Abstract
The present study was designed to examine whether lesions of the insular cortex (IC; Experiment 1), the basolateral amygdala (BLA) or medial amygdala (MeA; Experiment 2) influence the neophobic reactions to orally consumed liquid stimuli. Three different types of stimuli were used: taste (0.5% saccharin), olfactory (0.1% amyl acetate), and trigeminal (0.01 mM capsaicin). Rats with IC, BLA and MeA lesions showed normal responses to the olfactory and trigeminal stimuli. Each type of lesion, however, disrupted the initial occurrence of neophobia to the taste stimulus. The significance of these findings to conditioned taste aversion is discussed.
Keywords: Neophobia, insular cortex, amygdala, rat
1. Introduction
Postingestive consequences critically influence the amount of food consumed. When such knowledge is not available (i.e., when the food is novel), animals tend to eat less because of an innate fear of unfamiliar food, a phenomenon termed neophobia (Barnett, 1956, 1958; Corey, 1978). If no aversive gastrointestinal effects follow food intake, the initial fear response dissipates and intake increases (i.e., the attenuation of neophobia occurs) until consumption achieves asymptote for that particular food (Best, Domjan & Haskins, 1978; Domjan, 1977). On the other hand, if consumption is followed by gastrointestinal illness, intake of the food decreases, a phenomenon termed conditioned taste aversion (CTA; Garcia & Ervin, 1968; for recent reviews see Reilly & Schachtman, 2009). CTAs are typically viewed as examples of Pavlovian conditioning in which behavior is guided by an association between the mental representations of the taste (or conditioned stimulus, CS) of the food and the later negative postingestive consequences (or unconditioned stimulus, US).
In addition to the occurrence of neophobia, the novelty of a food also influences the acquisition of CTA. That is, CTAs are formed much more readily when the CS is novel compared to when it is familiar, an effect termed latent inhibition (for reviews see Lubow, 1989, 2009). Some of our recent research has examined the influence of permanent brain lesions on the acquisition of CTAs. For example, Roman, Nebieridze, Sastre, and Reilly (2006) found that excitotoxic lesions of the insular cortex (IC) attenuated but did not prevent CTA acquisition. In that study, the IC-lesioned (ICX) rats showed what was assumed to be diminished neophobic reactions by overconsuming the novel saccharin CS relative to neurologically intact animals on the first CTA conditioning trial, suggesting that ICX rats might perceive the novel CS as if it was familiar. To test this hypothesis, we conducted a latent inhibition study in which CTA acquisition was assessed when the CS was either novel or familiar. In accord with the prediction, ICX rats acquired CTAs at a rate significantly slower than normal animals when the saccharin CS was novel but at the same slow rate as normal animals when the saccharin CS was familiar (Roman & Reilly, 2007; see also Kiefer & Braun, 1977, Roman, Lin & Reilly, manuscript under review). Furthermore, a similar deficit was also found in rats with lesions of the basolateral amygdala (BLA; St. Andre & Reilly, 2007), an area containing rich reciprocal connections with the IC (Krettek & Price, 1977). These findings raise the possibility that the impaired CTAs in rats with IC or BLA lesions may result from a deficiency in the detection/recognition of the novelty of the CS. Thus, the direct examination of the roles of the IC and BLA in taste neophobia in the present study will inform our understanding of the involvement of these structures in CTA learning.
Neophobia is sometimes defined as a reduction in consumption of a novel solution relative to baseline water intake (e.g., Kesner, Berman & Tardif, 1992; Kolakowska, Larue-Achagiotis & Le Magnen, 1984; Yamamoto, Fujimoto, Shimura & Sakai, 1995). This single-trial definition, however, fails to account for the palatability of the test stimulus. Some stimuli, such as certain concentrations of alanine, sodium chloride, saccharin, or sucrose, are first avoided but are eventually consumed in greater amounts than water (Miller & Holzman, 1981; Reilly & Trifunovic, 2001). Other stimuli, such as certain concentrations of citric acid or quinine, are never consumed in the same amounts as water (Miller & Holzman, 1981). Therefore, the occurrence of neophobia is best determined in terms of intake of the novel stimulus on trial 1 relative to intake after additional trials. Minimally, one extra trial is needed to determine that intake on trial 1 was significantly lower than intake on trial 2 (when the CS is presumably less fear inducing because it is now more familiar than on trial 1). However, in order to determine the magnitude of the neophobic response more than two taste trials are needed. This is because it is necessary to compare intake of the taste stimulus when it is novel with intake when it is familiar and safe (i.e., when intake is at asymptote for that particular taste stimulus). Minimally, stable intake requires two consecutive taste trials when the amount consumed is not significantly different. Thus, even if neophobia occurs only on trial 1, in order to be confident about the magnitude of the neophobic response a minimum of three trials is needed. Of course, more trials may be needed if neophobia only partially dissipates after the first trial. Typically, then, multiple trials are needed to determine the occurrence and magnitude of the neophobic reaction to a novel taste stimulus.
As noted above, the primary goal of the present study was to examine whether lesions of the IC or BLA impair taste neophobia in order to better understand the nature of CTA deficits in rats with either type of brain lesion. Thus, all the subjects were first tested with a taste stimulus. To maintain comparability with the contemporary taste neophobia literature (e.g., Figueroa-Guzmán, Kuo, & Reilly, 2006; Figueroa-Guzmán & Reilly, 2008; Gutiérrez, Rodriguez-Ortiz, De La Cruz, Núnez-Jaramillo, & Bermúdez-Rattoni, 2003; Gutiérrez, Tellez & Bermudez-Rattoni, 2003; Koh, Wilkins & Bernstein, 2003; Wilkins & Bernstein, 2006), we used 0.5% sodium saccharin as the exemplar neophobia-inducing taste stimulus. The secondary goal of the present study was to examine the influence of IC lesions on odor neophobia. We have demonstrated that IC lesions, while disrupting CTA acquisition, have no influence on the acquisition of conditioned odor aversions (Roman et al., 2006). Thus, our expectation was that ICX rats would show a normal neophobic reaction to an odor stimulus. The selected olfactory stimulus was an aqueous solution of 0.1% amyl acetate, which Slotnick, Westbrook and Darling (1997) demonstrated was identifiable as an odor that does not also stimulate taste receptors. Finally, to determine the generality of any lesion effects that might be obtained, we also explored neophobia to a oral trigeminal stimulus. In this case, 0.01 mM capsaicin was used because it is an effective oral CS in aversive conditioning (e.g., Grigson, Reilly, Shimura & Norgren, 1998). Experiment 1 of the present study examined taste, olfactory and trigeminal neophobia in ICX rats. Experiment 2 examined whether lesions of the BLA or medial amygdala (MeA) influence neophobia for orally consumed stimuli. The MeA is a central relay of odor information (McDonald, 1998; Sah, Faber, Lopez de Armentia & Power, 2003; Shipley, McLean & Ennis, 1995) and lesions of the structure are known to disrupt some forms of olfactory-guided behaviors (e.g., Li, Maglinao & Takahashi, 2004; Petrulis & Johnston, 1999; Takahashi, Hubbard, Lee, Dar & Sipes, 2007).
2. Results
2.1 Experiment 1
2.1.2 Anatomical
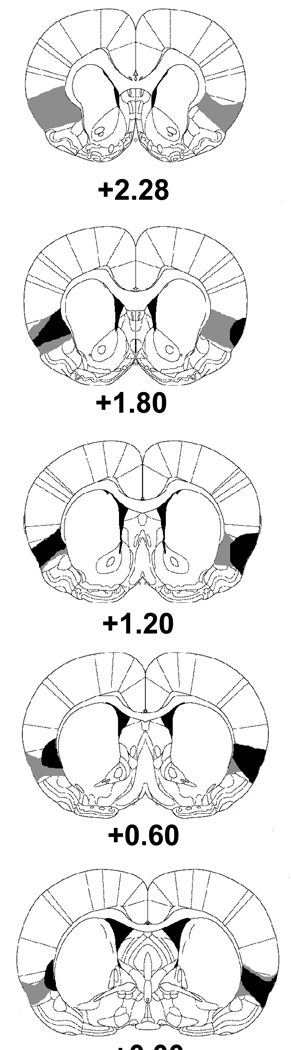
Figure 1 shows serial reconstructions of the IC lesions. According to Kosar, Grill and Norgren (1986), the gustatory portion of the IC is located on the dorsal bank of the rhinal fissure and extends dorsoventrally approximately 0.5 mm and anteroposterially approximately 2.5 mm. Histological analyses were conducted by examining for the presence of gliosis and the absence of cell bodies. Rats with subtotal or unilateral lesions were excluded from the behavioral analysis. In most cases the lesions were confined to the IC. There were some case in which the lesions showed minor encroached into surrounding areas, including somatosensory cortex, claustrum, and piriform cortex. After histological examination, the final numbers of rats in the SHAM and ICX groups were 11 and 10, respectively.
Fig. 1.
Serial reconstructions of the largest (gray) and smallest (black) lesions of the insular cortex are shown at five levels (2.28, 1.80, 1.20, 0.60, 0.00 mm anterior to bregma) on diagrams that were adapted with permission from the Paxinos & Watson (2005) atlas.
2.2.3 Behavioral
Taste stimulus: As shown in the left-side panel of Figure 2, neurologically intact (SHAM) subjects showed a profound neophobic reaction to the 0.5% saccharin solution on Trial 1. This innate response was fully habituated by Trial 3 when intake achieved asymptote. IC lesions attenuated the magnitude of the initial response to the novel taste solution but had no influence on the level of saccharin consumption at asymptote. This characterization of the data was supported by statistical analysis. A two-way ANOVA revealed a significant main effect of Lesion, F(1,19) = 5.41, p < .05, a significant main effect of Trial, F(3,57) = 78.91, p < .001, and a significant Lesion x Trial interaction, F(3,57) = 7.66, p < .001. Post hoc comparisons (simple main effects) further indicated that the difference between SHAM and ICX rats was significant on Trial 1, F(1,57) = 43.86, p < .001, and on Trial 2, F(1,57) = 10.08, p < .01, but not on Trials 3 or 4 (ps > .05).
Fig. 2.
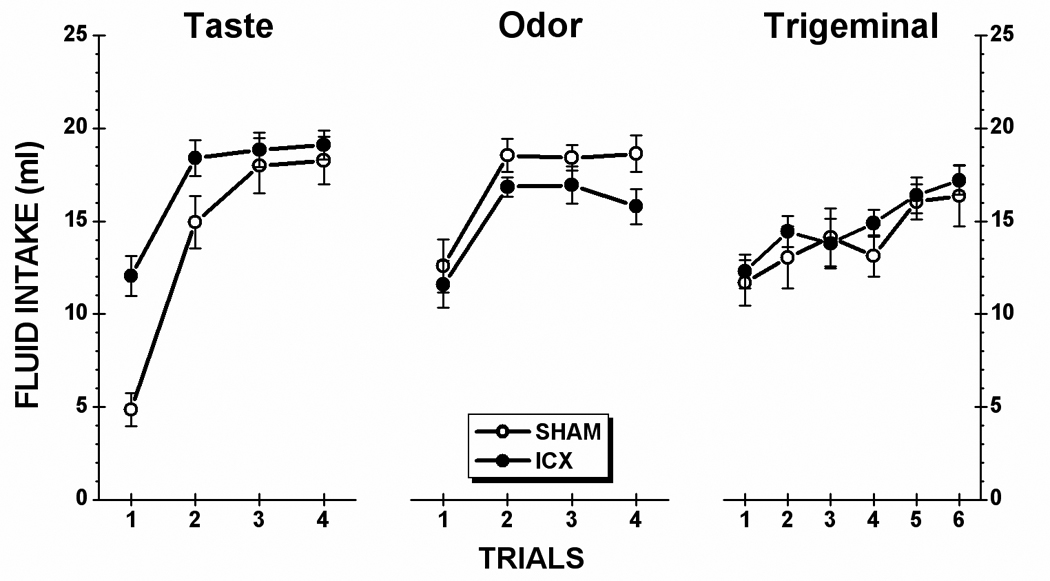
Mean (±SE) fluid intake of each stimulus for the control (SHAM) subjects and rats with insular cortex lesions (ICX). During each trial, animals were allowed 15 minutes unrestricted access to each solution: taste (0.5% saccharin), olfactory (0.1% amyl acetate), trigeminal (0.01 mM capsaicin).
Olfactory stimulus: Inspection of the data in the center panel of Figure 2 suggests that IC lesions influenced neither the initial neophobic response to, nor the asymptotic level of intake of, amyl acetate. A Lesion X Trial ANOVA found no significant main effect of Lesion (F < 1) and no significant Lesion x Trial interaction (F < 1). There was, however, a significant main effect of Trial, F(3,57) = 25.95, p < .001. Post hoc comparisons of this main effect indicated that the rats consumed significantly less amyl acetate solution on Trial 1 than on each of the other three trials (ps < .05); no other pairwise comparisons were significant (ps >.05).
Trigeminal stimulus: Intake over the six capsaicin trials is displayed in the right-side panel of Figure 2. Analysis of the data summarized in the figure found a significant main effect of Trial, F(5,95) = 9.77, p < .05, but no main effect of Lesion (F < 1) and no Lesion x Trial interaction (F < 1). Follow-up comparisons with simple main effects revealed that the consumption of capsaicin increased gradually across trials and that it was not until Trial 5 that intake was significantly greater than that of Trial 1 (p < .05). Furthermore, there was no significant difference between intake on Trial 5 and Trial 6 (p >.05). Although neophobia was present, it is less clear that asymptote was achieved for capsaicin intake despite six exposures to the stimulus.
2.2 Experiment 2
2.2.1 Anatomical
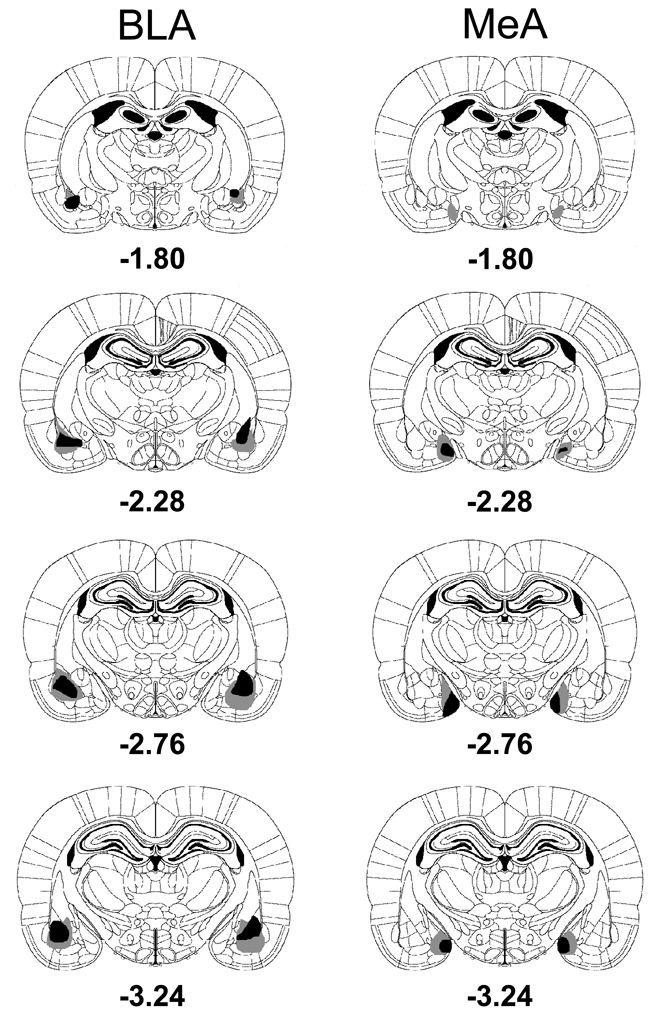
Figure 3 shows schematic representations of the histological results from rats with BLA or MeA lesions. The NMDA-induced BLA lesions were centered in the basolateral amygdala and spread out dorsally to part of the lateral amygdala. Although some damage also occurred in the central amygdala, this was not consistently found. Some minimal lesions also occurred to the dorsal endopiriform nucleus, lateral to the BLA. For the MeA lesions, the damage occurred to both dorsal and ventral nuclei of the MeA. Rats with undersized or unilateral lesions were excluded from the behavioral analysis. After histological examination, 5 BLAX and 3 MeAX rats were eliminated and left the group sizes as follows: SHAM, n = 11; BLAX, n = 9; MeAX, n = 10.
Fig. 3.
Serial reconstructions of the largest (gray) and smallest (black) lesions of the basolateral amygdala (BLA) or the medial amygdala (MeA) are shown at four levels (1.80, 2.28, 2.76, 3.24 mm posterior to bregma) on diagrams that were adapted with permission from the Paxinos & Watson (2005) atlas.
2.2.2 Behavioral
Taste stimulus: Figure 4 (left panel) shows the saccharin consumption of the SHAM, BLA-lesioned (BLAX) and MeA-lesioned (MeAX) rats during each of the five taste test trials. From inspection of the figure, it is evident that BLAX and MeAX rats each consumed more of the novel saccharin solution on Trial 1 than the SHAM subjects. Furthermore, these intergroup differences were sustained over the first three taste trials. Unsurprisingly, then, an ANOVA conducted on saccharin intake data found a significant main effect of Lesion, F(2,27) = 9.71, p < .001, a significant main effect of Trial, F(4,108) = 245.88, p < .001, and a significant Lesion x Trial interaction, F(8,108) = 7.74, p < .001. Post hoc comparisons of the interaction revealed that both BLAX and MeAX rats drank significantly more saccharin than SHAM animals on Trial 1, Trial 2, and Trial 3 (ps < .05). The lesion-induced difference dissipated on Trial 4 and Trial 5. The analysis also found that MeAX rats consumed more saccharin than BLAX rats on Trial 1 and Trial 2 (ps < .05); each group drank comparable amounts of saccharin on Trials 3 – 5 (ps > .05).
Fig. 4.
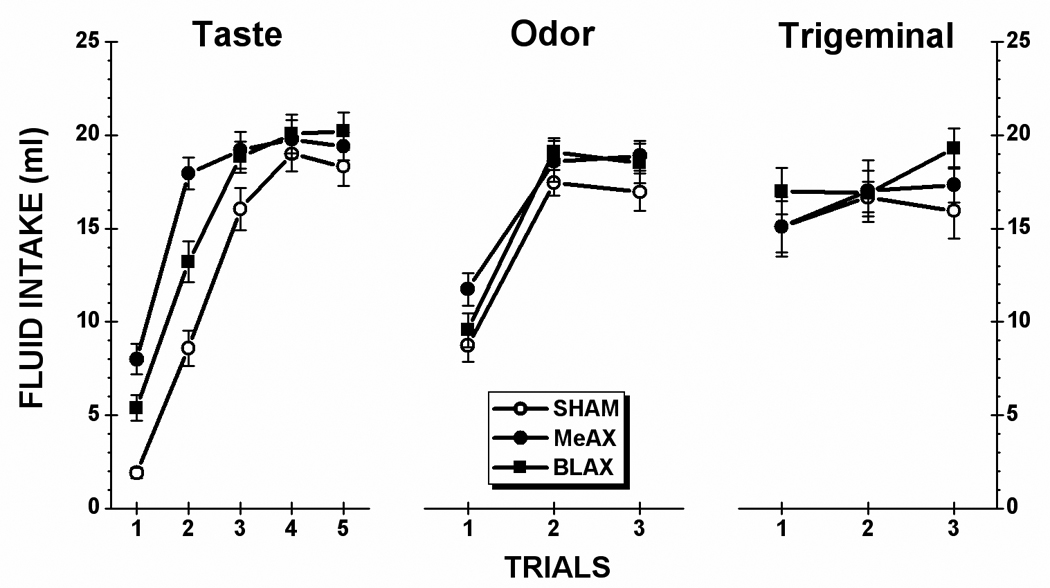
Mean (±SE) fluid intake of each stimulus for the control (SHAM) subjects and rats with lesions of either the basolateral amygdala (BLAX) or medial amygdala (MeAX). During each trial, animals were allowed 15 minutes unrestricted access to each solution: taste (0.5% saccharin), olfactory (0.1% amyl acetate), trigeminal (0.01 mM capsaicin).
Olfactory stimulus: Intake data for amyl acetate is presented in the center panel of Figure 4. As shown in the figure, all three groups drank similar amounts of the aqueous odor stimulus. An ANOVA conducted on these data found a significant main effect of Trial, F(2,52) = 123.87, p < .001. However, there was no significant main effect of Lesion, F(2,26) = 2.37, p >.05, and no significant Lesion x Trial interaction (F < 1). The rats drank significant lower amounts of amyl acetate on Trial 1 than on Trial 2 or on Trial 3 (ps <.05). There was, moreover, no significant difference between Trial 2 and Trial 3 intake (p >.05).
Trigeminal stimulus: The intake data are presented in the right-side panel of Figure 4. An ANOVA conducted on these data found no significant main effect of Lesion (F < 1), and no significant Lesion x Trial interaction (F < 1). Although it may seems that capsaicin intake changed across trials, the main effect of Trial approached but did not achieve significance, F(2,54) = 2.59, p = 0.08.
3. Discussion
The present study was designed to examine whether lesions of the IC (Experiment 1), the BLA or MeA (Experiment 2) influence the occurrence of neophobia to orally consumed liquid stimuli. SHAM (i.e., neurologically intact) rats demonstrated neophobic reactions to each of the three target stimuli: saccharin, amyl acetate, and capsaicin. None of the forebrain lesions had any significant influence on the consumption of either the olfactory stimulus or the trigeminal stimulus. However, each type of lesion disrupted the initial occurrence of taste neophobia. That is, ICX, BLAX and MeAX rats consumed significantly more saccharin on the first 2 or 3 trials and therefore unsurprisingly took fewer trials to reach asymptote than the SHAM subjects. Of further note, none of the forebrain lesions influenced the level of saccharin intake at asymptote. This latter finding is important because it indicates that that neophobia deficit cannot be attributed to a lesion-induced alteration in the perceived intensity of the taste stimulus, which would also be expected to influence the level of intake at asymptote.
As alluded to earlier, few studies have examined the effects of brain lesions on neophobia to orally consumed liquid stimuli. Most of the knowledge on this topic is extrapolated from intake on the first acquisition trial (i.e., prior to administration of the US) of CTA, conditioned odor aversion and conditioned capsaicin aversion studies. In more recent years, a new tool, the assessment of immediate early gene (e.g., c-fos) expression, has been used to examine the neural substrates of neophobia, at least with regard to taste stimuli. For example, Koh et al. (2003; for a review see Bernstein, Wilkins & Barot, 2009), using either voluntary drinking or intraoral infusions, found elevated expression of c-fos in the IC and the central nucleus of the amygdala when 0.5% saccharin was novel relative to familiar saccharin. These investigators did not report data for the MeA. There was, however, no difference in the magnitude of expressed c-fos activation in the BLA after novel taste consumption. In Experiment 2 of the present study we found that BLAX rats showed elevated intake of novel saccharin, which is consistent with previous lesion-CTA studies (e.g., Aggleton Petrides & Iversen, 1981; Fitzgerald & Burton, 1983; Kolakowska et al., 1984 Nachman & Ashe, 1974; Shimai & Hoshishima, 1982). The lack of novel taste-induced c-fos expression in the BLA in the Koh et al. (2003) study serves to highlight one of the limitations of using c-fos expression to map the neural substrates of behavior. That is, c-fos imaging can only be used to detect neuronal activation; the c-fos gene is not expressed during inhibitory neural activity (Hughes & Dragunow, 1995; Sheng & Greenberg, 1990). On the other hand, given these converging lines of evidence from lesion and c-fos studies, one might speculate that a normal neophobic reaction to a novel taste stimulus involves inhibitory activity in the BLA. The merits of this hypothesis will be evaluated in future studies.
Olfactory information reaches the MeA (McDonald, 1998; Sah et al., 2003; Shipley et al., 1995). Moreover, there is a growing literature indicating a role for the MeA in responsivity to certain innate fear-inducing stimuli such as the odor of predators (e.g., Chen, Shemyakin & Wiedenmayer, 2006; Li et al., 2004; Takahashi et al., 2007). There was, then, good reason to expect that the MeA might be involved in neophobic responsivity to a novel odor cue. However, this prediction found no support in the present results. Although MeAX rats showed a normal neophobic reaction to the odor stimulus, they showed impaired taste neophobia. It appears, then, that both the MeA and BLA are components in the same neural circuit responsible for taste neophobia. The nature of this MeA-BLA interaction cannot be determined from the present results and must await further research. However, it seems clear that these structures (as well as the IC) are not performing identical functions. If this were the case, the loss of one component would not be expected to lead to a neophobia deficit because of the presence of the other, intact components. Presumably, these three structures (MeA, BLA and IC) are performing inter-dependent, not identical, functions.
The present results are important for our understanding of the neural underpinning of taste aversion learning. Although CTA is a seemingly simple learning phenomenon, which can be acquired in a single CS-US trial, it is comprised of at least 5 different stages, each of which is integral to the final expression of the aversion: detection/processing of the taste stimulus that will become the CS, detection/processing of the illness US, association of the neural representations of the CS and US, retrieval of the information embodied in the CS-US association, and expression of that knowledge in performance (for a review see Reilly, 2009). It will be apparent, then, that CTA can be attenuated or abolished by interrupting one or more of these stages. Using procedures involving a single taste-illness pairing, some studies have suggested that the role of the IC or amygdala in CTA is to acquire or store the neural representation of the taste-illness association (e.g., Bermúdez-Rattoni & McGaugh, 1991; Nishijo, Uwano, Tamura & Ono, 1999; Sakai, & Yamamoto, 1999). However, the present results indicate that caution must be exercised with regard to this analysis. If the attenuated taste neophobia is a consequence of a lesion-induced disruption in the perception of taste novelty then an alternate interpretation of the one-trial CTA deficit in such rats becomes viable. By this alternate analysis neither the BLA nor the IC is involved in the associative mechanism that links the taste CS with the illness US. Rather, the apparent CTA deficit found in one-trial learning procedures reflects a retardation in acquisition due to a latent inhibition-like effect. That is, the CTA deficit is a secondary consequence of the taste neophobia deficit in which rats with BLA or IC lesions treat the novel taste as familiar. To clarify the nature of the deficits, we examined the effects of BLA (St Andre & Reilly, 2007) or IC (Roman & Reilly, 2007) lesions on CTA using a multiple-trial design and found that lesion-induced deficits only occurred when a novel taste served as the CS but not when a familiar taste was used. Even in the novel taste condition, the lesions did not prevent CTA but simply delayed acquisition, indicating roles for the BLA and IC in the processing of the taste CS. That is, we believe that the lesioned animals failed to recognize the CS as a novel taste and consequently treated it as a familiar one, which in turn produced a latent inhibition-like retardation of CTA acquisition.
Given that lesions of the BLA or IC each disrupt taste neophobia (the present results) and attenuate CTA acquisition (Roman & Reilly, 2007; St Andre & Reilly, 2007), it is easy to assume that the two nuclei may share the same behavioral function. This is, however, not likely to be the case because, as argued earlier with regard to the neophobia deficits, there is no evidence of behavioral compensation consequent to lesions of one or other structure. Two recent findings from our laboratory provide further evidence that the BLA and IC are each involved in taste neophobia but in somewhat different ways. In these two studies, rats were given 6 trials to an initially novel saccharin solution, with each 15-min exposure spaced 3 days apart as in the present study. Before each of the first three trials, MK801 (a non-competitive NMDA receptor antagonist) was infused into the BLA (Figueroa-Guzmán & Reilly, 2008) or IC (Figueroa-Guzmán et al., 2006); no intracranial infusions were administered prior to each of the final three saccharin trials. Like the SHAM subjects of the present study, control subjects showed maximal taste neophobia on Trial 1, partial recovery on Trial 2 and complete recovery from neophobia on the third saccharin trial. Indicating that NMDA receptors in the BLA have no influence on the perception of saccharin or the detection of taste novelty, MK801-infused rats displayed a normal neophobic reaction on Trial 1. However, the neophobic response remained maximal on each trial that the drug was infused. Equally important, on Trial 4 (the first saccharin exposure in the absence of a pre-trial infusion of MK801) the rats drank as much saccharin as the control subject (i.e., complete recovery from neophobia occurred). A similar pattern emerged from the study involving pre-trial infusions of MK801 into the IC, except for one notable difference. That is, instead of an immediate and complete recovery from neophobia on Trial 4, after the termination of MK801 infusions into the IC these rats habituated to saccharin at the same rate as that previously shown by the control subjects (albeit offset by 3 trials). Results from these MK801 studies suggest that NMDA receptors in the BLA are important for the retrieval of a consolidated taste memory whereas NMDA receptors in the IC are implicated in the proper consolidation of a taste memory.
The results of the present study not only provide confirmation that the BLA and IC are essential for taste neophobia but also identify a new component of the neurocircuitry of this system: the MeA. Furthermore, the results clearly demonstrate that these three structures (MeA, BLA and IC) have roles that are specifically relevant to taste neophobia; normal olfactory and trigeminal neophobia occurs in the absence of an intact MeA, BLA or IC. The goal for future research will be to define the neural circuits and the pharmacological substrates that underlie the initial expression of, and the recovery from, taste neophobia. As we have argued, knowledge about this important component of the feeding system will also inform a more complete understanding of the brain mechanisms that govern taste aversion learning.
4. Experimental Procedures
4.1 Animals
A total of 63 naïve male Sprague-Dawley rats (25 in Experiment 1 and 38 in Experiment 2) were obtained from Charles River Laboratories (Wilmington, MA). The rats were individually housed in the stainless hanging cages in a room with 12:12 light/dark cycle with light on at 7:00 am. The rats weighed between 280 and 300 g at the time of surgery and were maintained on ad libitum food and water except as noted below for experimental purposes. The experimental protocols were approved by the Animal Care and Use Committee at University of Illinois at Chicago and the rats were treated in accordance with the National Institutes of Health’s (1986) Guide for the care and Use of Laboratory Animals and the American Psychological Association’s guidelines for animal research.
4.2 Surgery
4.2.1 Experiment 1
The rats were divided into two groups based upon the surgical treatment administered: IC-lesioned (ICX; n=14), and non-surgical control (SHAM; n=11). All rats were anesthetized with an intraperitoneal injection of sodium pentobarbital (50 mg/ml). SHAM rats did not undergo any additional surgical manipulations. ICX rats were fixed in a stereotaxic apparatus (ASI; Warren, MI) using nontraumatic earbars. Body temperature was monitored with a rectal thermometer and regulated with a heating pad (Harvard Apparatus, Holliston, MA). A subcutaneous scalp injection of 0.25% bupivacaine (Hospira, Lake Forest, IL) provided analgesia during and after the surgical procedures. The skull was exposed with a midline incision, and the skin and periosteum were retracted to expose the cranial sutures. The skull was leveled between bregma and lambda, and trephine holes were drilled over the area to be lesioned. A glass capillary micropipette (tip diameter ~70 µm) was filled with N-methyl-D-aspartic acid (NMDA) and lowered to the lesion sites, of which there were two per hemisphere. The drug was then infused iontophoretically using a Midgard Precision Current Source (Stoelting, Wood Dale, IL) into the target area. Taken from Roman and Reilly (2007), lesion coordinates and infusion durations were as follows: Site 1, AP +1.2, ML ±5.2, DV −5.0 for 10 min; Site 2, AP +1.2, ML ±5.2, DV −4.7 for 6 min. Following the lesions, the incision was closed with wound clips and the rats were returned to their home cages once they recovered from the anesthesia.
4.2.2 Experiment 2
A second set of rats was randomly assigned into three groups: BLA-lesioned (BLAX; n = 14), MeA-lesioned (MeAX; n = 13), and sham control (SHAM; n = 11). The general surgical procedures were the same as those described in Experiment 1 except for the location of the infusion sites and lesion parameters. For BLA lesions, three 6-min infusions were made in each hemisphere at the following coordinates: Site 1, AP −2.0, ML ±4.6, DV −6.9; Site 2, AP −2.64, ML ±4.8, DV −7.5; Site 3, AP −3.12, ML ±5.2, DV −7.4. For MeA lesions, two 8-min infusions per hemisphere were made at the following coordinates: Site 1, AP −2.04, ML ±3.1, DV −8.25; Site 2, AP −3.0, ML ±3.4, DV −8.5.
4.3 Apparatus
Both Experiment 1 and Experiment 2 were conducted in the home cages. Liquid stimuli were presented by attaching a cylinder with silicone stopper and steel drinking tube to the front of the cage. Fluid intake was measured to the nearest 0.5 ml.
4.4 Procedure
4.4.1 Experiment 1
Once the rats recovered from surgery (~10 days), they were placed on a water deprivation schedule that limited their intake to 15 min daily, until water consumption stabilized. On the next day, all rats received a taste neophobia trial consisting of 15 min exposure to 0.5% sodium saccharin (w/v) instead of water. This was repeated every third day with 15 min water access on each of the intervening days. The tests continued until rats consumed comparable amounts of saccharin for at least two consecutive trials. Following this criterion, there were four taste trials. The same procedure was repeated with 15 min exposures to an aqueous solution of 0.1% amyl acetate (v/v; Fisher Chemical; Fairlawn, NJ) for four odor trials, and, finally, for six trials with the oral trigeminal stimulus 0.01 mM capsaicin (Sigma, St. Louis, MO).
4.4.2 Experiment 2
The procedure was identical to that described in Experiment 1 except that there were five taste trials, three odor trials and three trigeminal trials as determine by asymptote intake for each type of stimulus.
4.5 Histology
After all behavioral testing, each rat was given a lethal injection of sodium pentobarbital and then perfused intracardially with physiological saline followed by 10% formalin. The brain was extracted and stored in 4% formalin at least two days and then in the 20% sucrose for another two days. Subsequently, the brain was blocked and sectioned coronally at 50 µm using a cryostat. Consecutive sections through the lesion site were mounted and stained for cell bodies with cresyl violet. Reconstructions of the lesions were made with the aid of a microscope (Zeiss Axioskop 40) connected to a computer with Q-capture software (Quantitative Imaging Corporation, Burnaby, British Columbia, Canada).
4.6 Statistics
For each stimulus type, the fluid intake in each experiment was analyzed with a two-way mixed design ANOVA with Lesion as the between-subject variable and Trial as the within-subject variable. Post hoc comparisons, if needed, were conducted by examining simple main effects. All analyses were conducted with the help of Statistica software (6.0; StatSoft, Tulsa, OK). The significant value was set at α = .05.
Acknowledgments
This research was supported by grants DC04341 and DC06456 from the National Institute of Deafness and Other Communication Disorders.
Abbreviations
- ANOVA
analysis of variance
- BLA
basolateral amygdala
- BLAX
basolateral amygdala-lesioned
- CS
conditioned stimulus
- CTA
conditioned taste aversion
- IC
insular cortex
- ICX
insular cortex-lesioned
- NMDA
N-methyl-D-aspartic acid
- MeA
medial amygdala
- MeAX
medial amygdala-lesioned
- SHAM
non-lesioned control
- US
unconditioned stimulus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
REFERENCES
- Aggleton JP, Petrides M, Iversen SD. Differential effects of amygdaloid lesions on conditioned taste aversion learning by rats. Physiol. Behav. 1984;27:397–400. doi: 10.1016/0031-9384(81)90322-x. [DOI] [PubMed] [Google Scholar]
- Barnett SA. Behaviour components in the feeding of wild and laboratory rats. Behavior. 1956;9:24–43. [Google Scholar]
- Barnett SA. Experiments on “neophobia” in wild and laboratory rats. Brit. J. Psychol. 1958;49:195–201. doi: 10.1111/j.2044-8295.1958.tb00657.x. [DOI] [PubMed] [Google Scholar]
- Bermúdez-Rattoni F, McGaugh JL. Insular cortex and amygdala lesions differentially affect acquisition on inhibitory avoidance and conditioned taste aversion. Brain Res. 1991;549:165–170. doi: 10.1016/0006-8993(91)90616-4. [DOI] [PubMed] [Google Scholar]
- Bernstein IL, Wilkins EE, Barot SK. Mapping conditioned taste aversion associations through patterns of c-Fos expression. In: Reilly S, Schachtman TR, editors. Conditioned taste aversion: Behavioral and neural processes. New York: Oxford University Press; 2009. pp. 328–340. [Google Scholar]
- Best MR, Domjan M, Haskins WL. Long-term retention of flavor familiarization: Effects of number and amount of prior exposures. Behav. Biol. 1978;23:95–99. doi: 10.1016/s0091-6773(78)91212-9. [DOI] [PubMed] [Google Scholar]
- Chen SWC, Shemyakin A, Wiedenmayer CP. The role of the amygdala and olfaction in unconditioned fear in developing rats. J. Neurosci. 2006;26:233–240. doi: 10.1523/JNEUROSCI.2890-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey DT. The determinants of exploration and neophobia. Neurosci. Biobehav. Rev. 1978;2:235–253. [Google Scholar]
- Domjan M. Attenuation and enhancement of neophobia for edible substances. In: Barker LM, Best MR, Domjan M, editors. Learning mechanisms in food selection. New York: Baylor University Press; 1977. pp. 151–179. [Google Scholar]
- Figueroa-Guzmán Y, Kuo J, Reilly S. NMDA receptor antagonist MK-801 infused into insular cortex prevents the attenuation of gustatory neophobia in rats. Brain Res. 2006;1114:183–186. doi: 10.1016/j.brainres.2006.07.036. [DOI] [PubMed] [Google Scholar]
- Figueroa-Guzmán Y, Reilly S. NMDA receptors in the basolateral amygdala and gustatory neophobia. Brain Res. 2008;1210:200–203. doi: 10.1016/j.brainres.2008.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald RE, Burton MJ. Neophobia and conditioned taste aversion deficits in the rats produced by undercutting temporal cortex. Physiol. Behav. 1983;30:203–206. doi: 10.1016/0031-9384(83)90006-9. [DOI] [PubMed] [Google Scholar]
- Garcia J, Ervin ER. Gustatory-visceral and telereceptor-cutaneous conditioning: Adaptation in internal and external milieus. Comm. Behav. Biol. 1968;1:389–415. [Google Scholar]
- Grigson PS, Reilly S, Shimura T, Norgren R. Ibotenic acid lesions of the parabrachial nucleus and conditioned taste aversion: further evidence for an associative deficit in rats. Behav. Neurosci. 1998;112:160–171. [PubMed] [Google Scholar]
- Gutiérrez R, Rodriguez-Ortiz CJ, De LaCruz V, Núnez-Jaramillo L, Bermúdez-Rattoni F. Cholinergic dependence of taste memory formation: Evidence of two distinct processes. Neurobiol. Learn. Mem. 2003;80:323–331. doi: 10.1016/s1074-7427(03)00066-2. [DOI] [PubMed] [Google Scholar]
- Gutiérrez R, Téllez LA, Bermúdez-Rattoni F. Blockade of cortical muscarinic but not NMDA receptors prevents a novel taste from becoming familiar. Eur. J. Neurosci. 2003;17:1156–1162. doi: 10.1046/j.1460-9568.2003.02608.x. [DOI] [PubMed] [Google Scholar]
- Hughes P, Dragunow M. Induction of immediate-early genes and the control of neurotransmitter-regulated gene expression within the nervous system. Pharmacol. Rev. 1995;47:133–178. [PubMed] [Google Scholar]
- Kesner RP, Berman RF, Tardif R. Place and taste aversion learning: Role of basal forebrain, parietal cortex, and amygdala. Brain Res. Bull. 1992;29:345–353. doi: 10.1016/0361-9230(92)90066-7. [DOI] [PubMed] [Google Scholar]
- Kiefer SW, Braun JJ. Absence of differential associative responses to novel and familiar taste stimuli in rats lacking gustatory neocortex. J. Comp Physiol. Psychol. 1977;91:498–507. doi: 10.1037/h0077347. [DOI] [PubMed] [Google Scholar]
- Koh MT, Wilkins EE, Bernstein IL. Novel tastes elevate c-fos expression in the central amygdala and insular cortex: Implication for taste aversion learning. Behav. Neurosci. 2003;117:1416–1422. doi: 10.1037/0735-7044.117.6.1416. [DOI] [PubMed] [Google Scholar]
- Kolakowska L, Larue-Achagiotis C, Le Magnen J. Comparative effects of lesions of the basolateral and lateral nuclei of the amygdala on neophobia and conditioned taste aversion in rats. Physiol. Behav. 1984;32:647–651. doi: 10.1016/0031-9384(84)90320-2. [DOI] [PubMed] [Google Scholar]
- Kosar E, Grill HJ, Norgren R. Gustatory cortex in the rat. I. Physiological properties and cytoarchitecture. Brain Res. 1986;379:329–341. doi: 10.1016/0006-8993(86)90787-0. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. Projections from the amygdaloid complex to the cerebral cortex and thalamus in the rat and cat. J. Comp. Neurol. 1977;172:687–722. doi: 10.1002/cne.901720408. [DOI] [PubMed] [Google Scholar]
- Li C-I, Maglinao TL, Takahashi LK. Medial amygdala modulation of predator odor-induced unconditioned fear in the rat. Behav. Neurosci. 2004;118:324–332. doi: 10.1037/0735-7044.118.2.324. [DOI] [PubMed] [Google Scholar]
- Lubow RE. Latent inhibition and conditioned attention theory. Cambridge: Cambridge University Press; 1989. [Google Scholar]
- Lubow RE. Conditioned taste aversion and latent inhibition: A review. In: Reilly S, Schachtman TR, editors. Conditioned taste aversion: Behavioral and neural processes. New York: Oxford University Press; 2009. pp. 37–57. [Google Scholar]
- McDonald AJ. Cortical pathways to the mammalian amygdala. Prog. Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- Miller RR, Holzman AD. Neophobia: Generality and function. Behav. Neural Biol. 1981;33:17–44. doi: 10.1016/s0163-1047(81)92202-0. [DOI] [PubMed] [Google Scholar]
- Nachman M, Ashe JH. Effects of basolateral amygdala lesions on neophobia, learned taste aversions, and sodium appetite in rats. J. Comp. Physiol Psychol. 1974;87:622–643. doi: 10.1037/h0036973. [DOI] [PubMed] [Google Scholar]
- Nishijo H, Uwano T, Tamura R, Ono T. Gustatory and Multimodal Neuronal Responses in the Amygdala During Licking and Discrimination of Sensory Stimuli in Awake Rats. J. Neurophysiol. 1999;79:21–36. doi: 10.1152/jn.1998.79.1.21. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5th ed. San Diego, CA: Academic Press; 2005. [Google Scholar]
- Petrulis A, Johnston RE. Behav. Neurosci. 1999;113:345–357. doi: 10.1037//0735-7044.113.2.345. [DOI] [PubMed] [Google Scholar]
- Reilly S. Central gustatory system lesions and conditioned taste aversion. In: Reilly S, Schachtman TR, editors. Conditioned taste aversion: Behavioral and neural processes. New York: Oxford University Press; 2009. pp. 309–327. [Google Scholar]
- Reilly S, Schachtman TR, editors. Conditioned taste aversion: Behavioral and neural processes. New York: Oxford University Press; [Google Scholar]
- Reilly S, Trifunovic R. Lateral parabrachial nucleus lesions in the rat: Neophobia and conditioned taste aversion. Brain Res. Bull. 2001;55:359–366. doi: 10.1016/s0361-9230(01)00517-2. [DOI] [PubMed] [Google Scholar]
- Roman C, Nebieridze N, Sastre A, Reilly S. Effects of lesions of the bed nucleus of the stria terminalis, lateral hypothalamus, or insular cortex on conditioned taste aversion and conditioned odor aversion. Behav. Neurosci. 2006;120:1257–1267. doi: 10.1037/0735-7044.120.6.1257. [DOI] [PubMed] [Google Scholar]
- Roman C, Lin J-Y, Reilly S. Manuscript under review. Conditioned taste aversion and latent inhibition following extensive taste preexposure in rats with insular cortex lesions. doi: 10.1016/j.brainres.2008.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman C, Reilly S. Effects of insular cortex lesions on conditioned taste aversion and latent inhibition in the rat. Eur. J. Neurosci. 2007;26:2627–2632. doi: 10.1111/j.1460-9568.2007.05872.x. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ESL, Lopez de Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Sakai N, Yamamoto T. Possible routes of visceral information in the rat brain in formation of conditioned taste aversion. Neurosci. Res. 1999;35:53–61. doi: 10.1016/s0168-0102(99)00067-x. [DOI] [PubMed] [Google Scholar]
- Sheng M, Greenberg ME. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990;4:477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- Shimai S, Hoshishima K. Effects of bilateral amygdala lesions on neophobia and conditioned taste aversion in mice. Percept. Motor Skills. 1982;54:127–130. doi: 10.2466/pms.1982.54.1.127. [DOI] [PubMed] [Google Scholar]
- Shipley MT, McLean JH, Ennis M. Olfactory system. In: Paxinos G, editor. The rat nervous system. 2nd ed. San Diego, CA: Academic Press; 1995. pp. 899–928. [Google Scholar]
- Slotnick BM, Westbrook F, Darling FMC. What the rat’s nose tells the rat’s mouth: Long delay aversion conditioning with aqueous odors and potentiation of taste by odors. Anim. Learn. Behav. 1997;25:357–369. [Google Scholar]
- StAndre J, Reilly S. Effects of central and basolateral amygdala lesions on conditioned taste aversion and latent inhibition. Behav. Neurosci. 2007;121:90–99. doi: 10.1037/0735-7044.121.1.90. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Hubbard DT, Lee I, Dar Y, Sipes SM. Predator odor-induced conditioned fear involves the basolateral and medial amygdala. Behav. Neurosci. 2007;121:100–110. doi: 10.1037/0735-7044.121.1.100. [DOI] [PubMed] [Google Scholar]
- Wilkins EE, Bernstein IL. Conditioning method determines patterns of c-fos expression following novel taste-illness pairings. Behav. Brain Res. 2006;169:93–97. doi: 10.1016/j.bbr.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Fujimoto Y, Shimura T, Sakai N. Conditioned taste aversion in the rat with excitotoxic brain lesions. Neurosci. Res. 1995;22:31–49. doi: 10.1016/0168-0102(95)00875-t. [DOI] [PubMed] [Google Scholar]