Abstract
Background: Heat shock protein 70 (Hsp70) regulates protein biosynthesis and refolding of denatured proteins. Since Hsp70 participates in recovery from stress injury, we examined the effect of Hsp70 genetic deletion in the azoxymethane (AOM)/dextran sulfate sodium (DSS) model of inflammation and colon cancer. Methods: Hsp70 mutant mice (Hsp70.1−/−/70.3−/−) and wild-type (WT) littermates received AOM and three cycles of DSS and were killed 24 weeks later. Tumors were graded for histology and immunostained for p53, adenomatous polyposis coli, β-catenin, cyclooxygenase-2 (Cox-2) and inducible nitric oxide synthase (iNOS) and sequenced for p53 mutations. Results: Elevated adenomas developed in 4/10 WT mice with no dysplasia in adjacent mucosa. In contrast, 7/8 Hsp70 knock out (KO) mice developed chronic mucosal inflammation and multifocal areas of flat dysplasia and 4/8 progressed to invasive carcinomas arising in a background of flat dysplastic mucosa. These differences in the incidence of flat dysplasia and invasive cancers were significant (P < 0.05). Nuclear p53 was stronger in Hsp70 KO tumors compared with WT tumors, and sequencing confirmed p53 mutations in 2/5 tumors from Hsp70−/− versus 0/5 in WT mice. In Hsp70 WT tumors, β-catenin was predominantly nuclear, compared with membranous β-catenin in Hsp70−/− tumors, suggesting that Hsp70 regulates β-catenin in colonic tumorigenesis. Cox-2 and iNOS levels were increased in tumors from Hsp70−/− mice compared with Hsp70 WT tumors. Conclusions: Hsp70-deleted mice treated with AOM/DSS develop flat invasive colonic tumors that mimic many histological and molecular features of ulcerative colitis colon cancer. This model will be useful to dissect the role of Hsp70 in inflammatory bowel disease colon cancer.
Introduction
The colonic epithelium provides a critical barrier to protect the host from resident commensal and pathogenic microbes. Multiple homeostatic mechanisms maintain the integrity of this tissue including continuous cellular regeneration to replace apoptotic cells, epithelial tight junctions and supporting innate and adaptive immune systems. Derangements in these systems can lead to barrier defects resulting in inflammation. If inflammation is not contained and the barrier is not repaired, sepsis and even death can result. Heat shock proteins participate in barrier function to protect against cell damage induced by a variety of stresses including chemical, physical and infectious injuries (1). These proteins are molecular chaperones that participate by protein–protein interactions in synthesis, folding, translocation, macromolecular assembly and degradation of other proteins. Through adenosine triphosphate-dependent mechanisms, heat shock proteins refold damaged proteins or target irreversibly denatured proteins for degradation. These chaperones are also critical components of many signal transduction pathways (2).
Heat shock protein 70 (Hsp70) is an inducible member of this family of proteins. In addition to protecting cellular proteins from stress-induced damage, Hsp70 has been implicated in regulating diverse signaling pathways involving nuclear factor-kappa B, Src, Akt and Raf (2). We have shown that Hsp70 protects epithelial cells against Clostridium difficile toxin-induced injury as well as oxidant or thermal stress (3,4). We also showed that glutamine is required for the heat shock response, which appeared to mediate Hsp70 protection against oxidant-induced stress (5). More recently, we demonstrated that Hsp70 was downregulated in actively inflamed mucosa from individuals with ulcerative colitis (UC) and Crohn’s disease (6). Proinflammatory cytokines tumor necrosis factor-α and interferon-γ, which are increased in inflamed bowel mucosa, also downregulated Hsp70 in vitro and in vivo by inhibiting Hsp70 protein translation (6). Taken together, these results suggest that inflammation-related signals occurring in inflammatory bowel disease (IBD) might exacerbate colitis at least in part by downregulating Hsp70 and thereby interfering with its vital role in maintaining intestinal homeostasis.
Colon cancer, a dreaded complication of chronic IBD, differs from typical polypoid sporadic colon cancers in that they frequently arise in a background of dysplasia and are often multifocal, flat and deeply invasive. A growing body of evidence supports an important role for inflammation in the pathogenesis of IBD cancer (7). In this regard, the extent and degree of inflammation are increasingly recognized as important risk factors for colonic malignant transformation in IBD (8–10). The molecular features of IBD cancers also differ in a number of important respects from sporadic colon cancers, including frequency of adenomatous polyposis coli (apc) mutations, timing of p53 mutations and levels of inflammatory mediators (11). Since Hsp70 exerts cytoprotective effects following stress but is downregulated by inflammation in IBD, we asked what would be the consequence of Hsp70 loss in inflammation-associated colon cancer (12).
Several animal models have been described to investigate colitis-associated colon cancer. The combination of azoxymethane (AOM), a colon-specific carcinogen, and repeated cycles of non-mutagenic dextran sulfate sodium (DSS) strongly promotes colitis-associated colon cancer (13). To directly test the role of Hsp70 in colitis-associated colon cancer, we have used a genetic approach to examine AOM/DSS-induced tumorigenesis in mice deleted of this chaperone. In prior studies with these mice, other investigators have demonstrated that Hsp70 protects against ischemia reperfusion and radiation-induced injuries (14,15). In this study, we found that DSS induced a more severe injury and eventually chronic self-sustaining colitis in Hsp70-deleted mice, whereas the injury rapidly healed in their wild-type (WT) counterparts. We then showed that AOM/DSS induces histological features of human IBD-associated colon cancer in mice lacking Hsp70. To further characterize this model, we also investigated several proto-oncogenes and inflammatory mediators. We identified a number of molecular changes in AOM/DSS-induced malignant transformation in Hsp70-deleted mice that mimic important features of human UC-associated colon cancer.
Materials and methods
Materials
AOM was purchased from Midwest Research Institute (Kansas City, Missouri). DSS molecular weight = 36 000–50 000 g/mol was purchased from MP Biomedicals, LLC (Solon, OH). Mice heterozygous for Hsp70 deletion (Hsp70.1+/− and 70.3+/− mice), on a mixed 129S/C57Bl6/J background, were a generous gift from Dr David Dix (National Institutes of Health). Mice were interbred and WT and double-mutant Hsp70−/− littermates used for experiments. APC (Cat# SC-896) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal antibodies to inducible nitric oxide synthase (iNOS) (Cat#610329) were obtained from BD PharMingen (San Jose, CA). Rabbit polyclonal p53 antibodies (Cat# NCL-p53-CM5p) were obtained from Novocastra (Bannockburn, IL) (UK). Rabbit anti-Cox-2 antibodies (Cat#160106) were purchased from Cayman Chemicals (Ann Arbor, MI). Hsp70 antibodies (SPA810) and Hsc70 antibodies (SPA-815F) were obtained from Stressgen (Vancouver, Canada). Electrophoretic grade acrylamide, bisacrylamide, Tris, sodium dodecyl sulfate and prestained molecular weight markers were from Bio-Rad Laboratories (Richmond, CA). Kodak (Rochester, NY) supplied the X-OMAT AR film. Polyvinylidene difluoride membranes (Immobilon-P) were purchased from Millipore (Bedford, MA). All other reagents were of the highest quality available and were obtained from Sigma–Aldrich Corp. (St Louis, MO), unless otherwise noted.
Methods
DSS protocol.
Animals were housed in the Carlson Animal Care Facility at 22–25°C and fed standard Purina rodent chow. All procedures were done under approved animal care and use protocols that followed National Institutes of Health guidelines for humane animal care. Mice were genotyped as described (15). WT and Hsp70-deleted mice (n = 11 per group) initially weighing 19–23 g were provided 2.5% DSS in the drinking water for 5 days followed by 3 weeks of regular water. This DSS cycle was repeated a total of four times. Animals were weighed daily and stool consistency and bleeding monitored during and after DSS administration until weight loss, bleeding and diarrhea resolved and then twice weekly thereafter. Clinical severity of colitis was scored as described with disease activity index calculated from the combined scores of weight loss, stool consistency and bleeding (16). Animals were killed 180 days after initial DSS treatment to assess colitis and proinflammatory cytokine profiles.
Proteomic cytokine arrays.
Colonic mucosa from WT or Hsp70-deleted mice treated with four cycles of DSS and killed after 180 days were scrape isolated and proteins quantified by bicinchoninic acid assay (Bio-Rad Laboratories). Lysates (250 μg protein) were incubated with arrayed antibody supports for 2 h. Arrays were then incubated with biotinylated secondary antibodies for 2 h followed by incubation with horseradish peroxidase-conjugated streptavidin for 2 h. Antigen–antibody complexes were detected by chemiluminescence and captured by xerography.
AOM/DSS protocol.
To examine the role of Hsp70 in colonic carcinogenesis, we used a recently described modification of AOM tumor induction that involved addition of repeated cycles of DSS (13). AOM is a mutagen that induces G to A transitions and when used alone induces polypoid tumors in rodents with clinical, histological and molecular features that mimic human sporadic colon cancer (17). When DSS is given after AOM administration, tumorigenesis is strongly promoted (13). WT and Hsp70-deleted mice that were 6 weeks of age were given AOM (7.5 mg/kg body wt, intraperitoneally). One week later, mice received 2.5% DSS in the drinking water for 5 days followed by 3–4 weeks of water without DSS. Three cycles of DSS were administered and colitis was scored as described (16). Mice were killed 24 weeks after AOM treatment.
Tissue harvest.
Colons were harvested and lengths measured. Colons were then divided longitudinally and grossly visible tumors isolated. Colons were fixed flat in 10% buffered formalin for 24 h and paraffin embedded as Swiss rolls. Tumors were fixed in formalin. Sections were stained with hematoxylin and eosin and histological features, including inflammation, dysplasia and neoplasia, were evaluated by a gastrointestinal pathologist (J.H.) blinded to mouse genotype. Inflammation was assessed by the cellular infiltration of polymorphonuclear and mononuclear leukocytes and scored as mild, moderate or severe. Dysplasia and neoplasia were graded using standard published criteria (18,19).
Western blotting.
Colonic and intestinal mucosa were isolated by scraping segments with a glass slide and homogenized with a Polytron (power setting 4 × 30 s) in 1.5 ml non-denaturing lysis buffer containing 25 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.5, 125 mM NaCl, 1% octylphenyl-polyethylene glycol, 10 mM MgCl2, 1 mM ethylenediaminetetraacetic acid and 2% glycerol. Proteins were extracted in sodium dodecyl sulfate-containing Laemmli buffer and measured by Bio-Rad protein assay. Proteins were subjected to quantitative western blotting analysis as described (20). Briefly, proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis on 4–20% resolving polyacrylamide gradient gels and electroblotted to polyvinylidene difluoride membranes. Blots were incubated overnight at room temperature with specific primary antibodies followed by 1 h incubation with appropriate peroxidase-coupled secondary antibodies and subsequent detection on X-OMAT film by enhanced chemiluminescence.
Immunohistochemistry.
Immunostaining was performed on 4 μm thick paraffin-embedded colonic sections. Slides were deparaffinized and rehydrated in decreasing concentrations of ethanol. Antigen retrieval for Hsp70, APC, β-catenin, cyclooxygenase-2 (Cox-2) and iNOS were performed by boiling slides in 10 mM sodium citrate (pH 6.0). For p53, antigen retrieval was performed using a steam pressure cooker (Cell Marque). Endogenous peroxidase activity was quenched by incubating slides for 5 min with Peroxidase Block (Dako, Glostrup, Denmark). Slides were incubated at 4°C overnight with primary antibodies at the following dilutions: APC at 1:500, p53 at 1:500, Cox-2 at 1:200 and iNOS at 1:100. Slides were then incubated with DAKO secondary antibodies for 45 min. Antibodies were detected by 5–30 s incubation with 3,3′-diaminobenzidine substrate-chromogen. Slides were counterstained with Mayer’s hematoxylin. For each of these antibodies, negative controls included isotype-matched monoclonal antibodies or omission of primary antibodies and showed no staining.
Laser capture microdissection.
Formalin-fixed paraffin-embedded 6 μ sections were mounted on Leica PEN-membrane slides (Leica Microsystems, Bannockburn, IL). Slides were deparaffinized with xylene for 5 min and rehydrated in a graded series of 100, 95 and 70% alcohol washes for 15 s. Slides were briefly stained in Mayer’s hematoxylin for 5 s and rinsed in a graded series of alcohol washes for 15 s followed by xylene for 5 min and air dried for laser microdissection. Control colonic epithelial cells from untreated WT and Hsp70-deleted mice and malignant colonocytes from AOM/DSS-treated mice were dissected using the Leica AS LMD instrument (Leica).
p53 sequencing.
DNA was extracted from microdissected tissue using QIAamp Micro Kit (Qiagen, Valencia, CA). Exons 5 and 6 of p53 were amplified by polymerase chain reaction (PCR) using Ex Taq with high-fidelity proofreading activity (TaKaRa Bio, Madison WI). The forward primer was 5′-ACACCT-GATCGTTACTCGGCTTGT-3′ and the reverse primer was 5′-AAATTACAGA-CCTGGTGGCTCA-3′ that yielded a predicted 442 bp product. The primer sequences were based on the published murine p53 complementary DNA sequence (GenBank accession no. X01237). PCR products were gel purified using the Qiaex II Agarose Gel Extraction kit (Qiagen), re-extracted and dissolved in 50 μl of double-distilled H2O. Ex Taq polymerase possesses a non-template-dependent terminal transferase activity that added 3′-deoxyadenosine to the PCR products that were subcloned into a TOPO TA vector (Invitrogen, Carlsbad, CA). Sequencing was performed on 10 clones from each PCR product using the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Carlsbad, CA) and an Applied Biosystems 3730 XL Sequencer. The p53 chromatographs were compared with GenBank sequences using the Sequencher software program (Gene Codes, Ann Arbor MI). Mutations were confirmed by sequencing the opposite strand and by examining three to five independent clones from separate PCRs. Only base changes consistently present in the sequencing analyses were accepted as p53 mutations.
Statistics.
Values were expressed as means ± SDs. Clinical colitis was scored using a previously validated disease activity index that included weight loss, diarrhea and bleeding as described (16). Differences in incidence in tumors or dysplasia were calculated using Fisher’s exact test, with differences having a P < 0.05 considered statistically significant. Nuclear immunostaining for p53 and β-catenin was graded on a semiquantitative scale of 0–3+: 0 (<5% positive staining), 1+ (5–10% positive cells), 2+ (11–30% positive cells) and 3+ (>30% positive cells). Cox-2 was both cytoplasmic and nuclear and intensity was scored on 0–3+ scale. APC and iNOS were cytoplasmic and scored on 0–3+ scale. In addition, the cell type (stromal versus epithelial cell) and subcellular localization (membranous, cytoplasmic and nuclear) were described.
Results
Loss of Hsp70 leads to persistent colitis following repeated cycles of DSS
Hsp70 (Hsp70.1 and Hsp70.3)-deleted mice were phenotypically normal, although slightly smaller than their WT littermates. Histologies of the small intestine and colon of Hsp70−/− mice were normal. We confirmed that these mice had no detectable Hsp70 expression by northern analysis (data not shown), as well as western blotting and immunostaining (Figure 1). It should be noted that Hsp70 is normally absent in the small bowel, but induced in the colon by immune and microbial signals (Figure 1). Prior studies demonstrated that stressed Hsp70-deleted mice suffered greater tissue injury and genomic instability, suggesting that colonic stress might uncover a critical role for Hsp70 (14,15). To examine this question, we treated Hsp70 WT and Hsp70 knock out (KO) mice with four cycles of DSS and examined changes in histology and proinflammatory cytokines. With each cycle, mice developed clinical colitis as manifested by weight loss, loose stools and bleeding (supplementary Figure 1S is available at Carcinogenesis Online). WT mice appeared to have recovered from colitis shortly after the fourth cycle of DSS as assessed by weight gain and resolution of diarrhea and bleeding. In contrast, Hsp70-deleted mice developed refractory colitis that persisted for up to the time of killing, as manifested by failure to gain weight, persistent diarrhea and bleeding. The mean disease activity index for this group was 3.7 ± 0.7, compared with 0 for the WT mice. DSS-induced colitis in Hsp70 KO mice was characterized by a mixed infiltrate of neutrophils and mononuclear cells and marked crypt architectural destruction and regeneration with crypt shortening and branching, similar to human IBD (supplementary Figure 1S is available at Carcinogenesis Online). Compared with DSS-treated WT mice, several proinflammatory cytokines were also increased in the colonic mucosa of DSS-treated Hsp70 KO mice, including interleukin (IL)-1α, IL-6, vascular cell adhesion molecule (VCAM) and macrophage-inflammatory protein-2 (supplementary Figure 2S is available at Carcinogenesis Online). A detailed analysis of chronic DSS-induced inflammation in Hsp70-deleted mice will be described in a separate report (J.Tao, in preparation).
Fig. 1.
Hsp70 expression. (A) WT colon. (B) Hsp70−/− colon. (C) Western blotting Hsp70 in colon and small intestine. Hsp70 is detected in WT colon, but absent in colonic epithelium of Hsp70−/− mouse. Constitutive Hsc70 is shown as a loading control.
Hsp70 determines the morphological features of AOM/DSS-induced colonic malignant transformation
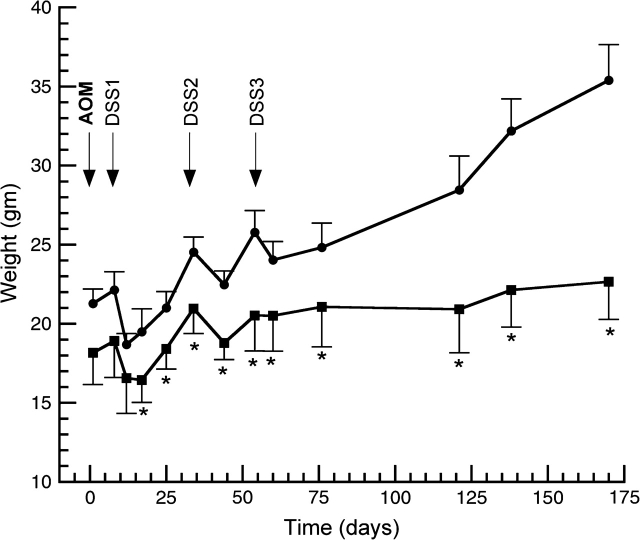
Since Hsp70-deleted mice developed persistent inflammation and increased inflammatory cytokines following multiple cycles of DSS, we asked whether these mice might be more susceptible to tumorigenesis. To address this question, we treated WT and Hsp70-deleted mice with a single dose of AOM, followed by three cycles of DSS. As shown in Figure 2, WT mice recovered clinically and gained weight within 1–2 weeks following each cycle of DSS. In WT mice, inflammation had resolved and ulcerations have healed by the time of killing. In contrast, Hsp70-deleted mice showed delayed weight gain and persistent clinical colitis with diarrhea and occult or gross colonic bleeding. At the termination of the experiment, Hsp70-deleted mice treated with AOM/DSS weighed 22.7 ± 1.8 g, compared with 30.2 ± 1.3 g for age-matched control Hsp70-deleted mice. At necropsy, Hsp70-deleted mice had persistent mucosal inflammation and ulcerations in the left colon. As shown in Table I, colons of AOM/DSS-treated Hsp70-deleted mice were significantly shorter than colons of Hsp70-deleted control mice, whereas colon lengths for Hsp70 WT mice did not differ between the AOM/DSS-treated and control (saline treated) group. AOM/DSS-treated Hsp70-deleted mice also exhibited microscopic signs of chronic colitis, characterized by crypt architectural distortion and lamina propria inflammation similar to changes observed in human UC.
Fig. 2.
Effects of Hsp70 genotype and AOM/DSS treatment on weight gain. WT and Hsp70-deleted mice were treated with AOM (7.5 mg/kg body wt) on day 1 and 1 week later given 2.5% DSS × 5 days in the drinking water. This cycle was repeated twice. Beginning of DSS cycles are indicated with arrows. Animals were killed 170 days after AOM. Shown are weights (mean ± SD) in grams. *P < 0.05 compared with WT mice.
Table I.
Effect of genotype and AOM/DSS on colon length
| Hsp70 genotype | Treatment | Mean ± SD (cm) |
| WT | Saline | 10.2 ± 1.2 |
| WT | AOM/DSS | 10.1 ± 0.6 |
| Hsp70 KO | Saline | 9.9 ± 0.5 |
| Hsp70 KO | AOM/DSS | 8.6 ± 0.4a |
Colon length was measured from distal cecum to rectum. n = 5 animals in each group.
P = 0.04, compared with control (saline treated) Hsp70 KO mice (Student’s t-test). Colon lengths of saline- and AOM/DSS-treated Hsp70 WT mice were not different.
The incidence of dysplastic lesions and tumors are summarized in Table II. Elevated colonic adenomas developed in 4/10 (40%) WT mice within 6 months that were similar in appearance to sporadic human colonic adenomatous polyps (Figure 3A and B). Aside from these polyps, there were no dysplastic foci in the mucosa of WT mice, including none in the background mucosa from which the polyps arose. In contrast and as shown in Figure 3C and D, AOM/DSS induced multiple areas of flat dysplastic mucosa in 7/8 (87.5%) Hsp70-deleted mice. In four of these mice (50%), dysplasia progressed to flat invasive carcinomas that arose in a background of dysplasia (Figure 3E and F). The incidence of flat dysplasia (P < 0.005) and invasive carcinomas (P < 0.05) were significantly greater in Hsp70−/− mice compared with WT mice (Table II). These features of malignant transformation in Hsp70-deleted mice mimicked the pattern of progression from flat dysplasia to invasive cancers in human UC-associated cancer (21).
Table II.
Hsp70 determines growth characteristics of AOM/DSS-induced colonic tumors
| Genotype | Mice | Polypoid adenoma | Flat dysplasiaa |
Cancersb | |
| Lesions/mouse | Incidence | ||||
| WT | 10 | 4 | 0 | 0 | 0 |
| Hsp70−/− | 8 | 0c | 2.8 ± 0.7c | 0.875c | 4c |
Flat dysplasia, mean number of lesions (mean ± SD) and incidence.
Flat intramucosal or invasive adenocarcinomas.
P < 0.05 compared with Hsp70 WT mice.
Fig. 3.
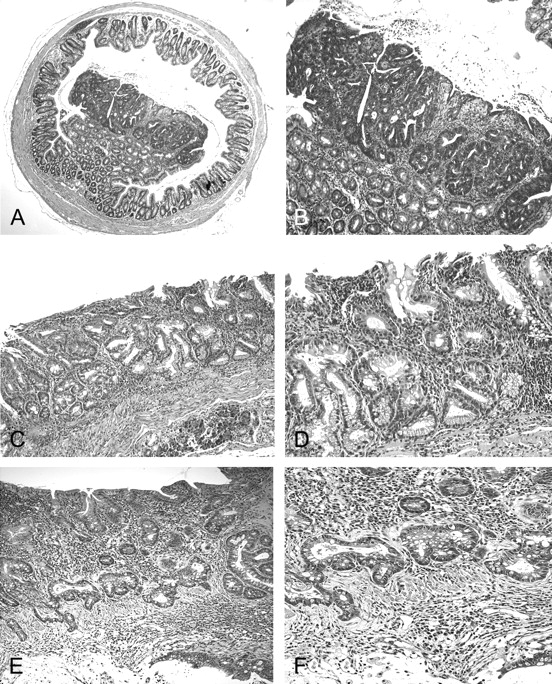
AOM/DSS induces polypoid lesions in WT mice and flat dysplasia and invasive cancers in Hsp70 KO mice. (A) Polypoid adenoma in WT animal, ×40. Note that tumor was elevated and limited to the epithelium. (B) Polypoid tumor, ×100. (C) Flat dysplastic mucosa from Hsp70−/− mouse, ×100. (D) Flat dysplastic mucosa from Hsp70−/− mouse, ×200. (E) Invasive adenocarcinoma from Hsp70−/− mouse, ×100. (F) Flat invasive adenocarcinoma from Hsp70−/− mouse, ×200.
Molecular features of AOM/DSS-induced tumors in WT and Hsp70−/− mice
To begin to assess molecular abnormalities in this model of persistent inflammation and invasive cancers, we immunostained tumors for several tumor proto-oncogenes and inflammatory mediators. Tumors in Hsp70 KO mice expressed nuclear p53 at levels of 2–3+ compared with 0–1+ for tumors in WT mice (Figure 4). The increased expression of p53 suggested that this tumor suppressor might be mutated, as occurs frequently in UC colon cancer (22–27). We screened mouse exons 5–6 that were most commonly mutated in another form of murine colitis (28). We identified three p53 mutations in two of five tumors from Hsp70−/− mice, but none in tumors from animals expressing WT Hsp70 (Table III). These p53 mutations were A to G transitions or T to C transversions in codon 159. Tumors with mutant p53 showed 3+ nuclear p53 staining. It should be noted that tumors in WT mice were adenomas, whereas those in the Hsp70-deleted mice were invasive carcinomas. Thus, differences in tumor stage could have contributed to differences in p53 mutations.
Fig. 4.

Expression levels of nuclear p53 are higher in tumors from Hsp70 KO mice. AOM/DSS-induced tumors from WT and Hsp70 KO mice were stained for p53. (A) Tumor from WT mouse, ×200. (B) Tumor from WT mouse, ×400. (C) Tumor from Hsp70 KO mouse, ×200. (D) Tumor from Hsp70 KO mouse, ×400. Note the prominent nuclear staining in tumor from Hsp70 KO mouse. Shown are representative tumors from three mice in each group.
Table III.
p53 mutations in tumors from Hsp70-deleted mice
| Tumors | Exon | Nucleotide position | Codon | Nucleotide | Predicted amino acid |
| KO4a | 5 | +8114 | 159 | atc → gtc | Iso → Thr |
| KO4b | 5 | +8114 | 159 | atc → gtc | Iso → Thr |
| KO5 | 5 | +8115 | 159 | atc → acc | Iso → Val |
No mutations were identified in 5/5 tumors from WT mice, whereas three p53 mutations were detected in two of five tumors assayed from Hsp70 KO mice.
The majority of sporadic human colon cancers possess a truncating non-sense mutation in the apc gene. The encoded mutant protein does not react with antibodies directed against the C-terminus of WT APC (29). We immunostained AOM/DSS-induced tumors from WT and Hsp70-deleted mice with antibodies directed to the C-terminus of Apc (Figure 5). As a control, we also stained intestinal Min adenomas that have mutant Apc. As shown in Figure 5A, Min adenomas failed to stain with this antibody, whereas adjacent intestine was positive. Colonic tumors in Hsp70 WT animals showed loss of APC staining compared with adjacent colonic crypts, similar to loss of APC staining in Min small intestinal adenomas (Figure 5D). In contrast, colonic tumors in Hsp70-deleted mice showed persistent cytoplasmic APC staining, consistent with WT APC (Figure 5G). It should be noted that the majority of UC colon cancers express WT APC (30). As expected, Min adenomas showed 3+ nuclear β-catenin staining (Figure 5C). In tumors from WT mice, nuclear β-catenin was 2–3+ (Figure 5F), whereas β-catenin was predominantly membranous (0–1+ nuclear) in tumors from Hsp70-deleted mice (Figure 5I). Hence, changes in β-catenin were inversely related to APC expression, consistent with the role of APC as a negative regulator of β-catenin. Thus, with respect to p53, APC and β-catenin, tumors from Hsp70-deleted mice more closely mimicked human UC tumors than those arising in Hsp70 WT animals. As noted for the p53 results, it is important to emphasize, however, that these comparisons in APC and β-catenin expression involved adenomas from Hsp70 WT mice and invasive carcinomas in Hsp70-deleted mice.
Fig. 5.

Effects of Hsp70 on APC and β-catenin in AOM/DSS-induced tumors. Min adenomas (A–C) and AOM/DSS-induced tumors from WT (D–F) and Hsp70−/− mice (G–I) were stained for APC (A, D and G) and β-catenin (B, C, E, F, H and I). (A) APC in Min adenoma. Note that loss of APC in Min adenoma compared with adjacent villi. (B) β-Catenin staining in Min adenoma, ×100. (C) β-Catenin staining in Min adenoma, ×400. Note the prominent nuclear staining. (D) APC in Hsp70 WT AOM/DSS tumor. Note that loss of APC compared with adjacent crypts. (E) β-Catenin staining in Hsp70 WT tumor, ×200. (F) β-Catenin staining in Hsp70 WT tumor, ×400. (G) APC staining in Hsp70−/− tumor. Note the persistent Apc expression in Hsp70−/− tumor. (H) β-Catenin staining in Hsp70−/− tumor, ×200. (I) β-Catenin staining in Hsp70−/− tumor, ×400. Note that nuclear β-catenin in Min adenoma and Hsp70 WT tumor compared with membranous staining in Hsp70−/− tumor. Shown are representative tumors from three to four mice in each group.
Inflammation is an important risk factor for the development of colon cancer in UC (8–10). Cox-2 and iNOS are two important proinflammatory mediators that are increased in UC and associated dysplasia (31,32). We, therefore, examined AOM/DSS tumors for Cox-2 and iNOS expression levels to assess their potential roles in this model of inflammation-associated colon cancer. As shown in Figure 6, 2+ to 3+ levels of nuclear and cytoplasmic Cox-2 and cytoplasmic iNOS were found in tumors from Hsp70-deleted mice, compared with 0–1+ levels in tumors from Hsp70 WT mice. Cox-2 was expressed in both epithelial and stromal cells, whereas iNOS was predominantly detected in epithelial cells.
Fig. 6.
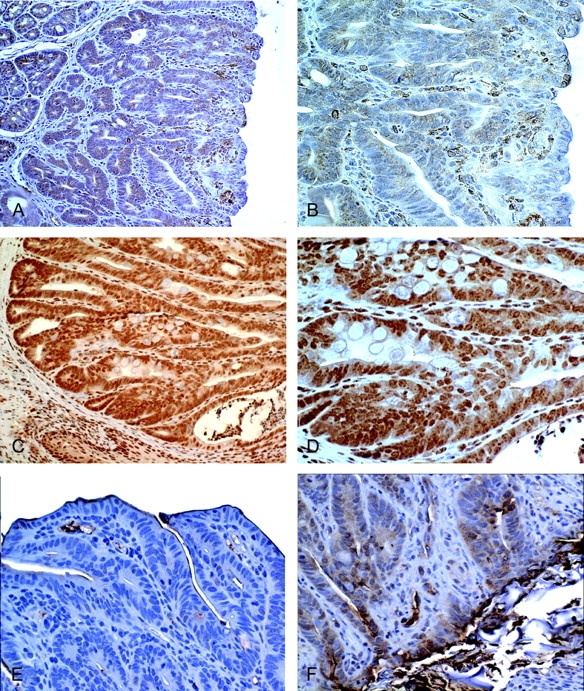
Expression levels of Cox-2 and iNOS are increased in tumors from Hsp70 KO mice. AOM/DSS-induced tumors from WT and Hsp70−/− mice were stained for Cox-2 and iNOS. (A) Cox-2 in tumor from WT mouse, ×200. (B) Cox-2 in tumor from WT mouse, ×400. (C) Cox-2 in tumor from Hsp70−/− mouse, ×200. (D) Cox-2 in tumor from Hsp70−/− mouse, ×400. (E) iNOS in tumor from WT mouse ×400. (F) iNOS in tumor from Hsp70−/− mouse, ×400. Note that increased epithelial and stromal Cox-2 and epithelial iNOS staining in tumors from Hsp70−/− mice compared with Hsp70 WT mice. Shown are representative tumors from three mice in each group.
Discussion
In prior studies, we demonstrated that Hsp70 has important functions in maintaining resistance to oxidant stresses or bacterial toxins (3–5). More recently, we showed that Hsp70 is downregulated in areas of active inflammation in IBD (6). The Hsp70-deleted mouse model has provided a powerful genetic tool to investigate the role of this chaperone in colonic function. Prior studies using these mice demonstrated that Hsp70 is not required for normal colonic homeostasis in the unchallenged host (14,15). Cellular injuries induced by ischemia and reperfusion or radiation, however, were exacerbated with loss of Hsp70 (14,15). In the current report, we first demonstrated that multiple cycles of DSS induced persistent colitis in Hsp70-deleted mice. The colitis was manifested clinically with failure to gain weight and continued bleeding and diarrhea. At killing, there were persistent histological abnormalities and increased proinflammatory cytokines in these mutant mice. Thus, Hsp70 regulates healing in repetitive DSS-induced injury. These findings were in agreement with complementary studies by Tanaka et al. (33), who demonstrated that upregulation of Hsp70 in transgenic mice inhibited DSS colitis, in contrast to increased colitis in Hsp70-deleted mice. Tanaka et al. also found in the DSS model that upregulated Hsp70 inhibited increases in several proinflammatory cytokines, including IL-6 and VCAM-1, consistent with our studies. Several of these cytokines are elevated in human IBD and causally implicated in DSS colitis or AOM/DSS tumorigenesis (34–37).
Since inflammation is a major risk factor for UC-associated colon cancer, these studies suggested that Hsp70 downregulation might contribute to neoplastic transformation. We, therefore, examined the effect of genetic deletion of Hsp70 on inflammation-associated colon cancer. We directly demonstrated that loss of Hsp70 dramatically alters the natural history of AOM/DSS injury and colonic malignant transformation. In the absence of Hsp70, AOM/DSS-treated mice developed multiple flat dysplastic colonic lesions and invasive cancers with histological and molecular features mimicking UC colon cancers. These results, together with our earlier findings that Hsp70 is downregulated in IBD, suggest that loss of Hsp70 might be causally linked to cancer in these inflammatory diseases.
Mouse strains vary in inflammatory response to DSS and in tumor induction by AOM/DSS (38,39). These differences in genetic background might account for some of the differences in our study and those reported in the literature. While we observed flat dysplastic lesions only in Hsp70−/− mice, others have noted the presence of flat dysplasia and invasive cancers in several WT mouse strains that presumably expressed Hsp70. Okayasu et al. (40) described flat and elevated dysplasia and invasive cancers in AOM/DSS-treated CBA/J mice. Suzuki et al. (41) reported that ICR mice developed multiple dysplastic foci and adenocarcinomas following one cycle of AOM/DSS. Tanaka et al. (42) also found frequent dysplastic lesions and 100% incidence of adenocarcinomas in this strain. In contrast, Cooper et al. (43) reported that two-thirds of cancers arose in colons without dysplasia and that dysplasia was flat in only one-third of DSS-treated Swiss-Webster mice. He also noted that inflammation was significantly greater in mice with flat compared with polypoid dysplastic lesions. Cooper’s results suggested that the duration and severity of mucosal inflammation might determine whether animals developed elevated tumors or flat dysplastic lesions. Our data are consistent with this concept. Hsp70 WT littermates in our study did not develop flat dysplastic lesions or invasive cancers, perhaps because inflammation was less intense or resolved more rapidly. Littermates lacking Hsp70, however, developed chronic colitis and an increased incidence of dysplasia and cancer. Thus, our studies demonstrate that Hsp70 plays a key role in determining the neoplastic potential of AOM/DSS injury. Our results are also consistent with studies showing that Hsp70 overexpression inhibits inflammation in the DSS model (33). Taken together, these studies emphasize the importance of genetic background and Hsp70 genotype in determining the natural history of AOM/DSS-induced colonic malignant transformation.
UC-associated tumors differ in pathogenesis, molecular features and natural history from sporadic colorectal cancers (44). Sporadic tumors generally originate from dysplastic crypts arising in a field of non-dysplastic mucosa that progress to visible adenomas and eventually carcinomas. In contrast, IBD-associated carcinomas frequently develop within flat areas of dysplastic mucosa and invade deeply often without polypoid features, similar to our findings in Hsp70-deleted mice. In sporadic tumorigenesis, transition to dysplasia often occurs with mutations in the apc. Subsequently other mutations occur that can include K-ras, p53 and DCC genes (45). Inactivating mutations and loss of WT apc occur in nearly 70% of sporadic cases, whereas apc mutations are present in fewer than 10% of UC colon cancers and occur later in disease progression (30). In our AOM/DSS model, loss of APC staining in tumors from WT animals suggested possible Apc mutations, whereas APC staining was preserved in Hsp70−/− tumors consistent with WT Apc. It should be noted that AOM can induce Apc mutations in rats and that β-catenin mutations have been demonstrated in mice treated with AOM or dimethylhydrazine/DSS (46–48). In future studies, it will be of interest to assess the mutation status of β-catenin in this model. Consistent with APC staining differences, nuclear β-catenin was greater in polypoid tumors from WT mice compared with flat tumors in Hsp70-deleted mice. Cooper et al. (43) also reported that β-catenin was frequently increased in polypoid but not flat dysplastic lesions. In addition to activating mutations, WT β-catenin can be activated by signals such as prostaglandins (49). Further study will be required to determine whether Hsp70 directly or indirectly modulates β-catenin mutations or signaling in the AOM/DSS model. Since inflammation was greater in mice with flat dysplastic lesions in Cooper’s report and in our studies, we speculate that the intensity of inflammation might control β-catenin localization and tumor morphogenesis. Consistent with this hypothesis, sporadic colon cancers have little inflammation and exhibit polypoid features, whereas inflammation-associated UC cancers frequently arise in flat dysplastic areas.
While p53 mutations are relatively late events in sporadic colon cancer, p53 loss of heterozygosity or mutations occur early in UC tumorigenesis even in non-dysplastic mucosa. These mutations might be related to increased inflammation-induced oxidant damage (23,26). In this regard, we identified mutations in tumors arising in an inflammatory background from Hsp70-deleted mice, but none in non-inflamed polypoid adenomas from mice with WT Hsp70. Nuclear p53 staining was also higher in tumors mutant for p53, compared with tumors from mice sufficient for Hsp70. In Hsp70−/− tumors without identified mutations, it is possible that increased nuclear p53 staining reflected mutations residing in p53 exons that were not sequenced or inflammation-induced upregulation of WT p53. In prior studies in p53-null mice, repeated DSS cycles increased the incidence of flat dysplasia and cancer, whereas mice with WT p53 developed polypoid lesions (50). Thus, loss of p53 function might also contribute to development of flat dysplasia in AOM/DSS-induced tumorigenesis in Hsp70−/− mice, a feature mimicking UC colonic carcinogenesis.
Hsp70 might regulate colonocyte growth independent of its effects on inflammation since the proliferating crypt cell compartment is expanded in Hsp70-deleted mice (L.Lichtenstein, unpublished observations). We believe, however, that inflammatory signals (e.g. increased proinflammatory cytokines and elevated Cox-2 and iNOS) are proximate promoters of IBD cancer and that Hsp70 plays a tumor suppressor role by limiting inflammation. Cox-2 and iNOS are upregulated in UC and AOM/DSS-induced tumors (31,42,51). These enzymes generate reactive oxygen and nitrogen species that are believed to contribute to tumor hyperproliferation, apoptotic resistance and angiogenesis (52,53). With regard to inflammation, Hsp70 is expressed in both epithelial cells and immune cells. Given its cytoprotective effects in epithelial cells and anti-inflammatory effects in immune cells, we speculate that Hsp70 loss in colonocytes and immune cells increases mucosal injury and exacerbates inflammation. Dissecting the epithelial and immune cell roles of Hsp70 in this colitis-associated colon cancer model will require further study. In prior AOM/DSS studies, cell-specific abrogation of nuclear factor-kappa B signals uncovered distinct epithelial cell and stromal cell contributions of nuclear factor-kappa B to colon cancer development (54).
A number of animal models have been employed to study UC, including mice with homozygous deletions of IL-10, IL-2 and β(2)-microglobulin or transforming growth factor-β1 (28,55,56). Compared with these models, there are several important advantages of the AOM/DSS model of colon cancer in Hsp70-deleted mice. Other colitis models exhibit either a rapidly fatal course or relatively low disease incidence and variable penetrance. Often in the latter case, there are long time intervals prior to disease onset and the disease occurs in patchy distributions. In contrast, AOM/DSS-treated Hsp70-deleted mice demonstrated nearly complete disease penetrance, with rapid onset and relatively homogenous features in the left colon that mimicked many histological and molecular features of UC, including development of flat invasive colon cancers. Based on observations in this study, we suggest that loss or downregulation of Hsp70 during chronic inflammation is permissive for IBD-associated colon cancer. Loss of Hsp70 permitted a more robust inflammatory response and increased epithelial cell injury that might increase the mutation load. It will be of interest to assess whether genetic factors that regulate human Hsp70 gene expression contribute to genetic susceptibility of IBD. Recent studies have identified a polymorphism in Hsp70 that was associated with more severe disease (57). Further studies will be required to assess whether this or other polymorphisms regulate Hsp70 expression or function. Our results suggest, moreover, that therapeutic strategies to restore Hsp70 might inhibit intestinal inflammation and reduce the risk of IBD-associated colon cancer (58,59).
Supplementary material
Supplementary Figures 1S and 2S can be found at http://carcin.oxfordjournals.org/
Funding
CCFA Research Training Award (SH); CCFA Senior Research Award (M.B.), (R37 DK47722 to E.B.C.); Digestive Diseases Research Core Center (P30DK42086 to E.B.C.); Samuel Freedman Research Laboratories for Gastrointestinal Cancer Research; Department of Pathology Research Core Facilities of the University of Chicago
Acknowledgments
We gratefully acknowledge the assistance of Dr Masha Kocherginsky with statistical analyses.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- AOM
azoxymethane
- Apc
adenomatous polyposis coli
- Cox-2
cyclooxygenase-2
- DSS
dextran sulfate sodium
- Hsp70
heat shock protein 70
- IBD
inflammatory bowel disease
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- PCR
polymerase chain reaction
- UC
ulcerative colitis
- WT
wild-type
References
- 1.Westerheide SD, et al. Heat shock response modulators as therapeutic tools for diseases of protein conformation. J. Biol. Chem. 2005;280:33097–33100. doi: 10.1074/jbc.R500010200. [DOI] [PubMed] [Google Scholar]
- 2.Pratt WB, et al. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp. Biol. Med. (Maywood) 2003;228:111–133. doi: 10.1177/153537020322800201. [DOI] [PubMed] [Google Scholar]
- 3.Liu TS, et al. Protective role of HSP72 against Clostridium difficile toxin A-induced intestinal epithelial cell dysfunction. Am. J. Physiol. Cell Physiol. 2003;284:C1073–C1082. doi: 10.1152/ajpcell.00134.2002. [DOI] [PubMed] [Google Scholar]
- 4.Musch MW, et al. Induction of heat shock protein 70 protects intestinal epithelial IEC-18 cells from oxidant and thermal injury. Am. J. Physiol. 1996;270:C429–C436. doi: 10.1152/ajpcell.1996.270.2.C429. [DOI] [PubMed] [Google Scholar]
- 5.Wischmeyer PE, et al. Glutamine protects intestinal epithelial cells: role of inducible HSP70. Am. J. Physiol. 1997;272:G879–G884. doi: 10.1152/ajpgi.1997.272.4.G879. [DOI] [PubMed] [Google Scholar]
- 6.Hu S, et al. Translational inhibition of colonic epithelial heat shock proteins by IFN-gamma and TNF-alpha in intestinal inflammation. Gastroenterology. 2007;133:1893–1904. doi: 10.1053/j.gastro.2007.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coussens LM, et al. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rutter M, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126:451–459. doi: 10.1053/j.gastro.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Gupta RB, et al. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology. 2007;133:1099–1105. doi: 10.1053/j.gastro.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Itzkowitz SH, et al. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;287:G7–G17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 11.Itzkowitz SH. Molecular biology of dysplasia and cancer in inflammatory bowel disease. Gastroenterol. Clin. North Am. 2006;35:553–571. doi: 10.1016/j.gtc.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Mosser DD, et al. Molecular chaperones and the stress of oncogenesis. Oncogene. 2004;23:2907–2918. doi: 10.1038/sj.onc.1207529. [DOI] [PubMed] [Google Scholar]
- 13.Okayasu I, et al. Promotion of colorectal neoplasia in experimental murine ulcerative colitis. Gut. 1996;39:87–92. doi: 10.1136/gut.39.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hampton CR, et al. HSP70.1 and -70.3 are required for late-phase protection induced by ischemic preconditioning of mouse hearts. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H866–H874. doi: 10.1152/ajpheart.00596.2002. [DOI] [PubMed] [Google Scholar]
- 15.Hunt CR, et al. Genomic instability and enhanced radiosensitivity in Hsp70.1- and Hsp70.3-deficient mice. Mol. Cell. Biol. 2004;24:899–911. doi: 10.1128/MCB.24.2.899-911.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper HS, et al. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab. Invest. 1993;69:238–249. [PubMed] [Google Scholar]
- 17.Takahashi M, et al. Gene mutations and altered gene expression in azoxymethane-induced colon carcinogenesis in rodents. Cancer Sci. 2004;95:475–480. doi: 10.1111/j.1349-7006.2004.tb03235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riddell RH, et al. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum. Pathol. 1983;14:931–968. doi: 10.1016/s0046-8177(83)80175-0. [DOI] [PubMed] [Google Scholar]
- 19.Boivin GP, et al. Pathology of mouse models of intestinal cancer: consensus report and recommendations. Gastroenterology. 2003;124:762–777. doi: 10.1053/gast.2003.50094. [DOI] [PubMed] [Google Scholar]
- 20.Bissonnette M, et al. Mutational and nonmutational activation of p21ras in rat colonic azoxymethane-induced tumors: effects on mitogen-activated protein kinase, cyclooxygenase-2, and cyclin D1. Cancer Res. 2000;60:4602–4609. [PubMed] [Google Scholar]
- 21.Ullman T, et al. Progression of flat low-grade dysplasia to advanced neoplasia in patients with ulcerative colitis. Gastroenterology. 2003;125:1311–1319. doi: 10.1016/j.gastro.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 22.Bruwer M, et al. Immunohistochemical expression of P53 and oncogenes in ulcerative colitis-associated colorectal carcinoma. World J. Surg. 2002;26:390–396. doi: 10.1007/s00268-001-0237-7. [DOI] [PubMed] [Google Scholar]
- 23.Hussain SP, et al. Increased p53 mutation load in noncancerous colon tissue from ulcerative colitis: a cancer-prone chronic inflammatory disease. Cancer Res. 2000;60:3333–3337. [PubMed] [Google Scholar]
- 24.Wong NA, et al. Immunohistochemical assessment of Ki67 and p53 expression assists the diagnosis and grading of ulcerative colitis-related dysplasia. Histopathology. 2000;37:108–114. doi: 10.1046/j.1365-2559.2000.00934.x. [DOI] [PubMed] [Google Scholar]
- 25.Chaubert P, et al. K-ras mutations and p53 alterations in neoplastic and nonneoplastic lesions associated with longstanding ulcerative colitis. Am. J. Pathol. 1994;144:767–775. [PMC free article] [PubMed] [Google Scholar]
- 26.Yin J, et al. p53 point mutations in dysplastic and cancerous ulcerative colitis lesions. Gastroenterology. 1993;104:1633–1639. doi: 10.1016/0016-5085(93)90639-t. [DOI] [PubMed] [Google Scholar]
- 27.Brentnall TA, et al. Mutations in the p53 gene: an early marker of neoplastic progression in ulcerative colitis. Gastroenterology. 1994;107:369–378. doi: 10.1016/0016-5085(94)90161-9. [DOI] [PubMed] [Google Scholar]
- 28.Sohn KJ, et al. Molecular genetics of ulcerative colitis-associated colon cancer in the interleukin 2- and beta(2)-microglobulin-deficient mouse. Cancer Res. 2001;61:6912–6917. [PubMed] [Google Scholar]
- 29.Oshima H, et al. Morphological and molecular processes of polyp formation in Apc(delta716) knockout mice. Cancer Res. 1997;57:1644–1649. [PubMed] [Google Scholar]
- 30.Tarmin L, et al. Adenomatous polyposis coli gene mutations in ulcerative colitis-associated dysplasias and cancers versus sporadic colon neoplasms. Cancer Res. 1995;55:2035–2038. [PubMed] [Google Scholar]
- 31.Agoff SN, et al. The role of cyclooxygenase 2 in ulcerative colitis-associated neoplasia. Am. J. Pathol. 2000;157:737–745. doi: 10.1016/S0002-9440(10)64587-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kimura H, et al. Increased expression of an inducible isoform of nitric oxide synthase and the formation of peroxynitrite in colonic mucosa of patients with active ulcerative colitis. Gut. 1998;42:180–187. doi: 10.1136/gut.42.2.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanaka K, et al. Genetic evidence for a protective role for heat shock factor 1 and heat shock protein 70 against colitis. J. Biol. Chem. 2007;282:23240–23252. doi: 10.1074/jbc.M704081200. [DOI] [PubMed] [Google Scholar]
- 34.Reinecker HC, et al. Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn's disease. Clin. Exp. Immunol. 1993;94:174–181. doi: 10.1111/j.1365-2249.1993.tb05997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Becker C, et al. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity. 2004;21:491–501. doi: 10.1016/j.immuni.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 36.Soriano A, et al. VCAM-1, but not ICAM-1 or MAdCAM-1, immunoblockade ameliorates DSS-induced colitis in mice. Lab. Invest. 2000;80:1541–1551. doi: 10.1038/labinvest.3780164. [DOI] [PubMed] [Google Scholar]
- 37.Siegmund B, et al. IL-1 beta -converting enzyme (caspase-1) in intestinal inflammation. Proc. Natl Acad. Sci. USA. 2001;98:13249–13254. doi: 10.1073/pnas.231473998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mahler M, et al. Differential susceptibility of inbred mouse strains to dextran sulfate sodium-induced colitis. Am. J. Physiol. 1998;274:G544–G551. doi: 10.1152/ajpgi.1998.274.3.G544. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki R, et al. Strain differences in the susceptibility to azoxymethane and dextran sodium sulfate-induced colon carcinogenesis in mice. Carcinogenesis. 2006;27:162–169. doi: 10.1093/carcin/bgi205. [DOI] [PubMed] [Google Scholar]
- 40.Okayasu I, et al. Dysplasia and carcinoma development in a repeated dextran sulfate sodium-induced colitis model. J. Gastroenterol. Hepatol. 2002;17:1078–1083. doi: 10.1046/j.1440-1746.2002.02853.x. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki R, et al. Sequential observations on the occurrence of preneoplastic and neoplastic lesions in mouse colon treated with azoxymethane and dextran sodium sulfate. Cancer Sci. 2004;95:721–727. doi: 10.1111/j.1349-7006.2004.tb03252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanaka T, et al. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 2003;94:965–973. doi: 10.1111/j.1349-7006.2003.tb01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cooper HS, et al. Dysplasia and cancer in the dextran sulfate sodium mouse colitis model. Relevance to colitis-associated neoplasia in the human: a study of histopathology, B-catenin and p53 expression and the role of inflammation. Carcinogenesis. 2000;21:757–768. doi: 10.1093/carcin/21.4.757. [DOI] [PubMed] [Google Scholar]
- 44.Eaden JA, et al. Colorectal cancer complicating ulcerative colitis: a review. Am. J. Gastroenterol. 2000;95:2710–2719. doi: 10.1111/j.1572-0241.2000.02297.x. [DOI] [PubMed] [Google Scholar]
- 45.Vogelstein B, et al. The multistep nature of cancer. Trends Gen. 1993;9:138–141. doi: 10.1016/0168-9525(93)90209-z. [DOI] [PubMed] [Google Scholar]
- 46.Femia AP, et al. Frequent mutation of Apc gene in rat colon tumors and mucin-depleted foci, preneoplastic lesions in experimental colon carcinogenesis. Cancer Res. 2007;67:445–449. doi: 10.1158/0008-5472.CAN-06-3861. [DOI] [PubMed] [Google Scholar]
- 47.Takahashi M, et al. Frequent mutations of the beta-catenin gene in mouse colon tumors induced by azoxymethane. Carcinogenesis. 2000;21:1117–1120. [PubMed] [Google Scholar]
- 48.Kohno H, et al. Beta-catenin mutations in a mouse model of inflammation-related colon carcinogenesis induced by 1,2-dimethylhydrazine and dextran sodium sulfate. Cancer Sci. 2005;96:69–76. doi: 10.1111/j.1349-7006.2005.00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang D, et al. Prostaglandin E(2) promotes colorectal adenoma growth via transactivation of the nuclear peroxisome proliferator-activated receptor delta. Cancer Cell. 2004;6:285–295. doi: 10.1016/j.ccr.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 50.Chang WC, et al. Loss of p53 enhances the induction of colitis-associated neoplasia by dextran sulfate sodium. Carcinogenesis. 2007;28:2375–2381. doi: 10.1093/carcin/bgm134. [DOI] [PubMed] [Google Scholar]
- 51.Rachmilewitz D, et al. Enhanced colonic nitric oxide generation and nitric oxide synthase activity in ulcerative colitis and Crohn's disease. Gut. 1995;36:718–723. doi: 10.1136/gut.36.5.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fosslien E. Molecular pathology of cyclooxygenase-2 in neoplasia. Ann. Clin. Lab. Sci. 2000;30:3–21. [PubMed] [Google Scholar]
- 53.Jenkins DC, et al. Roles of nitric oxide in tumor growth. Proc. Natl Acad. Sci. USA. 1995;92:4392–4396. doi: 10.1073/pnas.92.10.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Greten FR, et al. Ikkbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 55.Kuhn R, et al. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 56.Shull MM, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nam SY, et al. Heat shock protein gene 70-2 polymorphism is differentially associated with the clinical phenotypes of ulcerative colitis and Crohn's disease. J. Gastroenterol. Hepatol. 2007;22:1032–1038. doi: 10.1111/j.1440-1746.2007.04927.x. [DOI] [PubMed] [Google Scholar]
- 58.Hirakawa T, et al. Geranylgeranylacetone induces heat shock proteins in cultured guinea pig gastric mucosal cells and rat gastric mucosa. Gastroenterology. 1996;111:345–357. doi: 10.1053/gast.1996.v111.pm8690199. [DOI] [PubMed] [Google Scholar]
- 59.Ohkawara T, et al. Geranylgeranylacetone protects mice from dextran sulfate sodium-induced colitis. Scand. J. Gastroenterol. 2005;40:1049–1057. doi: 10.1080/00365520510023161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.