Abstract
The carcinogenic potential of dibenzo[a,l]pyrene (DBP) has been well characterized in numerous animal models. We have previously documented that a single dose of 15 mg/Kg DBP to pregnant mice late in gestation (GD 17) produces an aggressive T-cell lymphoma as well as lung and liver cancer in offspring. The current study examines the chemopreventative properties of chlorophyllin (CHL) and chlorophyll (Chl) in this transplacental carcinogenesis model. Pregnant B6129SF1 females, bred to 129S1/SvIm males, received purified diets incorporated with either 2000 p.p.m. CHL, 2000 p.p.m. Chl or 10% freeze-dried spinach beginning at gestation day 9. Lymphoma-dependent mortality was not significantly altered by maternal consumption of any of the diet and little effect on lung tumor burden in mice surviving to 10 months of age was observed. However, coadministration of CHL at 380 mg/Kg with DBP by gavage (molar ratio of 10:1, CHL:DBP) provided significant protection against DBP-initiated carcinogenesis. Offspring born to dams receiving CHL co-gavaged with DBP exhibited markedly less lymphoma-dependent mortality (P < 0.001). The degree of protection by CHL, compared with controls dosed with DBP in tricaprylin (TCP) as the vehicle, was less marked, but still significant. Coadministration of CHL (TCP as vehicle) also reduced lung tumor multiplicity in mice by ∼50% and this was observed throughout the study (P < 0.005). This is the first demonstration that CHL can provide potent chemoprotection in a transplacental carcinogenesis model and support a mechanism involving complex-mediated reduction of carcinogen uptake.
Introduction
Chlorophyllin (CHL) is a water-soluble derivative of chlorophyll (Chl) in which magnesium has been replaced with copper and the phytol chains lost. CHL has been safely used in human medicine (e.g. Derifil, primarily to control body odor in geriatric patients) for many years (1) and is available as a dietary supplement. Chl is present in our diet in green, leafy vegetables, reaching levels of 5.7% in spinach (2). Although the potential for CHL and Chl to act as antimutagens in vitro had been previously published (3), the cancer chemopreventive properties of CHL and Chl in vivo were first demonstrated in the aflatoxin B1 (AFB1) hepatocellular carcinoma (HCC) in rainbow trout model (4,5,6,7,8,9) and later in a rodent model (10). Physical complexation with the carcinogen reduces bioavailability to target organs (11,12), whereas extended preloading with dietary CHL prior to a single carcinogenic treatment with AFB1 is ineffective (6). This mechanism should be essentially species independent and, therefore, effective in humans. Indeed, in a human clinical intervention trial in Qidong, China, where dietary AFB1 exposure is a serous concern (13), a dose of 100–300 mg of CHL, given with meals, for only 3 months was effective at reducing the urinary biomarker of AFB1-dependent DNA adduction by more than half (14). CHL costs pennies a day with no significant side effects being reported, making it extremely attractive for intervention due to the high rate of compliance.
It has been difficult to conduct cancer chemoprevention studies in vivo, mainly due to prohibitive costs and chemical instability. A counter-current chromatography method was recently reported (15), enabling the production of 23 g of highly pure Chl a/b from 90 Kg of spinach leaves in a single run. In addition to demonstrating chemoprevention against AFB1-dependent HCC, in both trout and rat (9,10), the Chl purified via this method markedly reduced AFB1 exposure in humans following oral coadministration (Jubert, Bench, Dashwood, Mata, Pereira, Tracewell, Turtletaub, Williams, and Bailey, unpublished data), using 14C microdosing and accelerator mass spectrometry as the means of detection (16).
Our laboratory has developed a mouse transplacental cancer model (17,18,19,20) that has proven useful for chemoprevention studies. Incorporation of indole-3-carbinol (I3C, a major chemopreventive phytochemical from cruciferous vegetables) (20) or green tea (21) into the maternal diet or drinking water, respectively, provided marked protection for offspring with respect to development of dibenzo[a,l]pyrene (DBP)-dependent lymphoma and lung cancer. Although DBP-dependent lymphoma mortality is dependent upon fetal Cyp1b1 (18), chemoprevention by I3C was independent of the aryl hydrocarbon receptor (ahr) genotype (20). In the case of tea, it appeared that the major effect was from caffeine, as neither decaffeinated green tea nor epigallocatechin-3-gallate provided protection, whereas caffeine alone provided the greatest protection.
We now report that coadministration to pregnant mice of CHL by gavage with the potent polycyclic aromatic hydrocarbon (PAH), DBP, results in marked protection from mortality in offspring beginning at 3–6 months of age from an aggressive T-cell lymphoma and significantly reduced transplacental DBP-dependent lung tumor multiplicity as well. However, if CHL or Chl, either as pure compounds or as a component of freeze-dried spinach, was incorporated into the maternal diet before, during and after gavage with DBP alone, no chemoprotection toward the offspring was observed.
Materials and methods
Chemicals and diets
CHL, tricaprylin (TCP) and dichloromethane were purchased from Sigma Chemical Co. (St Louis, MO). The chlorin content of CHL was based on the manufacturer's assay of 4.5% copper and assertion that all copper was present as copper chlorins. DBP was provided by the National Cancer Institute sponsored Carcinogen Repository, at Midwest Research Institute (Kansas City, MO) and was at least 98% pure as determined by high-performance liquid chromatography. The semipurified diets, AIN93G and AIN93M, were purchased from Research Diets (New Brunswick, NJ). Chl was prepared as described below.
Preparation of Chl
The Chl used in this study was extracted from baby spinach purchased from local organic growers. A detailed description of the extraction process can be found elsewhere (15). Briefly, after removal of stems, the leaves were washed with cold water, freeze dried, washed twice with petroleum ether (boiling point 30–60°C) and solids extracted twice using methanol:petroleum ether (3:1, vol/vol). Combined extracts were transferred to a separatory funnel and washed with saturated NaCl. A repeat wash of the aqueous layer with petroleum ether was recombined to give the final extract and again washed with saturated NaCl, filtered and evaporated in vacuo (<30°C). On average, 30 g of freeze-dried spinach yielded 300 mg of Chl. This Chl extract (90% pure by high-performance liquid chromatography) contained trace amounts of other pigments (carotenoids), as well as some oils, fats and waxes derived from the spinach leaves. Separate testing of those non-Chl fractions has revealed no protection against DBP carcinogenesis (Bailey et al., unpublished data).
Preparation of test solutions
Concentrated stocks (>5 mg/ml) of DBP were first prepared in dichloromethane and reconstituted to working concentrations in corn oil or TCP gavage vehicles. CHL is virtually insoluble in corn oil; thus, CHL solutions were prepared and diluted to the administered concentration in TCP gavage vehicle.
Animals and treatment protocols
Eight-week-old B6129SF1 female and 129S1/SvImJ male mice (Jackson Laboratories, Bar Harbor, ME) were housed at the Laboratory Animal Resource Center at Oregon State University. Mice were allowed to acclimate for 1 week at 20 ± 1°C and 50 ± 10% humidity, with a light–dark cycle of 12 h in microisolator cages (Life Products, Seaford, DE) with CareFRESH bedding. During breeding, gestation and lactation, mice were fed powdered AIN93G diet ad libitum. Upon breeding, gestation day 0 was established by the appearance of the vaginal plug. Beginning on the 9th day of gestation, pregnant mice were randomly assigned to one of the following feeding regimens: 2000 p.p.m. CHL, 2000 p.p.m. Chl, 10% dietary spinach or control diet (powdered AIN93G). On the 17th day of gestation, pregnant mice were treated with vehicle (corn oil, 5 ml/kg body wt) or 15 mg/kg DBP. Another subset of mice on control diet were administered CHL (380 mg/kg) as a co-gavage with DBP (15 mg/kg) to give a molar ratio of CHL:DBP of 10. Pregnant mice were continued on the corresponding feeding regimens through the completion of nursing (21 days post-parturition). After weaning, offspring of each sex from the same litter were housed together (up to four per cage) and fed pelleted AIN93G for the first 3 months and then pelleted AIN93M diet ad libitum until euthanized. The number of dams and offspring in each experimental group is shown in Table I. We observed no overt adverse effects of any treatment regimen on dams or offspring (e.g. no difference in birth weight, litter size, etc.).
Table I.
Effect of treatment and genotype on survival of offspring
| Maternal treatment | No. of offspring | Gender ratio |
Genotype ratio |
% Survival |
||
| Male:female | b-1/d:d/d | Overall | b-1/d | d/d | ||
| Control-A | 51 (9) | 1.08:0.92 | 1.04:0.96 | 25.5 | 19.2 | 32.0 |
| Control-B | 60 (8) | 0.5:2.0 | 0.93:1.07 | 50.0 | 41.4 | 58.1 |
| Spinach | 108 (14) | 1.0:1.0 | 0.92:1.08 | 22.2 | 19.2 | 25.0 |
| CHL | 101 (13) | 0.94:1.06 | 0.98:1.02 | 16.8 | 16.0 | 17.6 |
| Chl | 96 (12) | 1.04:0.96 | 1.08:0.92 | 26.0 | 26.0 | 26.1 |
| co-CHL | 106 (15) | 0.83:1.21 | 1.01:0.99 | 76.6 | 75.9 | 77.4 |
Day 0 of gestation was set as the first day a vaginal plug was observed. The numbers of dams for each group are indicated in parenthesis. Dams were placed on the listed dietary regimens beginning at gestation day 9 as their sole diet source. Dams designated in the following groups received DBP (15 mg/kg) as a single dose by gavage (corn oil 5 ml/Kg) on day 17 of gestation (control-A, spinach, CHL and Chl). The remaining dams (control-B and co-CHL) received DBP at identical levels in the TCP gavage vehicle. The group (co-CHL) in which the CHL (in TCP) was administered with DBP had a significantly higher survival rate than the control groups employing either corn oil (P < 0.001) or TCP (P < 0.01) as the vehicle.
To monitor the health status of mice, sentinels were housed within the colony and periodically tested for viral or bacterial pathogens and parasites. All tests, conducted independently under contract to the University of Missouri Research Animal Diagnostic Laboratory (Columbia, MO), were negative throughout the course of the study. Any indication of morbidity, distress or pain resulted in immediate euthanization with an overdose of CO2, exsanguination and necropsy. Remaining survivors were euthanized and necropsied at 10 months of age, as in our prior work (17,18,19). All procedures used in the handling, treatment and husbandry of mice were approved in advance by the Oregon State University Institutional Animal Care and Use Committee.
Histopathology
At necropsy, the heart, thymus, lung, liver, spleen and kidney were removed, as well as other tissues if they appeared abnormal by gross pathology. The tissues were fixed in 10% formalin, stained with hematoxylin and eosin and analyzed by light microscopy. The previously identified T-cell lymphoblastic lymphoma produces high rates of mortality in this transplacental model (17,18,19). The lymphomas were very aggressive, resulting in invasion of numerous organs by transformed lymphocytes. In addition to lymphoma, mice surviving to 10 months of age developed lung tumors and most males had liver lesions, including foci, hepatocellular adenomas and, rarely, HCC. The lung lesions were initially scored by gross necropsy and a subset of each group submitted for histopathology. As identified previously, the lung lesions were diagnosed as hyperplasia, adenomas, adenoma with progression and carcinomas (17,18,19).
Genotyping for ahrb-1 and ahrd alleles
At necropsy, an ear punch was collected and lysed overnight at 55°C in a solution of DirectPCR Lysis Reagent containing proteinase K (Viagen Biotech, Los Angeles, CA). The resulting lysate was briefly centrifuged prior to undergoing a polymerase chain reaction with allele-specific primers to permit one-tube genotyping of the ahr alleles as described previously (17). Polymerase chain reaction products were separated and visualized on Novex 8% Tris-borate ethylenediaminetetraacetic acid gels (Invitrogen Technologies, Carlsbad, CA).
Statistical analysis
In statistical analysis of the offspring responses of various litter, we corrected for cluster (litter) effects if there was evidence of such an effect. This statistical approach is more completely described in (21).
Survival curves
To evaluate the survival curves, we used a log-rank test, also known as the Mantel–Haenszel or Mantel–Cox test (22). The survival curves of each group were evaluated (Figure 1); P-values that are significantly different at α = 0.05 from control A (diets, corn oil vehicle) and control B (co-gavage, TCP vehicle) are highlighted in Table I. In addition, in some cases it is relevant to adjust these P-values since multiple hypothesis tests are being performed.
Fig. 1.
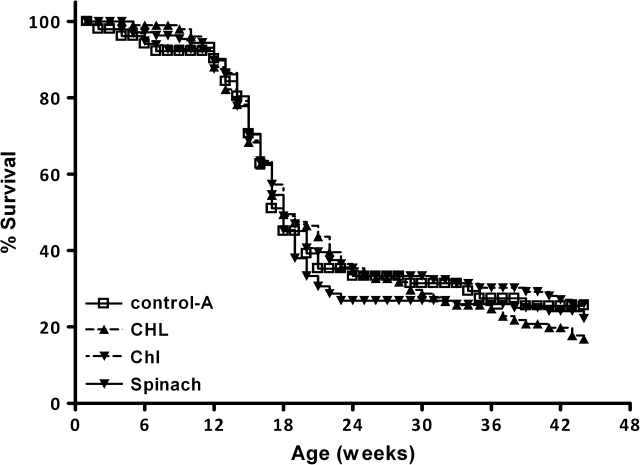
Effect of maternal dietary treatment on DBP-dependent mortality. DBP was administered at a dose of 15 mg/Kg by gavage (corn oil) on day 17 of gestation. The pregnant and nursing dams were administered powdered AIN93G diets supplemented with nothing [control (open squares)], 2000 p.p.m. CHL (filled triangles), 2000 p.p.m. Chl (inverted triangle-dashed line) or 10% freeze-dried spinach (inverted triangle-solid line).
Multiplicity data
To evaluate lung tumor data, we used a Wilcoxon rank sum test (alternatively known as the Mann–Whitney U test), which is a non-parametric version of a t-test for equal means. We used this because an initial evaluation of the multiplicity data for each group showed that, in many cases, the data could not be considered normally distributed. We compared the lung multiplicity information for all groups (Table II). Since we were performing multiple comparisons, which increases the likelihood of observing a significant comparison simply by chance, the P-value cutoffs for significance were modified by a Bonferroni correction.
Table II.
Effect of treatment and genotype on lung tumor multiplicity
| Maternal treatment | Overall | Responsive (b-1/d) | Non-responsive (d/d) |
| Control-A | 14.5 ± 2.6 | 15.2 ± 4.7 | 14.1± 3.0 |
| Control-B | 16.0 ± 1.3 | 13.8 ± 1.6 | 17.5 ± 1.8 |
| CHL | 14.8 ± 1.5 | 13.0 ± 1.5 | 16.3 ± 2.5 |
| Chl | 16.1 ± 1.2 | 16.5 ± 1.7 | 15.8 ± 1.8 |
| Spinach | 19.4 ± 1.6 | 16.5 ± 1.9 | 21.4 ± 2.2 |
| Co-CHL | 8.6 ± 0.7 | 8.9 ± 0.8 | 8.3 ± 1.0 |
Data are presented as mean ± SE for multiplicity (number of lung tumors per mouse), all groups are significantly different from co-CHL. Control-A, control-B, CHL, Chl and spinach are not significantly different from one another. co-CHL b-1/d and co-CHL d/d are not significantly different than one another.
Results
CHL and Chl effects on DBP-dependent lymphoma mortalities
As previously documented by our laboratory, administration of a single dose of DBP on day 17 of gestation did not elicit acute maternal or fetal toxicities (number of pups/litter, weight at birth, sex ratio, etc.) and no gender differences were observed (17,18,19,20,21). Offspring born to mothers treated with DBP exhibited lymphoma-dependent mortality beginning at 10–12 weeks of age (Figure 1). When B6129F1 dams are crossed with 129 sires, half the offspring should have the ahr ‘responsive’ phenotype (genotype, ahrb-1/d) and half the ‘non-responsive’ (genotype, ahrd/d). Consistent with our previous findings, offspring identified as ahr responsive typically had lower rates of survival compared with their non-responsive littermates (Table I). Interestingly, administration of Chl or CHL, in the diet or by coadministration, eliminated that genotypic difference in offspring survival irrespective of any overall survival effect.
Maternal dietary exposure to freeze-dried spinach, CHL or Chl (in AIN93G) did not significantly alter the overall survival rates of offspring born to mothers treated with DBP (Figure 1). It should be noted that, as in previous studies with this model (17,18,19,20,21), there were no mortalities in offspring born to dams administered the corn vehicle (rather than DBP) among all the treatments (data not shown).
When dams were coadministered CHL with DBP, the protection against lymphoma mortality in the offspring was highly significant (P < 0.001, Figure 2A). The impact of coadministered DBP with CHL was apparent irrespective of offspring genotype (Figure 2B and Table I). To our surprise, mortality was lower in offspring born to mothers dosed with DBP alone using TCP instead of corn oil as the vehicle (Figure 2A). The difference was significant (P < 0.01), although less than in the mothers that were dosed with DBP in corn oil (P < 0.001). This result highlights the importance of including appropriate vehicle controls in transplacental chemoprevention studies, as performed here, since the TCP vehicle alone apparently can alter DBP bioavailability to the fetus.
Fig. 2.
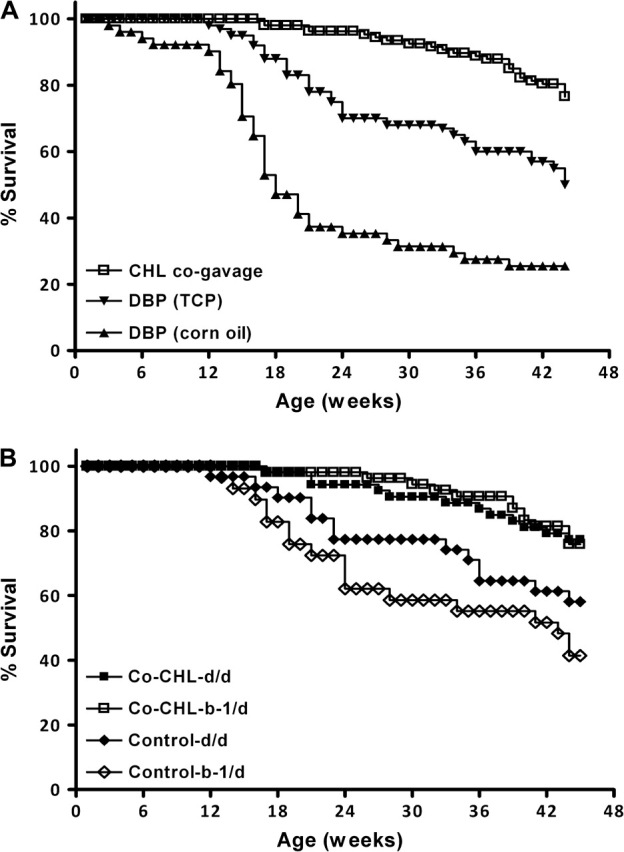
(A) Impact of coadministration of CHL on DBP-dependent mortality. Pregnant and nursing dams were administered control diets and gavaged at gestation day 17 with DBP in corn oil (filled triangles), TCP (filled inverted triangles) or DBP concurrently with 380 mg/kg CHL (open squares). CHL was coadministered at a dose of 380 mg/kg by co-gavage with DBP (15 mg/kg) to give a molar ratio of 10 (CHL:DBP). There is strong statistical evidence of vehicle differences (P < 0.001 for DBP in corn oil versus co-CHL and P < 0.01 for DBP in TCP versus co-CHL. Likewise, DBP in corn oil versus TCP was significant, P < 0.01) (B) Influence of genotype and CHL co-gavage on DBP-dependent mortality. Offspring born to dams receiving DBP by gavage (TCP as the vehicle) and genotyped as ahrb-1/d (open diamonds) or ahrd/d (filled diamonds); offspring born to dams receiving DBP concurrently with 380 mg/kg CHL (in TCP) and genotyped as ahrb-1/d (open squares) or ahrd/d (filled squares). P-values associated with treatment differences indicate significance regardless of genotype upon co-CHL administration (P < 0.01).
CHL and Chl effects on DBP-dependent transplacental lung cancer
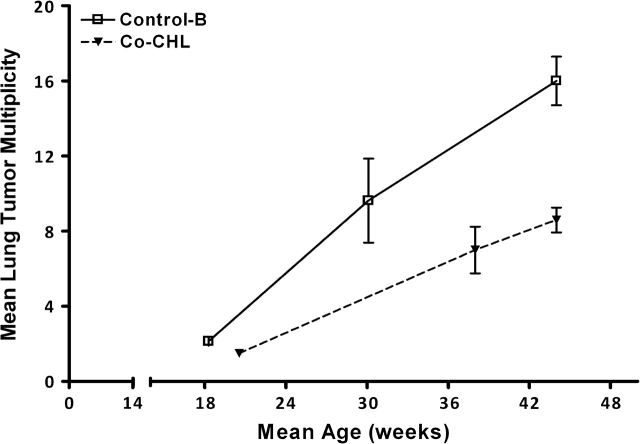
As we have previously reported with this model, all mice exposed in utero to DBP and surviving to 10 months of age exhibited multiple lung lesions. The chemoprotective properties of I3C and green tea have been examined in this model and effectively reduced lung tumor burden by 35 and 32% in mice reaching 10 months of age (20,21). In the current study, maternal consumption of spinach, CHL or Chl in the diet did not provide any significant protective effects against DBP-dependent lung cancer. However, as with DBP-dependent lymphoma mortality, the ability of CHL to protect against lung tumor burden did prove significant if it was coadministered with DBP. Mice born to these mothers receiving the co-gavage of CHL and surviving to 10 months of age had ∼51% fewer lung tumors (Table II, P < 0.01). Interestingly, although the vehicle used for delivery of DBP (corn oil versus TCP) had an impact on lymphoma mortality, mice surviving to 10 months of age had very similar levels of lung tumor multiplicity, 14.5 versus 15.2 for corn oil and TCP, respectively. Additionally, we also documented the time course of lung tumor multiplicity from 3 to 10 months of age. Cotreatment of CHL with DBP provided protection throughout the entire duration of the tumor study (Figure 3). If reduction in DBP bioavailability accounted for the extent of transplacental chemoprotection observed with corn oil versus TCP, one would have to account for the difference between fetal target tissues. The genotype of the offspring was not a significant factor in the degree of chemoprevention observed by coadministration of CHL (Table II). A potential explanation could lie in differences in DBP dose response among fetal target organs and end points in this model. As previously reported in a 10 000 animal dose–dose matrix experiment (8), DBP dose responses for liver tumor incidence and tumor multiplicity in the rainbow trout model were not linear, but instead reached a plateau or optimum at higher DBP doses. As a consequence, CHL chemoprevention in this organ was observed only at CHL doses sufficiently high to bring the ‘effective’ or bioavailable DBP dose below the plateau or optimum region. In contrast, tumor incidence in trout stomach was linear over the entire DBP dose range studied and CHL chemoprevention was observable at every DBP dose.
Fig. 3.
Effects of coadministered CHL on lung tumor burden throughout the study. Offspring were euthanized due to lymphoma-dependent morbidity (3–9 months) or at the conclusion of the study (10 months) and lung lesions (predominantly adenomas) quantified by histopathology. Data indicate offspring born to mothers given 15 mg/kg DBP alone by gavage (in TCP) (open squares) or concurrent with 380 mg/kg CHL (in TCP) (inverted filled triangles). Numbers euthanized in the first group (<21 weeks) were 7 and 2. For the second group (>21 weeks and <44 weeks), 8 and 16 were euthanized. Numbers surviving to the end were 30 and 82 for control-B and co-CHL, respectively. The bars indicate ± SE.
Discussion
Exposure during pregnancy and lactation to chemicals in the environment, including tobacco smoke, has been associated with increases in incidences of disease, malformations or behavior in offspring (23,24,25,26,27,28,29,30,31). A number of chemicals have been shown to be transplacental carcinogens in rodent models (reviewed in refs 32,33) and epidemiology studies suggest that this phenomenon occurs in exposed human populations as well (23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38).
PAHs are formed from the incomplete combustion of organic materials including the burning of coal, petroleum products or tobacco (reviewed in ref. 39) and have been listed as human carcinogens by international agency for research on cancer (40). Increasing energy requirements, especially in countries such as China, are resulting in greater use of coal for energy production; indeed, China derives 70% of its energy from burning coal and the consumption is greater than the USA, European Union and Japan combined (41,42,43,44). In recent years, Chinese physicians have noted an increased incidence of diseases, which they associate with increased fuel emission (44,45,46,47,48). The USA derives ∼50% of its energy from coal. China and the USA also lead the world in automobile use, another important source of environmental PAHs. With respect to environmental exposure to PAHs in the USA, it should also be noted that emissions from China reach the USA west coast in ∼6 days from the point of origin, during which they undergo a process of ‘aging’, reacting with sulfur, oxygen and nitrogen to yield more genotoxic PAH derivatives (49,50).
The fetus and the neonate are at increased risk from the toxicological effects of PAHs and exposure of women who are of childbearing age and women known to be pregnant or nursing should be of concern. We developed a mouse model in which a single treatment with DBP, a few days prior to parturition, produced a severe T-cell lymphoma between 3 and 6 months of age in offspring (17,18,19). If the mice do not succumb to the lymphoma, 100% develop multiple lung tumors and the majority of males also exhibit liver tumors (17,18). Utilizing Cyp1b1-null mice, we demonstrated that DBP-induced transplacental lymphoma mortality is dependent upon Cyp1b1 expression (18). In the human fetus, as in mice, the thymus exhibits the highest expression of CYP1B1 of any organ during late gestation, and among all CYP isoforms, CYP1B1 has the highest activity toward the conversion of DBP to carcinogenic metabolites (51).
We further developed this model for the study of transplacental chemoprevention by dietary agents. Feeding pregnant and lactating mice I3C (20), or providing green tea or caffeine in the drinking water (21), resulted in significant protection for offspring against DBP-dependent T-cell lymphoma mortality and lung tumor multiplicity. One caveat with this model, with respect to statistical analysis of the effect of chemopreventive agents on lung tumors, is the fact that, as many mice die at an early age from lymphoma, we are not statistically assessing a true representative population. In order to assess transplacental chemoprevention efficacy in lung without the confounding lymphoma mortality, we would have to conduct the studies in a different strain such as the A/J mouse. We find this remarkable given that, once weaned, offspring were never exposed to the chemopreventive agent. Therefore, all the chemopreventive benefits had to be from in utero exposure and/or through breast milk. The lymphoma is fatal to mice corresponding in human age to a young adult, with lung tumors developing approximately at the equivalent of human middle age. Thus, modification of the mother's diet during pregnancy (and perhaps lactation) may provide long-term protection from chemical carcinogenesis following in utero exposure, to middle age and beyond.
Previously, we demonstrated the chemopreventive potential of both CHL and Chl in the trout model and in the rat with AFB1 and DBP as the carcinogen (4,5,6,7,8,9,10,11). A preliminary clinical intervention trial in China, where dietary AFB1 exposure is high and HCC represents the major cause of cancer mortality, showed significant protective effects of CHL tablets taken orally at mealtime (14). Results to date point to the importance of simultaneous coadministration of CHL with the carcinogen, supporting the complexation theory for chemoprevention (11,12). Unpublished data in human volunteers given ultralow doses of AFB1 in studies employing accelerator mass spectrometry demonstrated a marked reduction in carcinogen bioavailability when coadministered with CHL or Chl (Bailey et al., unpublished data). These results do not exclude additional potential mechanisms of CHL chemoprevention, including modifications of carcinogen-metabolizing enzymes (52). The study in China documented that some CHL was systemically bioavailable in humans (53) and oral CHL was capable of inhibiting PAH-induced skin cancer in mice when the carcinogen was applied topically (54).
In our mouse model of transplacental cancer, coadministration of CHL provided marked protection against DBP-dependent T-cell lymphoma mortality and lung tumor burden. By design, CHL, Chl and freeze-dried spinach were incorporated into the synthetic AIN93G diet to test for possible systemic effects on tumor development. As mice are nocturnal, most diet would be consumed at night, and there would be little if any agent left in the stomach to interfere with DBP uptake when it was administered hours later. The results point to the importance of complexation as a mechanism of CHL chemoprevention, and presumably also for Chl. The latter possibility remains to be confirmed since we did not include a group with Chl coadministration. However, the results also do not exclude a transient effect, such as inhibition of phase I- or phase II xenobiotic-metabolizing enzymes, or epigenetic mechanisms of chemoprevention, such as alterations in DNA methyl transferase or histone deacetylase, that might return to baseline when DBP dosing took place. In a similar fashion, the administration of CHL by gavage could impact enzymes important for bioactivation/detoxication and potentially exert competitive inhibitory effects at such high concentrations. However, as the major route of PAH exposure (in non-smokers) is dietary (46), our results would indicate that CHL should be administered with each meal for maximum efficacy, as in the China intervention study (14,55). With respect to the impact of the ahr genotype, coadministration of CHL appeared to eliminate genotype sensitivity rather than making it more complex. There is no statistical difference between CHL cotreatment with respect to response by the b-1/d and d/d ahr genotypes.
In summary, CHL, which is inexpensive and appears to lack toxicity in humans, was demonstrated to be effective in the reduction of transplacental cancer risk if given with the PAH carcinogen DBP. This protection was evident even with tumors that appeared well into adult life and is a further example of the ‘fetal basis of disease’. Cancer is the number two cause of death in children/young adults (accidents being number one) and lymphoma/leukemias are the most common of these cancers. Lung cancer is the major cause of cancer mortality in both sexes in the USA and has a relatively poor prognosis (5 year survival rate of 15%). For these reasons, chemopreventive strategies that begin early in development have the potential to reduce the suffering (as well as the health care dollars) associated with cancer, and perhaps other chronic diseases.
Funding
Public Health Service (CA90890 to R.H.D., G.S.B., D.E.W.; ES07060 to D.J.C.; ES00210 to C.V.L.) from the National Institutes of Health and by the Cancer Chemoprotection Program of The Linus Pauling Institute at Oregon State University (R.H.D., G.S.B., D.E.W.); Laboratory-Directed Research and Development Program at the Pacific Northwest National Laboratory operated by Battelle for the U.S. Department of Energy under contract DE-AC06-76RLO1830.
Acknowledgments
The authors wish to thank Dr Michael Simonich for his technical help. In addition, we thank Mandy Louderback, Tracy Filley and Laura Magaña for their excellent animal care and for their provided assistance during necropsies. Thanks also go to the staff of Laboratory Animal Services at Oregon State University.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- AFB1
aflatoxin B1
- ahr
aryl hydrocarbon receptor
- Chl
chlorophyll
- CHL
chlorophyllin
- DBP
dibenzo[a,l]pyrene
- HCC
hepatocellular carcinoma
- I3C
indole-3-carbinol
- PAH
polycyclic aromatic hydrocarbon
- TCP
tricaprylin
References
- 1.Young RW, et al. Use of chlorophyllin in the care of geriatric patients. J Am. Geriatr. Soc. 1980;28:46–47. doi: 10.1111/j.1532-5415.1980.tb00124.x. [DOI] [PubMed] [Google Scholar]
- 2.Dashwood R. Chlorophylls as anticarcinogens. Int. J. Oncol. 1997;10:721–727. doi: 10.3892/ijo.10.4.721. [DOI] [PubMed] [Google Scholar]
- 3.Negishi T, et al. Antigenotoxic activity of natural chlorophylls. Mutat. Res. 1997;376:97–100. doi: 10.1016/s0027-5107(97)00030-4. [DOI] [PubMed] [Google Scholar]
- 4.Breinholt V, et al. Dietary chlorophyllin is a potent inhibitor of aflatoxin B1 hepatocarcinogenesis in rainbow trout. Cancer Res. 1995;55:57–62. [PubMed] [Google Scholar]
- 5.Dashwood R, et al. Chemopreventive properties of chlorophylls towards aflatoxin B1: a review of the antimutagenicity and anticarcinogenicity data in rainbow trout. Mutat. Res. 1998;399:245–253. doi: 10.1016/s0027-5107(97)00259-5. [DOI] [PubMed] [Google Scholar]
- 6.Breinholt V, et al. Chlorophyllin chemoprevention in trout initiated by aflatoxin B(1) bath treatment: an evaluation of reduced bioavailability vs. target organ protective mechanisms. Toxicol. Appl. Pharmacol. 1999;158:141–151. doi: 10.1006/taap.1999.8696. [DOI] [PubMed] [Google Scholar]
- 7.Reddy AP, et al. Inhibition of dibenzo[a,l]pyrene-induced multi-organ carcinogenesis by dietary chlorophyllin in rainbow trout. Carcinogenesis. 1999;20:1919–1926. doi: 10.1093/carcin/20.10.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pratt MM, et al. The importance of carcinogen dose in chemoprevention studies: quantitative interrelationships between, dibenzo[a,l]pyrene dose, chlorophyllin dose, target organ DNA adduct biomarkers and final tumor outcome. Carcinogenesis. 2007;28:611–624. doi: 10.1093/carcin/bgl174. [DOI] [PubMed] [Google Scholar]
- 9.Simonich MT, et al. Low-dose dietary chlorophyll inhibits multi-organ carcinogenesis in the rainbow trout. Food Chem. Toxicol. 2008;46:1014–1024. doi: 10.1016/j.fct.2007.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simonich MT, et al. Natural chlorophyll inhibits aflatoxin B1-induced multi-organ carcinogenesis in the rat. Carcinogenesis. 2007;28:1294–1302. doi: 10.1093/carcin/bgm027. [DOI] [PubMed] [Google Scholar]
- 11.Breinholt V, et al. Mechanisms of chlorophyllin anticarcinogenesis against aflatoxin B1: complex formation with the carcinogen. Chem. Res. Toxicol. 1995;8:506–514. doi: 10.1021/tx00046a004. [DOI] [PubMed] [Google Scholar]
- 12.Arimoto S, et al. Binding of polycyclic planar mutagens to chlorophyllin resulting in inhibition of the mutagenic activity. Mutat. Res. 1993;287:293–305. doi: 10.1016/0027-5107(93)90022-8. [DOI] [PubMed] [Google Scholar]
- 13.Yu SZ. Primary prevention of hepatocellular carcinoma. J. Gastroenterol. Hepatol. 1995;10:674–682. doi: 10.1111/j.1440-1746.1995.tb01370.x. [DOI] [PubMed] [Google Scholar]
- 14.Egner PA, et al. Chlorophyllin intervention reduces aflatoxin-DNA adducts in individuals at high risk for liver cancer. Proc. Natl Acad. Sci. USA. 2001;98:14601–14606. doi: 10.1073/pnas.251536898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jubert C, et al. Isolation of chlorophylls a and b from spinach by counter-current chromatography. J. Chromatogr. A. 2007;1140:95–100. doi: 10.1016/j.chroma.2006.11.063. [DOI] [PubMed] [Google Scholar]
- 16.Brown K, et al. Accelerator mass spectrometry for biomedical research. Meth. Enzymol. 2005;402:423–443. doi: 10.1016/S0076-6879(05)02014-8. [DOI] [PubMed] [Google Scholar]
- 17.Yu Z, et al. In utero exposure of mice to dibenzo[a,l]pyrene produces lymphoma in the offspring: role of the aryl hydrocarbon receptor. Cancer Res. 2006;66:755–762. doi: 10.1158/0008-5472.CAN-05-3390. [DOI] [PubMed] [Google Scholar]
- 18.Castro DJ, et al. Fetal mouse Cyp1b1 and transplacental carcinogenesis from maternal exposure to dibenzo(a,l)pyrene. Cancer Prev. Res. 2008;1:128–134. doi: 10.1158/1940-6207.CAPR-07-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castro DJ, et al. Mohan RM, et al., editors. A model for the study of maternal dietary inhibition of transplacental carcinogenesis. Recent Advances in Carcinogenesis. in press. [Google Scholar]
- 20.Yu Z, et al. Indole-3-carbinol in the maternal diet provides chemoprotection for the fetus against transplacental carcinogenesis by the polycyclic aromatic hydrocarbon dibenzo[a,l]pyrene. Carcinogenesis. 2006;27:2116–2123. doi: 10.1093/carcin/bgl072. [DOI] [PubMed] [Google Scholar]
- 21.Castro DJ, et al. Chemoprevention of dibenzo[a,l]pyrene transplacental carcinogenesis in mice born to mothers administered green tea: primary role of caffeine. Carcinogenesis. 2008;29:1581–1586. doi: 10.1093/carcin/bgm237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gibbons JD. Nonparametric Statistical Inference. Marcel Dekker Ltd., New York, NY; 1985. [Google Scholar]
- 23.Perera F, et al. DNA damage from polycyclic aromatic hydrocarbons measured by benzo[a]pyrene-DNA adducts in mothers and newborns from Northern Manhattan, the World Trade Center Area, Poland, and China. Cancer Epidemiol. Biomarkers Prev. 2005;14:709–714. doi: 10.1158/1055-9965.EPI-04-0457. [DOI] [PubMed] [Google Scholar]
- 24.Chang JS, et al. Parental smoking and the risk of childhood leukemia. Am. J. Epidemiol. 2006;163:1091–1100. doi: 10.1093/aje/kwj143. [DOI] [PubMed] [Google Scholar]
- 25.Choi H, et al. International studies of prenatal exposure to polycyclic aromatic hydrocarbons and fetal growth. Environ. Health Perspect. 2006;114:1744–1750. doi: 10.1289/ehp.8982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perera FP, et al. Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner-city children. Environ. Health Perspect. 2006;114:1287–1292. doi: 10.1289/ehp.9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perera FP, et al. Effects of transplacental exposure to environmental pollutants on birth outcomes in a multiethnic population. Environ. Health Perspect. 2003;111:201–205. doi: 10.1289/ehp.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jedrychowski W, et al. Maternal smoking during pregnancy and postnatal exposure to environmental tobacco smoke as predisposition factors to acute respiratory infections. Environ. Health Perspect. 1997;105:302–306. doi: 10.1289/ehp.97105302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keeler GJ, et al. Assessment of personal and community-level exposures to particulate matter among children with asthma in Detroit, Michigan, as part of Community Action Against Asthma (CAAA) Environ. Health Perspect. 2002;110(suppl. 2):173–181. doi: 10.1289/ehp.02110s2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flower KB, et al. Cancer risk and parental pesticide application in children of Agricultural Health Study participants. Environ. Health Perspect. 2004;112:631–635. doi: 10.1289/ehp.6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shu XO, et al. Parental exposure to medications and hydrocarbons and ras mutations in children with acute lymphoblastic leukemia: a report from the Children's Oncology Group. Cancer Epidemiol. Biomarkers Prev. 2004;13:1230–1235. [PubMed] [Google Scholar]
- 32.Anderson LM. Introduction and overview. Perinatal carcinogenesis: growing a node for epidemiology, risk management, and animal studies. Toxicol. Appl. Pharmacol. 2004;199:85–90. doi: 10.1016/j.taap.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 33.Anderson LM, et al. Critical windows of exposure for children's health: cancer in human epidemiological studies and neoplasms in experimental animal models. Environ. Health Perspect. 2000;108(suppl. 3):573–594. doi: 10.1289/ehp.00108s3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lightfoot TJ, et al. Causes of childhood leukaemia and lymphoma. Toxicol. Appl. Pharmacol. 2004;199:104–117. doi: 10.1016/j.taap.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 35.Alexander FE, et al. Transplacental chemical exposure and risk of infant leukemia with MLL gene fusion. Cancer Res. 2001;61:2542–2546. [PubMed] [Google Scholar]
- 36.Perera F, et al. In utero DNA damage from environmental pollution is associated with somatic gene mutation in newborns. Cancer Epidemiol. Biomarkers Prev. 2002;11:1134–1137. [PubMed] [Google Scholar]
- 37.Reynolds P, et al. Childhood cancer and agricultural pesticide use: an ecologic study in California. Environ. Health Perspect. 2002;110:319–324. doi: 10.1289/ehp.02110319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma X, et al. Critical windows of exposure to household pesticides and risk of childhood leukemia. Environ. Health Perspect. 2002;110:955–960. doi: 10.1289/ehp.02110955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luch A. Polycyclic aromatic hydrocarbon-induced carcinogenesis—an introduction. In: Luch A, editor. The Carcinogenic Effects of Polycyclic Aromatic Hydrocarbons. London: Imperial College Press; 2005. pp. 1–18. [Google Scholar]
- 40.International Agency for Research on Cancer. Polynuclear Aromatic Compounds. Lyon: International Agency for Research on Cancer; 1983. IARC monographs on the evaluation of the carcinogenic risk of chemicals on humans. [Google Scholar]
- 41.Xu S, et al. Emission of polycyclic aromatic hydrocarbons in China. Environ. Sci. Technol. 2006;40:702–708. doi: 10.1021/es0517062. [DOI] [PubMed] [Google Scholar]
- 42.Tao S, et al. Dispersion modeling of polycyclic aromatic hydrocarbons from combustion of biomass and fossil fuels and production of coke in Tianjin, China. Environ. Sci. Technol. 2006;40:4586–4591. doi: 10.1021/es060220y. [DOI] [PubMed] [Google Scholar]
- 43.Primbs T, et al. Atmospheric outflow of anthropogenic semivolatile organic compounds from East Asia in spring 2004. Environ. Sci. Technol. 2007;41:3551–3558. doi: 10.1021/es062256w. [DOI] [PubMed] [Google Scholar]
- 44.Zhang JJ, et al. Household air pollution from coal and biomass fuels in China: measurements, health impacts, and interventions. Environ. Health Perspect. 2007;115:848–855. doi: 10.1289/ehp.9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao Y, et al. Air pollution and lung cancer risks in China—a meta-analysis. Sci. Total Environ. 2006;366:500–513. doi: 10.1016/j.scitotenv.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 46.Yu IT, et al. Dose-response relationship between cooking fumes exposures and lung cancer among Chinese nonsmoking women. Cancer Res. 2006;66:4961–4967. doi: 10.1158/0008-5472.CAN-05-2932. [DOI] [PubMed] [Google Scholar]
- 47.Watts J. Doctors blame air pollution for China's asthma increases. Lancet. 2006;368:719–720. doi: 10.1016/S0140-6736(06)69267-2. [DOI] [PubMed] [Google Scholar]
- 48.Mumford JL, et al. Human exposure and dosimetry of polycyclic aromatic hydrocarbons in urine from Xuan Wei, China with high lung cancer mortality associated with exposure to unvented coal smoke. Carcinogenesis. 1995;16:3031–3036. doi: 10.1093/carcin/16.12.3031. [DOI] [PubMed] [Google Scholar]
- 49.Feilberg A, et al. Observations of the effect of atmospheric processes on the genotoxic potency of airborne particulate matter. Atmos Environ. 2002;36:4617–4625. [Google Scholar]
- 50.Reisen F, et al. Atmospheric reactions influence seasonal PAH and nitro-PAH concentrations in the Los Angeles basin. Environ. Sci. Technol. 2005;39:64–73. [PubMed] [Google Scholar]
- 51.Luch A, et al. Stable expression of human cytochrome P450 1B1 in V79 Chinese hamster cells and metabolically catalyzed DNA adduct formation of dibenzo[a,l]pyrene. Chem. Res. Toxicol. 1998;11:686–695. doi: 10.1021/tx970236p. [DOI] [PubMed] [Google Scholar]
- 52.Yun CH, et al. Non-specific inhibition of cytochrome P450 activities by chlorophyllin in human and rat liver microsomes. Carcinogenesis. 1995;16:1437–1440. doi: 10.1093/carcin/16.6.1437. [DOI] [PubMed] [Google Scholar]
- 53.Egner PA, et al. Identification and characterization of chlorin e(4) ethyl ester in sera of individuals participating in the chlorophyllin chemoprevention trial. Chem. Res. Toxicol. 2000;13:900–906. doi: 10.1021/tx000069k. [DOI] [PubMed] [Google Scholar]
- 54.Park KK, et al. Chemopreventive activity of chlorophyllin against mouse skin carcinogenesis by benzo[a]pyrene and benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide. Cancer Lett. 1996;102:143–149. doi: 10.1016/0304-3835(96)04173-0. [DOI] [PubMed] [Google Scholar]
- 55.Egner PA, et al. Chemoprevention with chlorophyllin in individuals exposed to dietary aflatoxin. Mutat. Res. 2003;523–524:209–216. doi: 10.1016/s0027-5107(02)00337-8. [DOI] [PubMed] [Google Scholar]