Abstract
Overexpression of cyclooxygenase 2 (COX2) and uncontrolled wingless and Int (Wnt)-signaling pathway have long been suggested to play crucial roles in colorectal cancer. Studies show that selective COX2 inhibitors possess great potential as chemopreventive agents for colon cancer. Recent studies suggest that targeting COX2 and epidermal growth factor receptor (EGFR) may provide better therapeutic strategy than inhibiting either single target and that this may alleviate the problem of COX2 inhibitor-associated side effects. Therefore, there have been intensive efforts to develop novel dietary substances that target COX2 and EGFR activation. Fisetin is a naturally occurring flavonoid commonly found in various vegetables and fruits. We found that the treatment of COX2-overexpressing HT29 human colon cancer cells with fisetin (30–120 μM) resulted in induction of apoptosis, downregulation of COX2 protein expression without affecting COX1 and inhibited the secretion of prostaglandin E2. Treatment of cells with fisetin also inhibited Wnt-signaling activity through downregulation of β-catenin and T cell factor 4 and decreased the expression of target genes such as cyclin D1 and matrix metalloproteinase 7. Fisetin treatment of cells also inhibited the activation of EGFR and nuclear factor-kappa B (NF-κB). Finally, the formation of colonies in soft agar was suppressed by fisetin treatment. Taken together, we provide evidence that the plant flavonoid fisetin can induce apoptosis and suppress the growth of colon cancer cells by inhibition of COX2- and Wnt/EGFR/NF-κB-signaling pathways. We suggest that fisetin could be a useful agent for prevention and treatment of colon cancer.
Introduction
Colorectal cancer is one of the common malignancies and represents the second leading cause of cancer deaths in the USA (1). According to World Health Organization, nearly one million people are diagnosed with colorectal cancer each year worldwide. Wingless and Int (Wnt)-signaling pathway plays a critical role during embryo development, and interestingly, its deregulation leads to carcinogenesis. Since its initial discovery in 1982, the research for the role of Wnt in cancer has been intensively investigated (2). In colon cancer, >90% of the tumor arises from activating mutations in the Wnt pathway (3). It is well known that β-catenin is a key effector in Wnt-signaling pathway since T cell factor (TCF) family members transcribe their target genes only when bound to β-catenin. The genetic predisposition such as mutations of adenomatous polyposis coli or β-catenin results in stabilization and activation of β-catenin that leads to uncontrolled proliferation of intestinal epithelial cells through the constitutively active Wnt-signaling pathway (4,5).
Cyclooxygenase (COX), known as prostaglandin (PG) H2 synthase, is the rate-limiting enzyme in the conversion of arachidonic acid into PGs. The two known forms of COX are referred to as COX1 and COX2. Overexpression of COX2 has been frequently observed in colon tumors and COX2 plays a major role in colon carcinogenesis (6). Many studies have implicated that PGE2, the metabolite of COX2 enzyme, is a potent mitogen and contributes to colon cancer development (7). Interestingly, when COX2 is targeted through either gene knockouts or COX2-specific inhibitors, a significant reduction of number of tumors is observed suggesting that COX2 plays a key role in colon tumorigenesis (8). Studies in humans indicate that use of specific COX2 inhibitors may be an effective approach for colorectal cancer prevention and treatment (9,10). However, the cardiovascular side effects limit the use of long-term treatment of COX2 inhibitors (11). An important downstream target of PGE2 is the epidermal growth factor receptor (EGFR) pathway that has also been implicated in colon carcinogenesis (12). EGFR is known to be required for establishment of intestinal tumors and is involved in development of advanced colorectal cancer (13). Recent studies have found that targeting COX2 and EGFR simultaneously can be more efficient than targeting a single pathway in suppressing cancer (14,15). These combinatorial strategies that target multiple pathways can provide not only improved therapeutic results over monotherapeutic regimens but also can relieve the problem of COX2 inhibitor-associated side effects through adjusting the dosing and/or scheduling (15).
Recently, there have been concentrated efforts to develop novel dietary substances as cancer preventive and/or therapeutic agents. Fisetin (3,7,3′,4′-tetrahydroxyflavone) (Figure 1A), a naturally occurring flavonoid commonly found in various vegetables and fruits such as onion, cucumber, apple, persimmon and strawberry (16), possesses antioxidative (17), anti-inflammatory (18) and antiproliferative effects in a wide variety of cancer cells including liver (19) and prostate cancer cells (20). We hypothesized that fisetin may provide chemopreventive as well as chemotherapeutic effects against colon cancer. The present study was designed to investigate whether fisetin confers inhibitory effects in HT29 human colon cancer cells. Here, we present the data showing that fisetin can induce apoptosis, inhibit COX2 expression without affecting COX1 and inhibit Wnt/β-catenin, EGFR and nuclear factor-kappa B (NF-κB) pathways in HT29 cells. These results suggest that fisetin could be a useful agent for prevention and treatment of colon cancer.
Fig. 1.
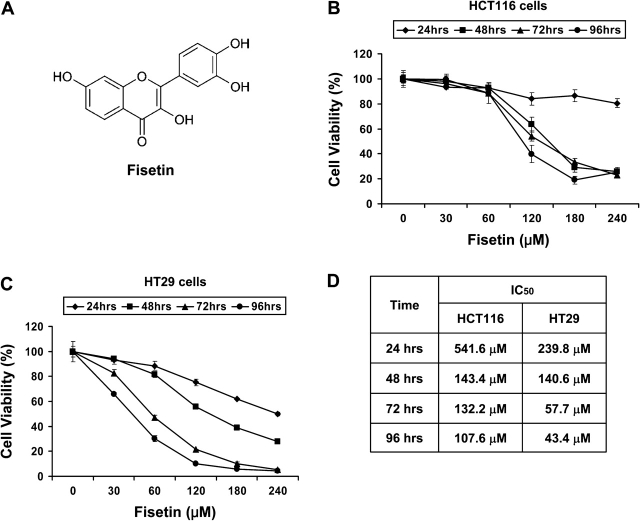
Effect of fisetin on viability of HCT116 and HT29 colon cancer cells. (A) The structure of fisetin is shown. Thiazolyl blue tetrazolium bromide assays of HCT116 (B) and HT29 (C) colon cancer cells. Cells were treated with fisetin up to 240 μM for 24, 48, 72 and 96 h. The absorbance was measured at 570 nm. All samples were done in triplicate and the data are presented as mean ± SEM. (D) The median inhibition concentration (IC50) values of fisetin for both cell lines are shown.
Materials and methods
Reagents and cell culture
Human colon carcinoma cell lines, HCT116 and HT29, were purchased from American Tissue Type Culture Collection (Manassas, VA). HCT116 cells were maintained in Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA) and HT29 cells were grown in RPMI-1640 (American Tissue Type Culture Collection). Both media were supplemented with 10% fetal bovine serum (HyClone, Logan, UT), 100 U/ml of penicillin and 100 μg/ml of streptomycin (Cambrex, Walkersville, MD). Cells were grown at 37°C in a humidified atmosphere consisting of 5% CO2. Fisetin was purchased from Sigma (St Louis, MO) and dissolved in dimethyl sulfoxide.
Thiazolyl blue tetrazolium bromide assay
Cells were seeded and cultured in 24-well plate with or without fisetin for the indicated time period. Media were removed and 0.05% thiazolyl blue tetrazolium bromide solution was added that was followed by incubation at 37°C for 2 h. Thiazolyl blue tetrazolium bromide solution was then replaced with dimethyl sulfoxide and incubated for 10 min. After incubation, the solution was aliquoted into 96-well plate in duplicate and the absorbance was measured.
Apoptosis assay
Two different assays were performed to measure apoptosis. First, APO-DIRECT system (Phoenix Flow System, San Diego, CA) was utilized according to the manufacturer's protocol using Becton Dickinson FACSCalibur at the McArdle Laboratory for Cancer Research, University of Wisconsin–Madison. Second, for immunofluorescent detection of apoptosis, Annexin-V-FLUOS staining kit was purchased from Roche (Indianapolis, IN) and Nikon Optiphot fluorescent microscope was used for analysis.
Plasmids, transfection and luciferase assay
COX2 luciferase constructs (pXP2-COX2-Luc) containing (−1796 to +104) region and empty pXP2-Luc were generously provided by Dr Miguel A.Iñiguez, Universidad Autónoma de Madrid-CSIC, Madrid, Spain. The pGL3 vector-based COX2 luciferase constructs wild-type (WT) and mutant (MT) that does not contain TCF-binding element were generous gifts from Dr S.Perwez Hussain, Laboratory of Human Carcinogenesis, National Cancer Institute, National Institutes of Health, Bethesda, MD. Basic pGL3-Luc and Renilla luciferase control vectors were purchased from Promega (Madison, WI). TOPflash plasmid construct was purchased from Millipore (Billerica, MA). Plasmids were amplified and purified using HiSpeed plasmid maxi kit from Qiagen (Valencia, CA). Lipofectamine 2000 (Invitrogen) was used for transient transfection. Vehicle or fisetin was added 1 day after the transfection for 24 h. Luciferase activities were determined using Dual Luciferase Reporter Assay System (Promega).
Nuclear and cytoplasmic extraction of cells
After the treatment of cells with fisetin, nuclear and cytoplasmic fractions of cells were extracted as described previously (21). The resulting fractions were subjected to bicinchoninic acid assay (Pierce, Rockford, IL) to measure the protein concentration.
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis and western blotting
Cells were harvested with lysis buffer supplemented with protease inhibitor cocktail (Calbiochem, San Diego, CA) and phosphatase inhibitor sodium orthovanadate. Total protein extraction and western blotting were performed as described previously (22).
Immunofluorescence imaging
Cells were seeded on a two-chamber tissue culture-treated glass slides. The next day, media were replaced with or without fisetin and cultured for 48 h. After removing the chamber, slides were rinsed with phosphate-buffered saline and cells were fixed with 2% paraformaldehyde and permeabilized in methanol. After washing with phosphate-buffered saline, slides were blocked with 2% donkey serum. Primary and secondary antibodies were incubated in 5% donkey serum. SlowFadeGold-DAPI (Invitrogen) was used as mounting and counterstaining media. For analysis, Bio-Rad Radiance 2100 MP Rainbow system in the W.M. Keck Laboratory for Biological Imaging at the University of Wisconsin–Madison was used.
PGE2 immunoassay
Equal number of cells were seeded and treated with fisetin for 48 h. Culture media were collected and debris was removed by centrifugation. PGE2 immunoassay kit was purchased from Cayman Chemicals (Ann Arbor, MI) and was carried out according to the manufacturer's instruction.
Colony formation assay
Briefly, cells were seeded in top agar containing 0.3% agar with RPMI-1640 media and 10% fetal bovine serum. Bottom agar consisted of 0.5% agar, RPMI-1640 media and 10% fetal bovine serum. Media with dimethyl sulfoxide or indicated doses of fisetin were added and replaced every 3 days. The plate was incubated at 37°C in 5% CO2 atmosphere.
Statistical analysis of the data
Microsoft Excel software was used to calculate the mean and the standard error of the mean. The t-test was used to compare the means of groups and P values <0.05 were considered significant. All statistic data obtained showed significance between the treatments as a whole as analyzed by analysis of variance.
Results
Fisetin induces growth inhibition of HCT116 and HT29 colon cancer cells
Employing HCT116 and HT29 colon cancer cell lines, we first evaluated the effect of fisetin on cell proliferation by thiazolyl blue tetrazolium bromide assay. In addition to aberrant Wnt signaling in both cell lines, HT29 cells are known to express COX2, whereas HCT116 cells lack COX2 expression. Both cell lines were sensitive to fisetin indicating that fisetin could inhibit proliferation of colon cancer cells regardless of COX2 status (Figure 1B and C). However, HT29 cells were more sensitive to fisetin as median inhibition concentration values were lower than that of HCT116 cells (Figure 1D). We selected HT29 cells to test whether fisetin could affect COX2- and Wnt-signaling pathways. For all downstream experiments, cells were treated with fisetin for 48 h unless otherwise indicated in ‘Materials and Methods’.
Fisetin induces apoptosis of HT29 colon cancer cells
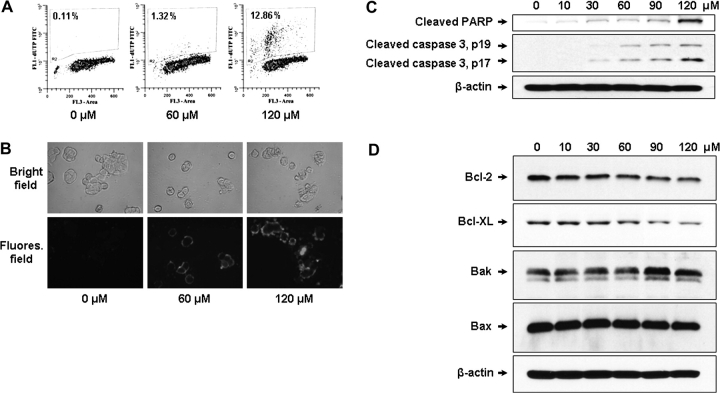
Resistance against apoptosis is critical for survival and also contributes to drug resistance of cancer cells. Therefore, the induction of apoptosis is an important mechanism of chemoprevention and chemotherapy of cancer (23). The fisetin-induced apoptosis was evaluated by different methods. First, dose-dependent increase in apoptosis was detected when HT29 cells were treated with fisetin (Figure 2A). Next, the induction of apoptosis was visualized by Annexin V assay. Treatment of cells with fisetin resulted in a dose-dependent increase in apoptosis as shown by increased green fluorescence (Figure 2B). Red fluorescence in 120 μM of fisetin-treated cells indicates late-stage apoptosis. Fisetin-induced apoptosis was further assessed by western blotting. Analysis of poly (ADP-ribose) polymerase cleavage, which is considered a biomarker of apoptosis, showed that the cleaved poly (ADP-ribose) polymerase fragment was increased after fisetin treatment (Figure 2C). Also, caspase 3 was cleaved and thus activated in fisetin-treated cells in a dose-dependent manner (Figure 2C). Since COX2 metabolite PGE2 is known to inhibit mitochondrial apoptotic pathway via inducing antiapoptotic Bcl-2 protein in colon cancer (24), we measured the levels of proteins involved in mitochondrial apoptotic pathway including Bcl-2. The treatment of cells with fisetin decreased the level of prosurvival Bcl-2 and Bcl-XL proteins, whereas the level of proapoptotic Bak protein was increased. Bax level remained unchanged upon fisetin treatment (Figure 2D).
Fig. 2.
Effect of fisetin on apoptosis of HT29 cells. For terminal dUTP nick end labeling assay (A), cells were treated with 0, 60 and 120 μM of fisetin. Fragmented DNA due to apoptosis was labeled by terminal deoxynucleotidyl transferase using fluorescein isothiocyanate– deoxyuridine triphosphate (FITC–dUTP) as substrate. For Annexin assay (B), cells were grown with or without fisetin in chamber glass slides. The exposed phosphatidylserine due to apoptosis induced by fisetin was recognized by Annexin-V-Fluorescein with high specificity. Green color denotes fluorescence from fluorescein and red color represents propidium iodide staining. The top panel shows bright field image of cells and bottom panel shows the fluorescent image of the same field. For western blot analysis of apoptosis, equal amount of protein was subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis followed by western blot analysis. (C) Anti-cleaved poly (ADP-ribose) polymerase (PARP) antibody and anti-cleaved caspase 3 antibody were utilized to detect apoptosis. (D) Antibodies specific for proteins that are involved in mitochondrial apoptosis pathway were tested. The membrane was stripped and reprobed with anti-β-actin antibody to verify equal loading.
Fisetin inhibits expression of COX2 in HT29 cells
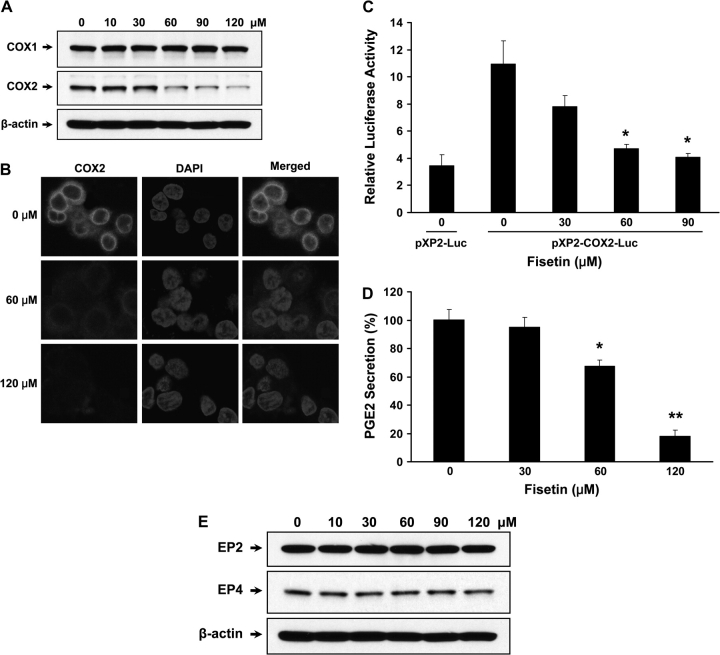
The role of COX2 in tumorigenesis of colon is well known and HT29 cells constitutively express COX2. Therefore, we investigated whether fisetin can modulate the expression of COX2 in these cells. We observed that fisetin inhibited the expression of COX2 protein in HT29 cells without affecting the level of COX1. As shown by western blot in Figure 3A, treatment of the cells with 60 μM of fisetin significantly diminished COX2 protein expression and almost abolished the COX2 expression at a dose of 120 μM of fisetin. To verify the downregulation of COX2, we performed immunofluorescent labeling. The bright red fluorescence from COX2 in control cells shows constitutive expression of COX2 in cytoplasm, whereas 60 μM of fisetin significantly decreased the COX2 expression. Finally, 120 μM of fisetin abrogated the expression of COX2 (Figure 3B).
Fig. 3.
Effect of fisetin on COX expression and PGE2 secretion. Cells were treated with indicated doses of fisetin and were subjected to analysis of COX expression. (A) Western blots of COX1 and COX2 were performed with specific antibodies. The membrane was stripped and reprobed with anti-β-actin antibody to verify equal loading. (B) Immunocytochemistry using COX2-specific antibody was performed. A total of 100 000 cells were seeded and treated with indicated doses of fisetin. For immunofluorescence labeling, anti-COX2 antibody (Cayman Chemicals) and Alexa Fluor 594 goat anti-mouse IgG (Invitrogen) were used as primary and secondary antibodies, respectively. 4′,6-Diamidino-2-phenylindole (DAPI) was used to counterstain the nucleus, ×600 (C) COX2 promoter activity with or without fisetin treatment was measured. Cells were transiently cotransfected with 1 μg of empty vector or COX2 luciferase construct containing Photinus pyralis (firefly) luciferase reporter and 50 ng of Renilla reniformis (sea pansy) luciferase control reporter plasmid. Luminescence from Renilla luciferase reporter serves as baseline response hence it was used as internal control. Results are represented as relative luciferase activity that was obtained by dividing the luminescence from firefly luciferase by the luminescence from Renilla luciferase. All samples were done in triplicate and the data are presented as mean ± SEM. *P < 0.05 versus pXP2-COX2-Luc control. (D) COX2 activity was measured by measuring PGE2 secretion. The plate was read at 414 nm using a microplate reader. The values were normalized according to cell number (1 × 106 cells) and expressed as % PGE2 secretion. The data shown are representative results of three independent experiments and are presented as mean ± SEM. *P < 0.05, **P < 0.01 versus control. (E) Western blots of EP2 and EP4 receptors were performed with specific antibodies and anti-β-actin antibody was used to verify equal loading.
Fisetin inhibits COX2 promoter activity and PGE2 secretion
Next, we measured the effect of fisetin on transcriptional regulation of COX2 using pXP2–COX2 promoter luciferase construct. The full-length COX2 promoter coupled to a luciferase reporter gene was transfected into HT29 cells. We found that fisetin significantly reduced the activity of COX2 promoter in a dose-dependent manner. Treatment of cells with 30, 60, 90 μM of fisetin inhibited COX2 promoter activity by 28.4, 57.0, 62.7%, respectively (Figure 3C). However, 120 μM of fisetin-treated cells transfected with COX2 promoter were detached and therefore luciferase activity was not measured. Since fisetin decreased the expression of COX2, we also assessed whether fisetin could modulate PGE2 secretion. As shown in Figure 3D, inhibition of PGE2 release by fisetin occurred dose dependently. When cells were treated with 60 and 120 μM of fisetin, the secretion of PGE2 was decreased by 32.6 and 82.0%, respectively. This suggests that fisetin-induced downregulation of PGE2 may be the mechanism responsible for induction of apoptosis through downregulation of Bcl-2 that we have shown previously. PGE2 enhances colon cancer progression through Gs-axin-β-catenin-signaling axis via its G protein-coupled receptor E-prostanoid (EP) 2 (25). However, fisetin did not affect the level of EP2 receptor indicating that fisetin inhibits the signaling through regulating ligand availability. Fisetin also did not affect the level of EP4 receptor (Figure 3E).
Fisetin inhibits β-catenin pathway in HT29 cells
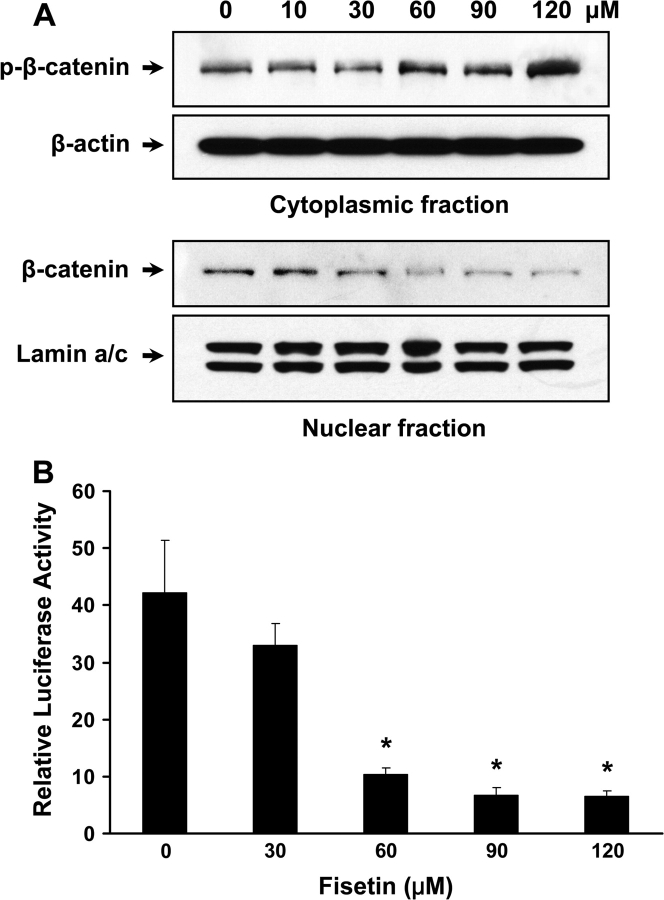
The key molecule that regulates Gs-axin-β-catenin- and Wnt-signaling pathway is β-catenin that is ubiquitinated for degradation when phosphorylated, whereas dephosphorylation leads to stabilization and translocation to nucleus for target gene expression together with TCF family members. In fisetin-treated cells, it is evident that phosphorylation of β-catenin is increased in a dose-dependent manner (Figure 4A).
Fig. 4.
Effect of fisetin on Wnt-signaling pathway. (A) Western blots of nuclear and cytoplasmic fractions using β-catenin antibodies were shown. Anti-phospho-β-catenin (Ser 45) antibody was used for western blotting of cytoplasmic fraction, whereas anti-β-catenin antibody was used to detect the level of β-catenin in the nucleus. Anti-β-actin antibody and anti-lamin a/c antibody were used to verify equal loading. (B) The activity of Wnt-signaling pathway was measured using TOPflash luciferase assay. Cells were transiently cotransfected with 1 μg of TOPflash reporter plasmid and 50 ng of Renilla luciferase control reporter plasmid. Results are represented as mentioned earlier. All samples were done in triplicate and the data are presented as mean ± SEM. *P < 0.05 versus control.
In nucleus, non-phospho-β-catenin is diminished accordingly (Figure 4A). These data indicate that fisetin decreases active form of β-catenin in these cells. To further verify the inhibition of these pathways induced by inactivation of β-catenin, we investigated whether fisetin could decrease TCF4–β-catenin transcriptional activity by using a TOPflash TCF reporter assay. As shown, fisetin-treated cells (30, 60, 90, 120 μM) exhibited inhibition of TCF4–β-catenin transcriptional activity by 21.9, 75.6, 84.3, and 84.5%, respectively (Figure 4B). At 60 μM of fisetin treatment, TOPflash luciferase activity was inhibited by 75.6% even though the nuclear level of TCF4 decreased marginally (Figure 5B). This can be explained by the significant decrease in nuclear β-catenin level at same dose of fisetin (Figure 4A). Therefore, at 60 μM of fisetin treatment, it is conceivable that the accumulated TCF4 in the nucleus is primarily not complexed with β-catenin making TCF4 inactive resulting in low signaling activity as shown by TOPflash luciferase assay.
Fig. 5.
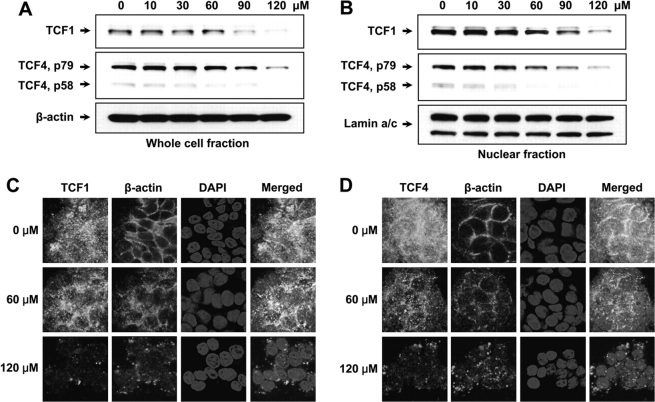
Effect of fisetin on TCF proteins. (A) Expression levels of TCF1 and TCF4 were measured through western blot experiment. Anti-β-actin antibody was used to verify equal loading. (B) Western blotting was performed with nuclear fraction of the cells to see whether the translocation of TCF1 and TCF4 were affected by fisetin. Anti-lamin a/c antibody was used to verify equal loading of nuclear proteins. (C) Immunofluorescent imaging of TCF1. For labeling, anti-TCF1 antibody (Cell Signaling Technology, Danvers, MA) and Alexa Fluor 488 goat anti-rabbit IgG (Invitrogen) were used as primary and secondary antibodies, respectively. (D) Immunofluorescent imaging of TCF4. Anti-TCF4 antibody (Cell Signaling Technology) and Alexa Fluor 488 goat anti-rabbit IgG (Invitrogen) were used for labeling. For both (C) and (D), 100 000 cells were seeded in chamber glass slides and treated with indicated doses of fisetin. For β-actin labeling, anti-β-actin antibody (Sigma) and Alexa Fluor 594 goat anti-mouse IgG were utilized. 4′,6-Diamidino-2-phenylindole (DAPI) was used to counterstain the nucleus. ×600.
Fisetin inhibits expression and translocation of TCF1 and TCF4 in HT29 cells
The fact that TCF4–β-catenin is a critical effector in colon carcinogenesis and that the transcriptional activity of TCF4–β-catenin was decreased upon fisetin treatment, we tested whether fisetin could downregulate the TCF4 expression itself. Western blot analysis of TCF4 revealed that fisetin downregulates not only the expression of TCF4 (Figure 5A) but also its translocation to the nucleus (Figure 5B). At the same time, the level of TCF1 was also measured. While TCF4 is predominantly expressed in colon cancer, HT29 cells also express TCF1. TCF1 is known to be one of the target genes of TCF4 (26) and is suggested to be involved in tumor progression (27). As shown, the expression and translocation of TCF1 was also inhibited by fisetin (Figure 5A and B). To confirm these results, we labeled fisetin-treated cells with TCF1- and TCF4-specific antibodies. As expected, the expression of both TCF1 and TCF4 were downregulated and the translocation of these two proteins to the nucleus were inhibited by fisetin as shown by diminished green fluorescence in TCF panel and Merged panel, respectively (Figure 5C and D).
Fisetin inhibits COX2 expression through downregulation of TCF4
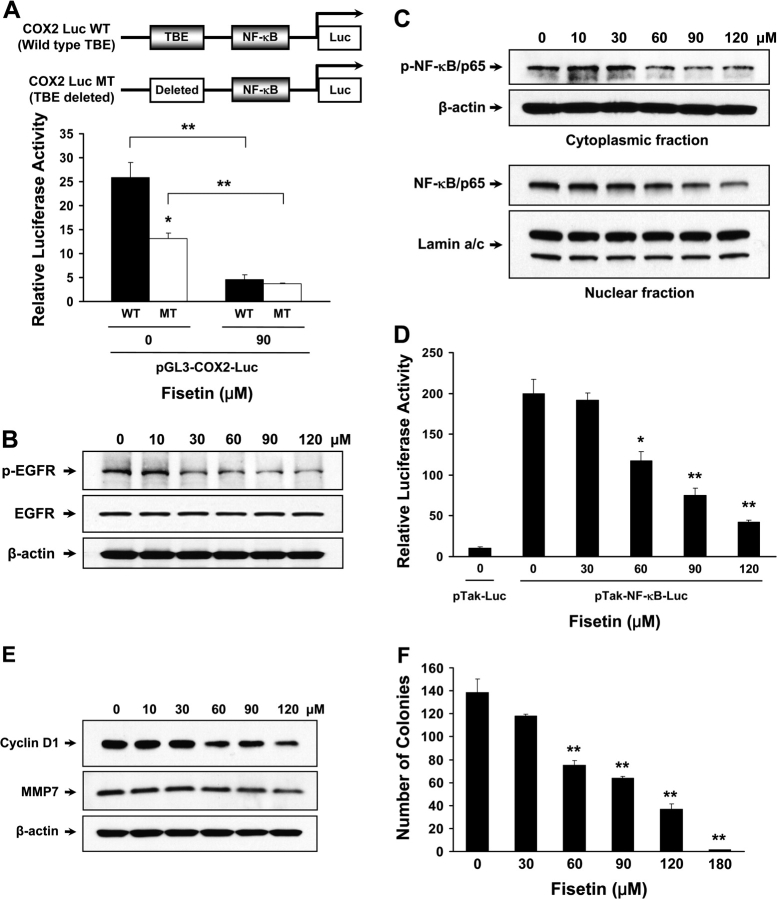
The promoter region of the COX2 gene contains binding sites for multiple transcription factors including TCF4, NF-κB, CCAAT/enhancer-binding protein, polyoma enhancer activator 3, cyclic adenosine 3′,5′-monophosphate response element binding and activating transcription factor (28). Since we observed downregulation of both TCF4 and β-catenin, we performed luciferase assay with MT COX2 promoter construct that does not contain TCF4-binding element (Figure 6A). Without fisetin treatment, we found that MT showed decrease in luciferase activity by 48.9% when compared with non-treated WT control (Figure 6A, WT versus MT at 0 μM). This indicates that TCF4, when bound to β-catenin, plays a major role in regulation of COX2 expression in HT29 cells. When WT at 90 μM is compared with WT at 0 μM, the luciferase activity was decreased by 82.5% (Figure 6A, WT, 0 versus 90 μM). At 90 μM of fisetin treatment, WT exhibited similar luciferase activity as MT that does not contain TCF4-binding site indicating that fisetin suppresses the expression of COX2 through inhibition of TCF4 (Figure 6A, WT versus MT at 90 μM). In MT transfected cells, 90 μM of fisetin decreased the relative luciferase activity from 13.2 to 3.65, which is 72.3% decrease when compared with non-treated cells suggesting that factors other than TCF4 is also involved in fisetin-regulated COX2 expression (Figure 6A, MT, 0 versus 90 μM).
Fig. 6.
Effect of fisetin on regulation of COX2 expression. (A) Top: schematic presentation of COX2 promoter luciferase constructs used in the assay. The promoter region of COX2 contains multiple transcription factor-binding sites including sites for TCF4 and NF-κB. WT constructs with TCF-binding element (TBE) and MT constructs with deletion of TBE are shown. Both constructs are pGL3 based. Bottom: effect of fisetin on WT and MT TBE-bearing COX2 promoter. Cells were transiently cotransfected with 1 μg of WT or MT COX2 luciferase construct and 50 ng of Renilla luciferase control reporter plasmid. Results are represented as before. The data shown are representative results of three independent experiments and are presented as mean ± SEM. *P < 0.05 versus paired control, **P < 0.01 versus indicated control. Effect of fisetin on EGFR and NF-κB activation. (B) The activation of EGFR was measured through western blot experiment. Anti-total EGFR and anti-β-actin antibodies were used as loading control. (C) The levels of cytoplasmic phospho-p65 NF-κB (Ser 536) and p65 NF-κB in the nucleus were measured by western blot. Anti-β-actin and anti-lamin a/c antibodies were used as loading control. (D) Transcriptional activity of NF-κB was measured. Cells were transiently cotransfected with 1 μg of pTak control vector or NF-κB luciferase construct and 50 ng of Renilla luciferase control reporter plasmid. Results are represented as stated before. All samples were done in triplicate and the data are shown as mean ± SEM. *P < 0.05, **P < 0.01 versus pTak-NF-κB-Luc control. Effect of fisetin on Wnt target gene expression and on colony formation. (E) For western blot analysis of Wnt target proteins, anti-cyclin D1 and anti-MMP7 antibodies were used. (F) Colony formation assay was performed with HT29 cells with or without fisetin. Assay was performed in six-well plates in triplicate. A total of 10 000 cells were seeded in top agar and grown for 4 weeks. At the end of the culture, colonies were stained and the number of colonies in the whole well was counted. The data are presented as mean ± SEM. **P < 0.01 versus control.
Fisetin inhibits activation of EGFR in HT29 cells
PGE2 has been known to transactivate EGFR thereby enhancing cancer growth and invasion (12,29). Since fisetin decreased secretion of PGE2, we tested whether fisetin could diminish EGFR activation. As shown, HT29 cells treated with fisetin exhibited decreased EGFR phosphorylation as low as at 30 μM (Figure 6B). We utilized an antibody that is specific to phosphorylated Tyr 1068 residue of EGFR. This residue, when phosphorylated, is a well-known binding site for growth factor receptor-bound protein 2, an adaptor protein that conveys the signal from EGFR to the well-established Ras–Raf–MAPK pathway.
Fisetin inhibits activation of NF-κB in HT29 cells
It is well known that NF-κB transcription factor is associated with cellular transformation and has been identified to be upregulated in colon cancer (30). Moreover, phosphorylation of p65 subunit of NF-κB is required to stimulate COX2 expression (31). In western blot analysis, we observed decreased level of phosphorylated p65 subunit of NF-κB in cytoplasm and concomitant decrease in p65 subunit in nuclear lysates of HT29 cells treated with fisetin (Figure 6C). This indicates that fisetin inhibits the expression of COX2 at least through dual manner, i.e. TCF4 and NF-κB, at transcriptional level. Next, we determined NF-κB transcriptional activity using luciferase reporter plasmid that contains NF-κB-binding site. We observed that fisetin treatment (30, 60, 90, 120 μM) resulted in decrease of NF-κB transcriptional activity by 3.7, 41.0, 62.3 and 78.6%, respectively, when compared with pTak-NF-κB-Luc control (Figure 6D). This suggests that fisetin could downregulate the NF-κB target genes including COX2 and Bcl-2.
Fisetin reduces expression of Wnt target genes and inhibits colony formation
Overexpression of proteins such as cyclin D1 and matrix metalloproteinase 7 (MMP7) has been implicated in colon carcinogenesis and are well-known targets of Wnt signaling. In our western blot analysis, we observed diminished levels of cyclin D1 and MMP7 when treated with fisetin (Figure 6E). Cyclin D1 is frequently overexpressed in human colon cancer and its increased expression is known to contribute to the abnormal growth and tumorigenicity (32). Mice that are lacking MMP7 have shown that intestinal tumorigenesis is suppressed suggesting that MMP7 plays a critical role in colon carcinogenesis (33). Colony formation assay was performed to test whether fisetin can inhibit the clonogenicity of the colon cancer cells. While the control group formed 138 colonies on average over 4 weeks, the clonogenicity of the fisetin-treated group showed dose-dependent decrease. The number of colonies formed decreased to 118, 75, 64 and 37 in 30, 60, 90 and 120 μM of fisetin-treated group and only 1 colony was formed in 180 μM fisetin-treated group, on average (Figure 6F).
Discussion
In this study, we demonstrate that the dietary constituent fisetin induces apoptosis and inhibits multiple signaling pathways that are involved in colon cancer growth. We observed that fisetin inhibits expression of COX2 in HT29. Many studies show that COX2 plays a critical role in colon carcinogenesis making COX2 an attractive target for prevention and treatment of colon cancer. The purpose of developing selective COX2 inhibitors (i.e. valdecoxib, rofecoxib, celecoxib) is to avoid various side effects of non-steroidal anti-inflammatory drugs. Inhibition of colon tumorigenesis by selective inhibition of COX2 is well known. Unfortunately, even though randomized clinical trials of selective COX2 inhibitors against colon cancer including the Adenoma Prevention with Celecoxib trial and the Adenomatous Polyp Prevention on Vioxx trial exhibited promising results, all trials were halted due to cardiovascular side effects (34). Therefore, the development of specific COX2 inhibitors without the risk of cardiovascular disease is warranted. Our data suggest that fisetin may be a good candidate for chemoprevention and chemotherapy of colon cancer. We showed that fisetin inhibits COX2 through downregulating its expression which is fundamentally different than the mechanism of the selective COX2 inhibitors which suppress enzyme activity via direct binding thereby decreasing the synthesis of PGs (35).
PGE2 enhances colon cancer progression through (i) activating Gs-axin-β-catenin signaling via EP2 (25); (ii) inhibiting apoptosis via inducing Bcl-2 protein (24) and (iii) transactivating EGFR (29). We observed that fisetin inhibits COX2 expression and concurrent decrease in PGE2 secretion that resulted in inactivation of β-catenin and induction of apoptosis. HT29 cells treated with fisetin also exhibited decreased EGFR phosphorylation.
Due to the mutations, Wnt-signaling pathway is hyperactive in colon cancer through constitutive activation of TCF4–β-catenin (5). Araki et al. (36) have shown that COX2 expression can be regulated by TCF4–β-catenin complex. This indicates that downregulation of TCF4 or β-catenin will suppress both Wnt and COX2 pathway. Moreover, this could lead to inhibition of PGE2-induced downstream signaling pathways such as EGFR activation. This suggests that TCF4 and β-catenin are the key regulators of colon carcinogenesis. Therefore, our data that fisetin downregulates both TCF4 and β-catenin are quite promising.
We observed decreased level of expression and nuclear translocation of both TCF1 and TCF4. TCF4 is the predominantly expressed form of TCF in colon cancer and its function is well understood as stated earlier. HT29 cells are known to express TCF1 and TCF4 (4,26) and it is shown that TCF1 is a target gene of TCF4 (26). TCF1 is suggested to be involved in tumor progression since it is overexpressed in metastatic site-derived COLO205 and COLO201 colon cancer cell lines but not in SW480 and SW620 cells, which are derived from the primary tumor site of the same patient (27). Moreover, TCF1 is not expressed in HT29M3 cell line, a highly differentiated derivative of HT29 cells, which becomes highly invasive when methylthioadenosine phosphorylase, whose expression is induced specifically by TCF1, is introduced (37).
Earlier studies have revealed that dietary agents epigallocatechin gallate and curcumin could inhibit the expression of COX2 through inhibition of NF-κB (38,39). We also found that fisetin can decrease the activation of NF-κB and can inhibit its transcriptional activity.
The majority of colon cancer cases are sporadic and a fraction of them has been suggested to be inherited. Familial adenomatous polyposis is the best-characterized inherited precursor of colon cancer where hundreds to thousands of polyps are formed during adolescence and if untreated, colon cancer is inevitable in these patients (40). The average age of colon cancer in familial adenomatous polyposis is 35 years which is >30 years earlier than the average age of diagnosis of colon cancer in the general population (41). This indicates that there is ample time and opportunity for early intervention in both cases of colon cancer. The majority of both types of colon cancer contain mutations in adenomatous polyposis coli leading to uncontrolled cell proliferation through signaling by activated β-catenin. Also, COX2 is elevated in 50% of adenomas and >80% of carcinomas of colon (42).
Our data show that fisetin inhibits the growth of not only COX2-positive HT29 cells but also COX2-negative HCT116 cells (Figure 1). HCT116 cell line harbors a mutation in β-catenin that results in uncontrolled cell growth through constitutive activation of Wnt-signaling pathway. Since fisetin induced downregulation of β-catenin and TCF4 in HT29 cells (Figures 4 and 5), the susceptibility of HCT116 cells to fisetin might also be due to the inhibition of Wnt-signaling pathway via downregulation of β-catenin and TCF4.
To date there have not been any human clinical trials or studies assessing the bioavailability of fisetin; however, multiple studies have shown that flavonoids with a similar structure to fisetin (e.g. quercetin, myricetin) are orally bioavailable (43).
Our data that fisetin can downregulate both β-catenin and COX2 provide a good rationale for further comprehensive research of this dietary compound against colon cancer. Taken together, our results suggest that fisetin could provide a multiprong strategy for targeting multiple signaling pathways leading to antitumorigenesis of colon.
Funding
United States Public Health Service (R01CA 120451).
Acknowledgments
We thank Dr Miguel A.Iñiguez (Universidad Autónoma de Madrid-CSIC, Madrid, Spain) and Dr S.Perwez Hussain (Laboratory of Human Carcinogenesis, National Cancer Institute, National Institutes of Health, Bethesda, MD) for providing their plasmids.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- COX
cyclooxygenase
- EGFR
epidermal growth factor receptor
- EP
E-prostanoid
- MMP7
matrix metalloproteinase 7
- MT
mutant
- NF-κB
nuclear factor-kappa B
- PG
prostaglandin
- TCF
T cell factor
- Wnt
wingless and Int
- WT
wild-type
References
- 1.Jemal A, et al. Cancer statistics, 2008. CA Cancer J. Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Klaus A, et al. Wnt signalling and its impact on development and cancer. Nat. Rev. Cancer. 2008;8:387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- 3.Giles RH, et al. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim. Biophys. Acta. 2003;1653:1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 4.Korinek V, et al. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 5.Morin PJ, et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 6.Gupta RA, et al. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat. Rev. Cancer. 2001;1:11–21. doi: 10.1038/35094017. [DOI] [PubMed] [Google Scholar]
- 7.Myung SJ, et al. 15-Hydroxyprostaglandin dehydrogenase is an in vivo suppressor of colon tumorigenesis. Proc. Natl Acad. Sci. USA. 2006;103:12098–12102. doi: 10.1073/pnas.0603235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oshima M, et al. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2) Cell. 1996;87:803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- 9.Arber N, et al. Chemoprevention of colorectal cancer: ready for routine use? Recent Results Cancer Res. 2005;166:213–230. doi: 10.1007/3-540-26980-0_14. [DOI] [PubMed] [Google Scholar]
- 10.Brown JR, et al. COX-2: a molecular target for colorectal cancer prevention. J. Clin. Oncol. 2005;23:2840–2855. doi: 10.1200/JCO.2005.09.051. [DOI] [PubMed] [Google Scholar]
- 11.Solomon SD, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N. Engl. J. Med. 2005;352:1071–1080. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 12.Buchanan FG, et al. Prostaglandin E2 regulates cell migration via the intracellular activation of the epidermal growth factor receptor. J. Biol. Chem. 2003;278:35451–35457. doi: 10.1074/jbc.M302474200. [DOI] [PubMed] [Google Scholar]
- 13.Roberts RB, et al. Importance of epidermal growth factor receptor signaling in establishment of adenomas and maintenance of carcinomas during intestinal tumorigenesis. Proc. Natl Acad. Sci. USA. 2002;99:1521–1526. doi: 10.1073/pnas.032678499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X, et al. Tumor growth inhibition by simultaneously blocking epidermal growth factor receptor and cyclooxygenase-2 in a xenograft model. Clin. Cancer Res. 2005;11:6261–6269. doi: 10.1158/1078-0432.CCR-04-2102. [DOI] [PubMed] [Google Scholar]
- 15.Buchanan FG, et al. Targeting cyclooxygenase-2 and the epidermal growth factor receptor for the prevention and treatment of intestinal cancer. Cancer Res. 2007;67:9380–9388. doi: 10.1158/0008-5472.CAN-07-0710. [DOI] [PubMed] [Google Scholar]
- 16.Arai Y, et al. Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J. Nutr. 2000;130:2243–2250. doi: 10.1093/jn/130.9.2243. [DOI] [PubMed] [Google Scholar]
- 17.Hanneken A, et al. Flavonoids protect human retinal pigment epithelial cells from oxidative-stress-induced death. Invest. Ophthalmol. Vis. Sci. 2006;47:3164–3177. doi: 10.1167/iovs.04-1369. [DOI] [PubMed] [Google Scholar]
- 18.Higa S, et al. Fisetin, a flavonol, inhibits TH2-type cytokine production by activated human basophils. J. Allergy Clin. Immunol. 2003;111:1299–1306. doi: 10.1067/mai.2003.1456. [DOI] [PubMed] [Google Scholar]
- 19.Chen YC, et al. Wogonin and fisetin induction of apoptosis through activation of caspase 3 cascade and alternative expression of p21 protein in hepatocellular carcinoma cells SK-HEP-1. Arch. Toxicol. 2002;76:351–359. doi: 10.1007/s00204-002-0346-6. [DOI] [PubMed] [Google Scholar]
- 20.Khan N, et al. Fisetin, a novel dietary flavonoid, causes apoptosis and cell cycle arrest in human prostate cancer LNCaP cells. Carcinogenesis. 2008;29:1049–1056. doi: 10.1093/carcin/bgn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Syed DN, et al. Green tea polyphenol EGCG suppresses cigarette smoke condensate-induced NF-kappaB activation in normal human bronchial epithelial cells. Oncogene. 2007;26:673–682. doi: 10.1038/sj.onc.1209829. [DOI] [PubMed] [Google Scholar]
- 22.Johnson JJ, et al. Carnosol, a dietary diterpene, displays growth inhibitory effects in human prostate cancer PC3 cells leading to G2-phase cell cycle arrest and targets the 5′-AMP-activated protein kinase (AMPK) pathway. Pharm. Res. 2008;25:2125–2134. doi: 10.1007/s11095-008-9552-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan N, et al. Apoptosis by dietary factors: the suicide solution for delaying cancer growth. Carcinogenesis. 2007;28:233–239. doi: 10.1093/carcin/bgl243. [DOI] [PubMed] [Google Scholar]
- 24.Sheng H, et al. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res. 1998;58:362–366. [PubMed] [Google Scholar]
- 25.Castellone MD, et al. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science. 2005;310:1504–1510. doi: 10.1126/science.1116221. [DOI] [PubMed] [Google Scholar]
- 26.Roose J, et al. Synergy between tumor suppressor APC and the beta-catenin-Tcf4 target Tcf1. Science. 1999;285:1923–1926. doi: 10.1126/science.285.5435.1923. [DOI] [PubMed] [Google Scholar]
- 27.Mayer K, et al. Ectopic activation of lymphoid high mobility group-box transcription factor TCF-1 and overexpression in colorectal cancer cells. Int. J. Cancer. 1997;72:625–630. doi: 10.1002/(sici)1097-0215(19970807)72:4<625::aid-ijc13>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 28.Tsatsanis C, et al. Signalling networks regulating cyclooxygenase-2. Int. J. Biochem. Cell Biol. 2006;38:1654–1661. doi: 10.1016/j.biocel.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 29.Pai R, et al. Prostaglandin E2 transactivates EGF receptor: a novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nat. Med. 2002;8:289–293. doi: 10.1038/nm0302-289. [DOI] [PubMed] [Google Scholar]
- 30.Lind DS, et al. Nuclear factor-kappa B is upregulated in colorectal cancer. Surgery. 2001;130:363–369. doi: 10.1067/msy.2001.116672. [DOI] [PubMed] [Google Scholar]
- 31.Manna SK, et al. Inhibition of RelA phosphorylation sensitizes apoptosis in constitutive NF-kappaB-expressing and chemoresistant cells. Cell Death Differ. 2007;14:158–170. doi: 10.1038/sj.cdd.4401929. [DOI] [PubMed] [Google Scholar]
- 32.Arber N, et al. Antisense to cyclin D1 inhibits the growth and tumorigenicity of human colon cancer cells. Cancer Res. 1997;57:1569–1574. [PubMed] [Google Scholar]
- 33.Wilson CL, et al. Intestinal tumorigenesis is suppressed in mice lacking the metalloproteinase matrilysin. Proc. Natl Acad. Sci. USA. 1997;94:1402–1407. doi: 10.1073/pnas.94.4.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bertagnolli MM. Chemoprevention of colorectal cancer with cyclooxygenase-2 inhibitors: two steps forward, one step back. Lancet Oncol. 2007;8:439–443. doi: 10.1016/S1470-2045(07)70139-0. [DOI] [PubMed] [Google Scholar]
- 35.Soliva R, et al. Theoretical studies on the inhibition mechanism of cyclooxygenase-2. Is there a unique recognition site? J. Med. Chem. 2003;46:1372–1382. doi: 10.1021/jm0209376. [DOI] [PubMed] [Google Scholar]
- 36.Araki Y, et al. Regulation of cyclooxygenase-2 expression by the Wnt and ras pathways. Cancer Res. 2003;63:728–734. [PubMed] [Google Scholar]
- 37.Bataille F, et al. Strong expression of methylthioadenosine phosphorylase (MTAP) in human colon carcinoma cells is regulated by TCF1/[beta]-catenin. Lab. Invest. 2005;85:124–136. doi: 10.1038/labinvest.3700192. [DOI] [PubMed] [Google Scholar]
- 38.Plummer SM, et al. Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF-kappaB activation via the NIK/IKK signalling complex. Oncogene. 1999;18:6013–6020. doi: 10.1038/sj.onc.1202980. [DOI] [PubMed] [Google Scholar]
- 39.Peng G, et al. Green tea polyphenol (-)-epigallocatechin-3-gallate inhibits cyclooxygenase-2 expression in colon carcinogenesis. Mol. Carcinog. 2006;45:309–319. doi: 10.1002/mc.20166. [DOI] [PubMed] [Google Scholar]
- 40.Galiatsatos P, et al. Familial adenomatous polyposis. Am. J. Gastroenterol. 2006;101:385–398. doi: 10.1111/j.1572-0241.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- 41.Lal G, et al. Familial adenomatous polyposis. Semin. Surg. Oncol. 2000;18:314–323. doi: 10.1002/(sici)1098-2388(200006)18:4<314::aid-ssu6>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 42.Williams CS, et al. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene. 1999;18:7908–7916. doi: 10.1038/sj.onc.1203286. [DOI] [PubMed] [Google Scholar]
- 43.Ross JA, et al. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002;22:19–34. doi: 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]