Abstract
Dysregulated innate responses, particularly excessive activation of interferon (IFN) pathways, have been implicated in the development of autoimmune pathologies. Autoreactivity frequently targets IFN-inducible genes such as the Ro autoantigens, which ubiquitinate and inhibit interferon regulatory factors (IRFs). A new study validates the role of these common autoantigens in preventing autoimmunity. The findings reveal that injury-induced systemic autoimmune disease is exacerbated in the absence of Ro52/Trim21 and is driven by the IL-23–Th17 pathway.
Systemic autoimmune diseases, such as systemic lupus erythematosus (SLE), systemic sclerosis, and Sjogren's syndrome (SS), are commonly characterized by circulating immunoglobulin G autoantibodies that include those specific for nuclear antigens (Sawalha and Harley, 2004; Routsias and Tzioufas, 2007; Koenig et al., 2008). It is generally accepted that these antibodies trigger pathogenic responses by forming immune complexes with ubiquitous antigens and consequently activating effector responses such as proinflammatory cytokine production. Indeed, the severity of these systemic rheumatic diseases correlates with high levels of inflammatory cytokines, particularly type I IFNs and IL-12/23p40 (Baechler et al., 2004; Crow, 2007; Wong et al., 2008).
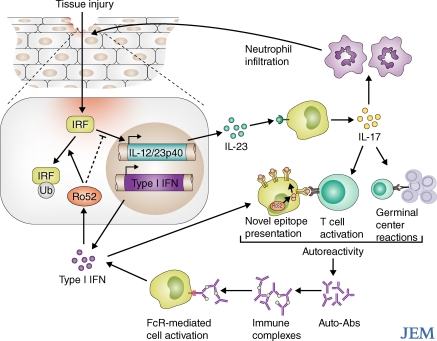
Given that the presence of autoantibodies is often the first sign of autoimmune disease, and that these antibody responses persist, lymphocyte autoreactivity is commonly viewed as the initiating event leading to chronic inflammatory conditions. In a strictly linear model, B cell autoreactivity against nuclear antigens is triggered by the synergistic activation of the B cell receptor and nucleic acid–binding TLRs. In this model, the subsequent inflammatory condition is a direct consequence of self-reactive antibodies produced by these activated B cells (Marshak-Rothstein, 2006). Yet, additional factors can complicate this simple linear model. B cell autoreactivity can be further augmented by the components of inflammation itself. For instance, IFN-α up-regulates TLR expression (Thibault et al., 2008), and, as a result, B cells remain sensitive to inflammatory signals and are more responsive to the adjuvant effect of TLR-binding nucleic acids. Additionally, inflammatory cytokines bolster multiple arms of the immune response, which helps sustain the proinflammatory state. For example, type I IFN can extend the activated T cell response, enhance humoral immunity, and promote antigen presentation (Blanco et al., 2001; Le Bon et al., 2001; Marrack et al., 1999). If unchecked, these normally beneficial responses can be pathological. Thus, systemic autoimmune disease could result from continuous inflammatory signals that create a feedback amplification loop of autoreactive pathological responses, resulting in systemic disease (Fig. 1). On p. 1661 of this issue, Espinosa et al. (2009) describe an animal model in which local injury–elicited inflammation initiates systemic autoreactivity, providing an example of the reciprocal nature of autoreactivity and inflammation.
Figure 1.
Amplification loop of inflammation and autoreactivity. Injury-induced inflammatory pathways, including IL-23/Il-17 and type I IFN, can amplify autoreactive conditions by increasing the likelihood of B cell–T cell interaction and by promoting the presentation of self-antigens. Autoantibodies that have been produced in these conditions further propagate the inflammatory pathology by forming immune complexes that activate effector cells. IRFs are essential for the IL-23–IFN pathway, and their activity can be down-regulated by IFN-inducible factors such as the Ro52 ubiquitin ligase. Ro52 and other IFN-inducible factors commonly provide novel epitopes for autoreactivity.
Cytokines in inflammation and autoimmunity
Both gene expression profiling and genetic studies have revealed an association between the type I IFN pathway and susceptibility to the autoimmune disease SLE. Microarray analysis of PBMCs from lupus patients demonstrated increased expression of a common set of IFN-inducible genes (Ly6E, Oasl, Ifit1,Stat1, Mx1, Mx2, Plscr1, and Irf7), which are called the “lupus IFN signature” (Baechler et al., 2003, 2004; Bennett et al., 2003). The IFN gene signature also appears to reflect an IFN protein signature, as severity of disease was shown to correlate with increased levels of type I IFN-inducible chemokines in the serum (Crow, 2007). The link between IFN pathways and autoimmune disease is reinforced by the strong genetic linkage between genes regulating the IFN signature and disease, the most prominent being Irf5 and Tyk2 (Shaw et al., 2003; Sigurdsson et al., 2005; Kozyrev and Alarcon-Riquelme, 2007). IRF-5 is required for TLR-mediated activation of inflammatory cytokines and type I IFN, and TYK2 is a tyrosine kinase associated with type I IFN signaling. The current view is that enhanced activity of either IRF5 or TYK2 accelerates type I IFN production and/or signaling and exacerbates autoreactive inflammatory pathology. The resultant high levels of type I IFN and of type I IFN-inducible genes in SLE patients may contribute to a vicious positive feedback loop that leads to chronic inflammation and autoimmunity. Other cytokines induced by IRFs, including IL-6, TNF, IL-12/IL-23p40, and ultimately IL-17 (Tailor et al., 2006), can subsequently amplify autoreactivity by way of T cell activation, germinal center expansion, B cell survival, neutrophil infiltration, and TLR up-regulation (Le Bon et al., 2001; Hsu et al., 2008; Thibault et al., 2008; Doreau et al., 2009).
The relevance of the type I IFN and IL-17 pathways in the development of systemic autoimmune disease has been revealed in several studies. In humans, IFN-α–treated subjects often test positive for antinuclear antibodies, although few develop autoimmune pathology (Kälkner et al., 1998). Mutations in genes encoding the antiviral nucleases TREX1 and RNaseH2 are associated with an increased production of IFN-α and are linked to both the Aicardi-Goutieres syndrome and early-onset SLE (Crow et al., 2006; Rice et al., 2007, 2009; Stetson et al., 2008). In lupus-prone mice, injecting IFN-α accelerates disease (Mathian et al., 2005). In addition, elevated levels of IL-17 and IL-23 have been found in lupus patients (Crispín et al., 2008; Doreau et al., 2009) and in animal models. However, definitive proof of the involvement of these cytokines in lupus pathology remains elusive, as genetic deletions of these cytokines in mice have generated conflicting results (Garrett-Sinha et al., 2008).
Although type I IFN is primarily associated with the induction of inflammatory cytokines, it also induces regulators that counteract inflammation. Among the IFN-inducible factors that provide negative feedback regulation in inflammation are some members of the tripartite motif (TRIM) family of proteins, which are important components of antiviral defense (Ozato et al., 2008). TRIM21, also called Ro52, is frequently a target autoantigen in rheumatic diseases (Schulte-Pelkum et al., 2009). The characterization of Ro52-deficient mice reported by Espinosa et al. (2009) in this issue connects Ro52 to an IFN-associated negative feedback loop that prevents unrestrained inflammation.
Ro/SS-A antigens: targets or culprits?
Autoantibodies to the Ro antigen (also called SS-A, for SS type A antigen), which are associated with UV hypersensitivity, are frequently detected serological autoimmune markers in rheumatoid diseases (Schulte-Pelkum et al., 2009). Anti-Ro sera may be directed against two different polypeptides with seemingly unrelated functions: Ro52 and Ro60. Ro52, an E3 ligase that ubiquitinates various members of the IRF family, is an IFN-inducible protein of the TRIM family that translocates to the nucleus upon IFN-α stimulation (Rhodes et al., 2002; Strandberg et al., 2008). Although its role in regulating the IFN pathway has been controversial, there is evidence that Ro52 can both promote and abrogate inflammation. Ubiquitination of IRF8 by Ro52 amplifies IL12p40 production and plays an essential role in promoting IFN I production during antiviral responses (Kong et al., 2007). On the other hand, Ro52 negatively regulates type I IFN by ubiquitin-mediated degradation of IRF3 (Higgs et al., 2008).
Ro60 is part of the Ro/La ribonucleoprotein (RNP) complex that associates with a small cytoplasmic RNA (RNA-Y) of unknown function. Analysis of its crystal structure suggests that Ro60 binds and stabilizes misfolded RNA (Stein et al., 2005). Mice deficient in Ro60 develop a lupus-like syndrome, although with a substantial genetic component (Xue et al., 2003). It has been hypothesized that misfolded RNA might accumulate in the absence of Ro60 and cause a breach of tolerance by exposing normally cryptic determinants to the immune system. It was also suggested that UV damage might induce the accumulation of misfolded RNA in the absence of Ro60, provoking an inflammatory response. Ro60's protective role in eliminating autoimmunity-inducing nucleic acids might parallel the preventive function suggested for the cytoplasmic nucleases TREX1 and RNaseH2, which are both strongly linked to autoimmune susceptibility (Crow et al., 2006; Rice et al., 2007, 2009; Stetson et al., 2008). Alternatively, Ro60 deficiency could result in immune dysregulation of the type now reported for Ro52-deficient mice.
Espinosa et al. (2009) show that Ro52-deficient mice develop uncontrolled inflammation and systemic autoimmunity as a consequence of minor tissue injury caused by ear tagging. The general autoimmune pathology and neutrophil recruitment to the site of injury were completely IL-23 dependent, as they were not observed when mice lacking Ro52 were crossed to IL-23p19–deficient animals. Characterization of immune cells derived from Ro52-deficient mice demonstrated that in addition to ubiquitinating the previously reported targets IRF3 and IRF8, Ro52 was required for polyubiquitination and degradation of IRF5.
The authors also report that Ro52-deficient bone marrow–derived macrophages and splenocytes released more inflammatory cytokines (IL-6, TNF, type I IFN, and IL-23) upon TLR activation. Overall, these data demonstrate that the ubiquitin ligase Ro52 is induced by IFN activation of immune cells, where it acts as a negative regulator of IFN signaling. A recent study by Yoshimi et al. supports these conclusions and also shows that Ro52 is a negative regulator of IFN I in vivo (Yoshimi et al., 2009).
The phenotype of Ro52-deficient mice thus provides a mechanistic view of how environmental factors could initiate autoimmune disease. For example, localized tissue injury from UV light damage or infection could provoke unrestricted IL-23/IL-17–mediated inflammation and subsequent autoimmunity in susceptible individuals.
Interestingly, the fact that Ro52 itself is a common autoantigen in rheumatic diseases and that other IFN-inducible proteins, including Ifi202, Ifi16, and Hsp70, have been identified as autoantigens in murine and human disease, reveals that IFN-inducible genes are often the targets of self-reactivity (Hueber et al., 2004; Zhuang et al., 2005). The autoantigen Ifi202 is both a lupus susceptibility gene and a regulator of nucleic acid-triggered inflammation by inhibiting the DNA sensor AIM2 (Roberts et al., 2009; Rozzo et al., 2001). TRIM68, also called SS-56, is another IFN-inducible autoantigen found in patients with SLE and Sjogren's disease that is structurally similar to Ro52 (Rice et al., 2009). Thus, TRIM antigens and Ifi202 not only control IFN responses and behave as autoimmune suppressors, but they are also common targets of autoreactivity. The regulatory role of Ro52 in inflammation provides a possible explanation for the correlation between genetic polymorphisms in Ro52 with disease and increased anti-Ro antibodies (Nakken et al., 2001).
It is possible that autoreactivity against IFN-inducible proteins could be a consequence of enhanced antigen presentation capacity of IFN-activated APCs. In fact, a major effect of type I IFN is to enhance dendritic cell maturation (Blanco et al., 2001; Le Bon et al., 2001; Longhi et al., 2009). Intriguingly, because some of these factors translocate to the nucleus and interact with RNA upon IFN activation, their antigenicity could be the result of RNA-mediated TLR activation combined with novel epitope presentation. RNP complexes contain RNA-Ys, which have been shown to promote dendritic cell maturation and IFN production (Kelly et al., 2006). These effects are dependent on the TLR adaptor protein MyD88 and endosome acidification, pointing to an endogenous adjuvant effect through TLR7 activation. The potential association of Ro52 with RNA-binding molecules in IFN-induced supramolecular complexes could promote the presentation of Ro52 epitopes upon translocation to the nucleus or other cellular compartments.
Although the association of Ro52 with Ro60 and RNPs has not been conclusively demonstrated, antibodies against Ro52 and Ro60 arise concurrently in SLE patients and epitope spreading between Ro52 and members of the RNP complex has been repeatedly observed after immunization in animal models (Deshmukh et al., 2005). It is still unclear how the epitope spreading occurs, although various pathways have been invoked that may act at various stages of disease. Cross-reactive antibodies toward epitopes within Ro52 and Ro60 could develop during the response, T cells could provide help to B cells presenting self-epitopes derived from macromolecular complexes, or immune complexes taken up through Fc receptors could be subsequently presented by an activated APC (Fig. 1). Any of these mechanisms could account for the fact that peripheral T cell tolerance against these Ro and RNP antigens seems to be particularly fragile both in human and mice.
TRIM factors and innate immunity
The TRIM superfamily includes >60 members whose functions are generally linked to the regulation of innate responses and viral defense (Ozato et al., 2008). The tripartite motif present in TRIM molecules comprises a RING domain, B-box domain, and a coiled–coil domain. RING domains mediate the conjugation of proteins with ubiquitin or other ubiquitin-like molecules, and coiled–coil domains promote self-aggregation. Similar to Ro52, many TRIM proteins are IFN-inducible E3 ubiquitin ligases. Interestingly, TRIM proteins can participate in viral innate immunity in different ways. Some have direct antiviral functions. For instance, TRIM5α interferes with HIV viral preintegration complex uncoating (Sayah et al., 2004; Stremlau et al., 2004), and TRIM19, or PML, inhibits the growth of numerous RNA and DNA viruses (Chelbi-Alix et al., 1998). Other TRIM proteins modulate antiviral responses by regulating IRF and NF-κB pathways. TRIM25's ability to ubiquitinate and subsequently activate the CARD domain in RIG-I activates NF-κB and induces IFN production. (Gack et al., 2007) The degradation of TAB2 and TAB3 by TRIM30, on the other hand, inhibits TRAF6 and inactivates NF-kB (Shi et al., 2008). The discovery by Espinosa et al. (2009) that Ro52 deficiency leads to autoimmunity expands our knowledge on the delicate balance orchestrated by TRIM factors to modulate immune responses and highlights its important regulatory function. Thus, although a robust induction of antiviral inflammatory responses is beneficial to combat viral infections, an unchecked response can lead to enhanced immunopathology and/or autoimmunity. In this respect, it will be interesting to investigate whether Ro52−/− animals are less susceptible to virus infection because of chronic activation of the type I IFN antiviral response.
Final thoughts
Systemic autoimmune diseases resemble responses to chronic viral infections that consequently result in an unrestrained, autoreactive amplification loop. It is possible that the main factor determining whether normally healthy immune responses turn into chronic autoimmune disease is not the intensity of the response or the incidence of autoreactivity at a given moment, but instead the ability to abort a self-propagating loop of inflammation and autoreactivity. This requires an exquisite balance between initiating effective immune responses and promptly terminating them once they are no longer beneficial, potentially explaining why genes involved in the regulation of inflammatory pathways, such as Ro52 and IRFs, are often found to be genetically associated with autoimmune disease. Modest alterations in gene dose or expression could disrupt this delicate balance and perpetuate a pathological feedback loop. Thus, targeting Ro52 activity may be useful for treating autoimmunity or even for enhancing antiviral vaccine responses.
Acknowledgments
Research in the Garcia-Sastre laboratory is supported by National Institutes of Health (NIH) grants R01AI46954, P01AI058113, P01AI082325, U01AI070469, U54 AI057158, and U19AI083025 and by a National Institutes of Allergy and Infectious Disease (NIAID)–funded Center for Research on Influenza Pathogenesis (HHSN266200700010C). Research in the Bolland laboratory is supported by the Intramural Research Program of the NIH, NIAID.
References
- Baechler E.C., Batliwalla F.M., Karypis G., Gaffney P.M., Ortmann W.A., Espe K.J., Shark K.B., Grande W.J., Hughes K.M., Kapur V., et al. 2003. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus.Proc. Natl. Acad. Sci. USA. 100:2610–2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baechler E.C., Gregersen P.K., Behrens T.W. 2004. The emerging role of interferon in human systemic lupus erythematosus.Curr. Opin. Immunol. 16:801–807 [DOI] [PubMed] [Google Scholar]
- Bennett L., Palucka A.K., Arce E., Cantrell V., Borvak J., Banchereau J., Pascual V. 2003. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood.J. Exp. Med. 197:711–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco P., Palucka A.K., Gill M., Pascual V., Banchereau J. 2001. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus.Science. 294:1540–1543 [DOI] [PubMed] [Google Scholar]
- Chelbi-Alix M.K., Quignon F., Pelicano L., Koken M.H., de Thé H. 1998. Resistance to virus infection conferred by the interferon-induced promyelocytic leukemia protein.J. Virol. 72:1043–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispín J.C., Oukka M., Bayliss G., Cohen R.A., Van Beek C.A., Stillman I.E., Kyttaris V.C., Juang Y.T., Tsokos G.C. 2008. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys.J. Immunol. 181:8761–8766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow M.K. 2007. Type I interferon in systemic lupus erythematosus.Curr. Top. Microbiol. Immunol. 316:359–386 [DOI] [PubMed] [Google Scholar]
- Crow Y.J., Leitch A., Hayward B.E., Garner A., Parmar R., Griffith E., Ali M., Semple C., Aicardi J., Babul-Hirji R., et al. 2006. Mutations in genes encoding ribonuclease H2 subunits cause Aicardi-Goutières syndrome and mimic congenital viral brain infection.Nat. Genet. 38:910–916 [DOI] [PubMed] [Google Scholar]
- Deshmukh U.S., Bagavant H., Lewis J., Gaskin F., Fu S.M. 2005. Epitope spreading within lupus-associated ribonucleoprotein antigens.Clin. Immunol. 117:112–120 [DOI] [PubMed] [Google Scholar]
- Doreau A., Belot A., Bastid J., Riche B., Trescol-Biemont M.C., Ranchin B., Fabien N., Cochat P., Pouteil-Noble C., Trolliet P., et al. 2009. Interleukin 17 acts in synergy with B cell-activating factor to influence B cell biology and the pathophysiology of systemic lupus erythematosus.Nat. Immunol. 10:778–785 [DOI] [PubMed] [Google Scholar]
- Espinosa A., Dardalhon V., Brauner S., Ambrosi A., Higgs R., Quintana F.J., Sjöstrand M., Eloranta M.-L., Ní Gabhann J., Winqvist O., et al. 2009. Loss of the lupus autoantigen Ro52/Trim21 induces tissue inflammation and systemic autoimmunity by disregulating the IL-23–Th17 pathway.J. Exp. Med. 206:1661–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gack M.U., Shin Y.C., Joo C.H., Urano T., Liang C., Sun L., Takeuchi O., Akira S., Chen Z., Inoue S., Jung J.U. 2007. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity.Nature. 446:916–920 [DOI] [PubMed] [Google Scholar]
- Garrett-Sinha L.A., John S., Gaffen S.L. 2008. IL-17 and the Th17 lineage in systemic lupus erythematosus.Curr. Opin. Rheumatol. 20:519–525 [DOI] [PubMed] [Google Scholar]
- Higgs R., Ní Gabhann J., Ben Larbi N., Breen E.P., Fitzgerald K.A., Jefferies C.A. 2008. The E3 ubiquitin ligase Ro52 negatively regulates IFN-beta production post-pathogen recognition by polyubiquitin-mediated degradation of IRF3.J. Immunol. 181:1780–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H.C., Yang P., Wang J., Wu Q., Myers R., Chen J., Yi J., Guentert T., Tousson A., Stanus A.L., et al. 2008. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice.Nat. Immunol. 9:166–175 [DOI] [PubMed] [Google Scholar]
- Hueber W., Zeng D., Strober S., Utz P.J. 2004. Interferon-alpha-inducible proteins are novel autoantigens in murine lupus.Arthritis Rheum. 50:3239–3249 [DOI] [PubMed] [Google Scholar]
- Kälkner K.M., Rönnblom L., Karlsson Parra A.K., Bengtsson M., Olsson Y., Oberg K. 1998. Antibodies against double-stranded DNA and development of polymyositis during treatment with interferon.QJM. 91:393–399 [DOI] [PubMed] [Google Scholar]
- Kelly K.M., Zhuang H., Nacionales D.C., Scumpia P.O., Lyons R., Akaogi J., Lee P., Williams B., Yamamoto M., Akira S., et al. 2006. “Endogenous adjuvant” activity of the RNA components of lupus autoantigens Sm/RNP and Ro 60.Arthritis Rheum. 54:1557–1567 [DOI] [PubMed] [Google Scholar]
- Koenig M., Dieudé M., Senécal J.L. 2008. Predictive value of antinuclear autoantibodies: the lessons of the systemic sclerosis autoantibodies.Autoimmun. Rev. 7:588–593 [DOI] [PubMed] [Google Scholar]
- Kong H.J., Anderson D.E., Lee C.H., Jang M.K., Tamura T., Tailor P., Cho H.K., Cheong J., Xiong H., Morse H.C., III, Ozato K. 2007. Cutting edge: autoantigen Ro52 is an interferon inducible E3 ligase that ubiquitinates IRF-8 and enhances cytokine expression in macrophages.J. Immunol. 179:26–30 [DOI] [PubMed] [Google Scholar]
- Kozyrev S.V., Alarcon-Riquelme M.E. 2007. The genetics and biology of Irf5-mediated signaling in lupus.Autoimmunity. 40:591–601 [DOI] [PubMed] [Google Scholar]
- Le Bon A., Schiavoni G., D'Agostino G., Gresser I., Belardelli F., Tough D.F. 2001. Type i interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo.Immunity. 14:461–470 [DOI] [PubMed] [Google Scholar]
- Longhi M.P., Trumpfheller C., Idoyaga J., Caskey M., Matos I., Kluger C., Salazar A.M., Colonna M., Steinman R.M. 2009. Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J. Exp. Med. 206:1589–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P., Kappler J., Mitchell T. 1999. Type I interferons keep activated T cells alive.J. Exp. Med. 189:521–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshak-Rothstein A. 2006. Toll-like receptors in systemic autoimmune disease.Nat. Rev. Immunol. 6:823–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathian A., Weinberg A., Gallegos M., Banchereau J., Koutouzov S. 2005. IFN-alpha induces early lethal lupus in preautoimmune (New Zealand Black x New Zealand White) F1 but not in BALB/c mice.J. Immunol. 174:2499–2506 [DOI] [PubMed] [Google Scholar]
- Nakken B., Jonsson R., Bolstad A.I. 2001. Polymorphisms of the Ro52 gene associated with anti-Ro 52-kd autoantibodies in patients with primary Sjögren's syndrome.Arthritis Rheum. 44:638–646 [DOI] [PubMed] [Google Scholar]
- Ozato K., Shin D.M., Chang T.H., Morse H.C., III 2008. TRIM family proteins and their emerging roles in innate immunity.Nat. Rev. Immunol. 8:849–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D.A., Ihrke G., Reinicke A.T., Malcherek G., Towey M., Isenberg D.A., Trowsdale J. 2002. The 52 000 MW Ro/SS-A autoantigen in Sjögren's syndrome/systemic lupus erythematosus (Ro52) is an interferon-gamma inducible tripartite motif protein associated with membrane proximal structures.Immunology. 106:246–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice G., Newman W.G., Dean J., Patrick T., Parmar R., Flintoff K., Robins P., Harvey S., Hollis T., O'Hara A., et al. 2007. Heterozygous mutations in TREX1 cause familial chilblain lupus and dominant Aicardi-Goutieres syndrome.Am. J. Hum. Genet. 80:811–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice G.I., Bond J., Asipu A., Brunette R.L., Manfield I.W., Carr I.M., Fuller J.C., Jackson R.M., Lamb T., Briggs T.A., et al. 2009. Mutations involved in Aicardi-Goutières syndrome implicate SAMHD1 as regulator of the innate immune response.Nat. Genet. 41:829–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts T.L., Idris A., Dunn J.A., Kelly G.M., Burnton C.M., Hodgson S., Hardy L.L., Garceau V., Sweet M.J., Ross I.L., et al. 2009. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA.Science. 323:1057–1060 [DOI] [PubMed] [Google Scholar]
- Routsias J.G., Tzioufas A.G. 2007. Sjögren's syndrome—study of autoantigens and autoantibodies.Clin. Rev. Allergy Immunol. 32:238–251 [DOI] [PubMed] [Google Scholar]
- Rozzo S.J., Allard J.D., Choubey D., Vyse T.J., Izui S., Peltz G., Kotzin B.L. 2001. Evidence for an interferon-inducible gene, Ifi202, in the susceptibility to systemic lupus.Immunity. 15:435–443 [DOI] [PubMed] [Google Scholar]
- Sawalha A.H., Harley J.B. 2004. Antinuclear autoantibodies in systemic lupus erythematosus.Curr. Opin. Rheumatol. 16:534–540 [DOI] [PubMed] [Google Scholar]
- Sayah D.M., Sokolskaja E., Berthoux L., Luban J. 2004. Cyclophilin A retrotransposition into TRIM5 explains owl monkey resistance to HIV-1.Nature. 430:569–573 [DOI] [PubMed] [Google Scholar]
- Schulte-Pelkum J., Fritzler M., Mahler M. 2009. Latest update on the Ro/SS-A autoantibody system.Autoimmun. Rev. 8:632–637 [DOI] [PubMed] [Google Scholar]
- Shaw M.H., Boyartchuk V., Wong S., Karaghiosoff M., Ragimbeau J., Pellegrini S., Muller M., Dietrich W.F., Yap G.S. 2003. A natural mutation in the Tyk2 pseudokinase domain underlies altered susceptibility of B10.Q/J mice to infection and autoimmunity.Proc. Natl. Acad. Sci. USA. 100:11594–11599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M., Deng W., Bi E., Mao K., Ji Y., Lin G., Wu X., Tao Z., Li Z., Cai X., et al. 2008. TRIM30 alpha negatively regulates TLR-mediated NF-kappa B activation by targeting TAB2 and TAB3 for degradation.Nat. Immunol. 9:369–377 [DOI] [PubMed] [Google Scholar]
- Sigurdsson S., Nordmark G., Göring H.H., Lindroos K., Wiman A.C., Sturfelt G., Jönsen A., Rantapää-Dahlqvist S., Möller B., Kere J., et al. 2005. Polymorphisms in the tyrosine kinase 2 and interferon regulatory factor 5 genes are associated with systemic lupus erythematosus.Am. J. Hum. Genet. 76:528–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein A.J., Fuchs G., Fu C., Wolin S.L., Reinisch K.M. 2005. Structural insights into RNA quality control: the Ro autoantigen binds misfolded RNAs via its central cavity.Cell. 121:529–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetson D.B., Ko J.S., Heidmann T., Medzhitov R. 2008. Trex1 prevents cell-intrinsic initiation of autoimmunity.Cell. 134:587–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandberg L., Ambrosi A., Espinosa A., Ottosson L., Eloranta M.L., Zhou W., Elfving A., Greenfield E., Kuchroo V.K., Wahren-Herlenius M. 2008. Interferon-alpha induces up-regulation and nuclear translocation of the Ro52 autoantigen as detected by a panel of novel Ro52-specific monoclonal antibodies.J. Clin. Immunol. 28:220–231 [DOI] [PubMed] [Google Scholar]
- Stremlau M., Owens C.M., Perron M.J., Kiessling M., Autissier P., Sodroski J. 2004. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys.Nature. 427:848–853 [DOI] [PubMed] [Google Scholar]
- Tailor P., Tamura T., Ozato K. 2006. IRF family proteins and type I interferon induction in dendritic cells.Cell Res. 16:134–140 [DOI] [PubMed] [Google Scholar]
- Thibault D.L., Chu A.D., Graham K.L., Balboni I., Lee L.Y., Kohlmoos C., Landrigan A., Higgins J.P., Tibshirani R., Utz P.J. 2008. IRF9 and STAT1 are required for IgG autoantibody production and B cell expression of TLR7 in mice.J. Clin. Invest. 118:1417–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C.K., Lit L.C., Tam L.S., Li E.K., Wong P.T., Lam C.W. 2008. Hyperproduction of IL-23 and IL-17 in patients with systemic lupus erythematosus: implications for Th17-mediated inflammation in auto-immunity.Clin. Immunol. 127:385–393 [DOI] [PubMed] [Google Scholar]
- Xue D., Shi H., Smith J.D., Chen X., Noe D.A., Cedervall T., Yang D.D., Eynon E., Brash D.E., Kashgarian M., et al. 2003. A lupus-like syndrome develops in mice lacking the Ro 60-kDa protein, a major lupus autoantigen.Proc. Natl. Acad. Sci. USA. 100:7503–7508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimi R., Chang T.H., Wang H., Atsumi T., Morse H.C., III, Ozato K. 2009. Gene disruption study reveals a nonredundant role for TRIM21/Ro52 in NF-kappaB-dependent cytokine expression in fibroblasts.J. Immunol. 182:7527–7538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang H., Narain S., Sobel E., Lee P.Y., Nacionales D.C., Kelly K.M., Richards H.B., Segal M., Stewart C., Satoh M., Reeves W.H. 2005. Association of anti-nucleoprotein autoantibodies with upregulation of Type I interferon-inducible gene transcripts and dendritic cell maturation in systemic lupus erythematosus.Clin. Immunol. 117:238–250 [DOI] [PubMed] [Google Scholar]