Abstract
Objective The purpose of this study was to clarify the nature of diurnal salivary cortisol dysregulation in youth who experience posttraumatic stress (PTS). Method Diurnal trends in salivary cortisol secretion were examined in a sample of 41 youth aged 10–16 years (26 youth exposed to interpersonal traumas and 15 control participants with no PTS) using hierarchical linear modeling. Results Cortisol levels were characterized by curvilinear trends in secretion (i.e., sharp declines from prebreakfast to prelunch followed by smaller decreases from prelunch to predinner with a leveling-off or slight increase from predinner to prebed assessment). Results further indicated that youth with PTS had sharper morning declines and relatively higher evening levels (i.e., a greater curve in the daily trend) than nontraumatized youth. Conclusions Findings help to elucidate the physiological basis for altered arousal patterns in youth with PTS. Traumatized youth showed wider daily fluctuations in cortisol levels when these trends were modeled in a curvilinear fashion. The findings help to describe the nature of stress dysregulation in trauma-exposed youth and may have implications for clarifying some of the apparent inconsistencies in the literature.
Keywords: cortisol, posttraumatic stress, hierarchical linear modeling
Activity in the hypothalamic–pituitary–adrenal (HPA) axis is an important part of the neurobiological response to stress and fear. Research aimed at understanding stress- and fear-related emotional problems, such as posttraumatic stress disorder (PTSD; APA, 1994), has often focused on cortisol secretion as a convenient index of HPA functioning. Fear reactions are associated with elevations in the secretion of cortisol, a corticosteroid hormone produced by the adrenal cortex that is easily assayed from blood, urine, or saliva samples (Gunnar, 2001 for a review). Cortisol is part of a system in which stressors activate the hypothalamus to release corticotropin-releasing hormone (CRH). CRH causes the pituitary gland to release adrenocorticotropic hormone (ACTH) into the bloodstream. ACTH then acts upon the adrenal glands resulting in the secretion of cortisol. Cortisol creates a negative feedback loop in which an excess of cortisol activates the brain's glucocorticoid receptors and suppresses the production of CRH. Dysregulation in this system has been associated with a number of emotional and behavioral disorders (Gunnar, 2001; Yehuda, 2006) and although cortisol secretion is an important part of the homeostatic response to stress, prolonged exposure to cortisol may be neurotoxic. Animal studies suggest cortisol-induced neurotoxicity in brain areas, such as the hippocampus (Sapolsky, 2000; Sapolsky, Uno, Rebert, & Finch, 1990). Youth who have experienced severe stress also are more likely to display smaller cerebral brain volumes than matched controls (De Bellis et al., 1999a) and research recently has shown that cortisol levels are associated with changes in the volume of the hippocampus over time in youth (Carrión, Weems, & Reiss, 2007).
Findings on the linkage between posttraumatic stress (PTS) and cortisol have been fairly consistent in showing dysregulation of cortisol; however, findings on the exact nature of the dysregulation have been inconsistent (De Bellis, 2001). Studies on adult samples have indicated, somewhat paradoxically considering the normative cortisol response, relatively low basal cortisol levels in Vietnam combat veterans, Holocaust survivors, and sexual abuse victims with PTSD, whereas other studies have reported increased levels of cortisol in adults with PTSD (Yehuda, 2006). A proposed mechanism for sensitization of the cortisol response is an increased number of glucocorticoid receptors in the HPA axis and, hence, facilitation of the negative feedback loop (Gunnar & Vazquez, 2001; Yehuda, 2006).
Research on the function of the HPA axis in youth who experience PTS has also shown some of the same inconsistencies (Cicchetti & Rogosch, 2001; De Bellis, 2001; Weems & Carrión, 2007). For example, research on youth exposed to traumatic stress and who have PTSD symptoms have suggested both relatively increased and decreased cortisol levels when compared to nontraumatized youth (De Bellis et al., 1999; Goenjian et al., 1996). Reviews of the literature on cortisol response in youth with PTS have theorized that there may be diurnal and developmental (both age-related and time-related) differences in the association (Gunnar & Vazquez, 2001; De Bellis, 2001). The time-related view postulates that PTS is associated with an initial increase in cortisol (Delahanty, Nugent, Christopher, & Walsh, 2005), but that eventually among those with PTSD, the HPA axis functioning may be altered to a condition of relatively lowered basal cortisol as time passes since the traumatic event (Weems & Carrión, 2007; Yehuda, 2006).
In addition to time-related differences, there are important diurnal trends in the secretion of cortisol (Carrión et al., 2002a; Gunnar & Vazquez, 2001) clarification of which may also help account for inconsistencies in the literature. This view suggests that stress dysregulation following trauma is not precisely tested through a comparison of mean differences between those with PTS and controls in their level of cortisol but by accurately modeling differences in the diurnal trends (e.g., sensitization of the HPA axis might show as wider deviations in the normal daily rhythm). Research on the normative pattern of diurnal cortisol excretion suggests a pattern of daily decreases in cortisol with morning levels higher relative to afternoon levels. Some evidence for differences in this daily rhythm have been reported using a comparison of mean differences at different points in the day (Bevans, Cerbone, & Overstreet, 2008; Carrión et al., 2002a). For example, Carrión et al. (2002a) compared youth with PTS to age- and gender-matched control youth and results indicated that the PTS group demonstrated a pattern of relatively elevated cortisol levels (assessed at prebreakfast, prelunch, predinner, and prebed) when compared to a control group. The differences were particularly evident in prebed time levels suggesting an earlier daily reascent in cortisol levels than nontrauma-exposed youth. Bevans et al. (2008) found that exposure to recent trauma coupled with a history of trauma earlier in life was related to relatively lower morning cortisol levels but higher afternoon cortisol levels. However, clarifying dysregulation in the daily rhythm may be best accomplished with novel analytic approaches for modeling trends. Hruschka, Kohrt, and Worthman (2005) have convincingly demonstrated the utility of multilevel analysis such as hierarchical linear modeling (HLM; Bryk & Raudenbush, 1987, 1992) in examining cortisol dysregulation within individuals and across groups.
The purpose of this study was to clarify the nature of salivary cortisol dysregulation in youth who experience PTS related to interpersonal traumas by examining daily trends in cortisol secretion. We predicted that cortisol levels would be characterized by curvilinear trends in secretion (i.e., sharp declines from prebreakfast to prelunch followed by smaller decreases from prelunch to predinner with a leveling-off or slight increase from predinner to prebed assessment). We further predicted that youth with exposure to trauma and PTSD symptoms would show differences in their daily trends in cortisol secretion. Based on our previous study (Carrión et al., 2002a) and the results of Bevans et al. (2008), we expected trauma-exposed youth to show sharper morning declines and relatively higher evening levels (i.e., a greater curve in the trend) than nontraumatized youth. The effects of age and gender on the trends were also examined for exploratory purposes. In our previous study (Carrión et al., 2002a), we found no effect of age and a modest effect of gender with girls demonstrating elevated cortisol levels relative to males among trauma-exposed youth.
Method
Sample characteristics for the trauma-exposed and non-exposed youth are presented in Table I. Additional methodological details of the assessment, measures, and procedures can be found in Carrión et al. (2002a); however, none of the sample participants in this study participated in our previous research. Briefly, in order to qualify for participation, youth were required to be between the ages of 10–16 years. Trauma-exposed individuals were recruited from social service agencies and mental health providers. These agencies and providers were asked to refer families of children with a history of interpersonal trauma and families called if they were interested. Thus, no data on participation rate is available; however, all families who contacted us and met inclusion criteria agreed to participate. Controls with no history of trauma and no psychiatric diagnosis were recruited through advertisements. Potential participants were excluded from the study if they were taking medications that could activate the central and autonomic nervous or HPA systems, if they reported a history of alcohol or drug dependence, mental retardation, gross obesity or growth failure, autism or schizophrenia, neurological disorder, fetal drug exposure, significant medical illness, or if they were currently experiencing trauma. The trauma group participants (hereafter referred to as the PTS group) were included in the study only if they reported a history of interpersonal trauma (i.e., physical abuse, witnessing violence, or sexual abuse) and reported a score of 10 or greater on the Clinician Administered PTSD Scale, Child and Adolescent Version (CAPS-CA; Nader et al., 1996). We sampled youth who were exposed to significant interpersonal trauma and who were experiencing PTSD symptoms; however, the trauma-exposed group did not have to meet full DSM-IV (APA, 1994) diagnostic criteria for inclusion. This was done to be consistent with our previous research (Carrión et al., 2002a) and because research suggests limitations to the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV; APA, 1994) criteria in youth (Carrión, Weems, Ray, & Reiss, 2002b).
Table I.
Descriptive Comparison of PTS and Controls
| Control (n = 15) | PTS (n = 26) | |
|---|---|---|
| Mean age (SD) | 13.1 (1.9) | 13.3 (2.2) |
| (range 10–16) | (range 10–16) | |
| Gender | ||
| Female (%) | 33.3 | 38.5 |
| Median Tanner stage | 4.0 | 4.0 |
| (range 2–5) | (range 1–5) | |
| Ethnicity (%) | ||
| White | 80.0 | 53.8 |
| African American | 0.0 | 3.8 |
| Hispanic | 0.0 | 30.7 |
| Pacific islander | 6.7 | 0.0 |
| Other | 13.3 | 11.5 |
| Median family income* | $100,000–$125,000 | $50,000–$75,000 |
*Significant difference (p <.05) between PTSD and control. Income ranges for both the PTS and control group was from “$10,000–$20,000” to “over $200,000”.
To assess PTS symptoms in the trauma-exposed group, we used the CAPS-CA (Nader et al., 1996). The CAPS-CA is a youth version of the adult instrument and is a semi-structured clinical interview for PTSD. Control youth completed the Child PTSD Symptom Scale (CPSS; Foa, Johnson, Feeny, & Treadwell, 2001) and their parents completed the Child Behavior Checklist (CBCL; Achenbach, 1991) to ensure that control participants were not exposed to trauma and relatively free of emotional problems (Control group CPSS mean score = 0.2, SD = 0.6). Participants’ pubertal development was determined by self-report. Participants selected from drawings with written descriptions representing the five Tanner Stages (Marshal & Tanner, 1970) of pubertal development. Salivary cortisol was obtained from the participant during home measurements (similar to procedures in Carrión et al., 2002a). It was collected four times a day (prebreakfast, prelunch, predinner, and prebed) over the course of 3 days producing 12 samples. Salivary samples were then refrigerated. Samples were returned within a week of collection on average. At that time the cotton swabs containing the specimen were centrifuged and saliva samples frozen in order to mail to the lab. Samples were processed using the Magic Cortisol radioimmunoassay kit produced by Ciba-corning (Giessen, Germany) as adapted for salivary cortisol analysis by the University of Minnesota Endocrine Laboratory. Inter- and intra-assay coefficients of variation were maintained at less than 12%. Cortisol is reported in μg/dl. Samples were averaged across days for increased reliability (Gunnar, 2001) in the main analyses. Stability across days was examined because while we predicted changes across time of day we expected relative stability across the 3 days in cortisol levels to justify aggregation across days in the HLM analyses. Individuals showed relative stability in their cortisol levels across the 3 days. Specifically, the correlation (Spearman) between day 1 and day 2 cortisol level was (ρ =.61, p <.001), between day 1 and day 3 (ρ =.55, p <.001), and day 2 and day 3 (ρ =.56, p <.001) (aggregating across time of day). Additional stability analyses are provided in the Results section.
All youth and their caretakers were presented with a written Institutional Review Board approved informed consent. Youth assent was also required for participation. A procedure was in place to report any suspected ongoing maltreatment; however, no cases were identified. Youth who participated in the project were currently in stable home environments and their guardians agreed to participate in the project. Hypotheses were tested via trend analyses of cortisol levels using HLM (Bryk & Raudenbush, 1987, 1992). In the first stage of the analysis, HLM was used to estimate the within-subject change (random effects, level 1) to test the appropriateness of quadratic (curvilinear) trends. The second stage HLM was used to compare PTS versus control participants in these trends as well as test age and gender (fixed effects, level 2) on the within-subject slopes for the daily cortisol levels across the four daily measurement points (prebreakfast, prelunch predinner, and prebedtime). The linear values were squared to create the quadratic curvilinear trend similar to previous research and as suggested by Bryk and Raudenbush (1992).
Results
The majority (62%) of the PTS group experienced multiple traumatic events including physical abuse (50%), witnessing interpersonal violence (58%), and sexual abuse (27%) with an average length of time since the CAPS-CA primary reported traumatic event of 4.86 (SD = 3.0; range 1–10) years; however, many of these youth (53%) experienced chronic traumatic stress. Within the PTSD group, the mean CAPS-CA total score was 31.4 (SD = 16.2). Comparison of the PTS and control samples on demographic variables and pubertal status is presented in Table I and indicated that the groups did not significantly differ on age, gender, ethnicity, or Tanner stage. However, the control participants had higher median family incomes. Given these findings, Spearman correlations were conducted examining the association between income and cortisol and indicated that cortisol levels were not significantly associated with income (largest ρ =.19, p =.27). Three day (aggregating across time of day) average measure interclass correlation coefficients (ICC) were calculated for the total sample (ICC =.79) and separately for the PTS (ICC =.83) and controls (ICC =.75) suggesting good stability in overall cortisol levels across days for both groups.
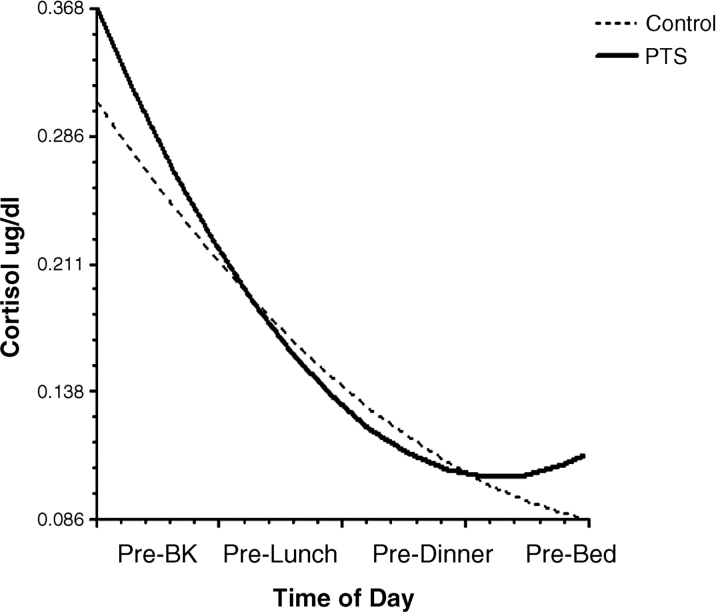
Level 1 HLM analyses tested linear and curvilinear trends in the individual growth curves using restricted maximum likelihood estimation. The results of the HLM analysis of the overall within-subject change curves indicated a significant linear [χ2 (41) = 148.06, p <.001] and quadratic component [χ2 (41) = 156.61, p <.001]. The significant curvilinear pattern is consistent with the expected sharp declines from prebreakfast to prelunch followed by smaller decreases from prelunch to predinner with a general leveling-off or slight increase from predinner to prebed assessment. The results of the level 2 HLM analyses on the effect of age, gender, and PTS (PTS versus control) on change curves are presented in Table II and depicted visually in Fig. 1. As shown in Table II, results indicated significant effects of PTS group status (p <.05) for the quadratic trend, indicating that there was a significant difference in the individual change curves in cortisol levels across the PTS and control youth. There were no significant effects of age or gender and there was not a significant difference in the y-intercept. As can be seen from Fig. 1 the trend shows a steeper initial decrease followed by a quicker return to increasing cortisol levels in the PTS group.
Table II.
HLM Estimation of the Influence of PTS Group, Age, and Gender on the Linear and Curvilinear with Robust Standard Errors
| Effect | Coefficient | Standard error | t-ratio | p-value |
|---|---|---|---|---|
| Intercept | ||||
| PTS vs.control | 0.16 | 0.10 | 1.68 | .101 |
| Sex | 0.16 | 0.11 | 1.42 | .165 |
| Age | 0.03 | 0.02 | 1.18 | .248 |
| Linear slope | ||||
| PTS vs. control | −0.13 | 0.06 | −2.13 | .033 |
| Sex | −0.07 | 0.07 | −0.94 | .348 |
| Age | −0.01 | 0.02 | −0.56 | .579 |
| Curvilinear slope | ||||
| PTS vs. control | 0.03 | 0.01 | 2.50 | .013 |
| Sex | 0.01 | 0.01 | 0.68 | .496 |
| Age | 0.00 | 0.00 | 0.41 | .683 |
Model random effects χ2(37) = 110.13, p < 0.001; PTS = Posttraumatic stress.
Figure 1.
Trends in cortisol levels across time of day.
Discussion
Findings from this study extend previous research by showing that patterns of diurnal salivary cortisol secretion were significantly different in youth with PTS related to interpersonal traumas as compared to nontraumatized control participants. As predicted, cortisol levels were characterized by curvilinear trends in secretion with sharp declines from prebreakfast to prelunch followed by smaller decreases from prelunch to predinner and a leveling-off from predinner to prebed assessment. However, when these trends were compared between the youth with PTS and controls, results suggest that youth with PTS had sharper morning declines and relatively higher predicted evening levels (PTS youth showed an increase from predinner to prebed) than nontraumatized youth. Results speak to the utility of trend fitting statistical analyses, such as HLM, in the study of variables that reflect biological rhythms such as cortisol levels because these analyses may better help to identify differences in the patterns of excretion.
Examining trends (versus mean levels) may help to integrate some of the apparent inconsistencies in the literature. For example, the trend analysis helps to show consistency between studies showing relatively decreased levels and studies showing relatively increased levels. For example, Bevans et al. (2008) found that exposure to trauma coupled with a history of trauma earlier in life was related to relatively lower morning cortisol levels but higher afternoon cortisol levels; whereas De Bellis et al. (1999b) found relatively elevated cortisol levels when averaged over 24 h. The trend analysis, as depicted in Fig. 1, does predict relatively lower levels at some points in the day but also relatively elevated cortisol levels among the PTS youth. Results are consistent with the idea that true diurnal changes (as opposed to levels) in cortisol excretion may better characterize the dysregulation of the HPA axis in PTS than a comparison of overall daily levels or levels at particular points in the day. This is because there are wide individual differences in normative cortisol levels in youth (e.g., what is elevated for one person may not be so for another and “optimal” levels of cortisol have not been established, see Gunnar, 2001 for further discussion) and so comparison of mean levels at any point of the day may continue to produce inconsistent results in terms of the PTS–cortisol link.
Explication of cortisol dysregulation in PTS may have clinical implications in terms of helping parents and children understand the altered arousal and emotional patterns they experience following a trauma. In particular, the findings help to elucidate the physiological basis of the emotional difficulties experienced by youth with PTSD. Drawing the past findings together with the present results, acute declines in cortisol levels from prebreakfast to prelunch may indicate a heightened response by glucocorticoid receptors to cortisol levels overtime. PTSD is defined by the somewhat paradoxical symptoms of, on one hand, hyperarousal and on the other, emotional numbing (APA, 1994). Clarifying the physiological changes that result from trauma may help explicate this complex picture of emotional dysregulation in posttraumatic stress reactions. In addition, examination of Fig. 1 points to the possible clinical relevance of cortisol levels to understanding sleep disturbance in youth with PTS. Specifically, extrapolating from after bed time to before waking, Fig. 1 suggests that relative elevations in cortisol levels for the PTS group may be occurring during the night. Anxiety disorder symptoms are often associated with disturbances in sleep (Alfano, Zakem, Costa, Taylor, & Weems, in press) and elevations in presleep and nighttime cortisol have been found among youth with anxiety disorders (Forbes et al., 2006).
Although this study may help to clarify the complex relation between PTS and cortisol response the study has several limitations. First, the sample size was relatively small and so findings of no differences (e.g., age and gender) should be interpreted with caution due to the possibility that power affected these particular findings. Conclusions about the progression of PTSD symptoms association with cortisol were not able to be studied in this sample since we did not have enough participants with recent traumatic events to evaluate time since the trauma associations. It will thus be important to further explore our findings using prospective designs with larger samples. However, research shows that after repeated measurement, PTSD symptoms tend to decline over time (Weems, Saltzman, Reiss, & Carrión, 2003) and so simple longitudinal designs may also be limited in showing time-related differences in the direction of the association between PTSD symptoms and cortisol levels. Cross-sequential designs may be useful in this regard (i.e., one that compares individuals with differences in their most recent trauma and follows them over time). In addition, while we matched PTS and control youth on Tanner stage we did not match female control and PTS participants on menstrual cycle. Menstrual cycle has been found to be related to cortisol levels (Abplanalp, Livingston, Rose, & Sandwisch, 1977) and so future studies may benefit from matching female participants on this variable as well. Finally, appropriate control groups are always a challenge in PTS studies; the current PTS sample has both a history of trauma and consequent PTS symptoms. Future studies might benefit from a comparison group with history of trauma and no PTSD symptoms in order to further clarify the PTS cortisol association. In this way, the relative contribution of trauma versus PTSD symptoms may be clarified.
Acknowledgment
This study was supported by NIMH grant MH63893 to Victor G. Carrión and by an Investing in Research Excellence grant awarded to Carl F. Weems.
Conflicts of interest: None declared.
References
- Abplanalp JM, Livingston L, Rose RM, Sandwisch D. Cortisol and growth hormone responses to psychological stress during the menstrual cycle. Psychosomatic Medicine. 1977;39:158–177. doi: 10.1097/00006842-197705000-00002. [DOI] [PubMed] [Google Scholar]
- Achenbach TM. Child behavior check-list. Burlington, VT: University of Vermont Department of Psychiatry; 1991. [Google Scholar]
- Alfano CA, Zakem A, Costa NM, Taylor LK, Weems CF. Depression and Anxiety. Sleep problems and their relation to cognitive factors, anxiety and depressive symptoms in children and adolescents. (in press) [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. Washington, DC: Author; 1994. [Google Scholar]
- Bevans K, Cerbone BA, Overstreet S. Relations between recurrent trauma exposure and recent life stress and salivary cortisol among children. Development and Psychopathology. 2008;20:257–272. doi: 10.1017/S0954579408000126. [DOI] [PubMed] [Google Scholar]
- Bryk AS, Raudenbush SW. Application of hierarchical linear models to assessing change. Psychological Bulletin. 1987;101:147–158. [Google Scholar]
- Bryk A, Raudenbush SW. Hierarchical linear models for social and behavioral research: Applications and data analysis methods. Newbury Park, CA: Sage; 1992. [Google Scholar]
- Carrión VG, Weems CF, Ray R, Glasser B, Hessl D, Reiss A. Diurnal salivary cortisol in pediatric posttraumatic stress disorder. Biological Psychiatry. 2002a;51:575–582. doi: 10.1016/s0006-3223(01)01310-5. [DOI] [PubMed] [Google Scholar]
- Carrión VG, Weems CF, Ray R, Reiss AL. Towards an empirical definition of pediatric PTSD: The phenomenology of PTSD symptoms in youth. Journal of the American Academy of Child and Adolescent Psychiatry. 2002b;41:166–173. doi: 10.1097/00004583-200202000-00010. [DOI] [PubMed] [Google Scholar]
- Carrión VG, Weems CF, Reiss AL. Stress predicts brain changes in children: A pilot longitudinal study on youth stress, PTSD, and the hippocampus. Pediatrics. 2007;119:509–516. doi: 10.1542/peds.2006-2028. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Diverse patterns of neuroendocrine activity in maltreated children. Development and Psychopathology. 2001;13:677–693. doi: 10.1017/s0954579401003145. [DOI] [PubMed] [Google Scholar]
- De Bellis MD. Developmental traumatology: The psychobiological development of maltreated children and its implications for research, treatment, and policy. Development and Psychopathology. 2001;13:539–564. doi: 10.1017/s0954579401003078. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Clark DB, Casey BJ, Giedd JN, Boring AM, et al. Developmental traumatology part II: Brain development. Biological Psychiatry. 1999a;45:1259–1270. doi: 10.1016/s0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Baum AS, Birmaher B, Keshavan MS, Eccard CH, Boring AM, et al. Developmental traumatology: I. Biological Stress Systems. Biological Psychiatry. 1999b;45:1259–1270. doi: 10.1016/s0006-3223(99)00044-x. [DOI] [PubMed] [Google Scholar]
- Delahanty DL, Nugent NR, Christopher NC, Walsh M. Initial urinary epinephrine and cortisol levels predict acute PTSD symptoms in child trauma victims. Psychoneuroendocrinology. 2005;30:121–128. doi: 10.1016/j.psyneuen.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Foa EB, Johnson KM, Feeny NC, Treadwell KRH. The Child PTSD Symptom Scale: A preliminary examination of its psychometric properties. Journal of Clinical Child Psychology. 2001;30:376–384. doi: 10.1207/S15374424JCCP3003_9. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Williamson DE, Ryan ND, Birmaher B, Axelson DA, Dahl RE. Peri-sleep-onset cortisol levels in children and adolescents with affective disorders. Biological Psychiatry. 2006;59:24–30. doi: 10.1016/j.biopsych.2005.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goenjian AK, Yehuda R, Pynoos RS, Steinberg AM, Tashjian M, Yang RK, et al. Basal cortisol, dexamethasone suppression of cortisol, and MHPG in adolescents after the 1988 earthquake in Armenia. American Journal of Psychiatry. 1996;153:929–934. doi: 10.1176/ajp.153.7.929. [DOI] [PubMed] [Google Scholar]
- Gunnar MR. The role of glucocorticoids in anxiety disorders: A critical analysis. In: Vasey MW, Dadds MR, editors. The developmental psychopathology of anxiety. New York, NY: Oxford University Press; 2001. pp. 143–159. [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of the expected daytime rhythm: Potential indices of risk in human development. Development and Psychopathology. 2001;13:516–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Hruschka DJ, Kohrt BA, Worthman CM. Estimating between-and within-individual variation in cortisol levels using multilevel models. Psychoneuroendocrinology. 2005;30:698–714. doi: 10.1016/j.psyneuen.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Marshal WA, Tanner JM. Variations in the pattern of pubertal changes in girls. Archives of Diseases in Childhood. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader KO, Kriegler JA, Blake DD, Pynoos RS, Newman E, Weather FW. Clinician administered PTSD Scale, child and adolescent version. White River Junction, VT: National Center for PTSD; 1996. [Google Scholar]
- Sapolsky R, Uno H, Rebert CS, Finch CE. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. Journal of Neuroscience. 1990;10:2897–2902. doi: 10.1523/JNEUROSCI.10-09-02897.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Archives of General Psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- Weems CF, Carrión VG. The associat between PTSD symptoms and salivary cortisol in youth: The role of the time since the trauma. Journal of Traumatic Stress. 2007;20:903–907. doi: 10.1002/jts.20251. [DOI] [PubMed] [Google Scholar]
- Weems CF, Saltzman K, Reiss AL, Carrión VG. A prospective test of the association between emotional numbing and hyperarousal in youth with a history of traumatic stress. Journal of Clinical Child and Adolescent Psychology. 2003;32:166–171. doi: 10.1207/S15374424JCCP3201_15. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Advances in understanding neuroendocrine alterations in PTSD and their therapeutic implications. Annals of the New York Academy of Sciences. 2006;1071:137–166. doi: 10.1196/annals.1364.012. [DOI] [PubMed] [Google Scholar]