nucleotide exchange factor Epac2 couples cyclic AMP and Ras signals at the plasma membrane. J. Biol. Chem. 2006;281:2506–2514. doi: 10.1074/jbc.M508165200. [DOI] [PubMed] [Google Scholar]
- 75.Liao Y, Satoh T, Gao X, Jin TG, Hu CD, Kataoka T. RA-GEF-1, a guanine nucleotide exchange factor for Rap1, is activated by translocation induced by association with Rap1-GTP and enhances Rap1-dependent B-Raf activation. J. Biol. Chem. 2001;276:28478–28483. doi: 10.1074/jbc.M101737200. [DOI] [PubMed] [Google Scholar]
- 76.Rebhun JF, Castro AF, Quilliam LA. Identification of guanine nucleotide exchange factors (GEFs) for the rap1 GTPase. Regulation of MR-GEF by M-Ras-GTP interaction. J. Biol. Chem. 2000;275:34901–34908. doi: 10.1074/jbc.M005327200. [DOI] [PubMed] [Google Scholar]
- 77.Gao X, Satoh T, Liao Y, Song C, Hu CD, Kariya Ki K, Kataoka T. Identification and characterization of RA-GEF-2, a Rap guanine nucleotide exchange factor that serves as a downstream target of M-Ras. J. Biol. Chem. 2001;276:42219–42225. doi: 10.1074/jbc.M105760200. [DOI] [PubMed] [Google Scholar]
- 78.Wang Z, Dillon TJ, Pokala V, Mishra S, Labudda K, Hunter B, Stork PJ. Rap1-mediated activation of extracellular signal-regulated kinases by cyclic AMP is dependent on the mode of Rap1 activation. Mol. Cell. Biol. 2006;26:2130–2145. doi: 10.1128/MCB.26.6.2130-2145.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huelsmann S, Hepper C, Marchese D, Knoll C, Reuter R. The PDZ-GEF dizzy regulates cell shape of migrating macrophages via Rap1 and integrins in the Drosophila embryo. Development. 2006;133:2915–2924. doi: 10.1242/dev.02449. [DOI] [PubMed] [Google Scholar]
- 80.Park YG, Zhao X, Lesueur F, Lowy DR, Lancaster M, Pharoah P, Qian X, Hunter KW. Sipa1 is a candidate for underlying the metastasis efficiency modifier locus Mtes1. Nat. Genet. 2005;37:1055–1062. doi: 10.1038/ng1635. [DOI] [PMC free article] [PubMed] [Google Scholar]