Abstract
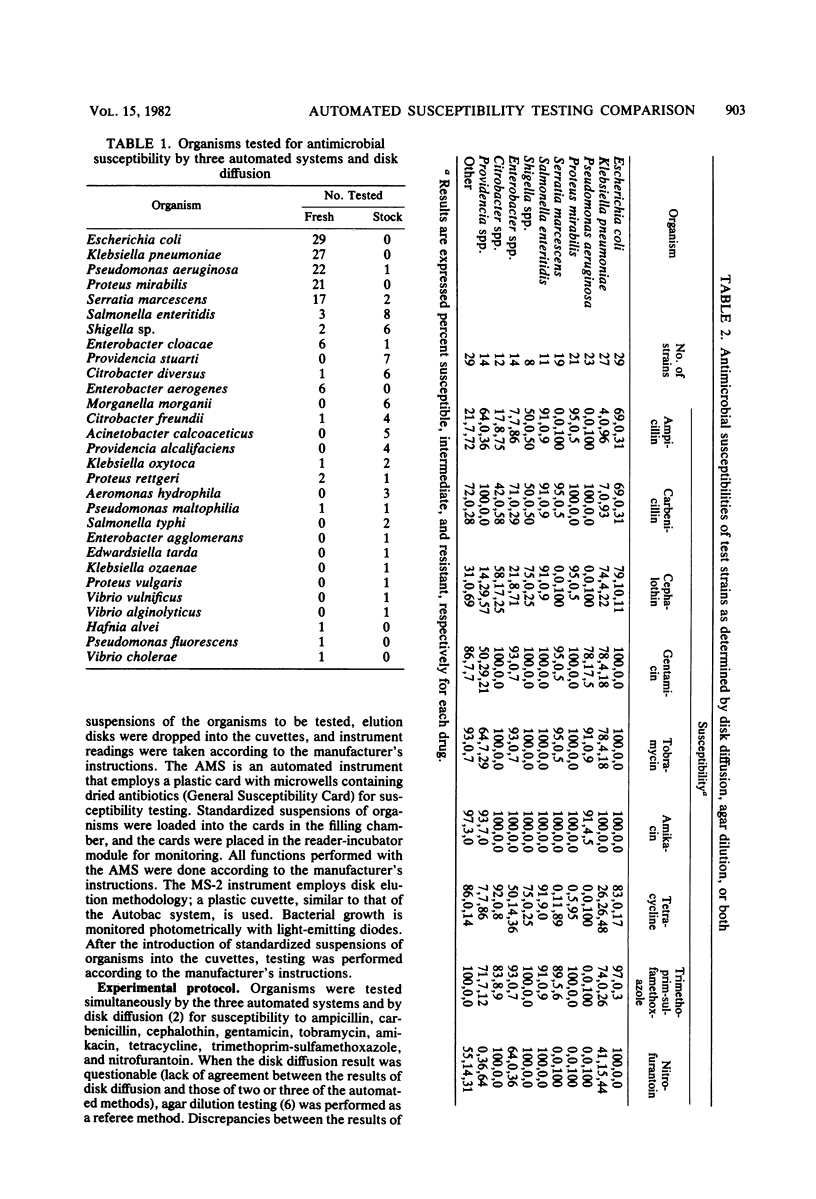
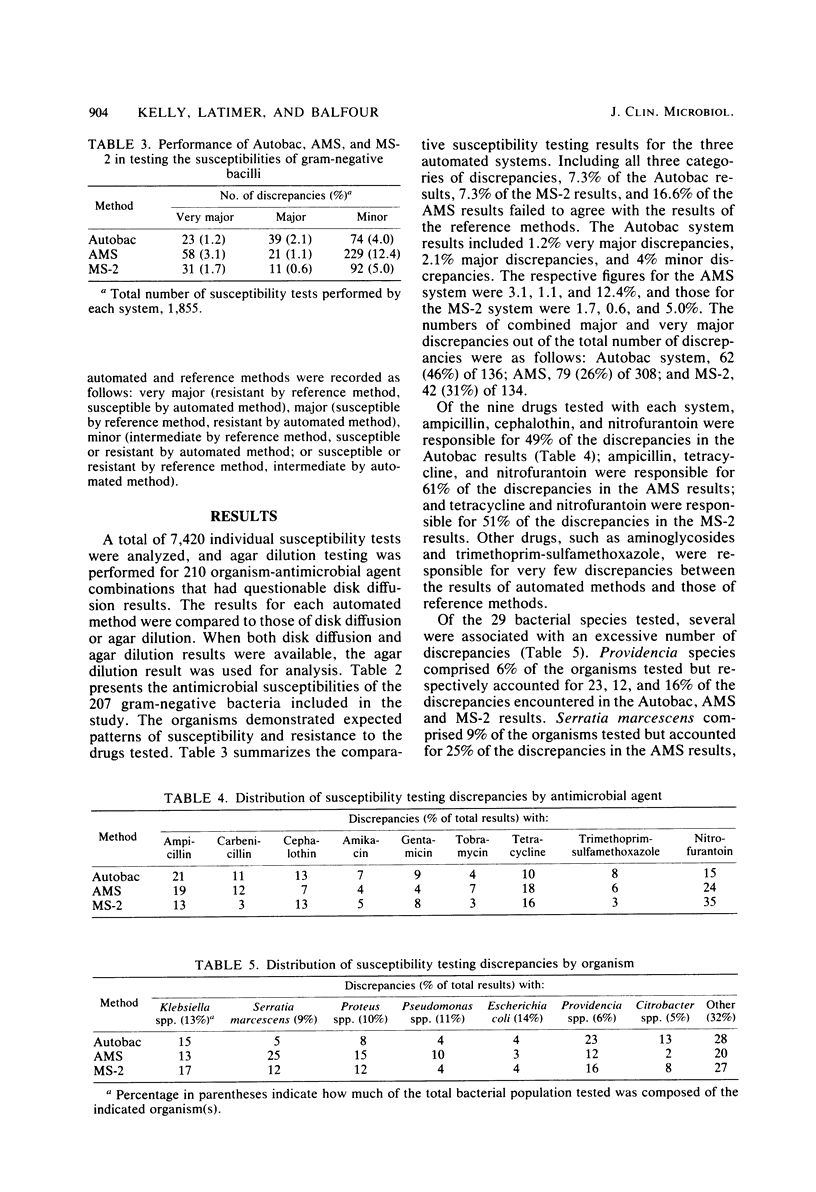
Several instruments for automated or semiautomated susceptibility testing are currently available. Three of these instruments, Autobac (General Diagnostics, Warner-Lambert Co., Morris Plains, N.J.), MS-2 (Abbott Laboratories, Dallas, Tex.), and AutoMicrobic system (AMS) (Vitek, Inc., Hazelwood, Mo.) were compared for antimicrobial susceptibility testing of gram-negative bacilli. A total of 207 isolates representing 29 species of gram-negative bacilli were tested simultaneously by each instrument and by a standardized disk diffusion reference method. Nine antimicrobial agents, including ampicillin, carbenicillin, cephalothin, gentamicin, tobramycin, amikacin, tetracycline, trimethoprim-sulfamethoxazole, and nitrofurantoin were tested. Discrepancies between the results of the automated and reference disk diffusion methods were resolved by agar dilution testing. Overall, 93% of the Autobac and MS-2 results and 83% of the AMS results were in agreement with the results obtained by the reference methods. The results of the Autobac, MS-2, and AMS systems respectively included 3.3, 2.3, and 4.2% major and very major discrepancies. Excessive testing discrepancies were found for certain drugs, including ampicillin, tetracycline, and nitrofurantoin, and for certain organisms, including species of Providencia, Serratia, and Citrobacter. The results of this comparison of three automated systems for antimicrobial susceptibility testing indicate that the Autobac and MS-2 instruments provided highly reliable results. The AMS need further development of its susceptibility testing capability to eliminate an unacceptably high number of minor discrepancies.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes W. G., Green L. R., Talley R. L. Clinical evaluation of automated antibiotic susceptibility testing with the MS-2 system. J Clin Microbiol. 1980 Oct;12(4):527–532. doi: 10.1128/jcm.12.4.527-532.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob C. V., Kleineman J. Comparison of drug sensitivity testing with microdilution quantitative minimum inhibitory concentration and the Autobac I system. J Clin Microbiol. 1980 May;11(5):465–469. doi: 10.1128/jcm.11.5.465-469.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornsberry C., Anhalt J. P., Washington J. A., 2nd, McCarthy L. R., Schoenknecht F. D., Sherris J. C., Spencer H. J. Clinical laboratory evaluation of the Abbott MS-2 automated antimicrobial susceptibility testing system: report of a collaborative study. J Clin Microbiol. 1980 Sep;12(3):375–390. doi: 10.1128/jcm.12.3.375-390.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornsberry C., Gavan T. L., Sherris J. C., Balows A., Matsen J. M., Sabath L. D., Schoenknecht F., Thrupp L. D., Washington J. A., 2nd Laboratory evaluation of a rapid, automatic susceptibility testing system: report of a collaborative study. Antimicrob Agents Chemother. 1975 Apr;7(4):466–480. doi: 10.1128/aac.7.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]


